Introduction
Renal ischemia/reperfusion injury (IRI) is a primary
cause of acute renal failure resulting from tubular dysfunction
following shock, sepsis or renal transplantation (1). Despite various advances in vascular
surgery, the rates of morbidity and mortality in patients with
post-operative IRI remain particularly high (2). As with other pathological conditions
of the kidney, renal ischemia may ultimately progress to chronic
advanced kidney disease, which is characterized by tubule and
capillary loss as well as regional interstitial fibrosis associated
with chronic hypoxic stress (3–6).
In the ischemic kidney, and during the subsequent
re-oxygenation, excessive reactive oxygen species (ROS) are
generated in the reperfusion phase, initiating a cascade of
deleterious cellular responses leading to inflammation, cell death
and acute kidney failure (7).
During the pathophysiological process, initiated in response to a
broad spectrum of pro-inflammatory mediators including tumor
necrosis factor (TNF)-α, interleukin-1 and ROS (8,9),
nuclear factor (NF)-κB is activated, mediating a vicious cycle of
further inflammatory responses, which contribute to cell apoptosis.
Therefore, targeting the NF-κB signaling pathway is a promising
novel therapeutic strategy to inhibit the inflammatory and innate
immune cascades responsible for the amplification of injury, which
may produce effective treatments or prevention strategies for IRI.
This concept was supported by a previous study that performed
treatments with in vivo transfection of NF-κB decoy
oligodeoxynucleotides, which significantly reduced the renal
dysfunction and damage associated with ischemic acute renal failure
(10).
Zinc finger protein A20 has been recognized as a
central regulator of inflammation and apoptosis due to its
ubiquitous inhibitory effect on NF-κB (8,11,12).
As an NF-κB dependent gene, A20 forms a negative feedback loop,
limiting NF-κB activation and inflammation in cells exposed to
hypoxia and re-oxygenation (8,13).
Lee et al (14) reported
that mice with A20 deficiency develop severe inflammation and
cachexia as a result of failure to terminate the TNF-induced
responses to NF-κB and cell death. Overexpression of A20 in rat
kidneys using recombinant adenovirus-mediated gene transfer,
protects rat kidneys from acute tubular necrosis following renal
ischemia (13).
Therefore, the authors hypothesized that A20 may
prevent the kidneys from IRI via a combination of anti-apoptotic
and anti-inflammatory effects. The aim of the present study was to
establish the A20 transfection model in rats and to evaluate the
protective effect of in vivo A20 transfection on renal IRI
by observing renal function, histopathological alterations, cell
apoptosis and the NF-κB signaling pathway.
Materials and methods
Construction of recombinant human A20
expression plasmid
Human A20 cDNA was cloned into a pcDNA3.1 vector
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) to
construct the expression plasmid, which was termed pcDNA3.1-A20.
The preparation of A20 cDNA and construction of pcDNA3.1-A20 were
performed by the Invitrogen Cn Service (Project No: KL120515006;
Shanghai, China). The plasmid DNA was transformed into E
coli DH5α competent cells (Invitrogen; Thermo Fisher
Scientific, Inc.) and grown in Luria Broth medium for propagation,
according to the manufacturer's protocol. The plasmid DNA was
subsequently extracted using the PureLink® HiPure Plasmid DNA
Purification kit (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The inserted sequences
were digested with the restriction endonucleases NheI and
XbaI (Promega Corporation, Madison, WI, USA) according to
manufacturer's protocol, and verified by DNA sequencing, performed
by the Invitrogen Cn Service, and separation by 1% gel
electrophoresis.
Experimental animals
The animal experiments were approved by the Animal
Ethics Committee of the Fujian University of Traditional Chinese
Medicine (permit no. SCXK Shanghai 2012–0002; Fuzhou, China).
Sprague-Dawley male rats (8–10 weeks in age and, 250–300 g in
weight) were provided by the Laboratory Animal Center of Fujian
University of Traditional Chinese Medicine. The rats received a
standard diet, and were housed individually with free access to tap
water. A 12 h light-dark cycle was provided. Room temperature was
maintained at 21–23°C with 30–60% humidity. Rats (n=24) were
randomly assigned into three groups: Saline (control), plasmid
control and A20 groups (n=8/group). The three groups were given
intravenous injections via the tail vein with saline (250 µl),
empty pcDNA3.1 plasmid (15 µl of Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) + 10 µg of pcDNA3.1 mixed with
saline to a final volume of 250 µl) or pcDNA3.1-A20 (15 µl of
Lipofectamine 2000 + 10 µg of pcDNA3.1-A20 mixed with saline to a
final volume of 250 µl), respectively. Following 48 h
post-injection, rats were anesthetized with sodium pentobarbital
(50 mg/kg; Pitman-Moore Inc., Washington Crossing, NJ, USA)
intraperitoneally. A ventral midline longitudinal incision (8–10
cm) was used to fully expose the left kidney, renal artery,
abdominal aorta and vena cava. The renal ischemia reperfusion
injury model was established by clamping the left renal artery and
vein for 45 min, then cutting the right kidney prior to reperfusion
of the left renal vasculature. At 24 h post-operation, at the time
of euthanasia, 2 ml blood samples were drawn from the mesenteric
vein and centrifuged at 1,500 × g for 10 min to obtain sera. For
paraffin histologic assessments and cell apoptosis analysis, one
part of the left kidney was fixed in 10% buffered formalin for 24
h, the remaining parts of the kidneys were snap-frozen in liquid
nitrogen for western blot, gene expression and electrophoretic
mobility shift assay analyses.
