Introduction
Colorectal carcinoma (CRC) is one of the most common
types of human malignancy and a leading cause of cancer-associated
mortality in developed countries (1). Identifying strategies to fight
against CRC is important, as it is an emerging health problem. It
is now well documented that phytochemicals, including flavonoids,
alkaloids and polysaccharides, are important in anticancer therapy
(2,3).
Zanthoxylum bungeanum, also known as Sichuan
pepper, is a common Chinese culinary herb, growing widely in the
Sichuan, Shaanxi, Shandong and Hebei provinces of China. Z.
bungeanum leaves are generally used as a vegetable (4). Previous studies have reported on
Z. bungeanum leaves, in which its antioxidant (5–7),
antimicrobial (8), antithrombotic
(9) and lipid
metabolism-regulating effects (10) were evaluated. However, there has
been limited focus on the anticancer activity of the components of
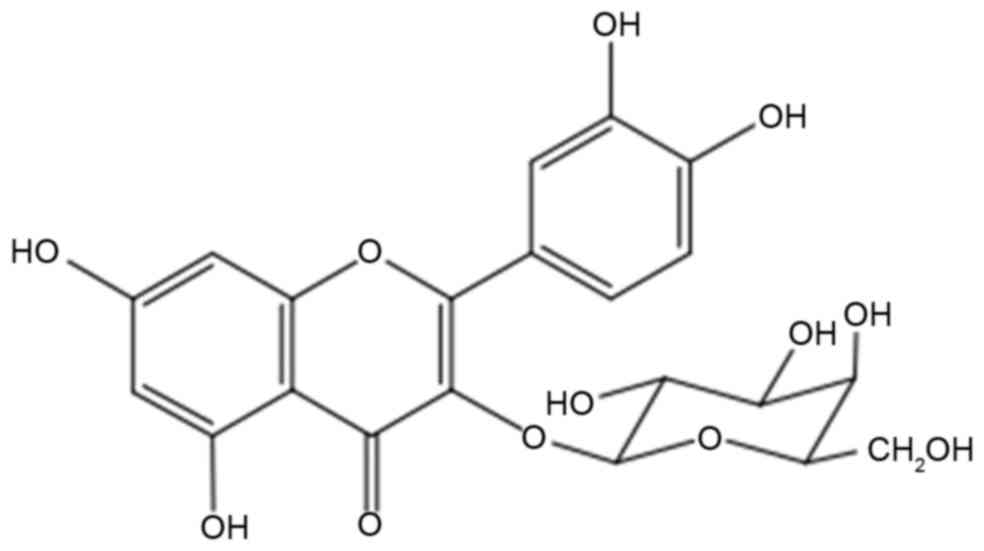
Z. bungeanum leaves. Hyperoside (quercetin-3-O-galactoside;
Fig. 1) is one of the primary
flavonoid compounds in Z. bungeanum leaves. In addition to
its potent efficacy for pain relief, anti-inflammatory and
antioxidant activity, and its potential for protecting the
cardiovascular system (11–13),
hyperoside shows antitumor activity in several tumor models,
including RL952 endometrial carcinoma cells (14), A549 lung adenocarcinoma cells
(15,16), 786-O renal cancer cells (17) and PC3 prostate cancer cells
(12). However, the molecular
mechanisms underlying hyperoside-induced growth inhibition and
apoptosis of CRC cells remain to be fully elucidated.
A number of flavonoids have been reported to exert
pro-oxidant actions, which may be an important mechanism underlying
their anticancer and apoptosis-inducing properties (18). It is well documented that
flavonoids generate reactive oxygen species (ROS) in cancer cells.
Enhanced ROS production leads to the disruption of cellular
antioxidant defense systems and the release of cytochrome c
from the mitochondria to the cytosol, resulting in apoptotic cell
death (19). Tumor cells with
higher levels of ROS are more susceptible to cell death, compared
with normal cells with lower levels of ROS. Therefore, novel
anticancer drugs may have high potential in promoting the
production of ROS. Accumulating evidence suggests that the tumor
suppressor, p53, is central to the process of ROS-mediated
apoptosis (20). In the present
study, hyperoside was isolated from Z. bungeanum leaves
(HZL) and its effect on caspase-dependent apoptosis and p53
signaling pathways in human SW620 CRC cells was investigated. The
results demonstrated that hyperoside induced cell cycle G2/M phase
arrest and apoptosis, which was associated with an increase in the
levels of p53, p21, B-cell lymphoma 2 (Bcl-2)-associated X protein
(Bax), cytochrome c, caspase-9, caspase-3 and apoptotic
protease activating factor 1 (Apaf-1). The antitumor potency of HZL
was also attributed to increasing ROS levels, a reduction in
mitochondrial membrane potential (∆Ψm), and
downregulation of the mRNA expression of glutathione peroxidase
(GSH-Px) and catalase (CAT).
Materials and methods
Materials
The leaves of Z. bungeanum were harvested in
Shaanxi, China in August 2015 and were identified according to the
identification standards of the Pharmacopeia of the People's
Republic of China (21).
Dulbecco's modified Eagle's medium (DMEM), dimethyl sulfoxide
(DMSO), 3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyltetrazolium
bromide (MTT) and 2′,7′-dichlorofluorescin diacetate (DCFH-DA) were
obtained from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Fetal
bovine serum (FBS) was purchased from Gibco; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA).
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl
carbocyanine iodide (JC-1) was purchased from Beyotime Institute of
Biotechnology (Shanghai, China). The primers for GSH-Px and CAT
were designed and synthesized by Takara Biotechnology Co., Ltd.
(Dalian, China). Reagents, including enzymes, cofactors and
nucleotides for internal standard construction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis, were obtained from Takara Biotechnology Co., Ltd.
Preparation of HZL
Z. bungeanum leaves (500 g) were soaked in
70% ethanol (1:10, w/v) for 2.5 h and were sonicated in an
ultrasonic bath at 200 kHz at 55°C for 45 min. The samples were
filtered, concentrated and then dried using a rotary evaporator.
The dried crude extract was added to distilled water and defatted
with petroleum ether. The residue was diluted in H2O and
extracted using ethyl acetate in triplicate. The resulting
fractions were chromatographed over acetonitrile-0.2% acetic acid
(15:85, v/v). The chemical identity of hyperoside was confirmed
using reverse phase high performance liquid chromatography by
comparison with authentic hyperoside (National Institute for the
Control of Pharmaceutical and Biological Products, Beijing,
China).
