Introduction
Skin flap transplantation is one of the most
commonly used, and effective methods to repair tissue defects and
reconstruct organs. However, the treatment is impeded due to the
occurrence of ischemia/reperfusion (I/R) injury in the process of
transplantation, which produces toxic compounds, including lipid
peroxide and reactive oxides. Thus, patients may require longer
hospitalization, multiple unexpected surgeries and increased costs
(1). A number of studies have
reported that I/R injury of skin flaps caused a series of complex
pathophysiological processes mediated by various cell types and
factors. The main mechanisms may involve the production of reactive
oxygen species (ROS), the adhesion and aggregation of neutrophils,
calcium overload, apoptosis and other processes (2–6).
Apoptosis is considered to be an important factor in I/R injury of
skin flaps (7). The mechanism of
I/R damage may involve the excessive production of oxygen free
radicals and the cross-links between proteins, resulting in
dysfunction of proteins with enzyme activity and altered gene
transcription (8,9).
Extracellular signal-regulated kinase (ERK) is an
important downstream mediator in the mitogen-activated kinase
(MAPK) pathway, with a crucial role in cellular signal transduction
pathways involved in cell proliferation, differentiation,
morphology, cytoskeleton construction, apoptosis, and
carcinogenesis (10,11). Previous research had demonstrated
that the ERK signaling pathway is closely associated with I/R
injury (12). Darling et al
(13) reported that the protective
effect of myocardial ischemia in the heart tissue of rabbits was
mediated by the ERK pathway, rather than the phosphoinositide
3-kinase pathway. Jung et al (14) reported that troglitazone induces
apoptosis of various types of cells, which may be linked to
inhibition of the ERK pathway. Therefore, I/R injury in skin flaps
may be mediated by the ERK pathway. Mitogen-activated protein
kinase phosphatase 1 (MKP-1), which is also termed dual specificity
phosphatase 1, is a specific negative regulator of the ERK pathway,
and can block substrate activity by promoting the serine/threonine
or tyrosine dephosphorylation (15).
The current treatment measures for I/R injury of
skin flaps include pretreatment of the skin flap, drug treatment
(growth factor, L-selection, calcium antagonists), biological
technology (antibodies, gene therapy) and sound wave therapy
(16). Drug treatments have the
best prospects for clinical application. The hormone estrogen has
received attention for its potential use in the therapy of I/R
injury. Currently, research findings have demonstrated that minute
quantities of estrogen can alleviate the I/R injury of skin flaps;
the effect may be mediated by NO production in vascular endothelial
cells, anti-oxygenation and anti-apoptosis (17). The underlying mechanism of
estradiol postconditioning protection remains incompletely
understood. In order to determine whether the application of
estradiol would be useful for the clinical treatment of I/R injury,
the current study was designed investigate the association between
estradiol, apoptosis and the ERK signaling pathway.
Materials and methods
Ethics
All experimental procedures and protocols used in
this investigation were reviewed and approved by the Animal Care
and Use Committee of the Experimental Animal Center of Soochow
University (Suzhou, China), and conformed to the Guide for the Care
and Use of Laboratory Animals published by the US National
Institutes of Health. The researchers that performed the animal
experiments, possessed qualified certification issued by the
Department of Science and Technology of Jiangsu Province.
Materials
Adult, male Wistar rats (250–350 g) were obtained
from the specific pathogen-free laboratory of the Experimental
Animal Center of Soochow University, were housed with 12/12 h
light/dark cycle in a temperature-controlled room and had free
access to food and water. Estradiol was purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). ERK1/2
rabbit monoclonal antibody mAb (cat. no. 4348S),
phospho-ERK1/2 rabbit mAb (cat. no. 4370S) and PD-98059
(cat. no. 9900L) were all obtained from Cell Signaling Technology,
Inc. (Danvers, MA, USA). MKP-1 antibody (cat. no. ab1351) was
obtained from Abcam (Cambridge, UK). Secondary antibodies were goat
anti-rabbit (cat. no. A0239), donkey anti-goat (cat. no. A0266),
goat anti-rat (cat. no. A0192) and GAPDH (cat. no. AG019) were all
obtained from Beyotime Institute of Biotechnology (Shanghai,
China). A terminal deoxynucleotidyl transferase dUTP nick end
labeling (TUNEL; cat. no. 11684795910) staining kit, which was used
to detect apoptotic index, was purchased from Roche Diagnostics
(Basel, Switzerland).
Animal model establishment
Wistar rats were anesthetized by intraperitoneal
injection of 2% pentobarbital sodium (20 ml/kg). The rats were put
into a supine position with the neck extended and the area was
shaved in preparation for the procedure. The operation area was
sterilized with iodophor and covered with sterile surgical towel.
As previously described by Manson et al (18), an axial pattern skin flap of 3×6 cm
in size was made of each rat, with the superficial artery and vein
in the abdominal wall acting as the pedicle. The blood flow of the
flap was blocked completely by a microvascular clip to clamp the
vessel pedicle for 6 h and the flap was sutured to their original
position with 5–0 stitches. During the period of the ischemia,
anesthesia with was maintained (2% pentobarbital sodium). After 6 h
of ischemia, the clamp was removed and the I/R injury model has
been established.
Experimental groups
Rats were fed to >250 g and divided randomly into
five groups (n=10 per group) as follows: Group I (control group),
after the flap was harvested, it was sutured to its own donor site
without clamps to reduce blood flow; Group II (I/R group), the I/R
injury model was established with no treatment; Group III (saline
group), the I/R injury model was established and normal saline (2
ml/kg) injected; Group IV (estradiol group), the I/R injury model
was established treated with estradiol (100 µg/kg) injected; Group
V (inhibitor group), I/R model rats were co-treated with estradiol
(100 µg/kg) and PD-98059 which may inhibit Mek-1, the upstream
protein of Erk (0.3 mg/kg). Rats of groups III–V were treated with
saline, estradiol, PD-98059 and estradiol by intraperitoneal
injection in the right lower abdomen after the surgery, and at day
2, 4 and 6 postoperatively.
