Introduction
Heart ischemia, with high morbidity and mortality
rates, is a public health concern worldwide. To reduce ischemic
myocardial injury and infarct size, the most effective therapy is
efficient myocardial reperfusion. However, this process can induce
further myocardial injury, known as myocardial ischemia/reperfusion
(MI/R) injury (1,2). MI/R injury was first described by
Jennings et al in 1960. It was observed that reperfusion
accelerated the development of necrosis in a canine coronary
ligation model and histological changes were observed following I/R
(3). I/R injury comprises distinct
phases of cellular injury. During ischemia, ATP depletion, lactate
accumulation and acidosis are observed, and during reperfusion, the
production of reactive oxygen and nitrogen species are observed
(4). Every year, ~1,000,000
individuals suffer from myocardial infarction in the United States
alone, and almost 50% of cases of myocardial infarction occur
following I/R (5). Therefore,
protecting the heart from MI/R injury is a primary goal of
therapeutic intervention.
It is now well established that oxidative stress
resulting from I/R is important in the initiation and development
of heart failure (6) Upon
reperfusion of heart ischemic tissue, rebound hyperoxia and the
oxidation of reduced intermediates causes a burst of reactive
oxygen species (ROS) generation (7). In this acute phase, primary sources
of ROS are predominantly from the mitochondrial respiratory chain
and xanthine oxidase reaction. Cytokines released from damaged
cells due to the inflammatory response also cause delayed and
amplified generation of ROS (8).
The protective effect of antioxidants has provided indirect
evidence for a role of oxyradicals in I/R-induced myocardial damage
(9). This evidences suggests that
antioxidant therapies may be effective in protecting against I/R
injury and that the administration of antioxidants may provide
potential benefit by attenuating oxidative stress (10,11).
Therefore, the screening of antioxidant agents in natural products
to mitigate oxidative stress is necessary.
The transcription factor, NFE2-related factor 2
(Nrf2), is a member of the cap ‘n’ collar family and is a master
regulator of cellular redox status (12). In oxidative stress, Nrf2 is free
from kelch-like ECH-associated protein 1, a rapid ubiquitination
causing the degradation of Nrf2, and then translocates from the
cytoplasm into the nucleus. It upregulates the expression of
numerous cytoprotective phase II detoxifying enzymes and
antioxidant genes, which together suppress oxidative stress in the
heart, and serve as a negative regulator of maladaptive cardiac
remodeling and dysfunction (13).
Based on the above, Nrf2 is important in maintaining the functional
integrity of the heart under oxidative stress.
As the active ingredients of traditional Chinese
medicine (TCM) have multiple targets, there has been increasing
interest in the treatment of MI/R injury with TCM (14). Among these, Xinji pill (XJP), a
compound of Chinese medicine, is a good example. It was approved by
the China State Food and Drug Administration in 1997 and is used
for the treatment of angina pectoris and cardiac dysfunction. XJP
has been commonly used clinically for the integrative treatment of
patients with viral or ischemic heart disease, and has been well
evaluated in the patients (15).
The active components of XJP are extracted from Codonopsis
pilosula, Herba epimedii, Salvia miltiorrhiza,
Carthamus tinctorius, Radix puerariae, Fructus
trichosanthis, Allium macrostemon and Coptis
chinensis. However, the therapeutic mechanism of XJP against
MI/R injury remains to be fully elucidated. The present study aimed
to verify the cardioprotective effect of XJP during MI/R and
elucidate the potential mechanisms.
Materials and methods
Experimental animals
Adult male Sprague-Dawley rats (n=60, 250±15 g body
weight) were obtained from the Animal Experimental Center of the
Fourth Military Medical University (Xi'an, China). The protocols of
the experiments were approved by the Ethics Committee for Animal
Experimentation, and performed according to the Revised Guidelines
for Animal Experimentation of the Fourth Military Medical
University and the National Institute of Health Guide for the Care
and Use of Laboratory Animals published by the National Academy of
Sciences (8th edition, Washington DC, 2011) (16). The rats were fed in an environment
with a 12-h light-dark cycle at 25°C.