The hyperoside was dissolved in DMSO immediately
prior to use, and the final concentration of DMSO was <0.1%
(v/v) in all experiments. The concentrations of hyperoside ranged
between 12.5 and 50 µM. DMSO at 0.1% was used as a control. All
determinations were performed in triplicate.
Cell culture and treatment
SW620 cells from the American Type Culture
Collection (ATCC; Manassas, VA, USA) were maintained in DMEM
supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin and
100 µg/ml streptomycin (Thermo Fisher Scientific, Inc.) in a
humidified 5% CO2 incubator at 37°C. The medium was
replaced every 2 days. The SW620 cells were cultured in 24- or
96-well plates at a density of 1–2×105 cells/ml and were grown to
~70% confluence.
Cell viability
The cell survival rate was quantified using a
colorimetric MTT assay. MTT was prepared at 2.5 mg/ml in
phosphate-buffered saline (PBS). Briefly, 20 µl aliquots of the MTT
stock solution were pipetted into each well, and the plates were
incubated at 37°C in a humidified 5% CO2 incubator.
After 4 h, the medium was removed and DMSO (200 µl) was added to
each well to dissolve the formazan. The optical density of each
well was measured 10 min later at 570 nm using a spectrophotometer
(Tecan Infinite M200 Pro; Tecan Group Ltd., Männedorf,
Switzerland).
Flow cytometric analysis for cell
cycle phase determination
Cell suspensions (0.5–1×105/ml) were prepared by
trypsinization and washed twice with PBS, followed by
centrifugation (200 × g) for 5 min at 4°C. The cells were fixed
with 70% ethanol at 4°C and resuspended in PBS, which contained
0.25 mg/ml of RNaseA (Thermo Fisher Scientific, Inc.). The
suspension was incubated for 30 min at 37°C, following which the
cells were labeled with 50 µg/ml propidium iodide (PI;
Sigma-Aldrich; Merck KGaA). The total DNA content was quantified by
fluorescence using a FACS flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA).
Flow cytometric analysis of
apoptosis
The prepared SW620 cells (1×106/ml) were washed
twice with ice-cold PBS and gently resuspended in 500 µl of binding
buffer. Subsequently, the cells were stained with 5 µl Annexin
V-FITC and agitated well. Finally, 5 µl PI was added to the cells,
incubated for 20 min in the dark and then analyzed using a FACS
flow cytometer (BD Biosciences).
Western blot analysis
Cell lysates were prepared from confluent 6-well
plates (Nalge Nunc International, Penfield, NY, USA) by scraping
into lysis buffer [0.05 M Tris-HCl, 10% (v/v) glycerol, 2% (w/v)
SDS]. The total protein content of cell lysates was determined by
the Bradford method (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Cell lysates (30 µg of total protein) were analyzed using
8–12% gradient SDS-PAGE. The proteins were transferred onto an
immunoblot PVDF membrane (EMD Millipore, Billerica, MA, USA), which
was blocked with 5% skimmed milk in TBS containing 0.1% Tween-20
(TBS-T) at room temperature for 1 h, and incubated overnight at 4°C
with the following primary antibodies: Rabbit anti-p53 (4667;
1:1,000), anti-p21 (2947; 1:2,000), anti-Bax (2772s; 1:1,000),
anti-cytochrome c (4280s; 1:1,000), anti-Apaf-1 (8969s; 1:1,000),
anti-caspase-9 (9508; 1:1,000), or anti-caspase-3 (9662s; 1:1,000)
(all from Cell Signaling Technology, Inc., Danvers, MA, USA). The
membranes were then washed with TBS-T buffer, incubated with the
horseradish peroxidase (HRP)-conjugated anti-mouse IgG (14709;
1:1,000; Cell Signaling Technology, Inc.) for 1 h at room
temperature, and enhanced using a chemiluminescence ECL assay kit
(Amersham; GE Healthcare Life Sciences, Chalfont, UK) according to
the manufacturer's protocol. Images of chemiluminescence were
captured using a Fujifilm LAS-3000 system (Fujifilm, Tokyo, Japan).
The basal levels of proteins were normalized to the protein level
of β-actin.
Measurement of ΔΨm
The ∆Ψm of the cells was analyzed using
fluorescence spectrophotometry using JC-1 dye. Briefly, the SW620
cells were stained with 5 mM JC-1 at 37°C for 20 min in a 5%
CO2 atmosphere. The cells were pelleted by
centrifugation (1,000 × g for 5 min at 4°C) and resuspended in PBS.
The JC-1 fluorescence of the cell suspensions and PBS controls were
measured in triplicate in Costar 96-well plates using a microplate
reader (Tecan Infinite M200 Pro; Tecan Group Ltd.). Ex/Em
(green)/Em (red) =485/538/590 nm; FL2/FL1 ratios were calculated
based on the fluorescence data. Each well was scanned by measuring
the intensity of each 25-square grid (1 mm2 area), which
was arranged in a 5×5 rectangular array (bottom scanning). A higher
red-to-green ratio indicated a more polarized or more negative and
hyperpolarized mitochondrial inner membrane.
Measurement of intracellular ROS
levels
The cellular ROS levels were measured using a
dichlorofluorescein assay. The cells were collected and incubated
with 100 µM DCF-DA (dissolved in DMSO) for 30 min at 37°C.
Subsequently, the cells were washed three times with PBS (pH 7.4)
and the relative levels of fluorescence were quantified in a
spectrophotofluorimeter (Tecan Infinite M200 Pro; Tecan Group,
Ltd.). The measured fluorescence values were expressed as a
percentage of the fluorescence in the control cells.
RT-qPCR analysis
The cells were washed with ice-cold PBS, and RNA was
extracted using TRIzol reagent (Bioteke Corporation, Beijing,
China) according to the manufacturer's protocol. cDNA was
synthesized from mRNA using a PrimeScript™ RT reagent kit (Takara
Biotechnology Co., Ltd.), followed by qPCR using a SYBR Premix Ex
Taq™ reagent kit (Takara Biotechnology Co., Ltd.) and the ABI Prism
7500 sequence detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The reactions were performed under the following
cycling conditions: 95°C for 30 sec, followed by 40 cycles of 95°C
for 5 sec and 56°C for 30 sec. The housekeeping gene β-actin was
used for normalization. All experiments were repeated at least
three times. The relative changes in gene expression were analyzed
using the 2−ΔΔCq method (22). The sequences for the RT-qPCR
primers were as follows: GSH-Px forward,
5′-CCTCTAAACCTACGAGGGAGGAA-3′ and reverse,
5′-GGGAAACTCGCCTTGGTCT-3′; CAT forward,
5′-TCCAAGGCAAAGGTATTTGAGCA-3′ and reverse,
5′-CAACGAGATCCCAGTTACCATCTTC-3′; β-actin forward,
5′-CATCCGTAAAGACCTCTATGCCAAC-3′ and reverse,
5′-ATGGAGCCACCGATCCACA-3′.