Western blot analysis
Cytoplasmic and nuclear proteins were extracted
using the Nucleoprotein Cytoplasm Protein Extraction kit (Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China) according to
manufacturer's protocol. The protein concentration was measured
using a Bicinchoninic Protein Assay (Beyotime Institute of
Biotechnology, Shanghai, China) according to manufacturer's
protocol. Proteins (50 µg) were separated by 10% SDS-PAGE and
transferred to nitrocellulose membranes. Following blocking in
phosphate-buffered saline (PBS) containing 5% skimmed milk
overnight at 4°C, the membranes were incubated with primary
antibodies against A20 (dilution, 1:1,000; rabbit; 5630; Cell
Signaling Technology, Inc., Danvers, MA, USA) and β-actin
(dilution, 1:2,000; rabbit; 4970; Cell Signaling Technology, Inc.)
for 2 h at room temperature. Bound antibodies were detected using
an anti-rabbit horseradish peroxidase-conjugated secondary antibody
(dilution, 1:5,000; goat; HS101-01; Beijing TransGen Biotech Co.,
Ltd.) for 1 h at room temperature. A chemiluminescent substrate
(Thermo Fisher Scientific, Inc.) was used to detect the
immunoreactive protein signals. Computerized image analysis was
performed using Image Lab™ version 5.1 and a Molecular Imager®
ChemiDoc™ XRS System (both from Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The values for the A20 protein were quantified
and normalized to β-actin abundance. All samples were performed in
duplicate.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from rat kidneys using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) and 5 µg RNA
was reverse-transcribed using the PrimeScript™ II First strand cDNA
Synthesis kit (Takara Bio, Inc., Otsu, Japan) according to the
manufacturer's protocol. The following primers for A20 were
designed to target the rat-specific mRNA region of the A20
gene: Forward, 5′-CGGCGCTAGATTCTACGTCTTCA-3′ and reverse,
5′-CTGTCTCGGATACTGCTTTGTCCA-3′. RT-qPCR was performed with the
SYBR® Premix Ex Taq™ (Takara Bio, Inc.) following the
manufacturer's protocol. Transcript levels were quantified by qPCR
using the following cycling conditions: 3 sec at 94°C, then 40
cycles of 3 sec at 95°C and 20 sec at 60°C. The expression levels
of A20 were normalized to the housekeeping gene (β-actin:
Forward, 5′-ACTTCGAGCAAGAGATGGC-3′ and reverse,
5′-ACGTCACACTTCATGATGGA-3′) and the initial amount of RNA used was
calculated using the 2−ΔΔCq method as described
previously (15). All samples were
performed in duplicate.
Renal function assay
Renal function was detected by measuring serum
creatinine (Scr) and blood urea nitrogen (BUN) using Raichem™
Enzymatic Creatinine Reagent and BUN Slow Rate Reagent (Cliniqa
Corporation, San Marcos, CA, USA) according to the manufacturer's
protocol, and measured on a Roche Automatic Biochemical Analyzer
(Roche Diagnostics, Basel, Switzerland).
Histological analysis
One portion of the renal tissue was fixed in 10%
buffered formalin for 24 h. Following fixation and dehydration,
sections (4 µm) were cut and stained with haematoxylin and eosin
for 1 min respectively at room temperature, followed by examination
with a light microscope. Renal injury scores were determined by
evaluating the degree of tubulointerstitial injury according to
tubular necrosis, tubular dilatation and/or atrophy, inflammatory
cell infiltration or cellar edema, as previously described
(16): 0, normal kidney; 1,
minimal necrosis (<5% involvement of the cortex or outer
medulla); 2, mild necrosis (5–25% involvement of the cortex or
outer medulla); 3, moderate necrosis (25–75% involvement of the
cortex or outer medulla); 4, severe necrosis (>75% involvement
of the cortex or outer medulla). Histologic examination was
performed in a blinded manner by a renal pathologist using a light
microscope (Zeiss AG, Oberkochen, Germany).
Evaluation of cell apoptosis
Cell apoptosis in the renal tissue sections (5-µm),
was quantified using a DeadEnd™ Colorimetric TUNEL System (Promega
Corporation), according to the manufacturer's protocol. For all
staining procedures, the terminal deoxynucleotidyl transferase dUTP
nick end labeling (TUNEL)-positive cells were counted in 8 random
high-power fields using a light microscope by two individual
evaluators. Disagreements in results were resolved by a third
evaluator.
Electrophoretic mobility shift assay
(EMSA)
The DNA binding activity of NF-κB was assessed on
nuclear extracts of kidney tissue, extracted as aforementioned,
using a Chemiluminescent EMSA kit (Beyotime Institute of
Biotechnology) according to the manufacturer's protocol.
Radio-labeled NF-κB consensus oligonucleotide was incubated with 5
µg nuclear extract in 10 µl binding buffer at room temperature for
20 min. The negative control reaction, cold probes competitive
reaction and the mutant cold probe competitive reaction were also
incubated with the same amount of samples as the controls and using
the exact same experimental conditions. Band shifts were resolved
on a non-denaturing 6.5% polyacrylamide gel. Radiographic signals
were quantified by densitometry using the Molecular imager
ChemiDoc™ XRS+ system with Image Lab™ software (Version
5.1, Bio-Rad Laboratories, Inc.).
Statistical analysis
The data were analyzed using SPSS 18.0 (SPSS, Inc.,
Chicago, IL, USA). Differences among multiple groups (>2) were
assessed using one-way analysis of variance, followed by the post
hoc Tukey-Kramer test. The data differences between two groups were
analyzed by an unpaired Student's t-test. P<0.05 was considered
to indicate a statistically significant difference. Data are
presented as mean ± standard deviation or as the median
(range).
