Introduction
The incidence of endometrial carcinoma increased
steadily in the last decade (1).
It has become the second most common gynecologic cancer in China
(2). A substantial body of
epidemiologic data indicated that metabolic syndrome, characterized
by obesity, hypertension, and diabetes is closely associated with
endometrial carcinoma (3). As
insulin resistance is the hallmark of metabolic syndrome, more
attention has been focused on the relationship between insulin-like
growth factors system and endometrial carcinoma (4,5).
The insulin-like growth factor binding protein
(IGFBP) superfamily serves essential roles in the IGF system by
modulating the bioavailability of IGFs and/or insulin (6–8).
IGFBP-related protein 1 (rP1) is a secreted protein belonging to
the IGFBP superfamily. IGFBP-rP1 may be associated with insulin
resistance by its high affinity with insulin (6,9). A
previous study of the authors indicated that elevated IGFBP-rP1 was
associated with decreased endometrial cancer risk (10). Insulin can promote cell growth
in vitro (11).
Accordingly, IGFBP-rP1 can function as a tumor-suppressor gene in
various cancers including colorectal cancer and breast cancer
(12–17). However, the biological role of
IGFBP-rP1 in endometrial cancer has not been investigated yet. In
the present study, the authors attempted to explore the role and
underlying molecular mechanisms of IGFBP-rP1 in endometrial cancer
cells in vitro.
Materials and methods
Materials
Human endometrial cancer cell lines Ishikawa and
HEC-1A were donated by the Key Laboratory of Women's Reproductive
Health of Zhejiang Province (Hangzhou, China). All cell lines were
cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
(v/v) heat-inactivated bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and grown at 37°C in an atmosphere of 95% air and
5% CO2. The mouse anti-human IGFBP-rP1 monoclonal
antibody was from R&D Systems, Inc. (cat no. MAB1334; 1:500;
Minneapolis, MN, USA). The rabbit anti-human polyclonal antibodies,
β-actin (cat no. 20536-1-AP; 1:1,000), Rb (cat no. 17218-1-AP;
1:800), p16 (cat no. 10883-1-AP; 1:500), p21 (cat no. 10355-1-AP;
1:500), p53 (cat no. 10442-1-AP; 1:500) and extracellular
signal-regulated kinase (ERK)1/2 (cat no. 16443-1-AP; 1:1,000) were
from ProteinTech Group, Inc. (Chicago, IL, USA). The rabbit
anti-human antibody, phospho-retinoblastoma (Thr826; p-RB; cat no.
AF0030; 1:1,000) was from Affinity Biosciences (Cincinnati, OH,
USA). Goat anti-human polyclonal antibody p-ERK (Tyr 204; cat no.
sc-7976; 1:800) was from Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA). The Cell Counting Kit (CCK)-8 was from Dojindo Molecular
Technologies, Inc. (Kumamoto, Japan). PD98059 was from Selleck
Chemicals (Houston, TX, USA).
Cell culture, transfection and small
interfering (si)RNA treatment
pcDNA 3.1 (IGFBP-rP1) containing full-length
IGFBP-rP1 coding sequence was donated by Department of Pathology,
School of Medicine, Zhejiang University (Hangzhou, China). DNA
sequencing analysis confirmed the fidelity of the constructs.
Transfection of pcDNA 3.1 (IGFBP-rP1) into HEC-1A cells, which did
not express IGFBP-rP1, was performed using Lipofectamine 2000
transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. HEC-1A cells with an
empty vector (HEC-1A-EV), produced by transducing with pcDNA
3.1/myc-His (−B) alone, was used as a negative control. Stable
transfectants (HEC-1A- IGFBP-rP1) were obtained following selection
with 500 µg/ml G418 for 2 weeks.
Three different sets of siRNAs (IGFBP-rP1 siRNA) and
irrelevant controls (negative control) (both from Invitrogen;
Thermo Fisher Scientific, Inc.) were transiently transfected into
Ishikawa cells (with IGFBP-rP1 expression) in 6-well culture dishes
(1×105 cells) using Lipofectamine 2000 transfection
reagent, according to the manufacturer's protocol. The IGFBP-rP1
siRNA#1 targets against the exon 5 of IGFBP-rP1
(5′-CAAUCCACUAACACUUUAGUUTT-3′, 5′-AACUAAAGUGUUAGUGGAUUGTT-3′),
IGFBP-rP1 siRNA#2 against the exon 2
(5′-CAGGUGUACUUGAGCUGUGAGGUCATT-3′,
5′-UGACCUCACAGCUCAAGUACACCUGTT-3′) and IGFBP7 siRNA#3 against the
exon 4 (5′-GCUGGAGAAUAUGAGUGCCAUGCAUTT-3′,
5′-AUGCAUGGCACUCAUAUUCUCCAGCTT-3′). The Ishikawa-siRNA (IGFBP-rP1)
cells were further cultured for 48 h until use.