Statistical analysis
All data are expressed as the mean ± standard
deviation of at least three independent determinations for each
experiment. Statistical analyses were performed using SPSS version
13.0 (SPSS, Inc., Chicago, IL, USA). One-way analysis of variance
with Duncan's multiple range test was used to examine differences
between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of HZL on SW620 cell growth
inhibition
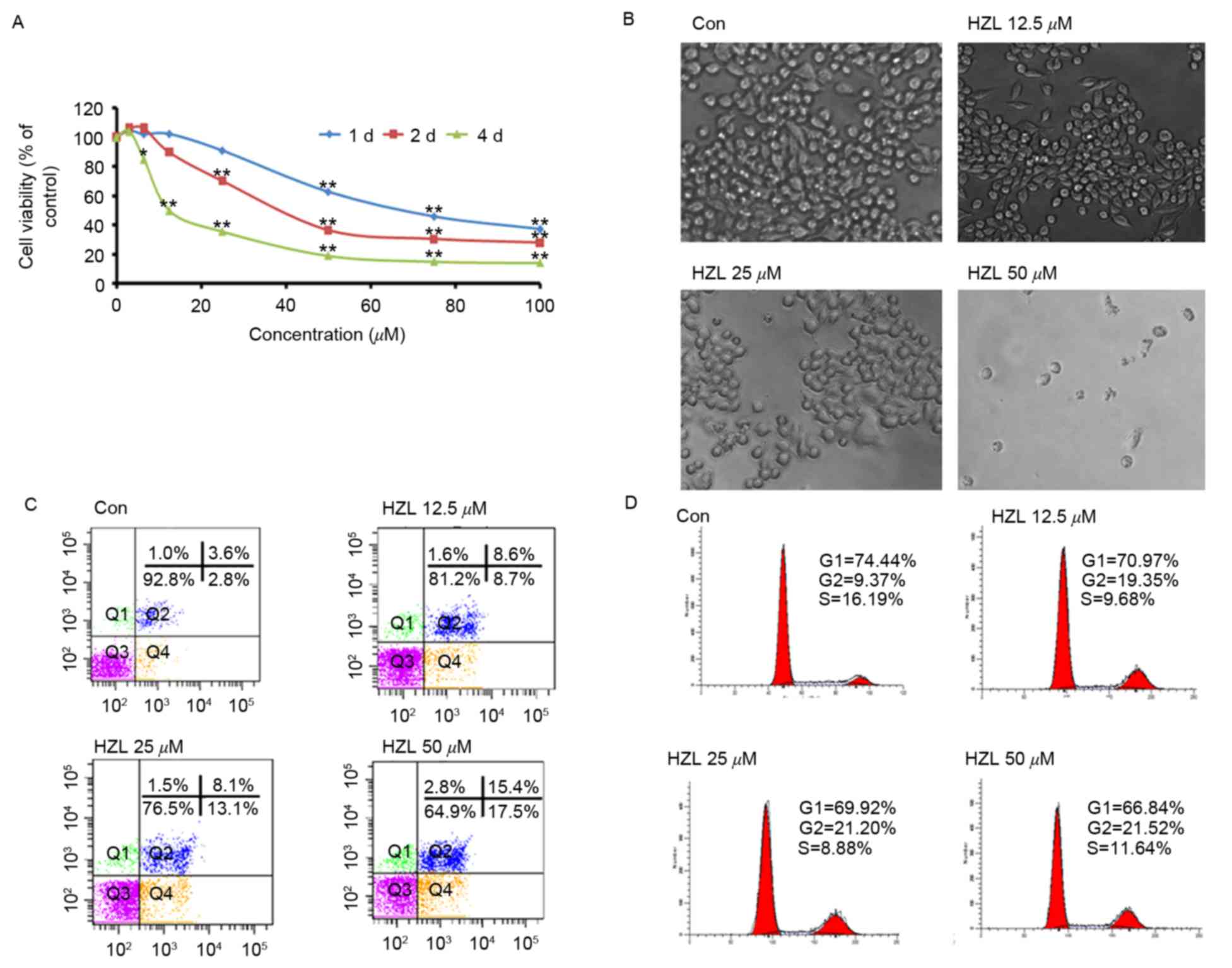
The growth inhibiting effect of HZL was assessed
using an MTT assay and microscopic analysis, as demonstrated in
Fig. 2A. Time- and
concentration-dependent growth inhibition was observed in the SW620
human CRC cell line. Following treatment with HZL for 24, 48 and 96
h, the half maximal inhibitory concentration values were
72.35±5.46, 36.41±1.36 and 19.51±4.95 µM, respectively. The
viabilities of cells treated with HZL (12.5, 25 and 50 µM) for 48 h
were significantly (P<0.01, P<0.01 and P<0.01,
respectively) lower, compared with cells in the control group
(Fig. 2A). Additionally,
microscopical analysis demonstrated prominent morphological changes
resembling cell swelling, irregularities in the plasma membrane and
the formation of membrane blebs and vacuoles, to a significantly
higher extent, compared with the control cells (Fig. 2B). This provided morphological
evidence to qualitatively demonstrate that HZL induced apoptosis,
which was in agreement with the results of the MTT assay described
above.
The present study then investigated whether the
cytotoxicity of HZL to SW620 cells is associated with the induction
of cell cycle arrest and apoptosis. Cell cycle arrest and apoptosis
were determined using flow cytometry following treatment of the
SW620 cells with 12.5, 25 and 50 µM HZL for 48 h. Characteristic
examples of the observations are demonstrated in Fig. 2C and D. The percentages of SW620
cell apoptosis were 17.3, 21.2 and 32.9%, in the12.5, 25 and 50 µM
HZL treatment groups, respectively (Fig. 2C). These results demonstrated that
HZL induced apoptosis in a dose-dependent manner, which was
consistent with the results of the MTT assay. The percentage of
G2/M phase cells in the groups treated with 12.5, 25 and 50 µM
HZLwas significantly elevated, whereas the percentages of G1/G0 and
S phase cells were decreased by HZL (Fig. 2D).