Results
A20 expression in renal tissue
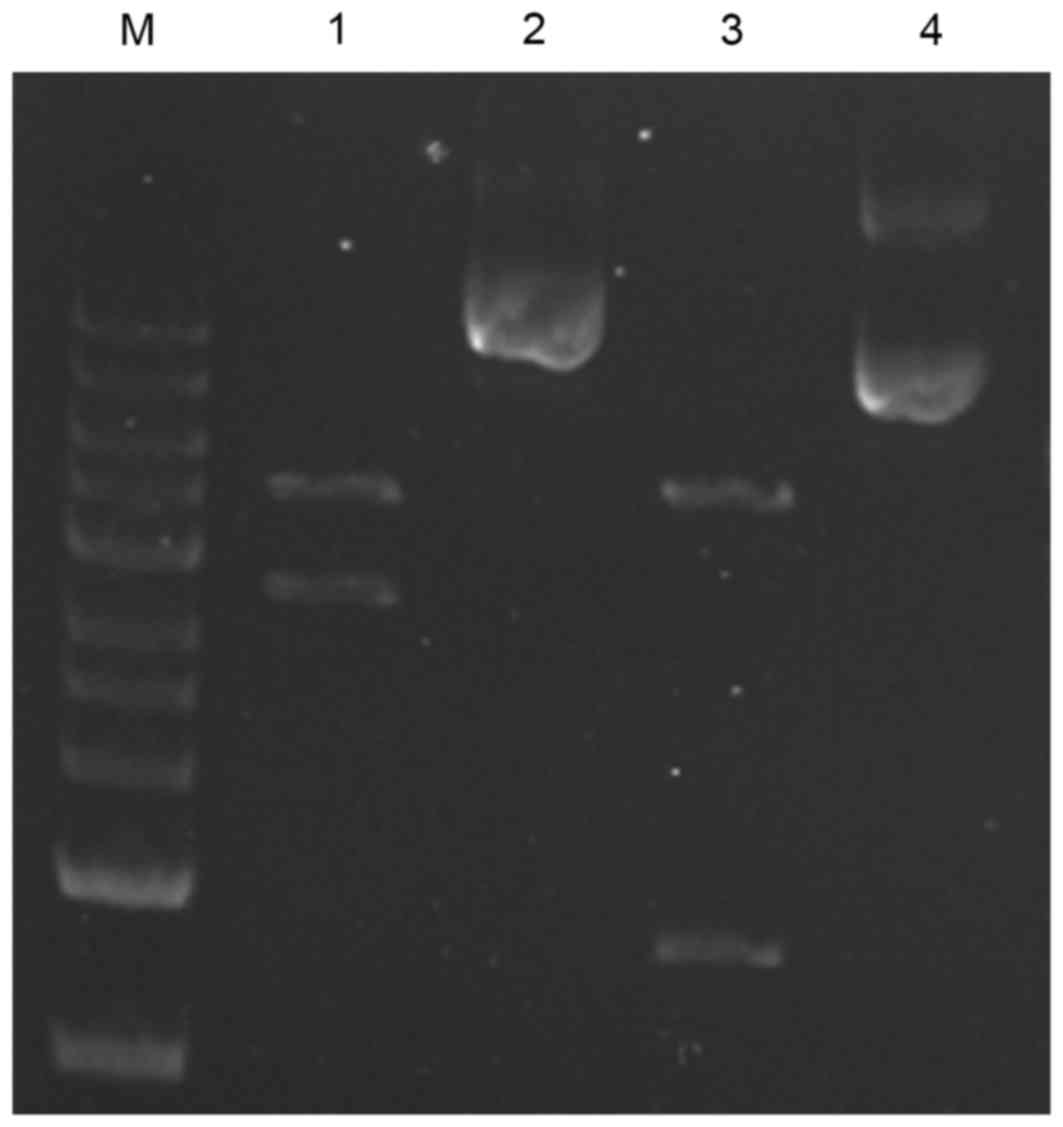
The recombinant pcDNA3.1-A20 plasmid was constructed
successfully as demonstrated by DNA sequence and restriction enzyme
analyses (Fig. 1). Subsequently
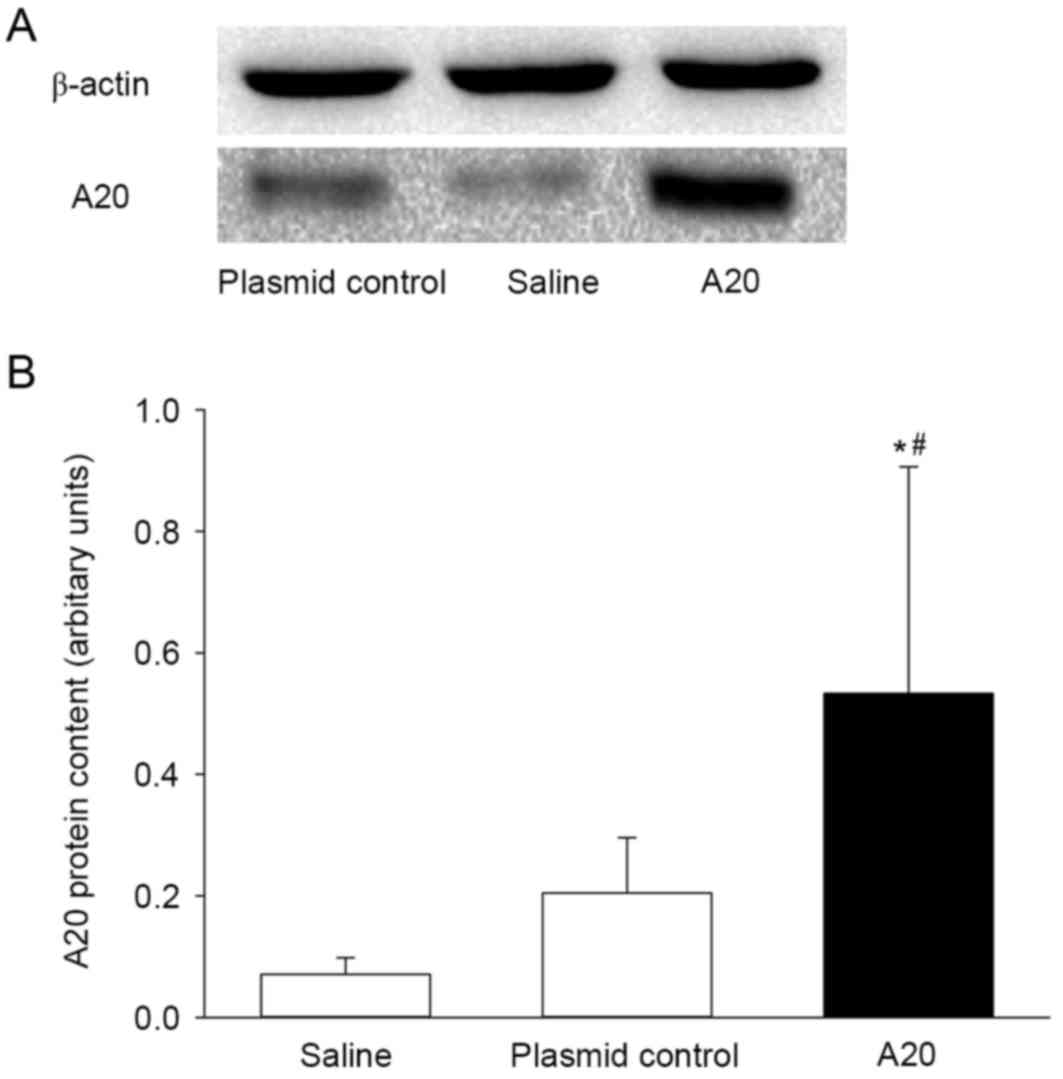
the plasmid DNA was wrapped by a liposome and transfected into the
rats. As expected, transfection with the pcDNA3.1-A20 significantly
increased the cytoplasmic A20 protein expression in vivo
(Fig. 2). When compared with the