Drug and reagents
XJP (batch no. 20151108) was obtained from Shaanxi
Hospital of Traditional Chinese Medicine (Xi'an, China), which was
prepared from water and ethanol extracts of Codonopsis
pilosula, Herba epimedii, Salvia miltiorrhiza,
Carthamus tinctorius, Radix puerariae, Fructus
trichosanthis, Allium macrostemon and Coptis
chinensis according to the guidelines of Good Manufacturing
Practice and Good Laboratory Practice (17,18).
The Hospital Agency and Shaanxi Provincial Food and Drug
Administration (Xi'an, China) determined the content of its major
components. Saline was used to dissolve XJP and produce solutions
at concentrations of 20, 40 and 80 mg/ml for experiments.
Chloral hydrate was purchased from Tianjin Kermel
Chemical Reagent Co., Ltd. (Tianjin, China), which was freshly
prepared prior to experiments in a 5% solution with saline. Kits
for the BCA protein assessment and detection of malondialdehyde
(MDA) were purchased from Nanjing Jiancheng Bioengineering
Institute (Nanjing, China). The antibodies against Nrf2 (cat. no.
sc-13032), heme oxygenase-1 (HO-1; cat. no. sc-10789), Akt (cat.
no. sc-8312) and phosphorylated (p) Akt (cat. no. sc-33437) were
obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
2′,7′-dichlorofluorescein diacetate (DCFH-DA) was purchased from
Molecular Probes; Thermo Fisher Scientific, Inc. (Waltham, MA,
USA).
Rat MI/R injury model and drug
administration
The rats were randomly divided into the following
groups: Sham, I/R, XJP 20 mg/ml + I/R, XJP 40 mg/ml + I/R and XJP
80 mg/ml + I/R. There were 6 rats in each group. The rats in the
treatment groups were intragastrically administered with XJP in
saline once daily for 14 days prior to I/R surgery. The rats in the
Sham group and I/R groups received saline solution alone at the
same time.
The rats were anesthetized by intraperitoneal
injection by 5% chloral hydrate at a dose of 350 mg/kg and all
experimental animal body temperatures were maintained at 37°C.
Following performing of left thoracotomy and exposure of the
hearts, a 6–0 silk suture was passed underneath the LAD (2–3 mm
inferior to the left auricle) and a slipknot was tied. MI/R was
induced by 30 min of ischemia followed by 24 h of reperfusion.
Significant changes, including widening of the QRS complex and
elevation of the ST segment detected using electroencephalography,
were indicators of successful coronary occlusion. The animals in
the Sham group underwent the same procedure but without ligation
with suture silk. Blood was collected from the abdominal aorta 24 h
following reperfusion, and the hearts were immediately removed and
rinsed with pre-cooled saline, prior to being rapidly frozen in
liquid nitrogen and preserved at −80°C.
Cardiac function and myocardial
infarct size determination
At 24 h post-reperfusion, cannulation, which was
connected to a biofunction experiment system (MP100-CE; Biopac
Systems, Inc., Santa Barbara, CA, USA), was performed to the left
ventricle through the right carotid artery following induction of
anesthesia with 5% chloral hydrate by intraperitoneal injection.
Heart rate (HR), left ventricular systolic pressure (LVSP), and
left ventricular maximum upstroke and descent velocity (+dp/dt
max/−dp/dt max) were measured by computer algorithms and an
interactive videographics program (Po-Ne-Mah Physiology Platform P3
Plus; Gould Instrument Systems, Valley View, OH, USA). The MI size
was determined using the Evans blue/TTC double staining method as
described previously (19)
following completion of functional determination.
Lactate dehydrogenase (LDH) and creatinine kinase
(CK) release evaluation. To isolate serum, blood samples were
collected from the abdominal aorta at 24 h post-reperfusion and
centrifuged at 3,250 × g for 10 min at 4°C. The activities of LDH
and CK in plasma were measured to evaluate myocardial damage using
commercially available assay kits (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China).