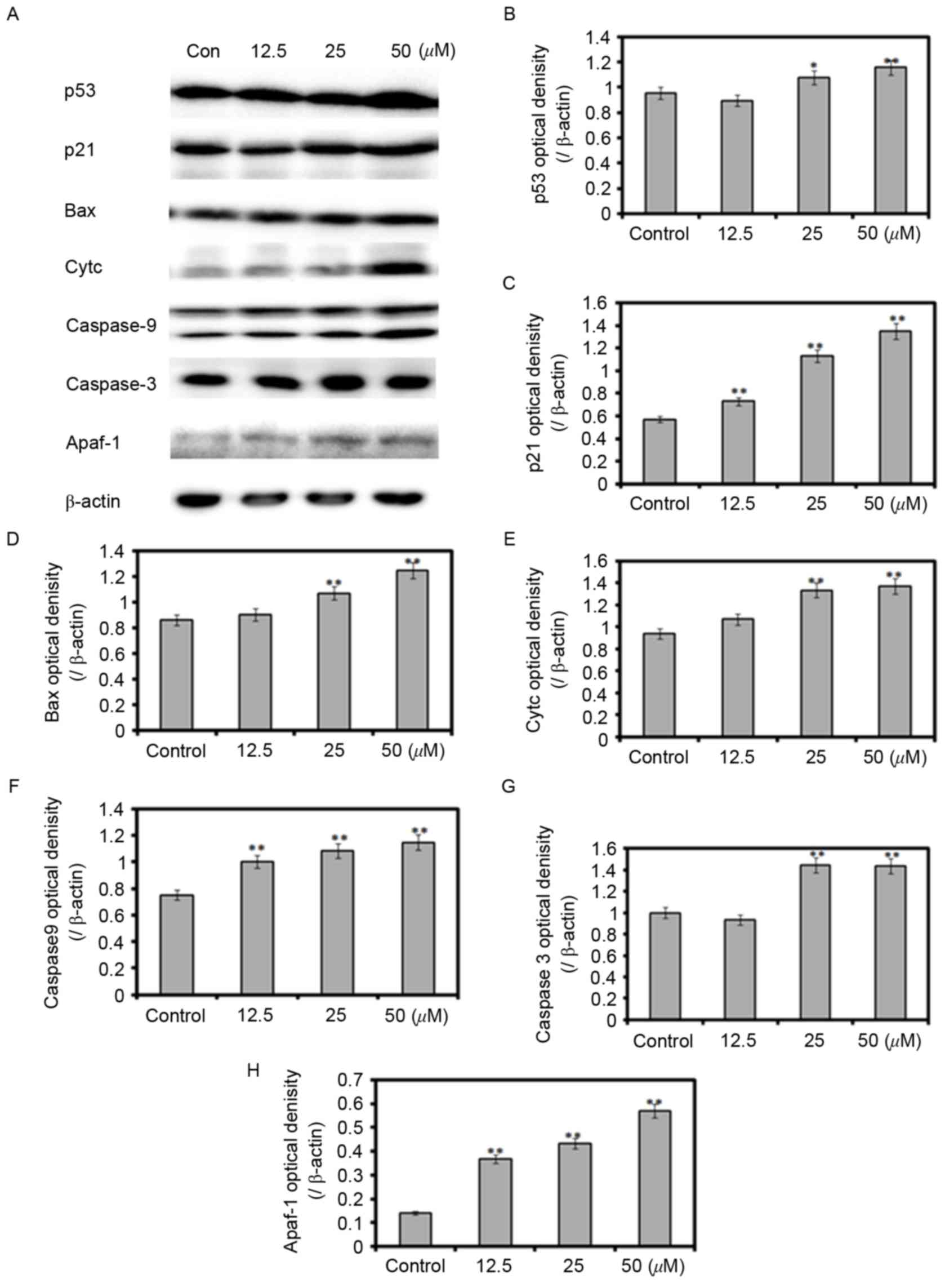
p53-mediated apoptosis
In order to examine the mechanisms underlying
HZL-induced cell cycle arrest and apoptosis, activation of the
p53-mediated signaling network in SW620 cells was investigated
using western blot analysis (Fig.
3A). Compared with the control group, the protein expression
levels of p53 and p21 were increased by 116 and 198%, respectively,
in the SW620 cells treated with 25 µM HZL for 48 h (Fig. 3B and C). The expression levels of
pro-apoptotic proteins Bax, cytochrome c, Apaf-1, caspase-9 and
caspase-3 were increased in a dose-dependent manner (Fig. 3D-H). These data suggested that HZL
induced apoptosis of the SW620 cells through p53- and
caspase-dependent apoptotic pathways.
ROS-mediated apoptosis
The enhancement of ROS production has long been
associated with the apoptotic response induced by anticancer
agents. Mitochondria are key organelles for cell survival and area
source of ROS generation during apoptosis. In the present study,
JC-1 staining was applied to detect changes in the ∆Ψm
of SW620 cells. The ratios of JC-1 red-to-green fluorescence
intensities decreased by 21.8, 41.6 and 43.7% when the SW620 cells
were treated with 12.5, 25 and 50 µM HZL for 48 h. These results
revealed that HZL induced apoptosis through the mitochondrial
pathway (Fig. 4A). The generation
of ROS was increased to 139.1% of the untreated control when
treated with 25 µM HZL for 48 h (Fig.
4B). This indicated that HZL caused the production of ROS. ROS
are created through aerobic metabolism and are rapidly removed by
endogenous antioxidants, including superoxide dismutase (SOD), CAT
and GSH-Px. The results of the present study demonstrated that the
mRNA expression levels of CAT and GSH-Px were decreased following
treatment with 25 µM HZL, whereas the ROS levels were increased
(Fig. 4C and D).