Observation of the skin flap
The gross condition of the skin flap was observed at
days 2, 4 and 6 postoperatively. The color, hair growth, survival
area and the inflammation exudation of the flaps were monitored by
daily observation.
Determination of flap survival
Images of the flaps were captured using a digital
camera at 7 days postoperatively, and computerized planimetry was
performed using Image-Pro Plus v6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA). The necrotic area was recorder, which was
obviously different in color compared with the normal tissue. The
survival rate of the flap was obtained by dividing the survival
area of the skin flap by the total area of the flap.
Histology analysis
The rats were anesthetized at 7 days
postoperatively. A full-thickness skin sample of 0.5×0.5 cm in size
near the vascular pedicle was obtained and fixed in 10% formalin
overnight. The sections were dehydrated in alcohol, rehydrated in
xylene, and embedded in wax. 10 µm consecutive section, label for
each slice, and standby, a normal slice was manufactured. The
sections were stained with hematoxylin and eosin. The histological
characteristics of the tissues were evaluated under a light
microscope, 10 fields of view were captured per slice.
Apoptosis index
Apoptosis was determined by TUNEL assay in each rat.
The skin flap tissues were sectioned into 10 µm by using a
microtome. Sections were dewaxed in xylene for 10 min and
dehydrated in an ascending ethanol gradient. After preparation of
washing, the slices were soaked in the TUNEL reaction solution at
room temperature for 1 h. Subsequently, the slices were washed in
PBS for 3 times for 5 min, fixed in 4% paraformaldehyde for 15 min
and sealed by methanol containing 3% H2O2 for
10 min at room temperature. Finally, after being immersed in the
diaminobenzidine for 5 min, the sections were stained with
hematoxylin and observed under a light microscope. For each
TUNEL-stained section, three randomly selected high power fields
were imaged. Apoptotic nuclei were deeply brown stained. Three
random high-power fields were imaged from each section. Apoptotic
and non-apoptotic cells were identified by Image Pro Plus version
6.0 software (Media Cybernetics, Inc., Rockville, MD, USA). The
apoptotic index was equal to apoptotic cells divided by the total
cells.
Western blot analysis
At 7 days after the surgery, an area of the skin
flap near the vascular pedicle was harvested. The skin layer and
adipose tissue were removed, and the tissue was cut into pieces and
stored in a cryogenic refrigerator (−80°C). RIPA lysis buffer and
protease inhibitors were added to the tissues (100:1), and the
samples were incubated on ice, centrifuged at 12,000 × g for 10 min
at 4°C and the supernatant extracted 30 min after centrifugation.
Subsequently, the protein lysates (50 mg/lane) were loaded into 12%
SDS-PAGE gels (Beyotime Institute of Biotechnology), separated by
electrophoresis and transferred to a nitrocellulose membrane. The
membrane was blocked in 5% non-fat powdered milk at room
temperature with gentle shaking for 2 h and then washed in
TBS-Tween 20 three times for 10 min each. Subsequently, the
membrane was incubated with diluted primary antibody Erk (1:1,000;
cat. no. 4348S; Cell Signaling Technology, Inc.), p-Erk (1:1,000;
cat. no. 4370S; Cell Signaling Technology, Inc.), MKP-1 (1:1,000;
cat. no. ab1351; Abcam), GAPDH (1:1,000; cat. no. AG019; Beyotime
Institute of Biotechnology) in 5% BSA at 4°C overnight with gentle
shaking. The membrane was washed by TBS-Tween for three times for
10 min each, then incubated with secondary antibody (Erk, p-Erk
with goat anti-rabbit; 1:1,000; cat. no. A0239; Beyotime Institute
of Biotechnology; MKP-1 with donkey anti-goat; 1:1,000; cat. no.
A0266; Beyotime Institute of Biotechnology; GAPDH with goat
anti-rat; 1:1,000; cat. no. A0192; Beyotime Institute of
Biotechnology) for 1.5 h at room temperature. Finally, the proteins
were detected by the method of ECL color (BeyoECL Plus, cat. no.
P0018; Beyotime Institute of Biotechnology) and all the target
protein bands were analyzed using Image J software (National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All the statistics were analyzed with SPSS software
17.0 (SPSS, Inc., Chicago, IL, USA). Experimental results are
presented as the mean ± standard deviation. One-way analysis of
variance was used to determine the statistical difference among the
groups, and Levene test was used for the evaluation of homogeneity
of variances. The Tukey-Kramer test was used for pairwise
comparison and the Bonferroni test was used for multiple
comparisons. Pearson coefficient was used to analysis the flap
survival rate and apoptosis index correlation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Observation of the skin flap
Following the surgery to establish I/R, different
degrees of swelling were observed in the I/R skin flap model rats
and the skin appeared a violet color. Necrosis gradually emerged in
the skin flaps. Necrotic areas appeared that were black, hard and
had no hair growth in the I/R group and saline group. Compared to
the original skin, the thickness of skin flap of I/R group and
saline group increased significantly. However, the degree of
swelling gradually declined in the control group, estradiol group
and inhibitor group. Only a small are of necrosis appeared in the
suture edge and the skin flap had no obvious differences to the
normal skin in these three groups.