Western blot analysis
Cells were separated from 6-well plates by
phosphate-buffered saline (PBS) containing 0.25% trypsin-EDTA and
washed 3 times with PBS, then lysed in 1 ml lysis buffer consisting
of 7 M urea, 2 M thiourea, 4% CHAPS, 65 mM DTT and 0.2% Bio-lyte
(pH 3–10; cat no. 1632094; Bio-Rad Laboratories, Inc., Hercules,
CA, USA) by sonication on ice. The lysates were centrifuged at
13,500 × g for 1 h at 4°C. Subsequently, the protein concentration
of the supernatants was measured by the Bradford method (Bradford
Protein Assay kit, cat no. PC0010; Solarbio Science &
Technology Company, Beijing, China), and aliquots of the protein
samples were stored at −80°C.
Aliquots of protein extracts (50 µg) were separated
on an 8% SDS-PAGE according to the protein molecular weight.
Subsequently, the protein was electrophoretically transferred onto
a polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc.).
Following blocking with TBS-Tween-20 (0.2%; TBST) containing 5%
non-fat milk, the membranes were incubated with primary antibodies
(see above) in TBST overnight at 4°C, followed by
peroxidase-conjugated second antibody [goat anti-mouse IgG (H+L);
cat no. 626520; 1:5,000; Thermo Fisher Scientific, Inc.; goat
anti-rabbit IgG (H+L); cat no. 31460, 1:5,000; Thermo Fisher
Scientific, Inc.; peroxidase-conjugated affinipure rabbit anti-goat
IgG (H+L); cat no. SA00001-4; 1:5,000; ProteinTech Group, Inc.]
diluted in 1:5,000 in TBST for 1 h at room temperature. Finally,
blots were developed with the Odyssey system version 3 (LI-COR
Biosciences, Lincoln, NE, USA). As a control for equal protein
loading, blots were re-stained using anti-β-actin antibody.
Cell proliferation assay
Cell proliferation of stable HEC-1A and Ishikawa
cells was measured using the CCK-8 (Dojindo Molecular Technologies,
Inc.). In brief, cells were plated in 96-well plates at 5×103/well.
A volume of 10 µl CCK-8 solutions were added during the last 4 h of
the culture. Optical density of the wells was measured at 450 nm
using the Multiska FC microplate reader (Thermo Fisher Scientific,
Inc.).
Flow cytometry assay
Cells were collected at 48 h following treatment for
DNA content analysis. The adherent cells were harvested with PBS
containing 0.25% trypsin-EDTA. The harvested cells were washed
twice with PBS. Then the cells were treated with PBS containing
0.25 mg/ml RNase at 37°C for 15 min and incubated with 50 mg/ml
propidium iodide at 4°C for 15 min in the dark. The stained cells
were analyzed by flow cytometry (Beckman Coulter, Inc., Brea, CA,
USA) with Cell Quest software version 6.0 (BD Biosciences, Franklin
Lakes, NJ, USA).
Senescence-associated β-galactosidase
(SA-β-gal) staining
The senescence status of cells was verified by in
situ staining for SA-β-galactosidase, as described previously
(18). Briefly, cells that were
grown on 60 mm cell culture dishes were washed three times with PBS
and fixed with 2% formaldehyde/0.2% glutaraldehyde in PBS for 10
min. Then, they were washed again and incubated with
β-galactosidase substrate staining solution [150 mM NaCl, 2 mM
MgCl2, 5 mM potassium ferricyanide, 5 mM potassium
ferrocyanide, 40 mM citric acid and 12 mM sodium phosphate; pH 6.0;
containing 1 mg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactoside
(X-gal)] for 24 h at 37°C. The cells were washed with PBS. Greenish
cytoplasmic staining was regarded as positive. The positive cells
were counted in three high power fields with a diameter of 4.4 mm
(Leica DMR 2000; Leica Microsystems GmbH, Wetzlar, Germany) and
recorded as a percentage of positive cells to the total. The
experiment was conducted in triplicate.
Statistical analysis
Statistical analysis was performed using SPSS
software (version, 19.0; IBM SPSS, Armonk, NY, USA) for Windows.
The paired-sample t-test was conducted to compare protein levels
and other data between groups. P<0.05 was considered to indicate
a statistically significant difference.