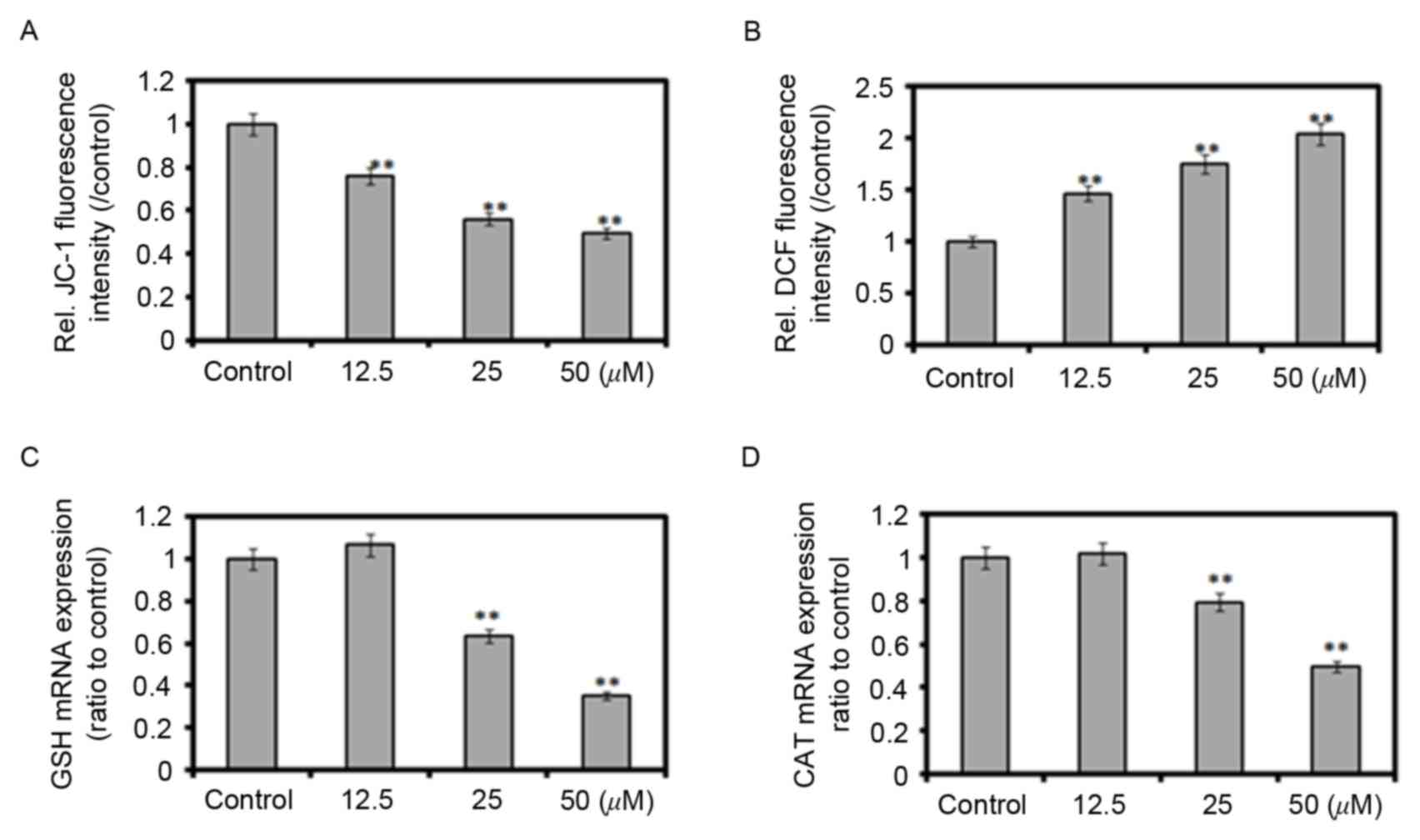 | Figure 4.Effect of Zanthoxylum
bungeanum on ΔΨm, ROS and the gene expression of antioxidant
enzymes. Cells were treated with 12.5, 25 and 50 µM hyperoside for
48 h. (A) ∆Ψm measured using a JC-1 assay; (B) Reactive
oxygen species production determined using the DCFH-Da probe; mRNA
expression of (C) GSH-Px and (D) CAT determined using reverse
transcription-quantitative polymerase chain reaction analysis.
**P<0.01 vs. control. GSH-Px, glutathione peroxidase; CAT,
catalase; DCFH-Da, 2′,7′-dichlorofluorescin diacetate; Rel.,
relative; JC-1,
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl
carbocyanine iodide. |
Discussion
Flavonoids are polyphenolic compounds, which are
found ubiquitously in plants. The role of dietary flavonoids in
cancer prevention has been widely discussed. Several mechanisms of
action of flavonoids have been identified, including carcinogen
inactivation, antiproliferative activity, cell cycle arrest,
induction of differentiation and apoptosis, inhibition of
angiogenesis, antioxidant activity and reversal of multidrug
resistance, or a combination of these mechanisms (23). Hyperoside is one of the major
active compounds of Z. bungeanum leaves; however, the
anticancer activity of hyperoside has not been reported previously.
To the best of the authors' knowledge, the present study is the
first to investigate the molecular effects of HZL on human SW620
CRC cells.
Inhibition of the cell cycle has become an important
target for the management of cancer. In the present study, the data
obtained on the cell cycle distribution of the cell population
indicated that HZL may have mediated the growth inhibition of SW620
cells via perturbation in the G2/M phase of the cell cycle. It has
been demonstrated that flavonoids induce the inhibition of growth
via cell cycle arrest in the G2/M phase of several cancer cell
lines, including ovarian carcinoma (SKOV3) and osteosarcoma (U2OS)
(24), anaplastic thyroid
carcinoma (25), and several
digestive system cancer cell lines (26,27),
which in itself is inconsistent with the results obtained in the
present study. However, Zhang et al (28) investigated the effect of hyperoside
on human osteosarcoma cells and found that, following treatment
with hyperoside, cells were arrested in the G0/G1 phase. Several
studies have also indicated that arrest of the cell cycle in the
G0/G1 phase is induced by flavonoids in human osteosarcoma MG-63,
HeLa, prostate and breast cancer cells (29–31).
Therefore, the cellular changes referred to above suggest that the
cell cycle arrest caused by flavonoids is cell type- and
concentration-dependent.
Tumor suppressor p53 is important in mediating cell
responses to various stresses, predominantly by inducing or
suppressing a number of genes involved in cell processes, including
cell death, cell cycle arrest, apoptosis, senescence and DNA-repair
(32,33). p21 is an important checkpoint gene
in the cell cycle, and it is also regulated by the transcription of
p53; it contributes to the repair of damaged cells by terminating
DNA synthesis and inactivating the nuclear antigen in proliferating
cells (34). As demonstrated in
Fig. 3, the present study detected
upregulation in the protein expression levels of p53 and p21,
suggesting that the upregulation of p53 and p21 was involved in
HZL-induced G2/M cell-cycle arrest.