Flap survival rate determination
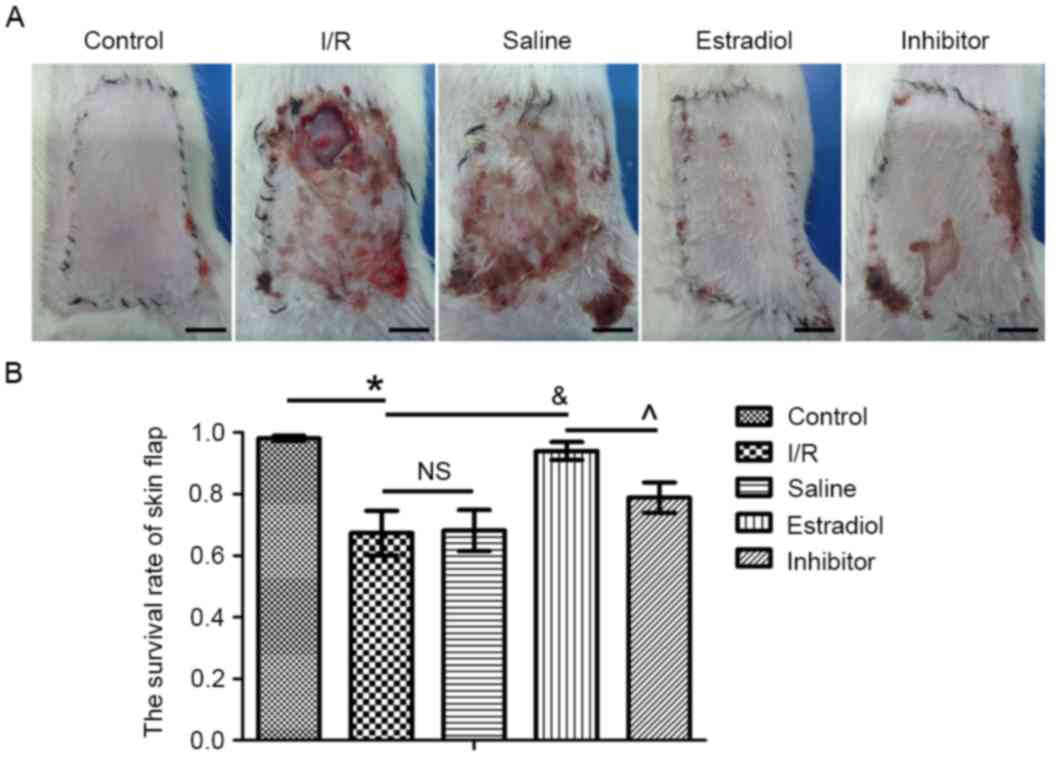
On postoperative day 7, the skin flaps were images
(Fig. 1A). Quantification of the
skin flap survival rate is presented in Fig. 1B. The survival rates of the flap
were 98.13±2.67% in the control group, 67.36±22.94% in the I/R
group, 68.19±21.11% in saline group, 94.05±9.27% in the estradiol
group and 78.87±15.65% in the inhibitor group. Compared with the
control group, the survival rate of the flap was significantly
decreased in the I/R group (P<0.05). There was no significant
change in the survival rate in the I/R skin flap model rats that
received saline (saline group) and those that were untreated (I/R
group; P>0.05). The survival rate of the estradiol group was
significantly higher than the I/R group (P<0.05). Additionally,
the survival rate was significantly higher in the estradiol group
than in the inhibitor group (P<0.05).
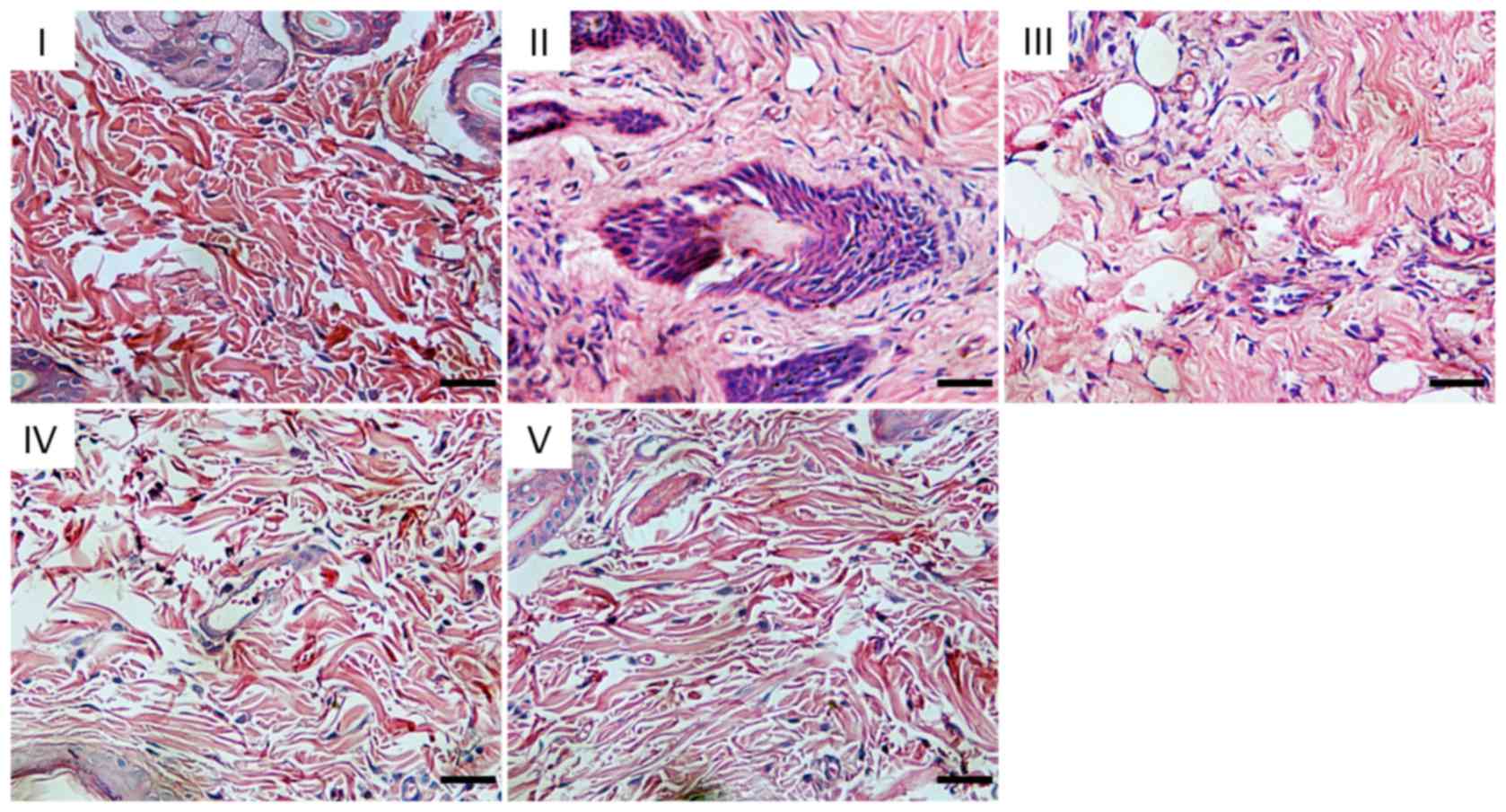
Histological analysis
At 7 day the I/R model was established, the tissue
structure of the control group was normal, with cells arranged
orderly and almost no observable infiltration of inflammatory
cells. The following features were observed in the I/R and saline
groups: Stratum corneum exfoliation, tissue edema, massive
neutrophil aggregation, adhesion, loss of normal tissue structure
and skin tissue necrosis. In the estradiol and inhibitor groups,
the flap tissue inflammation and edema were mild, and the tissue
structure was normal (Fig. 2).