Results
IGFBP-rP1 inhibited cell growth and
induced cellular senescence in endometrial carcinoma cells
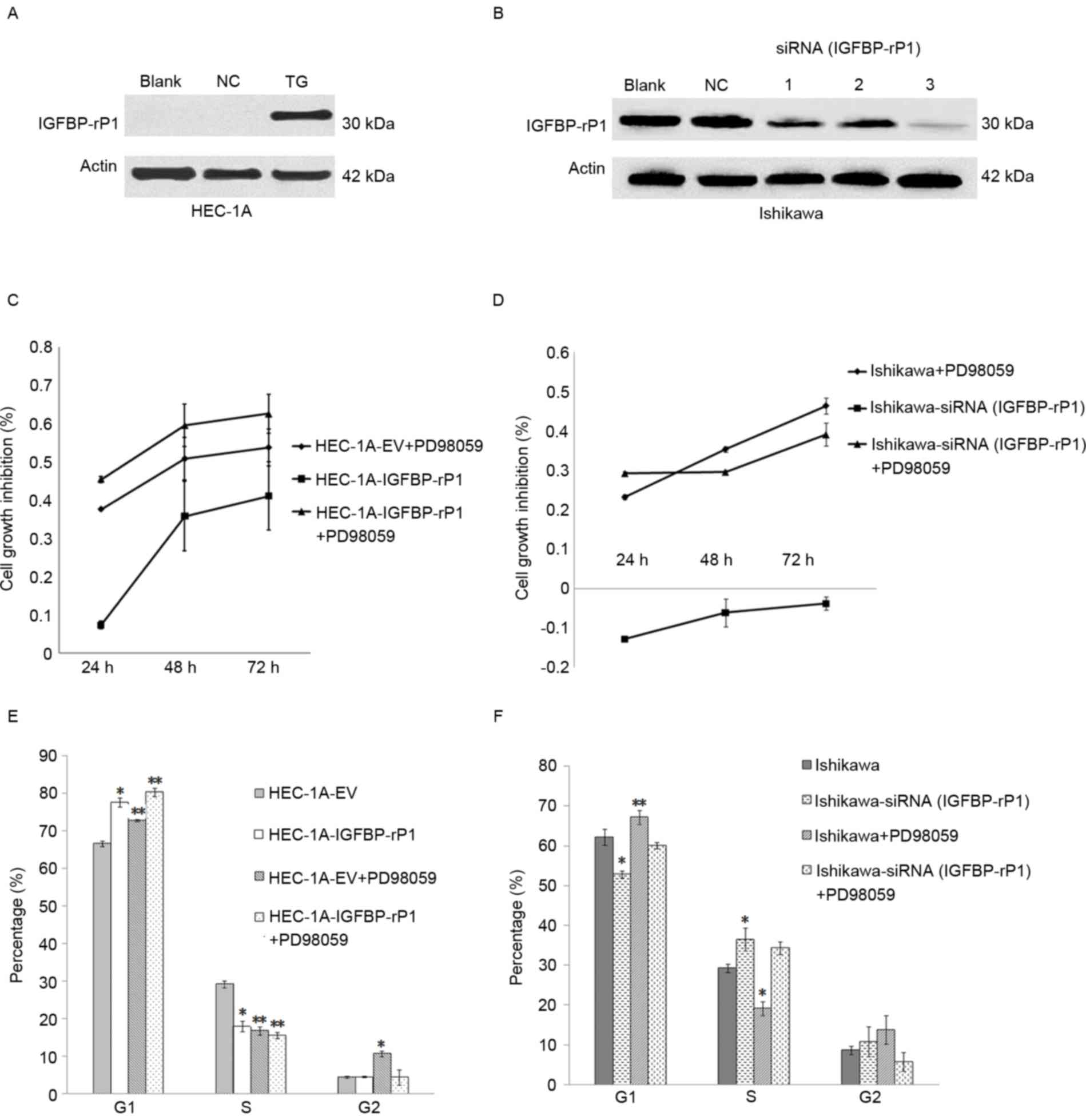
Expression of IGFBP-rP1 was detected by western
blotting following cell transfection in HEC-1A or significantly
inhibited by siRNA in Ishikawa cells (Fig. 1A and B). IGFBP-rP1 siRNA (#3)
presented the highest inhibitory effects and was used in all
subsequent experiments (Fig. 1B).
IGFBP-rP1 transfection suppressed the growth of HEC-1A cells
compared with negative controls whereas IGFBP-rP1 silence promoted
the growth of Ishikawa cells (Fig. 1C
and D). IGFBP-rP1 induced cell cycle arrest by demonstrating a
higher proportion of cells in the G1 phase in the HEC-1A-IGFBP-rP1
cells (77.59±1.275%) than in the controls (66.54±0.68%; P<0.05)
by flow cytometry with propidium iodide staining (Fig. 1E). In contrast, IGFBP-rP1 silence
promoted cell cycle in Ishikawa cells as indicated by decreased
cells in the G1 phase (52.76±0.88% in IGFBP-rP1-siRNA cells vs.
62.17±1.96% in control cells, P<0.05; Fig. 1F), and increased cells in the S
phase (36.45±2.89% in IGFBP-rP1-siRNA cells vs. 29.22±0.99% in
control cells; P<0.05).