ROS levels in the myocardial
mitochondria
A DCFH-DA fluorescent probe detection kit was used
to measure the intramitochondrial ROS levels. Mitochondria in
myocardial tissue were extracted and purified by a tissue
mitochondria isolation kit from Beyotime (Beyotime Institute of
Biotechnology, Haimen, China) Purified mitochondria were incubated
with DCFH-DA (1 µM) at 37°C for 30 min and then washed three times
with phosphate-buffered saline. The DCFH-DA-loaded mitochondria
were excited at 488 nm, and the fluorescence emission was collected
at 525 nm.
Tissue MDA content analysis
For the determination of MDA content, cardiac
tissues were collected as described above and measured using a
commercial kit from Nanjing Jiancheng Bioengineering Institute.
Briefly, tissue homogenate was added to phosphate-buffered saline
solution and ultra-sonication was used for complete homogenization.
Following centrifugation at 1,500 × g at 4°C for 15 min, the
supernatants were collected into glass tubes. The supernatants were
reacted with sodium acetate solution containing thiobarbituric acid
at 95°C for 40 min. Following centrifugation at 1,500 × g for 15
min at room temperature, the supernatants were collected and the
reaction products were measured spectrophotometrically at 532 nm
absorbance and expressed as MDA equivalents (pmol) per milligram of
proteins.
Western blot analysis
Following reperfusion, ~50 mg of myocardial tissue
was collected and stored at −80°C. Radioimmunoprecipitation assay
lysis buffer was used to extract the whole protein, and the
concentration was determined using a BCA protein kit according to
the manufacturer's protocol instructions, with the mean
concentration values computed. The assessment was performed twice
for each sample, and the values were averaged. The samples were
stored at −80°C.
Equal quantities (30 µg) of protein was separated on
a 10% Tris-glycine SDS-PAGE polyacrylamide gel and transferred onto
polyvinylidene fluoride membranes (Invitrogen; Thermo Fisher
Scientific, Inc.). Following blocking for 1.5 h with a 5% solution
of skim milk, membranes were incubated with primary antibodies
against Nrf2, HO-1, Akt, p-Akt and GAPDH at 1:1,000 dilution at 4°C
overnight, followed by the horseradish peroxidase-linked anti-goat
antibody for 60 min at 37°C. Following washing with Tris-buffered
saline, the relative intensity of protein signals were normalized
to the corresponding GAPDH intensity and quantified using
densitometric analysis with QuantityOne software version 4.62
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
TRIzol lysis buffer was used to extract total RNA
and concentrations were measured using a ND-1000 spectrophotometer
(Nano-Drop Technologies; Thermo Fisher Scientific, Inc.). cDNA was
synthesized, according to the manufacturer's protocol. The mRNA
expression levels of superoxide dismutase (SOD), glutathione
reductase (GSR), catalase (CAT) and glutathione peroxidase (GSH-Px)
were determined using RT-qPCR analysis in a Step One Real-Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). For
each sample, a 14 µl mix containing 1 µg total RNA, 1 µl of a 50 µM
Oligo (dT), 1 µl of a 10 mM dNTP mix and RNase-free dH2O
was heated at 65°C for 5 min, and cooled on ice. After this, a 6 µl
mixture containing 1 µl 200 U/µl SuperScript™ III Reverse
Transcriptase, 4 µl 5X First-Strand Buffer (Invitrogen; Thermo
Fisher Scientific, Inc.) and 1 µl of 40 U/µl RNase inhibitor was
added. Subsequently, reverse transcription was performed with an
initial priming step at 30°C for 10 min, followed by cDNA synthesis
at 42°C for 50 min. Activation was stopped at 70°C for 15 min.