saline group, the A20 protein level increased 7-fold following 72 h
of A20 transfection (P<0.05). Although the concentration of A20
appeared to be elevated in the animals transfected with the plasmid
control, when compared to the saline control the difference was not
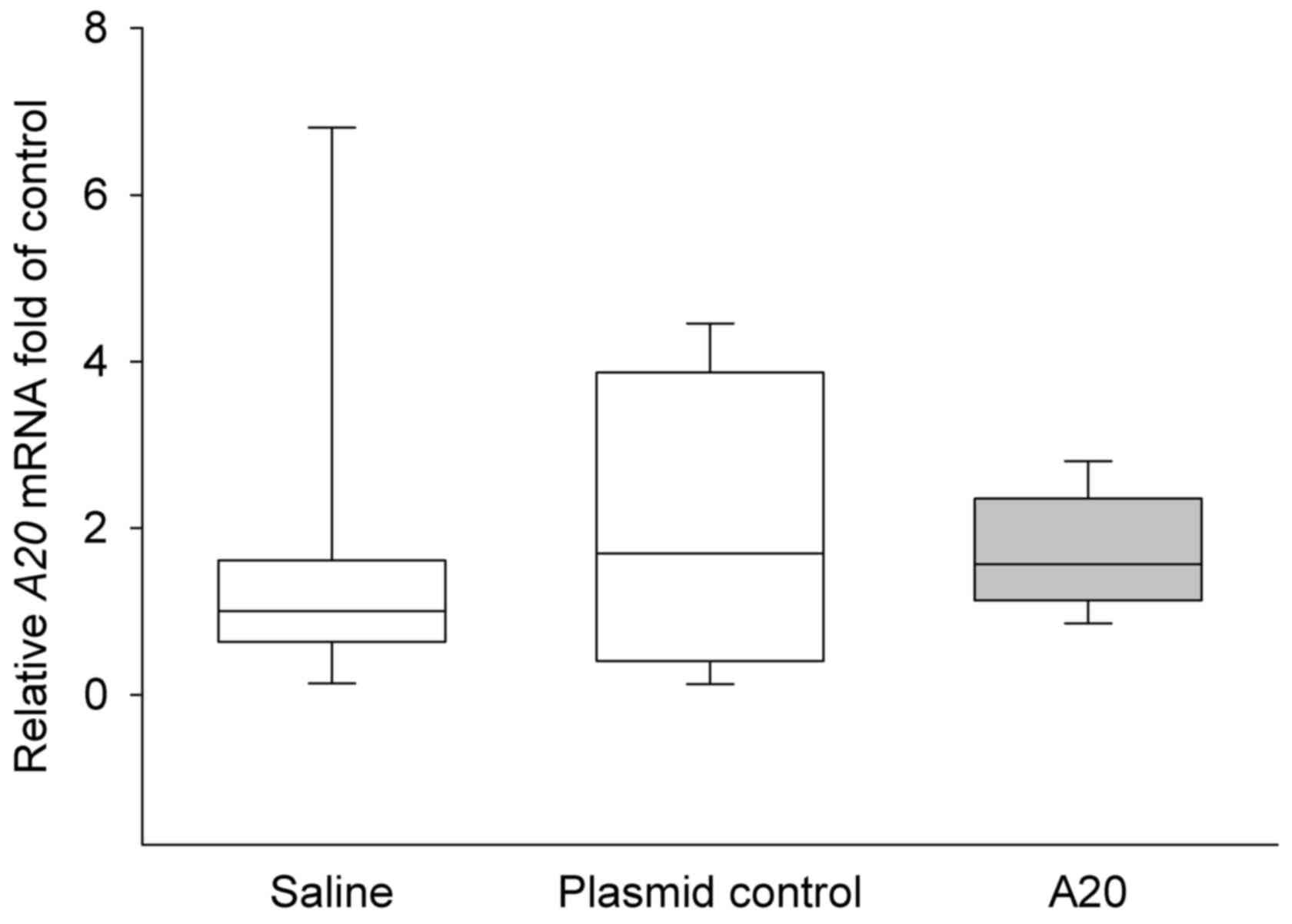
statistically significant (P>0.05). The A20 mRNA level was
further quantified in the three experimental groups, however, no
significant differences were identified in mRNA expression of A20
between groups (P>0.05; Fig.
3).