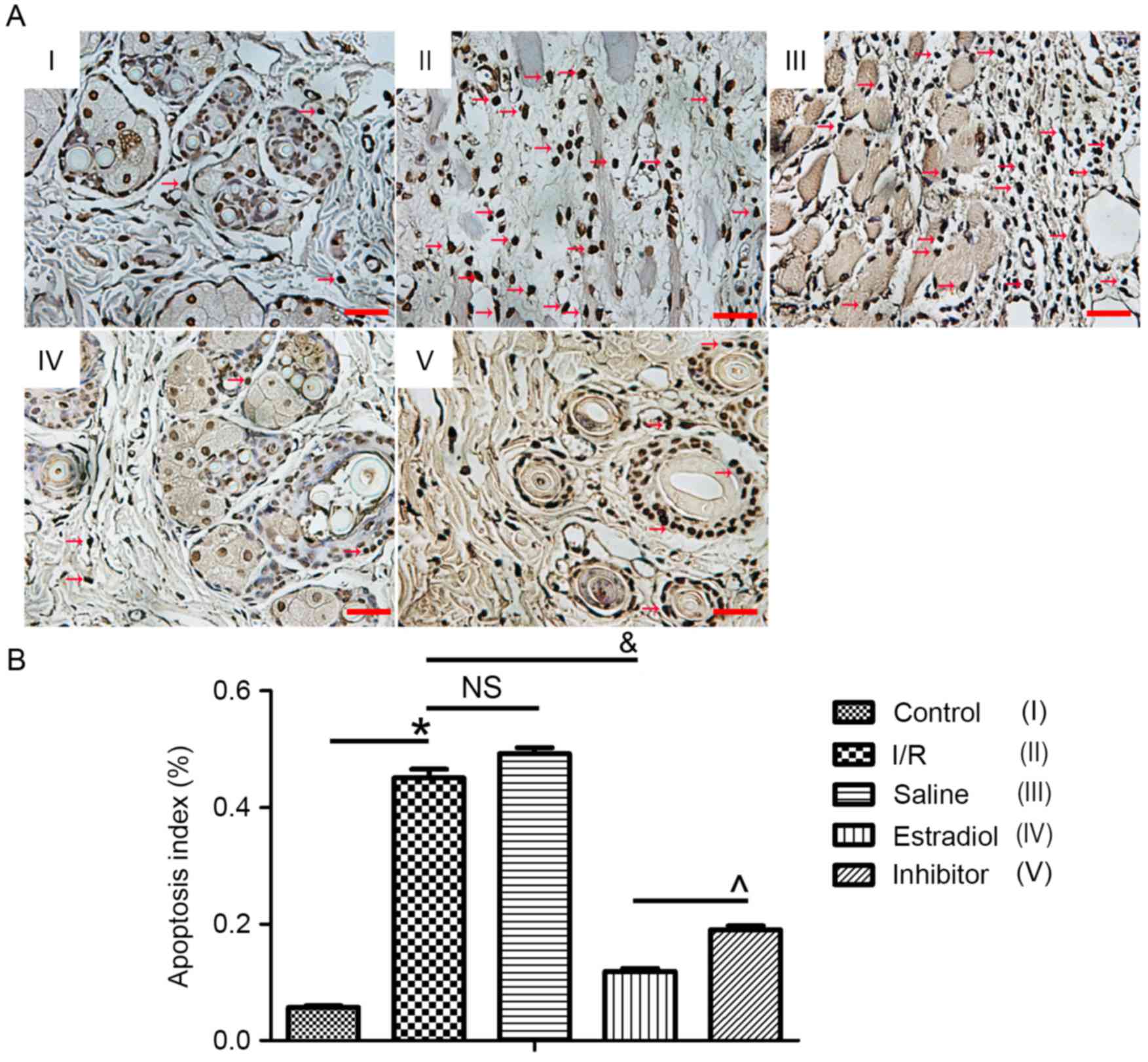
Apoptosis index
TUNEL staining was used to detect apoptosis and the
TUNEL-positive cells were stained brown. The apoptotic index was
5.69±1.475% in the control group, 45.02±8.128% in the I/R group,
49.18±5.801% in the saline group, 11.89±2.721% in the estradiol
group and 18.95±4.04% in the inhibitor group. Compared with the
control group, the apoptosis index was significantly increased in
flap sections from the I/R group (P<0.05). Compared with the
index in the I/R group, apoptosis was obviously decreased by
estradiol treatment, and furthermore, the ERK inhibitor PD-98059
partially eliminated the effect of estradiol (Fig. 3).