RT-qPCR was performed with SYBR-Green-based detection reagents
(Roche Applied Science, Penzberg, Germany). Thermal cycling
conditions were as follows: An initial predenaturation step at 94°C
for 10 min, followed by 35 cycles of denaturation at 94°C for 1
min, annealing at 58°C for 50 sec, extension at 72°C for 1 min and
a final extension step at 72°C for 7 min. Serial dilutions of cDNA
from the Sham surgery group were used to produce standard curves.
cDNA was run in duplicate. To account for differences in cDNA
preparation and cDNA amplification efficiency, the mRNA expression
was normalized to that of GAPDH. To reliably estimate changes in
mRNA levels, a standard curve for each mRNA was generated using
serial dilutions of a standard cDNA, as described previously
(20). The relative data for each
gene were calculated by the 2−ΔΔCq method (21). A value of 1 was attributed to sham
and all mRNA levels were expressed as an n-fold difference relative
to the sham. The sequences of the primers used were as follows: SOD
sense, 5′AGA TGA CTT GGG CAA AGG TG3′ and antisense, 5′CAA TCC CAA
TCA CAC CAC AA3′; CAT sense, 5′ACA TGG TCT GGG ACT TCT GG3′ and
antisense, 5′CCA TTC GCA TTA ACC AGC TT3′; GAPDH sense, 5′CCA TCA
CTG CCA CTC AGA AGA C3′ and antisense, 5′TCA TTG GCA GGT TTC TCC
A3′; GSH-Px sense, 5′GAT TCG TTC CAA ACT TCC TGC TA3′ and
antisense, 5′GCT CCC AGA ACA GCC TGT TG3′; GSR sense, 5′GGA AGT CAA
CGG GAA GAA GTT CAC TG3′ and antisense, 5′CAA TGT AAC CGG CAC CCA
CAA TAA C3′.
Caspase-3 activity
A fluorometric assay kit was used to measure
caspase-3 activity according to the manufacturer's protocol. In
brief, heart tissues were lysed in 50 µl of ice-cold lysis buffer
at 4°C for 30 min, and then centrifuged at 12,000 g at 4°C for 10
min. A BCA protein assay kit was used to quantify the protein
concentrations in the supernatants. Reaction buffer (50 µl) and
caspase-3 substrate (5 µl) were added to the lysates (40 µg per
assay). Following incubation for 4 h at 37°C, the fluorescence was
measured using a microplate reader with excitation at 400 nm
(Fluoroskan Ascent; Thermo Labsystems, Santa Rosa, CA, USA).
Statistical analysis
All values are expressed as the mean ± standard
deviation and analyzed using SPSS 18.0 statistical software (SPSS,
Inc., Chicago, IL, USA. The difference between two groups was
analyzed using one-way analysis of variance followed by Tukey's
test for multiple comparisons. P<0.05 was considered to indicate
a statistically significant difference.
Results
XJP ameliorates myocardial
function
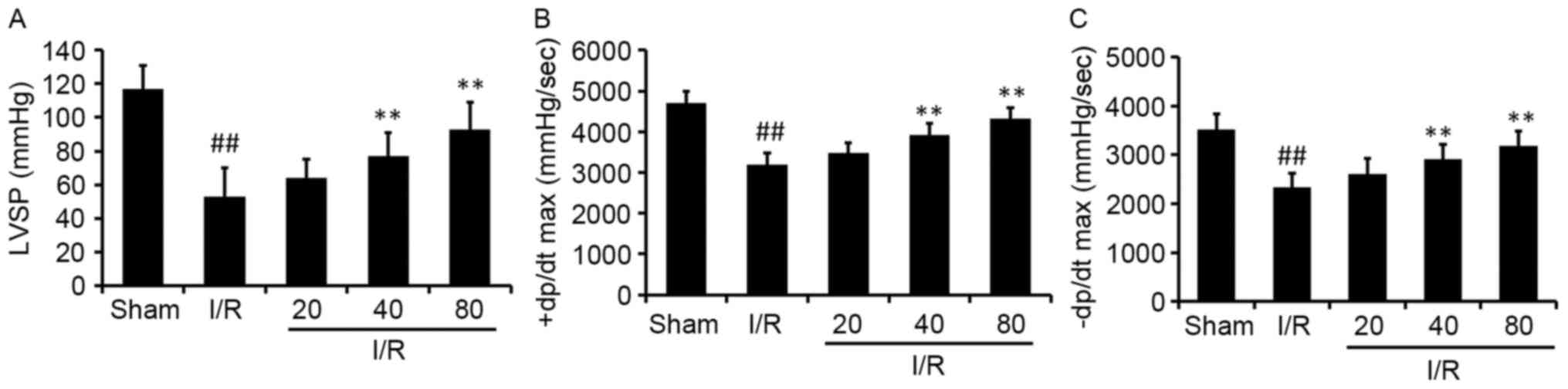
The effect of XJP on LVSP and the ±dp/dt max of the
left ventricle following MI/R for 24 h were evaluated. The rats
treated with XJP had significantly improved LVSP, +dp/dt max and
-dp/dt max 24 h following I/R, compared with I/R-treated animals
(Fig. 1). The HR and mean arterial
blood pressure (MABP) among groups were also evaluated, with no
significant differences in the changes. These results suggested
that XJP treatment provided benefits for myocardial function
recovery following MI/R.