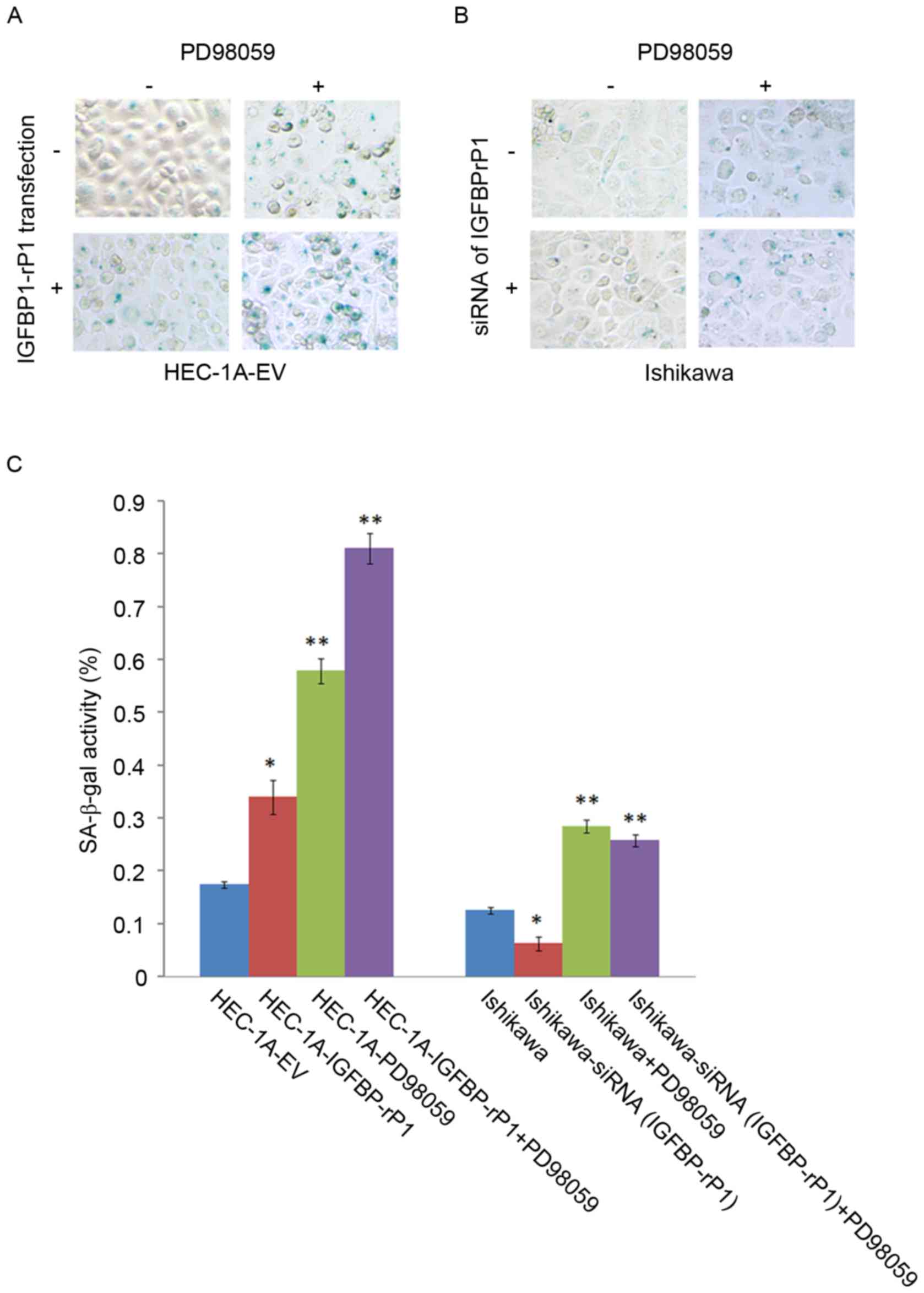
SA-β-galactosidase staining, a golden standard for
cellular senescence, indicated that HEC-1A-IGFBP-rP1 cells had a
higher proportion of SA-β-galactosidase positive cells
(34.04±3.24%) than the control cells (17.48±0.63%; P<0.01;
Fig. 2A). On the contrary,
SA-β-galactosidase activity significantly decreased in
Ishikawa-siRNA (IGFBP-rP1) cells (6.27±1.32%) compared with
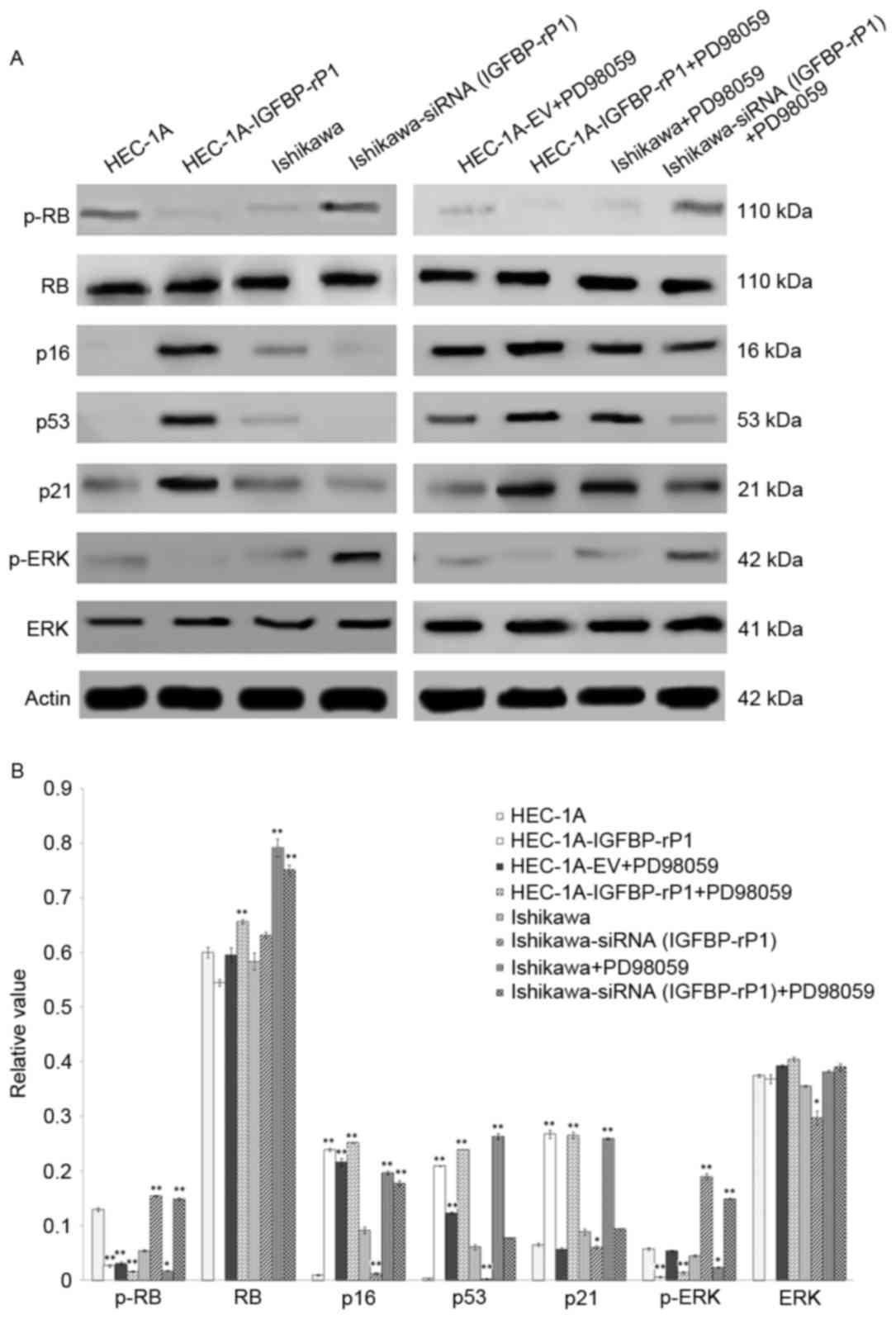
negative controls (12.57±0.63%; P<0.05; Fig. 2B and C). Moreover, p-RB, a key
regulator in cellular senescence, was significantly reduced in
HEC-1A-IGFBP-rP1 cells than control cells (2.64±0.21% vs.
12.95±0.31%; P<0.01) while other senescence-related proteins,
p21, p53 and p16, increased ~four-fold, 55-fold and 24-fold in
HEC-1A-IGFBP-rP1, respectively (P<0.01; Fig. 3A). In contrast, Ishikawa-siRNA
(IGFBP-rP1) cells presented a 2.8-fold increased expression of p-RB
(P<0.01) and a decrease level of p16, p21 and p53 protein than
control cells (p16, 1.28±0.17% vs. 9.15±0.59%; P<0.01; p53,
0.27±0.05% vs. 6.11±0.48%; P<0.01; p21, 6.09±0.31% vs.
8.88±0.56%; P<0.05; Fig. 3B).
The levels of total RB protein demonstrated no significant changes
between transfectants and control cells.
The suppression of p-ERK/ERK pathway
is associated with the biological functions of IGFBP-rP1
To explore the underlying cell growth inhibition
mechanisms of IGFBP-rP1, the authors analyzed the activities of the
ERK pathway in endometrial cells with forced or deprived IGFBP-rP1
expression. A 97.5% reduction of p-ERK was present in
HEC-1A-IGFBP-rP1 cells vs. control cells (P<0.01) and a
3.21-fold increase in Ishikawa-siRNA (IGFBP-rP1) cells vs. control
cells (P<0.01; Fig. 3). The
total ERK protein levels remained stable between transfectants and
control cells.