Apoptosis is a regulated programmed cell death
process, which provides an effective non-inflammatory mechanism for
the removal of redundant or damaged cells from tissues, thereby
attaining tissue homeostasis (35). Tumor suppressor p53 is also an
important activator of the intrinsic apoptotic pathway. The
upregulation of p53 is commonly observed following exposure to
DNA-damaging agents, including oxidative stress or UV light
(36). Tumor suppressor p53 has
been demonstrated to differentially regulate the levels of Bcl-2
and Bax in vitro and in vivo (37). The increased production of Bax
following p53 activation is due to p53 being a direct
transactivator of the gene expression of Bax (37). The decreased expression of Bax in
the present study was concomitant with the decreased expression of
p53 and suggested that the apoptosis induced by HZL in SW620 cells
may be controlled by hyperoside via the p53 pathway.
The collapse of ∆Ψm is considered to
coincide with the opening of mitochondrial permeability transition
pores, leading to the release of cytochrome c into the
cytosol. In the cytoplasm, cytochrome c combines with
caspase-9, Apaf-1 and dATP to form the apoptosome complex, which in
turn activates caspases-9, 3 and 7 (38). Caspase-9 and its cofactor, Apaf-1,
are essential downstream components of p53 in Myc-induced apoptosis
(39). In the present study, HZL
upregulated the expression levels of cytochrome c, Apaf-1,
caspase-9 and caspase-3, compared with the control group (Fig. 3). These findings suggested that p53
and the caspase-dependent signaling pathway were involved in
HZL-induced apoptosis.
Reduced ∆Ψm can lead to increased
generation of ROS and apoptosis. In this context, Zeng et al
(40) suggested that hyperoside
alters the ∆Ψm and induces mitochondrial collapse.
Similar results were obtained in the present study when SW620 cells
were treated with HZL. These findings, along with other those of
others, suggested that hyperoside acts as an antiproliferative
agent through the overproduction of ROS, induction of apoptosis and
loss of ∆Ψm. The p53 protein has been suggested as a
critical regulator of intracellular ROS levels. Upon activation
following DNA damage, p53 can activate several genes, which results
in increased ROS generation, contributing to the induction of
apoptosis in cells with unrepaired DNA damage (41).
It has been found that human colorectal tumors
(adenomas and carcinomas) have increased levels of different
markers of oxidative stress, including increased levels of ROS,
nitric oxide (NO) (42), lipid
peroxides, GSH-Px and CAT (43).
Alternative mechanisms for flavonoid pro-oxidant toxicity involve
numerous peroxidases, which catalyze the oxidation of polyphenols.
The intracellular phenoxyl radicals (redox-cycling phenols) formed
by myeloperoxidase in neutrophils also induce lipid peroxidation
and can co-oxidize GSH to form thiol radicals with concomitant
oxygen release. The levels of CAT may be critical in cell-induced
resistance to the effects of anticancer drugs, which upregulate p53
(44). Kang et al (45) demonstrated that p53 upregulates ROS
generation by suppressing the activity of CAT in response to DNA
damage. p53-mediated GSH depletion was found to enhance the
cytotoxicity of NO in silibinin-treated human cervical carcinoma
and HeLa cells (46). In the
present study, HZL inhibited the mRNA expression levels of CAT and
GSH-Px in a dose-dependent manner, suggesting that p53 was
associated with the expression of genes encoding antioxidant
enzymes.
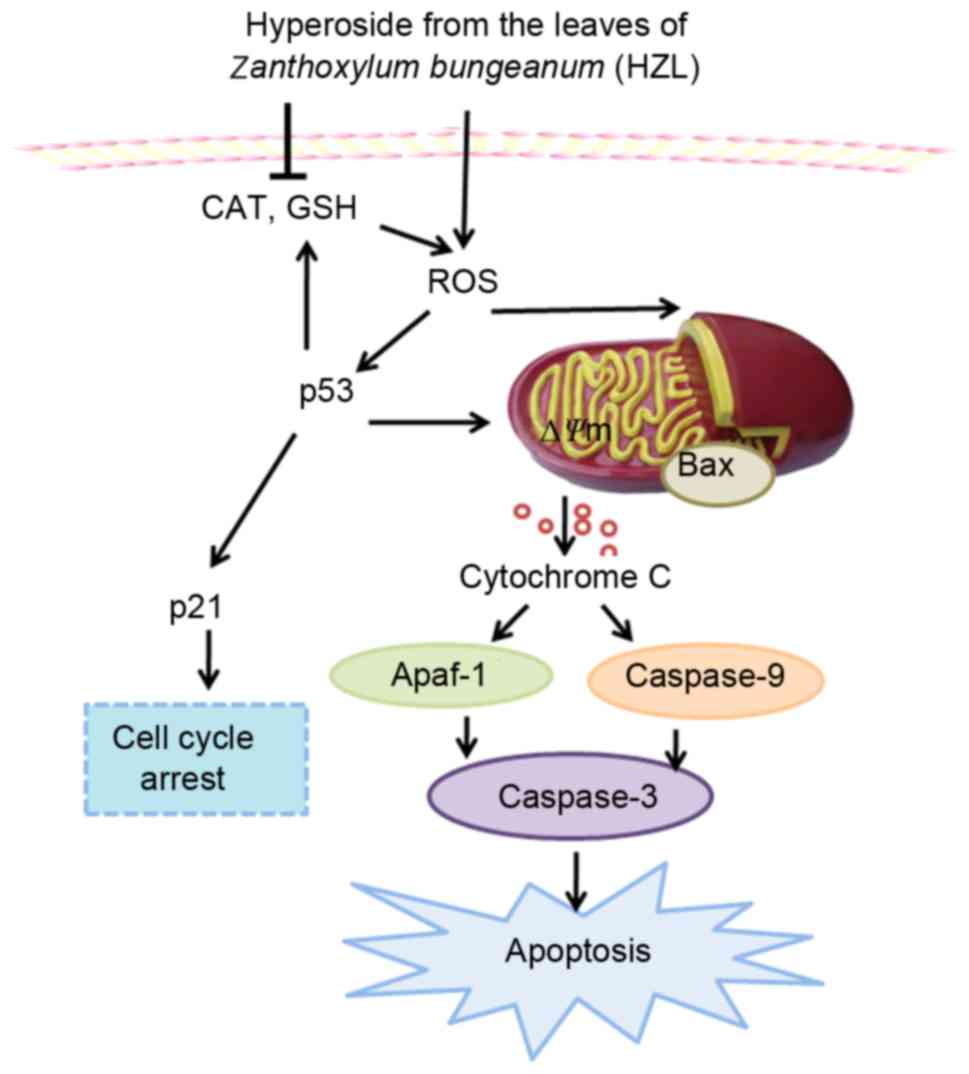
The complex mechanism of HZL-induced cell cycle
arrest and apoptosis in SW620 cells is demonstrated in Fig. 5. The results revealed that HZL
increased the accumulation of ROS via inhibition of the gene
expression of CAT and GSH, which resulted in cell cycle G2/M phase
arrest and apoptosis. Further mechanistic investigations revealed
that the induction of cell cycle G2/M phase arrest and apoptosis
was associated with the upregulated expression of p53, p21 and Bax
in the SW620 cells. Consequently, the ∆Ψm was reduced,
which accelerated the release of cytochrome c into the
cytoplasm leading to apoptosis of the SW620 cells via the
caspase-dependent pathway. These findings suggested that hyperoside
may be a potent chemopreventive agent against human CRC, acting
through induction of the caspase-dependent apoptosis and p53
signaling pathways. Further investigations are warranted to
determine the clinical efficacy and precise molecular mechanism by
which hyperoside inhibits cancer cell growth.