Renal injury
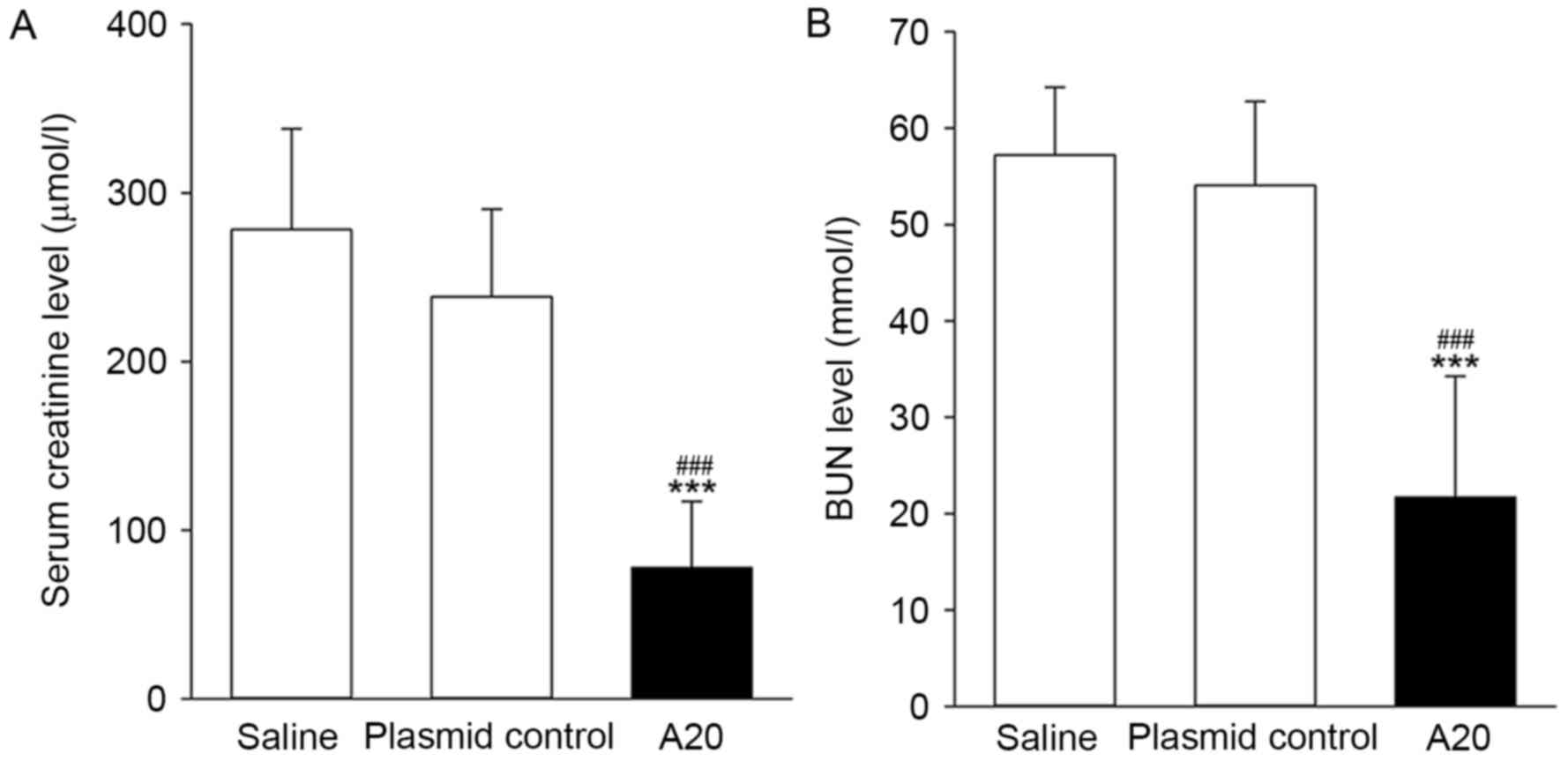
The present study determined the levels of the renal
injury indicators, Scr and BUN following IRI. When compared with
the animals pretreated with pcDNA3.1 or saline, overexpression of
A20 induced an ~3-fold decrease in the levels of Scr and BUN
(P<0.001; Fig. 4). The extent
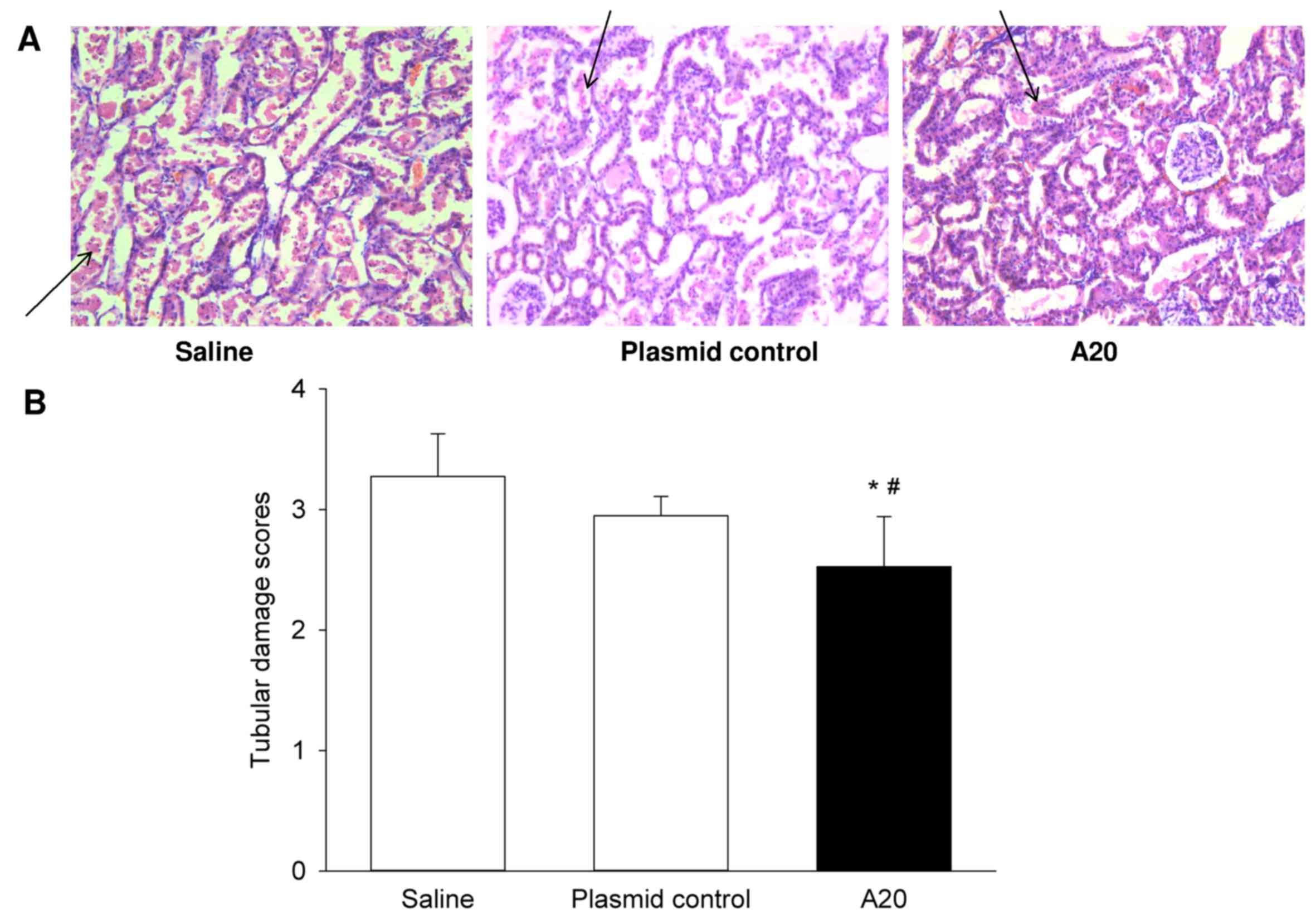
of renal tissue damage following IRI was evaluated using the
histological approach. The saline and plasmid control groups
exhibited a similar degree of pathological damage (Fig. 5), including, renal tubular
dilatation, cracks in cells and cells flattening, injured tubular
epithelial cells or loss of brush border, tubular epithelial cell
shedding or necrosis and protein casts or cell debris observed in
the tube cavity, and tubular epithelial cell shedding was observed
to be the most serious feature. Conversely, in the A20 group,
damage to the tubular epithelial cells brush border was the
fundamental alteration. However, changes in renal glomerulus
structures, including the endothelial cell lining and basement
membrane, were not significantly different amongst the three
groups. When analyzing the tubular damage scores, the A20 group was
significantly lower compared with the two control groups
(P<0.05; Fig. 5).