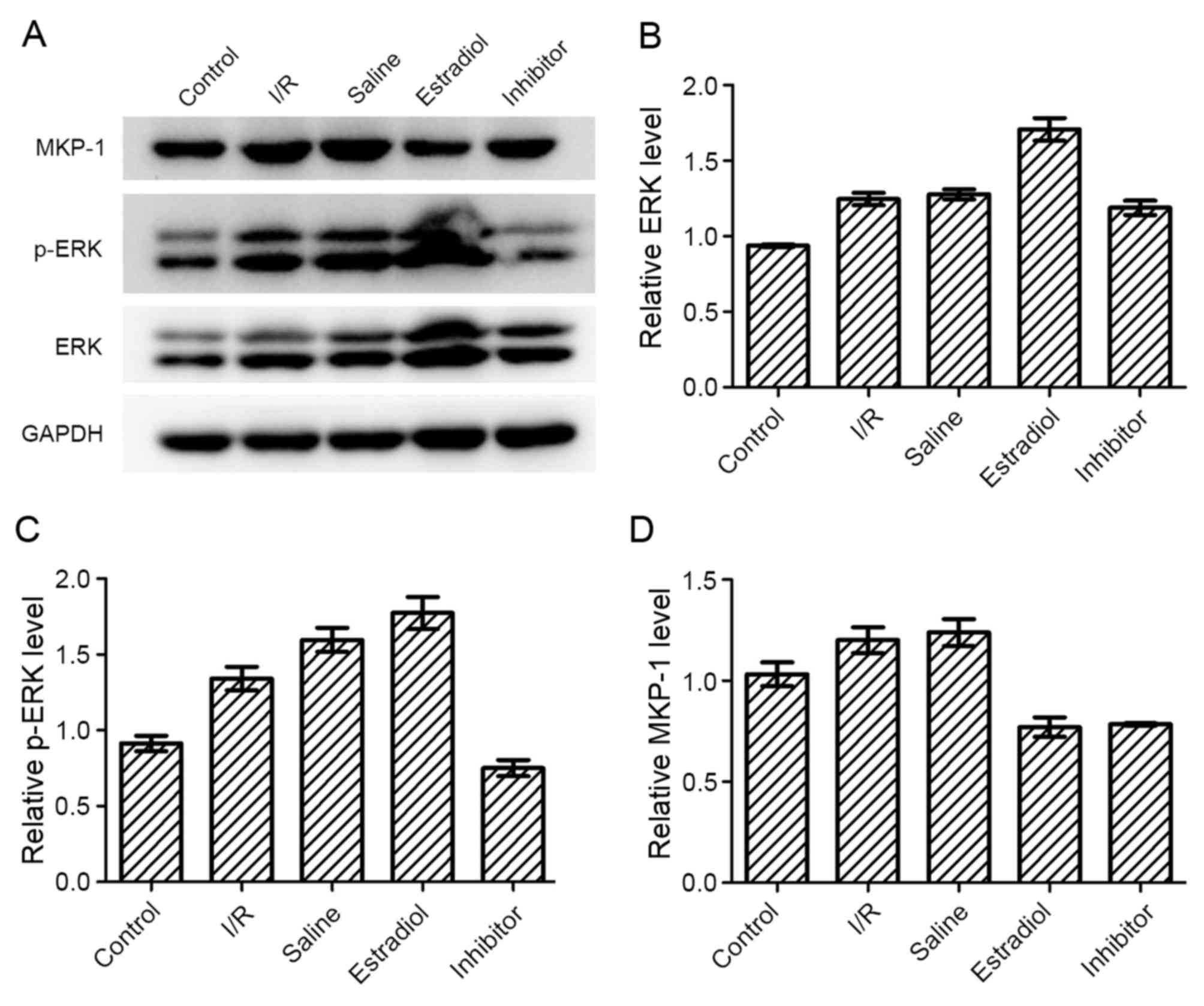
Western blot analysis
The levels of ERK pathway proteins, ERK, p-ERK and
MKP-1, were detected by western blotting. Compared with the I/R
group, the levels of ERK were increased in the estradiol group,
while the level of MKP-1 was decreased. PD98059 (inhibitor group)
inhibited the increased activation of ERK (phosphorylation), but
did not affect the protein expression of ERK and MKP-1.
Unexpectedly, the levels of ERK and MKP-1 in the I/R group and
saline group were marginally increased compared with the control
group, which may be cause by the stress reaction to I/R injury
(Fig. 4).