Acknowledgements
The present study was financed by the Special Fund
for Forest Scientific Research in Public Welfare (grant no.
201304811), the National Natural Science Foundation of China (grant
no. 31101266) and the Scientific and Technical Foundation of
Shaanxi Province (grant no. 2014JM4100). It was also partly
supported by the Institute of Mitochondrial Biology and Medical,
Xi'an Jiaotong University, Xi'an, China.
References
|
1
|
Amado NG, Predes D, Moreno MM, Carvalho
IO, Mendes FA and Abreu JG: Flavonoids and Wnt/β-catenin signaling:
Potential role in colorectal cancer therapies. Int J Mol Sci.
15:12094–12106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Luo X, Yu X, Liu S, Deng Q, Liu X, Peng S,
Li H, Liu J and Cao Y: The role of targeting kinase activity by
natural products in cancer chemoprevention and chemotherapy
(Review). Oncol Rep. 34:547–554. 2015.PubMed/NCBI
|
|
3
|
Miura K, Satoh M, Kinouchi M, Yamamoto K,
Hasegawa Y, Kakugawa Y, Kawai M, Uchimi K, Aizawa H, Ohnuma S, et
al: The use of natural products in colorectal cancer drug
discovery. Expert Opin Drug Discov. 10:411–426. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiong QB and Shi DW: Morphological and
histological studies of Chinese traditional drug ‘hua jiao’
(Pericarpium Zanthoxyli) and its allied drugs. Yao Xue Xue Bao.
26:938–947. 1991.(In Chinese). PubMed/NCBI
|
|
5
|
Zhang Y, Luo Z and Wang D: Efficient
quantification of the phenolic profiles of Zanthoxylum bungeanum
leaves and correlation between chromatographic fingerprint and
antioxidant activity. Nat Prod Res. 29:2024–2029. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Y, Wang D, Yang L, Zhou D and Zhang
J: Purification and characterization of flavonoids from the leaves
of Zanthoxylum bungeanum and correlation between their structure
and antioxidant activity. PLoS One. 9:e1057252014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang LC, Li R, Tan J and Jiang ZT:
Polyphenolics composition of the leaves of Zanthoxylum bungeanum
Maxim. Grown in Hebei, China, and their radical scavenging
activities. J Agric Food Chem. 61:1772–1778. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Luo Z, Wang D, He F and Li D:
Phytochemical profiles and antioxidant and antimicrobial activities
of the leaves of Zanthoxylum bungeanum. ScientificWorldJournal.
2014:1810722014.PubMed/NCBI
|
|
9
|
Yang Q, Cao W, Zhou X, Cao W, Xie Y and
Wang S: Anti-thrombotic effects of α-linolenic acid isolated from
Zanthoxylum bungeanum Maxim seeds. BMC Complement Altern Med.
14:3482014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu T, Zhong L, Hong Z, Li Y, Liu X, Pan L,
Xin H and Zhu Y: The effects of Zanthoxylum bungeanum extract on
lipid metabolism induced by sterols. J Pharmacol Sci. 127:251–259.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sukito A and Tachibana S: Isolation of
hyperoside and isoquercitrin from Camellia sasanqua as antioxidant
agents. Pak J Biol Sci. 17:999–1006. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang FQ, Liu M, Li W, Che JP, Wang GC and
Zheng JH: Combination of quercetin and hyperoside inhibits prostate
cancer cell growth and metastasis via regulation of microRNA21. Mol
Med Rep. 11:1085–1092. 2015.PubMed/NCBI
|
|
13
|
Huo Y, Yi B, Chen M, Wang N, Chen P, Guo C
and Sun J: Induction of Nur77 by hyperoside inhibits vascular
smooth muscle cell proliferation and neointimal formation. Biochem
Pharmacol. 92:590–598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li FR, Yu FX, Yao ST, Si YH, Zhang W and
Gao LL: Hyperin extracted from Manchurian rhododendron leaf induces
apoptosis in human endometrial cancer cells through a mitochondrial
pathway. Asian Pac J Cancer Prev. 13:3653–3656. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee JH, Ahn J, Kim JW, Lee SG and Kim HP:
Flavonoids from the aerial parts of Houttuynia cordata attenuate
lung inflammation in mice. Arch Pharm Res. 38:1304–1311. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fu T, Wang L, Jin XN, Sui HJ, Liu Z and
Jin Y: Hyperoside induces both autophagy and apoptosis in non-small
cell lung cancer cells in vitro. Acta Pharmacol Sin. 37:505–518.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li W, Liu M, Xu YF, Feng Y, Che JP, Wang
GC and Zheng JH: Combination of quercetin and hyperoside has
anticancer effects on renal cancer cells through inhibition of
oncogenic microRNA-27a. Oncol Rep. 31:117–124. 2014.PubMed/NCBI
|
|
18
|
Khan HY, Zubair H, Ullah MF, Ahmad A and
Hadi SM: A prooxidant mechanism for the anticancer and
chemopreventive properties of plant polyphenols. Curr Drug Targets.
13:1738–1749. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bayir H, Fadeel B, Palladino MJ, Witasp E,
Kurnikov IV, Tyurina YY, Tyurin VA, Amoscato AA, Jiang J, Kochanek
PM, et al: Apoptotic interactions of cytochrome c: Redox flirting
with anionic phospholipids within and outside of mitochondria.
Biochim Biophys Acta. 1757:648–659. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li PF, Dietz R and von Harsdorf R: p53
regulates mitochondrial membrane potential through reactive oxygen
species and induces cytochrome c-independent apoptosis blocked by
Bcl-2. EMBO J. 18:6027–6036. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
The Ninth Chinese Pharmacopoeia,
Commission of the People's Republic of China, . Pharmacopoeia of
the People's Republic of China. The Medicine Science and Technology
Press of China; 2010
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Procházková D, Bousová I and Wilhelmová N:
Antioxidant and prooxidant properties of flavonoids. Fitoterapia.