PD98059, an inhibitor of the MAP/ERK kinase
(MEK)/ERK pathway, significantly suppressed cell proliferation and
induced cellular senescence in endometrial cancer cells (Figs. 1C and D and 2A and B). Moreover, HEC-1A-IGFBP-rP1
cells with PD98059 treatment reported an additive effect on cell
growth inhibition compared to control cells with PD98059 treatment
alone or HEC-1A-IGFBP-rP1 (Fig. 1C and
D). G1 phase arrest was more apparent in HEC-1A-IGFBP-rP1 cells
with PD98059 treatment compared to control cells with PD98059
treatment or IGFBP-rP1 transfection alone (cells in G1 phase:
PD98059+IGFBP-rP1, 80.27±1.165% vs. PD98059, 72.76±0.35% or
IGFBP-rP1, 77.59±1.275%; P<0.01; cells in S phase:
PD98059+IGFBP-rP1, 15.45±0.86% vs. PD98059, 16.68±1.06% or
IGFBP-rP1, 17.93±1.41%; P<0.05; Fig. 1E). In Ishikawa cells,
IGFBP-rP1-siRNA alleviated G1 phase arrest of PD98059 treatment
(cells in G1 phase: IGFBP-rP1-siRNA+PD98059, 60.02±0.84% vs.
PD98059, 67.12±1.70%; P<0.01; Fig.
1F).
HEC-1A-IGFBP-rP1 cells with PD98059 presented more
SA-β-galactosidase positive cells than HEC-1A with PD98059
treatment (81.11±2.94% vs. 57.89±2.44%; P<0.01) or
HEC-1A-IGFBP-rP1 cells (81.11±2.94% vs. 34.04±3.24%; P<0.01;
Fig. 2C). SA-β-galactosidase
activity significantly decreased in Ishikawa-siRNA (IGFBP-rP1)
cells with PD98059 compared with controls (PD98059 alone,
25.80±1.15% vs. 28.48±1.16%; P<0.05). PD98059 can significantly
downregulate p-RB expression, and upregulate the expression of p16
and p53 in both HEC-1A and Ishikawa cells (P<0.01; Fig. 3), but did not influence the level
of total RB protein in endometrial cancer cells. PD98059 presented
a lower level of p-RB and p-ERK (p-RB, 1.62±0.12% vs. 3.08±0.29%;
P<0.01; p-ERK, 1.44±0.24% vs. 5.39±0.11%; P<0.01), and
upregulated expression of the p16, p53 and p21 proteins in
HEC-1A-IGFBP-rP1 cells than in control HEC-1A cells (p16,
25.20±0.09% vs. 21.62±0.67%; P<0.05; p53, 23.96±0.01% vs.
12.31±0.23%; P<0.05; p21, 26.55±0.59% vs. 5.70±0.28%;
P<0.01). On the contrary, PD98059 significantly upregulated the
expression of p-RB and p-ERK (p-RB, 14.90±0.21% vs. 1.75±0.08%;
P<0.01; p-ERK, 14.89±0.16% vs. 2.39±0.09%; P<0.01), and
significantly downregulated the expressions of p16, p53 and p21 in
Ishikawa-siRNA (IGFBP-rP1) cells than in control Ishikawa cells
(p16, 17.77±0.46% vs. 19.71±0.31%; P<0.05; p53, 7.76±0.11% vs.
26.34±0.54%; P<0.01; p21, 9.42±0.09% vs. 25.98±0.12%;
P<0.01).
Discussion
Accumulative evidence indicates that metabolic
syndrome is closely associated with endometrial carcinoma (3,19,20).
Insulin resistance remains the pillar of metabolic syndrome. The
dysfunction of the insulin/IGF/IGFBP axis serves essential roles in
insulin resistance. Numerous research has been focused on the role
of the insulin/IGF/IGFBPs axis in metabolic syndrome and related
diseases including endometrial carcinoma (4–8).
IGFBP-rP1 is a secreted factor belonging to the IGFBP family.
IGFBP-rP1 may play a tumor-suppressor role in various tumors
(12–17), but its biological functions in
endometrial carcinoma have not been fully investigated yet. In the
present study, the authors found that IGFBP-rP1 can inhibit cell
proliferation and induce cell senescence in endometrial cancer cell
lines. These findings supported the idea that IGFBP-rP1 functioned
as a tumor suppressor in endometrial carcinoma.
IGFBP-rP1, also known as MAC25 or IGFBP7, was
initially isolated from the senescent breast cancer cells (21). A number of studies validated the
role of IGFBP-rP1 in cellular senescence in various carcinomas
(13–15,17,22).
The p-RB/p53 pathway is well known to be the classical pathway in
the process of cellular senescence (23–26).
In the current study, IGFBP-rP1-transfection was shown to
downregulate the expression of p-RB and upregulate the expression
of p53, p16 and p21 in endometrial carcinoma cells, while IGFBP-rP1
silence demonstrated the opposite roles. These results clearly
demonstrated that IGFBP-rP1 can activate key factors, such as p-RB,
p53 and p21, in the p-RB/p53 pathway to trigger cellular senescence
in endometrial carcinoma. These results are in-keeping with several
reports from other cancers (13–15,17,22).