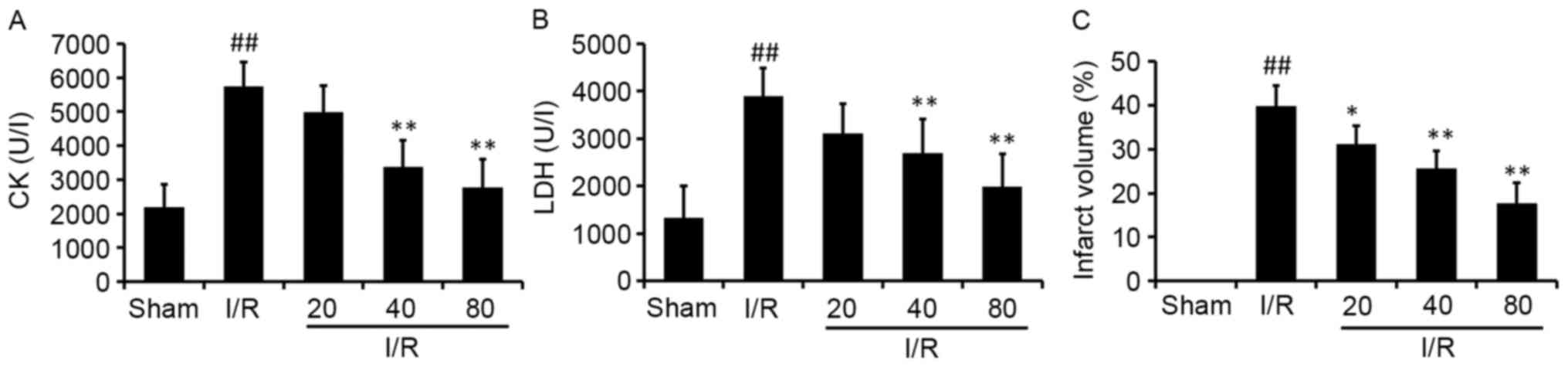
XJP reduces MI/R injury
To determine the cardioprotective effects of XJP
pretreatment on the injury induced by I/R, cardiac enzyme levels
(CK and LDH) were determined using a microplate reader. The results
showed that the rats in the model group had significantly higher
levels of CK and LDH, compared with those in the Sham group
(Fig. 2A and B). Compared with the
I/R group, the XJP treatment groups had significantly decreased
levels of CK and LDH in a dose-dependent manner. This indicated
that XJP may have protected the cardiomyocytes from injury
following MI/R.
The effect of XJP on infarct size was also examined
(Fig. 2C). It was found that the
infarct size was significantly increased by I/R treatment, compared
with the sham group, and the size was significantly reduced by XJP
treatment, compared with that in the I/R group. The results
revealed for infarct size and cardiac enzymes suggested that XJP
was useful in attenuating MI/R injury.