Renal tubular epithelial cell
apoptosis
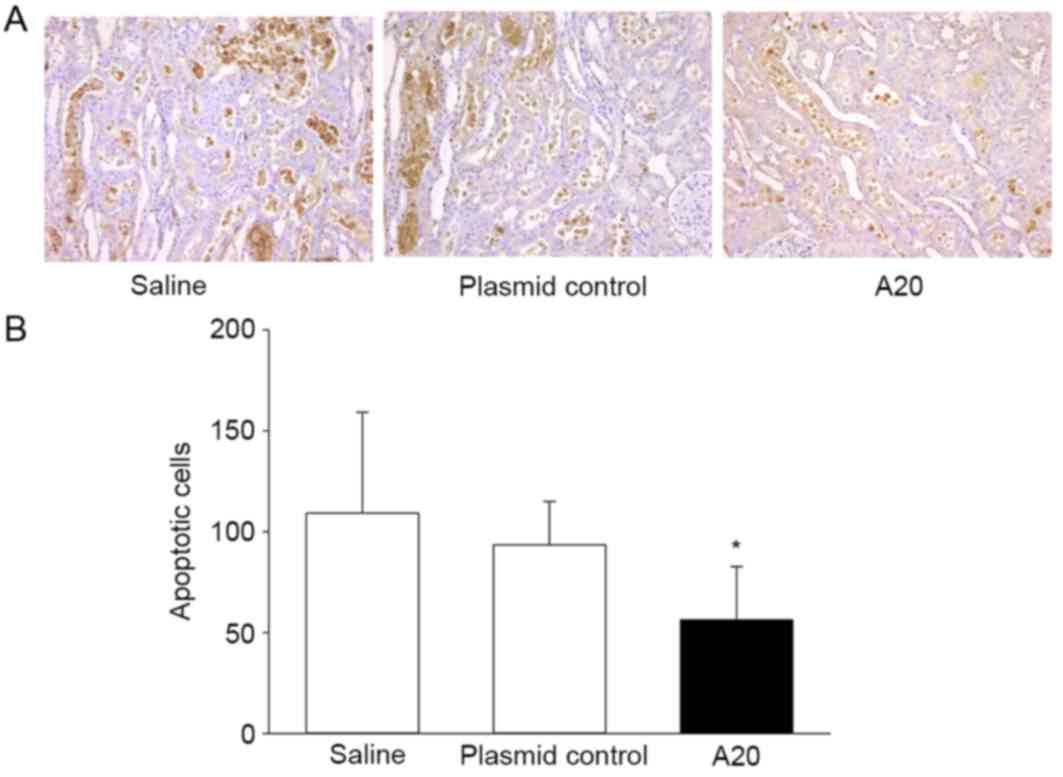
The number of apoptotic cells in the kidney tissues
of experimental rats was determined using a TUNEL assay. Apoptotic
cells were primarily present in the renal tubules, particularly in
the distal tubule, while few were identified in the renal
interstitium and glomerular. The number of TUNEL-positive cells was
significantly lower in the A20 group (56.25±26.47) when compared
with the saline group (109.25±49.91) at 24 h of IRI (P<0.05;
Fig. 6). In addition, the total
number of apoptotic cells accumulated in the renal tubular
epithelium was similar between the groups administrated saline or
empty plasmid control.