Wajapeyee et al (22)
reported that IGFBP7, had a central role in BRAFV600E-mediated
senescence and apoptosis in melanoma cells. Early work of the
authors indicated that IGFBP-rP1 could promote cellular senescence
in colorectal cancer cells (17).
IGFBP-rP1 binds to IGF-1 and IGF-2 with 100-fold
lower affinity than IGFBP1 to IGFBP6, which infers that IGFBP-rP1
may act in both an IGF-dependent and an IGF-independent way
(9). The IGF-dependent route is
well established as the central role in insulin resistance by the
activation of the PI3K/AKT signaling pathway. In contrast, the
IGF-independent manner of IGFBP-rP1 remains largely unknown at
present. ERK is generally considered to be anti-apoptotic, and ERK
signaling pathway is essential to cellular growth and survival.
Sustained activation of ERK1/ERK2 is necessary for G1- to S-phase
progression and is associated with induction of positive regulators
of the cell cycle and inactivation of anti-proliferative genes
(27). In the present study, the
authors' results clearly demonstrate that IGFBP-rP1 functions
through inhibition of the ERK signaling pathway. IGFBP-rP1 could
downregulate the p-ERK protein. The combination of IGFBP-rP1 and
PD98059, an inhibitor of the MEK/ERK pathway, had synergistic
effects on cell proliferation suppression and cellular senescence
in endometrial cancer cells. Moreover, IGFBP-rP1-siRNA alleviated
cell growth inhibition and cellular senescence, which were caused
by the blockage of ERK signaling pathway by PD98059 in endometrial
cancer cells. A previous study in breast cancer cells also
indicated that IGFBP-7 (IGFBP-rP1) strongly suppressed the
phosphorylation of ERK1/2, suggesting that IGFBP-7 mediates its
anti-proliferative effects through negative feedback signaling
(28). In addition, Wajapeyee
et al (22) indicated that
IGFBP7 induced senescence and apoptosis through autocrine/paracrine
pathways to inhibit BRAF-MEK-ERK signaling in BRAFV600E-expressing
melanoma cells.
In summary, the present study demonstrated that
IGFBP-rP1 acts as a potential tumor suppressor via the suppression
of the ERK signaling pathway in endometrial cancer cells. These
findings suggested that IGFBP-rP1 may be a potential therapeutic
target for cancer intervention. However, further investigation is
required to clarify the detailing of the IGFBP-rP1 and ERK networks
and their roles in vivo.
Acknowledgments
The present work was supported by grants from the
National Natural Science Foundations of China (grant nos. 81202067
and 81372790) and the Zhejiang Provincial Natural Science
Foundation of China (grant no. LQ12H16008).
References
|
1
|
Kitson SJ, Evans DG and Crosbie EJ:
Identifying high-risk women for endometrial cancer prevention
strategies: Proposal of an endometrial cancer risk prediction
model. Cancer Prev Res (Phila). 10:1–13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wei KR, Chen WQ, Zhang SW, Zheng RS, Wang
YN and Liang ZH: Epidemiology of uterine corpus cancer in some
cancer registering areas of China from 2003–2007. Zhonghua Fu Chan
Ke Za Zhi. 47:445–451. 2012.(In Chinese). PubMed/NCBI
|
|
3
|
Esposito K, Chiodini P, Capuano A,
Bellastella G, Maiorino MI and Giugliano D: Metabolic syndrome and
endometrial cancer: A meta-analysis. Endocrine. 45:28–36. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bruchim I, Sarfstein R and Werner H: The
IGF hormonal network in endometrial cancer: Functions, regulation,
and targeting approaches. Front Endocrinol (Lausanne).
5:762014.PubMed/NCBI
|
|
5
|
McGrath M, Lee IM, Buring J and De Vivo I:
Common genetic variation within IGFI, IGFII, IGFBP-1, and IGFBP-3
and endometrial cancer risk. Gynecol Oncol. 120:174–178. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamanaka Y, Wilson EM, Rosenfeld RG and Oh
Y: Inhibition of insulin receptor activation by insulin-like growth
factor binding proteins. J Biol Chem. 272:30729–30734. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ricort JM: Insulin-like growth factor
binding protein (IGFBP) signalling. Growth Horm IGF Res.
14:277–286. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hwa V, Oh Y and Rosenfeld RG: The
insulin-like growth factor-binding protein (IGFBP) superfamily.
Endocr Rev. 20:761–787. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oh Y, Nagalla SR, Yamanaka Y, Kim HS,
Wilson E and Rosenfeld RG: Synthesis and characterization of
insulin-like growth factor-binding protein (IGFBP)-7. Recombinant
human mac25 protein specifically binds IGF-I and -II. J Biol Chem.