Correlation analysis
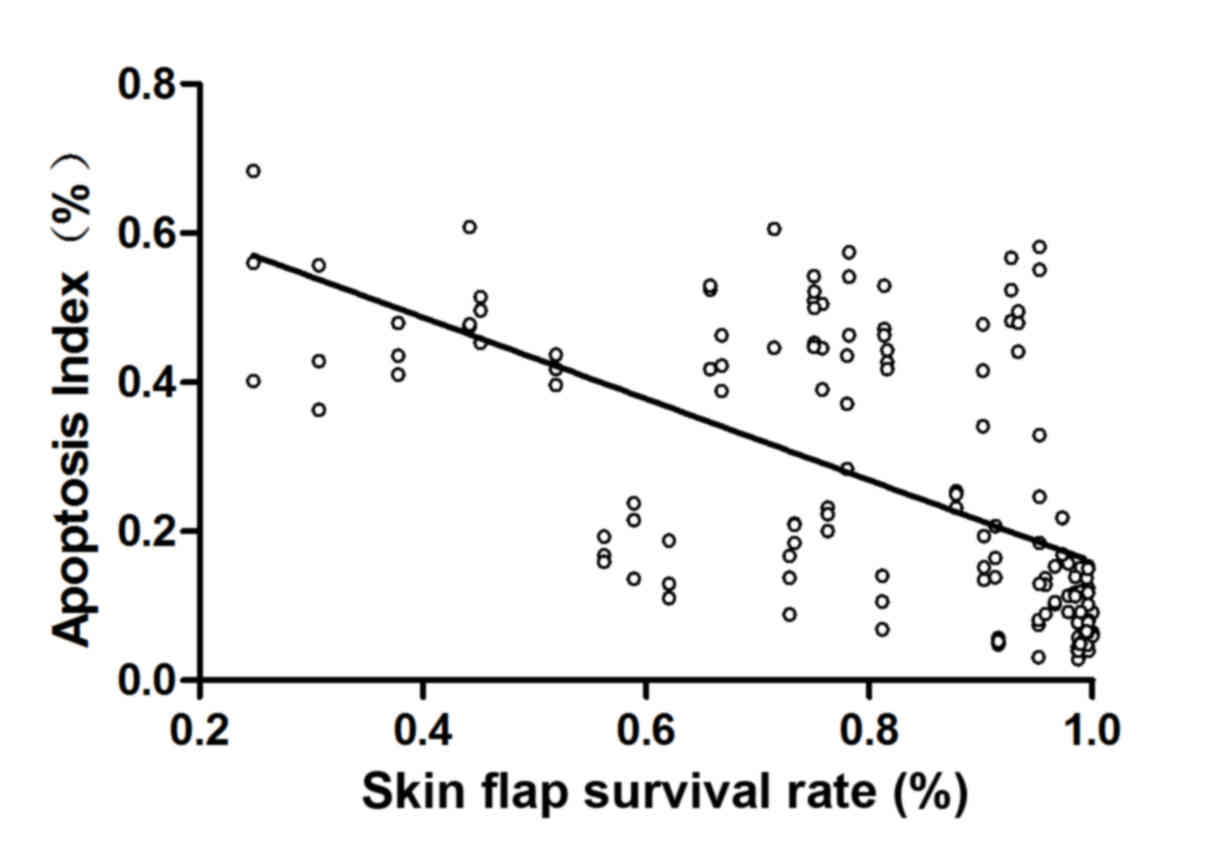
The Pearson coefficient of correlation analysis
(r=−0.595, P=0.01) showed a significant negative correlation
between apoptosis index and survival rate of skin flap (Fig. 5).
Discussion
The current study demonstrated that estradiol
therapy reduced the I/R injury of an axial flap. Additionally,
inhibition of ERK signaling partially abolished the effect of
estradiol, which suggested that the protective effect of estradiol
may be mediated through the ERK pathway. The findings also
demonstrated that apoptosis has an important role in I/R injury and
inhibition of apoptosis may be an important strategy for the
treatment of I/R injury.
I/R injury is the dysfunction and structural damage
to tissues or organs caused flowing the loss and recovery of blood
flow (19). A previous report
indicated that the necrosis rate in pedicle flaps following I/R
injury was 5–10% (20). Lipid
peroxides, reactive oxygen species, toxic substances and severe
inflammatory reaction in patients produced by I/R injury may
prolong the duration of patient hospitalization, increase the
number of operations require and increase treatment costs, which
directly influences the treatment effects of surgery and increases
the medical burden of patients (1). However, not all of the ischemic
tissue or organs may be damaged after the recovery of blood flow.
Various factors affect the occurrence and development of I/R
injury, including ischemia duration, collateral circulation, oxygen
demand and reperfusion conditions (21). The process of skin flap I/R injury
involves a variety of mechanisms to cooperate with each other,
which forms a series of linked cascade reaction, resulting in the
increased I/R injury and the tissue damage of the skin flap. If the
chain reaction is not terminated in a timely manner, the survival
rate of the flap is greatly threatened. Therefore, how to use
multifactor interventions or drug preconditioning methods to
terminate or inhibit certain pathways involved in I/R injury is
currently a focus of basic and clinical research in this area.
Estrogen, a female hormone, not only dominates the
development and maintenance of female reproductive organs and
secondary sex characteristic, but also promotes the retention of
water and sodium, the deposition of calcium in bones, and
influences the endocrine system, cardiovascular system, metabolism
and bone growth. Previous research has demonstrated that estrogen
reduces I/R injury in the brain, heart and liver (22–24).
Furthermore, estrogen may to improve the level of NO and increase
the survival area of random skin flaps (25). In the current study, inflammation
was reduced, apoptosis was inhibited and the survival area of the
axial flap was increased in the rats that were treated with
estradiol. The results suggested that estradiol had the similar
effect in the axial flap as in previous studies. Thus, similar
experiments should be performed applying estrogen to free
flaps.
Apoptosis is an independent, orderly and natural
death process. The occurrence of apoptosis in tissues following I/R
is associated with the severity of ischemia and reperfusion time.
Numerous reports have demonstrated that apoptosis is mediated by
multiple mechanisms during I/R injury (26–28).
ROS generated in the I/R-injured area may damage the DNA and
mitochondria, and induce the lipid peroxidation of the cell
membrane, which affects signal transduction. Additionally, protein
cross-linking causes reduce enzyme activities and altered gene
transcription, thus resulting in cell apoptosis. Furthermore,
inactivation of Bcl-2 protein is induced by calcium overload and a
variety of Ca2+-dependent endonucleases that induce the
degradation of DNA, activate tumor necrosis factor signal
transduction pathway, mediate the p53 pathway and promote the
apoptosis (29). An association
between apoptosis and the survival rate of the axial flap was
observed in the current study. Almost no hair growth was observed
on the flap surface in groups that had high levels of apoptosis
after I/R injury, which was detected by TUNEL. Skin ulceration and
necrosis were observed at the flap edge following I/R injury.