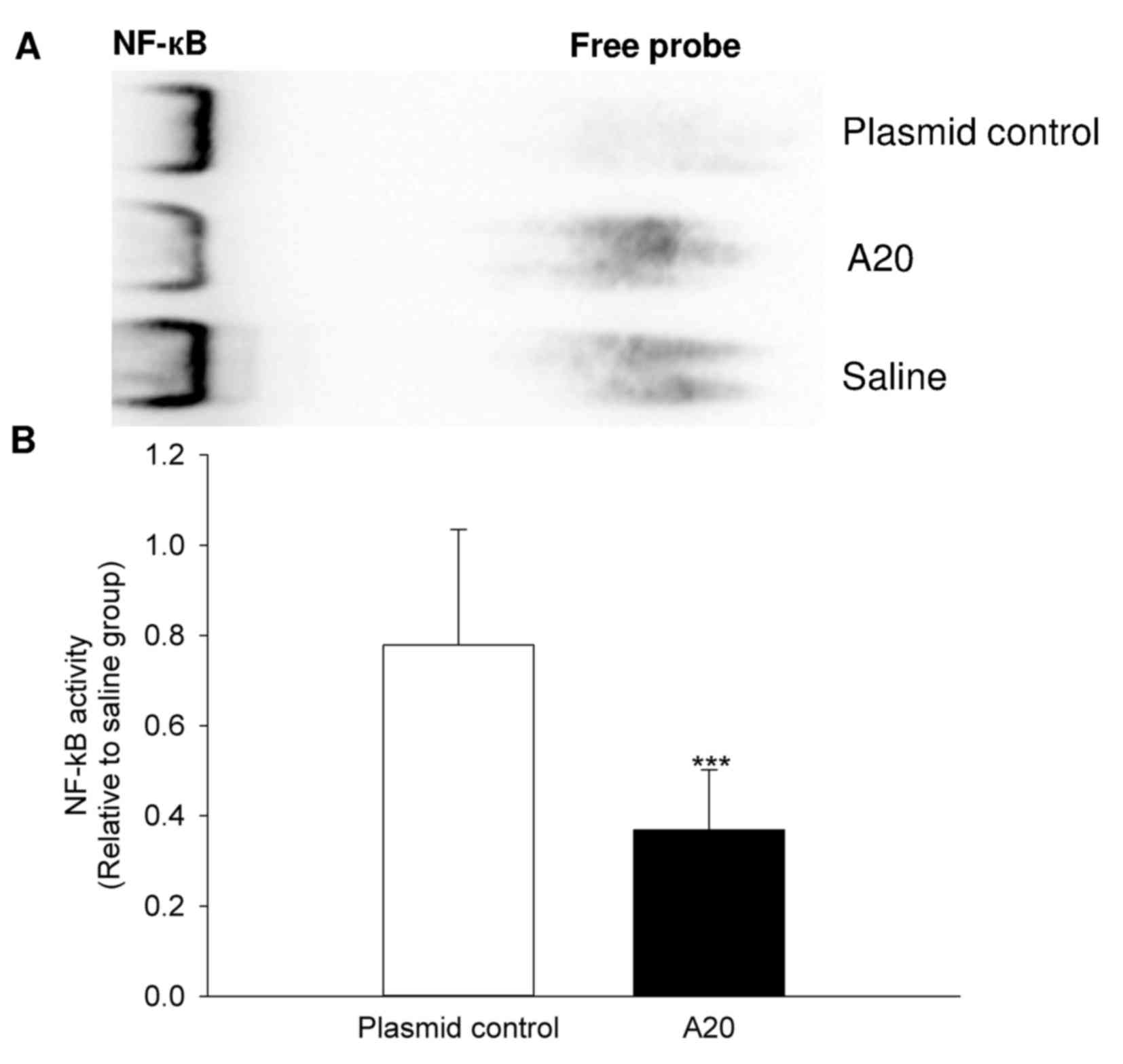
Analysis of NF-κB DNA-binding
activity
A significant reduction in nuclear DNA binding
activity of NF-κB was observed in the experimental rats transfected
with pcDNA3.1-A20. This activity was reduced in the A20 group by
53% when compared with the plasmid control group (P=0.001; Fig. 7).
Discussion
In the present study, a liposomal pcDNA3.1-A20
plasmid was successfully transfected into rats and a transient
expression of A20 was produced in kidney tissues. Overexpression of
A20 attenuated renal injury, improved histological features,
decreased cell apoptosis and inhibited NF-κB activation in this rat
model of renal IRI. Collectively, these results suggest that
transfection of the A20 gene may be a simple and highly
efficient method of protecting the kidneys from renal IRI.
To evaluate the therapeutic potential of the
A20 gene, a liposome-encapsulated plasmid DNA was used for
gene delivery as this method is a reliable and simple technique for
gene transfection (17,18). Notably, non-viral transfection
methods have the advantage of safe application with minimal risk of
replication or viral incorporation and a lower chance of
immunological rejection. In addition, plasmid DNA can be produced
stably and at a low cost, to a high level of purity. As
demonstrated by the western blot analysis, in vivo delivery
of the liposome pcDNA3.1-A20 complex promoted A20 protein
expression in kidney tissues 72 h following injection, suggesting
that this method of delivery may be an efficient way of
upregulating A20 expression in vivo. In contrast to A20
protein expression, the A20 mRNA expression levels did not
demonstrate a significant change 72 h following transfection. This
apparent discrepancy may be due to transient A20 gene expression
induced by pcDNA3.1-A20 and differences in mRNA and protein
turnover rates.
The present study determined whether A20
overexpression protected rat kidneys from IRI by conducting renal
function tests and observing histological alterations. Increased
blood levels of Scr and BUN are indicators of renal injury. The
results indicated that A20 transfection markedly decreased the Scr
and BUN levels following IRI. In response to IRI, the pathological
damage was primarily evident in tubular epithelial cells. This
damage was localized to the epithelial cells brush border following
A20 treatment, and the tubular injury scores were attenuated. These
results provided evidence of the beneficial effect of A20
transfection on renal IRI.
The functional and morphological improvement in the
A20 group is likely to be attributable to the inhibitory effect of
A20 on NF-κB activation and the resulting suppression of cell
apoptosis. Activation of NF-κB occurs rapidly in response to
ischemia/reperfusion in a number of tissues including kidney cells,
brain, liver and myocardium (9,19–22).
A20, as a key negative regulator of NF-κB, exhibits
anti-inflammatory and anti-apoptotic effects in cultured cells via
inhibition of NF-κB (11,12,23).
In the rat model of the present study, the number of apoptotic
cells was significantly reduced in animals pretreated with
pcDNA3.1-A20. In addition, a large proportion of TUNEL-positive
cells were predominantly observed in the distal tubular epithelium
in response to renal IRI, coinciding with the morphological
features observed. The nuclear NF-κB binding activity was examined
to analyze NF-κB signaling. Under normal conditions, NF-κB is
retained in the cytoplasm by binding to its specific inhibitor
protein, inhibitor of NF-κB (IκB). Upon stimulation, IκB is
phosphorylated, degraded and is subsequently separated from NF-κB.
The activated NF-κB is then translocated into the nucleus to
activate transcriptional expression of downstream genes associated
with inflammatory responses and apoptosis (21,24).
The results demonstrated that A20 transfection resulted in a 53%
reduction in NF-κB DNA-binding activity, further confirming the
efficacy of this in vivo transfection model and explaining
the protective mechanisms of A20 in renal injury.