271:30322–30325. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhan Y, Wang J, Ma Y, Liu Z, Xu H, Lu S
and Lu B: Serum insulin-like, growth factor binding protein-related
protein 1 (IGFBP-rP1) and endometrial cancer risk in Chinese women.
Int J Cancer. 132:411–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Serafim MK, Silva GM, Duarte AB, Araújo
VR, Silva TF, Lima AK, Chaves RN, Campello CC, Silva LD and
Figueiredo JR: High insulin concentrations promote the in vitro
growth and viability of canine preantral follicles. Reprod Fertil
Dev. 25:927–934. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Y, Pacyna-Gengelbach M, Ye F, Knösel
T, Lund P, Deutschmann N, Schlüns K, Kotb WF, Sers C, Yasumoto H,
et al: Insulin-like growth factor binding protein-related protein 1
(IGFBP-rP1) has potential tumour-suppressive activity in human lung
cancer. J Pathol. 211:431–438. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vizioli MG, Sensi M, Miranda C, Cleris L,
Formelli F, Anania MC, Pierotti MA and Greco A: IGFBP7: An
oncosuppressor gene in thyroid carcinogenesis. Oncogene.
29:3835–3844. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Benatar T, Yang W, Amemiya Y, Evdokimova
V, Kahn H, Holloway C and Seth A: IGFBP7 reduces breast tumor
growth by induction of senescence and apoptosis pathways. Breast
Cancer Res Treat. 133:563–573. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen D, Yoo BK, Santhekadur PK, Gredler R,
Bhutia SK, Das SK, Fuller C, Su ZZ, Fisher PB and Sarkar D:
Insulin-like growth factor-binding protein-7 functions as a
potential tumor suppressor in hepatocellular carcinoma. Clin Cancer
Res. 17:6693–6701. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Verhagen HJ, de Leeuw DC, Roemer MG,
Denkers F, Pouwels W, Rutten A, Celie PH, Ossenkoppele GJ,
Schuurhuis GJ and Smit L: IGFBP7 induces apoptosis of acute myeloid
leukemia cells and synergizes with chemotherapy in suppression of
leukemia cell survival. Cell Death Dis. 5:e13002014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma Y, Lu B, Ruan W, Wang H, Lin J, Hu H,
Deng H, Huang Q and Lai M: Tumor suppressor gene insulin-like
growth factor binding protein-related protein 1 (IGFBP-rP1) induces
senescence-like growth arrest in colorectal cancer cells. Exp Mol
Pathol. 85:141–145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dimri GP, Lee X, Basile G, Acosta M, Scott
G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O,
et al: A biomarker that identifies senescent human cells in culture
and in aging skin in vivo. Proc Natl Acad Sci USA. 92:9363–9367.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rosato V, Zucchetto A, Bosetti C, Dal Maso
L, Montella M, Pelucchi C, Negri E, Franceschi S and La Vecchia C:
Metabolic syndrome and endometrial cancer risk. Ann Oncol.
22:884–889. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ni J, Zhu T, Zhao L, Che F, Chen Y, Shou H
and Yu A: Metabolic syndrome is an independent prognostic factor
for endometrial adenocarcinoma. Clin Transl Oncol. 17:835–839.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Swisshelm K, Ryan K, Tsuchiya K and Sager
R: Enhanced expression of an insulin growth factor-like binding
protein (mac25) in senescent human mammary epithelial cells and
induced expression with retinoic acid. Proc Natl Acad Sci USA.
92:4472–4476. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wajapeyee N, Serra RW, Zhu X, Mahalingam M
and Green MR: Oncogenic BRAF induces senescence and apoptosis
through pathways mediated by the secreted protein IGFBP7. Cell.
132:363–374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xue W, Zender L, Miething C, Dickins RA,
Hernando E, Krizhanovsky V, Cordon-Cardo C and Lowe SW: Senescence
and tumour clearance is triggered by p53 restoration in murine
liver carcinomas. Nature. 445:656–660. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dimova DK and Dyson NJ: The E2F
transcriptional network: Old acquaintances with new faces.
Oncogene. 24:2810–2826. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rowland BD and Bernards R: Re-evaluating
cell-cycle regulation by E2Fs. Cell. 127:871–874. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gil J and Peters G: Regulation of the
INK4b-ARF-INK4a tumour suppressor locus: All for one or one for
all. Nat Rev Mol Cell Biol. 7:667–677. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meloche S and Pouysségur J: The ERK1/2
mitogen-activated protein kinase pathway as a master regulator of
the G1- to S-phase transition. Oncogene. 26:3227–3239. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Amemiya Y, Yang W, Benatar T, Nofech-Mozes
S, Yee A, Kahn H, Holloway C and Seth A: Insulin like growth factor
binding protein-7 reduces growth of human breast cancer cells and
xenografted tumors. Breast Cancer Res Treat. 126:373–384. 2011.
View Article : Google Scholar : PubMed/NCBI
|