However, less apoptosis was detected in the flaps of rats that had
receive estradiol injection, and the hair growth on its surface was
close to normal, suggesting that estradiol reduces the apoptosis
induced by I/R injury in skin flaps.
With the development of molecular biology, more
research on estradiol has provided a deeper understanding of the
signaling pathway. It was previously reported that estrogen
stimulation causes rapid and transient activation of the MAPK
protein kinase, that occurred within a few min (30). Another study reported that the
estradiol receptor is closely associated with the activity of p38
MAPK, and estradiol can regulate the inflammatory response by
regulating the activity of the p38 pathway (31). In brain astrocytes, osteoblasts and
glioma cells, ERK signaling is rapidly activated by estradiol
(32,33). By complaining between the control
group and the other I/R groups, the present demonstrated that the
ERK pathway was activated in this experiment. The level of ERK also
was increased by estradiol, which had a direct impact on the
survival rate of skin flap. In addition, when the ERK pathway was
inhibited by PD98059, the survival rate of skin flap was partially
reduced and the apoptosis rate was increased. It demonstrated that
ERK pathway has an important role in the effect of estradiol.
It has also been reported that an imbalance in
expression between MKP-1 and ERK may be one of the important
mechanisms that mediate the occurrence and development of tumors
(34). Serini et al
(35) reported that the mechanism
of apoptosis in lung cancer cells induced by docosahexaenoic acid
was associated with the increased expression of MKP-1 and
downregulation of the ERK pathway. The expression of MKP-1 in the
I/R group and saline group were increased in the current model,
which may be caused by the stress of I/R injury. Whereas the
expression of MKP-1 in the estradiol group was reduced compared
with the other three groups, and the levels of ERK and p-ERK were
increased, which indicates that the effect of estrogen on ERK
pathway may be mediated through MKP-1.
In summary, the findings of the current study
suggest that estradiol reduces I/R injury in an axial skin flap. It
was also demonstrated that the ERK pathway and apoptosis have
important roles in the effect of estradiol in this model. However,
inhibition of the ERK pathway combined with estradiol treatment
only partially reduced the effect of estradiol on the survival rate
of the flap, indicating that there are other factors and pathways
involved in the effect of estradiol on the survival the skin flap
in this model. Further studies are required to determine the
precise mechanisms, and to provide novel methods for clinical
treatment of I/R injury.
Acknowledgements
We would like to thank Mrs Jing Wang (Laboratory
Animal Center, Soochow University, Suzhou, China) for supplying the
experimental animals, and Dr Shiliang Wu (Molecular and Biochemical
Institute, Soochow University) for his technical guidance.
References
|
1
|
Rand-Luby L, Pommier RF, Williams ST,
Woltering EA, Small KA and Fletcher WS: Improved outcome of
surgical flaps treated with topical dimethylsulfoxide. Ann Surg.
224:583–590. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ozmen S, Ayhan S, Demir Y, Siemionow M and
Atabay K: Impact of gradual blood flow increase on
ischaemia-reperfusion injury in the rat cremaster microcirculation
model. J Plast Reconstr Aesthet Surg. 61:939–948. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cetin C, Kse AA, Aral E, Colak O, Erçel C,
Karabağli Y, Alataş O and Eker A: Protective effect of fucoidin (a
neutrophil rolling inhibitor) on ischemia reperfusion injury:
Experimental study in rat epigastric island flaps. Ann Plast Surg.
47:540–546. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Molski M, Groth A, Allison AC, Hendrickson
M and Siemionow M: Diannexin treatment decreases
ischemia-reperfusion injury at the endothelial cell level of the
microvascular bed in muscle flaps. Ann Plast Surg. 63:564–571.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu X, Xu W and Jiang H: HMGB1/IL-17A axis:
An important mechanism for myocardial ischemia-reperfusion injury.
Int J Cardiol. 174:447–448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen B, Zhou S, He Y, Zhao H, Mei M and Wu
X: Revealing the underlying mechanism of ischemia reperfusion
injury using bioinformatics approach. Kidney Blood Press Res.
38:99–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Burns AT, Davies DR, McLaren AJ, Cerundolo
L, Morris PJ and Fuggle SV: Apoptosis in ischemia/reperfusion
injury of human renal allografts. Transplantation. 66:872–876.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rossing L, Haendeler J, Mallat Z, Hugel B,
Freyssinet JM, Tedgui A, Dimmeler S and Zeiher AM: Congestive heart
failure induces endothelial cell apoptosis: Protective role of
carvedilol Journal. Am Coll Cardiol. 36:2081–2089. 2003. View Article : Google Scholar
|
|
9
|
Messmer UK, Briner VA and Pfeilschifter J:
Basic fibroblast growth factor selectively enhance TNF-alpha
induced apoptotic cell death in glomerular endothelial cells:
Effects on apoptotic signaling pathways. J Am Soc Nephrol.
11:2199–2211. 2000.PubMed/NCBI
|
|
10
|
Chong H, Vikis HG and Guan KL: Mechanisms
of regulating the Raf kinase family. Cell Signal. 15:463–469. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mercer KE and Pritchard CA: Raf proteins
and cancer: B-Raf is identified as a mutational target. Biochim
Biophys Acta. 1653:25–40. 2003.PubMed/NCBI
|
|
12
|
Abe J, Baines CP and Berk BC: Role of
mitogen-activated protein kinases in ischemia and reperfusion
injury: The good and the bad. Circ Res. 86:607–609. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Darling CE, Jiang R, Maynard M, Whittaker
P, Vinten-Johansen J and Przyklenk K: Postconditioning via
stuttering reperfusion limits myocardial infarct size in rabbit
hearts: Role of ERK1/2. Am J Physiol Heart Circ Physiol.