In conclusion, the results demonstrated that the
liposome-encapsulated plasmid DNA vector for gene delivery
upregulated the expression of A20 in vivo, conferring
protection against IRI which was mediated by NF-κB. These results
offer a potential prophylactic treatment strategy for renal injury
and support the use of A20 as a potential therapeutic target in
renal IRI. However, further studies are required to assess whether
there are side effects in other tissues, including the liver and
heart, due to the systemic delivery of A20.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Fujian Province (grant no. 2010J0101).
References
|
1
|
Agrawal M and Swartz R: Acute renal
failure. Am Fam Physician. 61:2077–2088. 2000.PubMed/NCBI
|
|
2
|
Giulini SM, Bonardelli S, Portolani N,
Giovanetti M, Galvani G, Maffeis R, Coniglio A, Tiberio GA, Nodari
F, de Lucia M, et al: Suprarenal aortic cross-clamping in elective
abdominal aortic aneurysm surgery. Eur J Vasc Endovasc Surg.
20:286–289. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Basile DP: Rarefaction of peritubular
capillaries following ischemic acute renal failure: A potential
factor predisposing to progressive nephropathy. Curr Opin Nephrol
Hypertens. 13:1–7. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Basile DP, Donohoe D, Roethe K and Osborn
JL: Renal ischemic injury results in permanent damage to
peritubular capillaries and influences long-term function. Am J
Physiol Renal Physiol. 281:F887–F899. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bonventre JV and Weinberg JM: Recent
advances in the pathophysiology of ischemic acute renal failure. J
Am Soc Nephrol. 14:2199–2210. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nangaku M: Chronic hypoxia and
tubulointerstitial injury: A final common pathway to end-stage
renal failure. J Am Soc Nephrol. 17:17–25. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Malek M and Nematbakhsh M: Renal
ischemia/reperfusion injury; from pathophysiology to treatment. J
Renal Inj Prev. 4:20–27. 2015.PubMed/NCBI
|
|
8
|
Beyaert R, Heyninck K and Van Huffel S:
A20 and A20-binding proteins as cellular inhibitors of nuclear
factor-kappa B-dependent gene expression and apoptosis. Biochem
Pharmacol. 60:1143–1151. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsui N, Kasajima K, Hada M, Nagata T,
Senga N, Yasui Y, Fukuishi N and Akagi M: Inhibiton of NF-kappaB
activation during ischemia reduces hepatic ischemia/reperfusion
injury in rats. J Toxicol Sci. 30:103–110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao CC, Ding XQ, Ou ZL, Liu CF, Li P, Wang
L and Zhu CF: In vivo transfection of NF-kappaB decoy
oligodeoxynucleotides attenuate renal ischemia/reperfusion injury
in rats. Kidney Int. 65:834–845. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cooper JT, Stroka DM, Brostjan C,
Palmetshofer A, Bach FH and Ferran C: A20 blocks endothelial cell
activation through a NF-kappaB-dependent mechanism. J Biol Chem.
271:18068–18073. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Longo CR, Arvelo MB, Patel VI, Daniel S,
Mahiou J, Grey ST and Ferran C: A20 protects from CD40-CD40
ligand-mediated endothelial cell activation and apoptosis.
Circulation. 108:1113–1118. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lutz J, le A Luong, Strobl M, Deng M,
Huang H, Anton M, Zakkar M, Enesa K, Chaudhury H, Haskard DO, et
al: The A20 gene protects kidneys from ischaemia/reperfusion injury
by suppressing pro-inflammatory activation. J Mol Med (Berl).
86:1329–1339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee EG, Boone DL, Chai S, Libby SL, Chien
M, Lodolce JP and Ma A: Failure to regulate TNF-induced NF-kappaB
and cell death responses in A20-deficient mice. Science.
289:2350–2354. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Partridge J, Carlsen H, Enesa K, Chaudhury
H, Zakkar M, Luong L, Kinderlerer A, Johns M, Blomhoff R, Mason JC,
et al: Laminar shear stress acts as a switch to regulate divergent
functions of NF-kappaB in endothelial cells. FASEB J. 21:3553–3561.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Furuichi K, Wada T, Iwata Y, Sakai N,
Yoshimoto K, Ki K Kobayashi, Mukaida N, Matsushima K and Yokoyama
H: Administration of FR167653, a new anti-inflammatory compound,
prevents renal ischaemia/reperfusion injury in mice. Nephrol Dial
Transplant. 17:399–407. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nicolau C, Le Pape A, Soriano P, Fargette
F and Juhel MF: In vivo expression of rat insulin after intravenous
administration of the liposome-entrapped gene for rat insulin I.
Proc Natl Acad Sci USA. 80:1068–1072. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Levine RM, Pearce TR, Adil M and Kokkoli
E: Preparation and characterization of liposome-encapsulated
plasmid DNA for gene delivery. Langmuir. 29:9208–9215. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kis A, Yellon DM and Baxter GF: Role of
nuclear factor-kappa B activation in acute ischaemia-reperfusion
injury in myocardium. Br J Pharmacol. 138:894–900. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Donnahoo KK, Shames BD, Harken AH and
Meldrum DR: Review article: The role of tumor necrosis factor in
renal ischemia-reperfusion injury. J Urol. 162:196–203. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nichols TC: NF-kappaB and reperfusion
injury. Drug News Perspect. 17:99–104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen WH, Zhang CY and Zhang GY:
Antioxidants attenuate reperfusion injury after global brain
ischemia through inhibiting nuclear factor-kappa B activity in
rats. Acta Pharmacol Sin. 24:1125–1130. 2003.PubMed/NCBI
|
|
23
|
Zou XL, Pei DA, Yan JZ, Xu G and Wu P: A20
overexpression inhibits lipopolysaccharide-induced NF-kappaB
activation, TRAF6 and CD40 expression in rat peritoneal mesothelial
cells. Int J Mol Sci. 15:6592–6608. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mazière C and Mazière JC: Activation of
transcription factors and gene expression by oxidized low-density
lipoprotein. Free Radic Biol Med. 46:127–137. 2009. View Article : Google Scholar : PubMed/NCBI
|