82:513–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Catanzaro D, Ragazzi E, Vianello C,
Caparrotta L and Montopoli M: Effect of quercetin on cell cycle and
cyclin expression in ovarian carcinoma and osteosarcoma cell lines.
Nat Prod Commun. 10:1365–1368. 2015.PubMed/NCBI
|
|
25
|
Celano M, Maggisano V, de Rose RF, Bulotta
S, Maiuolo J, Navarra M and Russo D: Flavonoid fraction of citrus
reticulata juice reduces proliferation and migration of anaplastic
thyroid carcinoma cells. Nutr Cancer. 67:1183–1190. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song H, Bao J, Wei Y, Chen Y, Mao X, Li J,
Yang Z and Xue Y: Kaempferol inhibits gastric cancer tumor growth:
An in vitro and in vivo study. Oncol Rep. 33:868–874.
2015.PubMed/NCBI
|
|
27
|
Li C, Yang X, Chen C, Cai S and Hu J:
Isorhamnetin suppresses colon cancer cell growth through the
PI3K-Akt-mTOR pathway. Mol Med Rep. 9:935–940. 2014.PubMed/NCBI
|
|
28
|
Zhang N, Ying MD, Wu YP, Zhou ZH, Ye ZM,
Li H and Lin DS: Hyperoside, a flavonoid compound, inhibits
proliferation and stimulates osteogenic differentiation of human
osteosarcoma cells. PLoS One. 9:e989732014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu M, Huang W, Bao N, Zhou G and Zhao J:
The flavonoid ampelopsin inhibited cell growth and induced
apoptosis and G0/G1 arrest in human osteosarcoma MG-63 cells in
vitro. Pharmazie. 70:388–393. 2015.PubMed/NCBI
|
|
30
|
Yi JL, Shi S, Shen YL, Wang L, Chen HY,
Zhu J and Ding Y: Myricetin and methyl eugenol combination enhances
the anticancer activity, cell cycle arrest and apoptosis induction
of cis-platin against HeLa cervical cancer cell lines. Int J Clin
Exp Pathol. 8:1116–1127. 2015.PubMed/NCBI
|
|
31
|
Wang P, Wang B, Chung S, Wu Y, Henning SM
and Vadgama JV: Increased chemopreventive effect by combining
arctigenin, green tea polyphenol and curcumin in prostate and
breast cancer cells. RSC Adv. 4:35242–35250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu Z, Wu L, Li L, Tashiro S, Onodera S and
Ikejima T: p53-mediated cell cycle arrest and apoptosis induced by
shikonin via a caspase-9-dependent mechanism in human malignant
melanoma A375-S2 cells. J Pharmacol Sci. 94:166–176. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu F, Dollé ME, Berton TR, Kuiper RV,
Capps C, Espejo A, McArthur MJ, Bedford MT, van Steeg H, de Vries A
and Johnson DG: Mouse models for the p53 R72P polymorphism mimic
human phenotypes. Cancer Res. 70:5851–5859. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Waga S, Hannon GJ, Beach D and Stillman B:
The p21 inhibitor of cyclin-dependent kinases controls DNA
replication by interaction with PCNA. Nature. 369:574–578. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fiers W, Beyaert R, Declercq W and
Vandenabeele P: More than one way to die: Apoptosis, necrosis and
reactive oxygen damage. Oncogene. 18:7719–7730. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pallardy M, Perrin-Wolff M and Biola A:
Cellular stress and apoptosis. Toxicol In Vitro. 11:573–578. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miyashita T, Krajewski S, Krajewska M,
Wang HG, Lin HK, Liebermann DA, Hoffman B and Reed JC: Tumor
suppressor p53 is a regulator of bcl-2 and bax gene expression in
vitro and in vivo. Oncogene. 9:1799–1805. 1994.PubMed/NCBI
|
|
38
|
Ni CH, Yu CS, Lu HF, Yang JS, Huang HY,
Chen PY, Wu SH, Ip SW, Chiang SY, Lin JG and Chung JG:
Chrysophanol-induced cell death (necrosis) in human lung cancer
A549 cells is mediated through increasing reactive oxygen species
and decreasing the level of mitochondrial membrane potential.
Environ Toxicol. 29:740–749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Soengas MS, Alarcón RM, Yoshida H, Giaccia
AJ, Hakem R, Mak TW and Lowe SW: Apaf-1 and caspase-9 in
p53-dependent apoptosis and tumor inhibition. Science. 284:156–159.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zeng KW, Wang XM, Ko H, Kwon HC, Cha JW
and Yang HO: Hyperoside protects primary rat cortical neurons from
neurotoxicity induced by amyloid β-protein via the
PI3K/Akt/Bad/Bcl(XL)-regulated mitochondrial apoptotic pathway. Eur
J Pharmacol. 672:45–55. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu S, Yan B, Lai W, Chen L, Xiao D, Xi S,
Jiang Y, Dong X, An J, Chen X, et al: As a novel p53 direct target,
bidirectional gene HspB2/αB-crystallin regulates the ROS level and
Warburg effect. Biochim Biophys Acta. 1839:592–603. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Haklar G, Sayin-Ozveri E, Yüksel M, Aktan
AO and Yalcin AS: Different kinds of reactive oxygen and nitrogen
species were detected in colon and breast tumors. Cancer Lett.
165:219–224. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rainis T, Maor I, Lanir A, Shnizer S and
Lavy A: Enhanced oxidative stress and leucocyte activation in
neoplastic tissues of the colon. Dig Dis Sci. 52:526–530. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bai J and Cederbaum AI: Catalase protects
HepG2 cells from apoptosis induced by DNA-damaging agents by
accelerating the degradation of p53. J Biol Chem. 278:4660–4667.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kang MY, Kim HB, Piao C, Lee KH, Hyun JW,
Chang IY and You HJ: The critical role of catalase in prooxidant
and antioxidant function of p53. Cell Death Differ. 20:117–129.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fan S, Yu Y, Qi M, Sun Z, Li L, Yao G,
Tashiro S, Onodera S and Ikejima T: P53-mediated GSH depletion
enhanced the cytotoxicity of NO in silibinin-treated human cervical
carcinoma HeLa cells. Free Radic Res. 46:1082–1092. 2012.
View Article : Google Scholar : PubMed/NCBI
|