289:1618–1626. 2005. View Article : Google Scholar
|
|
14
|
Jung JY, Yoo CI, Kim HT, Kwon CH, Park JY
and Kim YK: Role of mitogen-activated protein kinase (MAPK) in
troglitazone-induced osteoblastic cell death. Toxicology.
234:73–82. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nunes-Xavier C, Romá-Mateo C, Ríos P,
Tárrega C, Cejudo-Marín R, Tabernero L and Pulido R:
Dual-specificity MAP kinase phosphatases as targets of cancer
treatment. Anticancer Agents Med Chem. 11:109–132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Diederich RS, Mowlavi A, Meldrum G,
Medling B, Bueno RA and Neumeister MW: Local cooling provides
muscle flaps protection from ischemia-reperfusion injury in the
event of venous occlusion during the early reperfusion period. Hand
(N Y). 4:19–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Z, Yuhanna IS, Galcheva-Gargova Z,
Karas RH, Mendelsohn ME and Shaul PW: Estrogen receptor alpha
mediates the nongenomic activation of endothelial nitric oxide
synthase by estrogen. J Clin Invest. 102:401–406. 1999. View Article : Google Scholar
|
|
18
|
Manson GF, Anthenelli RM, Im MJ, Bulkley
GB and Hoopes JE: The role of oxygen-free radicals in ischemia
tissue injury in island skin flaps. Ann Surg. 198:87–90. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zingarelli B: Ischemia-reperfusion
injuryWheeler DS, Wong HR and Shanley TP: Science and Practice of
Pediatric Critical Care Medicine. London: Springer-Verlag; pp.
181–192. 2009
|
|
20
|
Harder Y, Amon M, Laschke MW, Schramm R,
Rücker M, Wettstein R, Bastiaanse J, Frick A, Machens HG, Küntscher
M, et al: An old dream revitalised: Preconditioning strategies to
protect surgical flaps from critical ischaemia and
ischaemia-reperfusion injury. J Plast Reconstr Aesthet Surg.
61:503–511. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Montalvo-Jave EE, Escalante-Tattersfield
T, Ortega-Salgado JA, Piña E and Geller DA: Factors in the
pathophysiology of the liver ischemia-reperfusion injury. J Surg
Res. 147:153–159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu HL, Baughman VL and Pelligrino DA:
Estrogen replacement treatment in diabetic ovariectomized female
rats potentiates poetischemic leukocyte adhesion in cerebral
venules. Stroke. 35:1974–1978. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carswell HV, Dominiczak AF and Macrae IM:
Estrogen status affects sensitivity to focal cerebral ischemia in
stroke-prone spontaneously hypertensive rats. Am J Physiol Heart
Cire Physiol. 278:290–294. 2000.
|
|
24
|
Shen SQ, Zhang Y and Xiong CL: The
protective effects of 17beta-estradiol on hepatic
ischemia-reperfusion injury in rat model, associated with
regulation of heat-shock protein expression. J Surg Res. 140:67–76.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shafighi M, Fathi AR, Brun C, Huemer GM,
Wirth R, Hunger R, Banic A and Constantinescu MA: Topical
application of 17β-estradiol (E2) improves skin flap survival
through activation of endothelial nitric oxide synthase in rats.
Wound Repair Regen. 20:740–747. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jia D, Han B, Yang S and Zhao J: Anemonin
alleviates nerve injury after cerebral ischemia and reperfusion
(i/r) in rats by improving antioxidant activities and inhibiting
apoptosis pathway. J Mol Neurosci. 53:271–279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koshinuma S, Miyamae M, Kaneda K, Kotani J
and Figueredo VM: Combination of necroptosis and apoptosis
inhibition enhances cardioprotection against myocardial
ischemia-reperfusion injury. J Anesth. 28:235–241. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tao T, Chen F, Bo L, Xie Q, Yi W, Zou Y,
Hu B, Li J and Deng X: Ginsenoside Rg1 protects mouse liver against
ischemia-reperfusion injury through anti-inflammatory and
anti-apoptosis properties. J Surg Res. 191:231–238. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Scarabelli TM and Gottlieb RA: Functional
and clinical repercussions of myocyte apoptosis in the multifaceted
damage by ischemia/reperfusion injury: Old and new concepts after
10 years of contributions. Cell Death Differ. 11 Suppl 2:S144–S152.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Collins P and Webb C: Estrogen hits the
surface. Nat Med. 5:1130–1131. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bhatt S, Xiao Z, Meng Z and
Katzenellenbogen BS: Phosphorylation by p38 mitogen-activated
protein kinase promotes estrogen receptor a tumor and functional
activity via the SCF (Skp2) proteasomal complex. Mol Cell Biol.
32:1928–1943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ivanova TV, Ivanov VN and Nadezhdina ES:
Transcription factors NFkappaB and AP-1/c-fos incell response to
nocodazol. Membr Cell Biol. 14:727–741. 2001.PubMed/NCBI
|
|
33
|
Endoh H, Sasaki H, Maruyama K, Takeyama K,
Waga I, Shimizu T, Kato S and Kawashima H: Rapid activation of MAP
kinase by estrogen in the bone cell line. Biochem Biophys Res
Commun. 235:99–102. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guo X, Zhang X, Li Y, Guo Y, Wang J, Li Y,
Shen B, Sun D and Zhang J: Nocodazole increases the ERK activity to
enhance MKP-1 expression which inhibits p38 activation induced by
TNF-α. Mol Cell Biochem. 364:373–380. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Serini S, Trombino S, Oliva F, Piccioni E,
Monego G, Resci F, Boninsegna A, Picci N, Ranelletti FO and
Calviello G: Docosahexaenoic acid induces apoptosis in lung cancer
cells by increasing MKP-1 and down-regulating p-ERK1/2 and p-p38
expression. Apoptosis. 13:1172–1183. 2008. View Article : Google Scholar : PubMed/NCBI
|