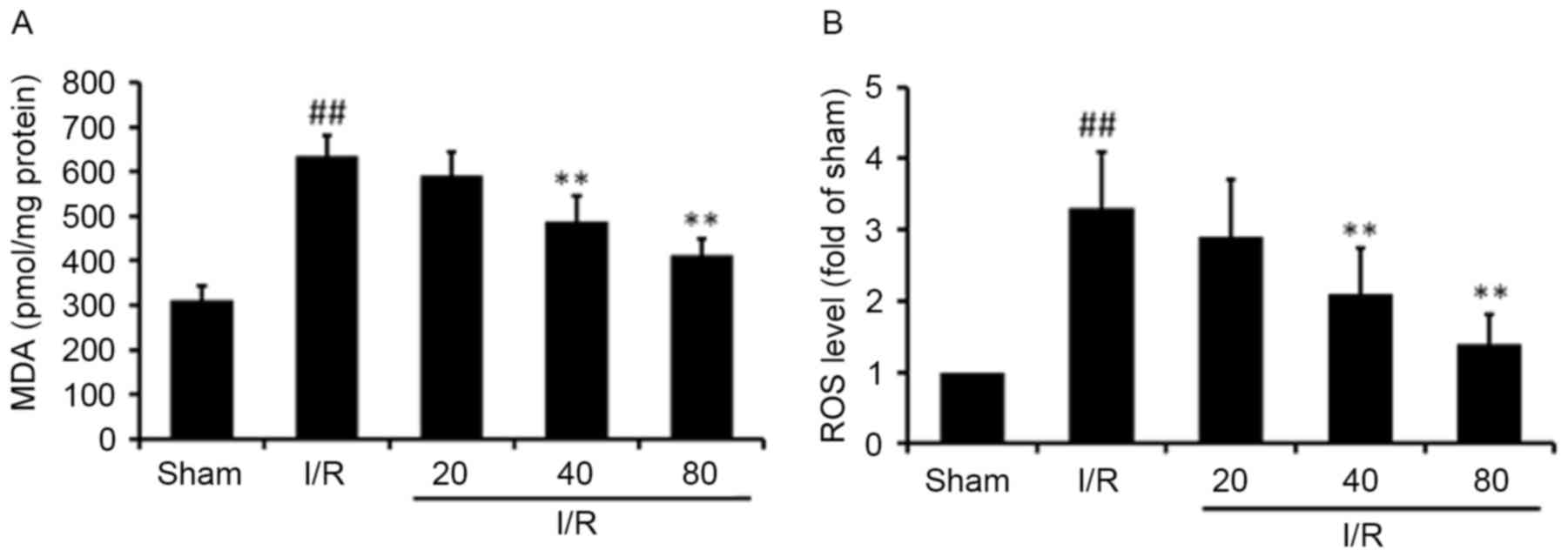
XJP reduces myocardial ROS and MDA
levels
Acute I/R can induce the enhanced generation of high
ROS; therefore, cardiac levels of ROS and MDA were measured. As
shown in Fig. 3, MDA levels in the
I/R-treated rats were significantly higher, compared with those in
the Sham rats (P<0.01). Pretreatment of the rats with XJP at
concentrations of 40 or 80 mg/kg significantly decreased MDA
levels, compared with those in the model group (Fig. 3A). In addition, in the I/R group,
ROS levels were higher, compared with those in the Sham group in
myocardial mitochondria, as shown in the fluorescence intensity
assay, however, in the rats pretreated with XJP, ROS levels were
significantly decreased, compared with those in the I/R group
(Fig. 3B).
XJP increases the mRNA expression of antioxidant
proteins. At the end of reperfusion, the mRNA levels of SOD, GSR,
CAT and GSH-Px were quantified using RT-qPCR analysis (Fig. 4). In the I/R group, the mRNA levels
of SOD, GSR, CAT and GSH-Px were significantly decreased
(P<0.01), compared with those in the Sham group, whereas the
mRNA expression of the antioxidant proteins in the XJP-treated
groups were significantly higher, compared with those in the I/R
group (P<0.01).
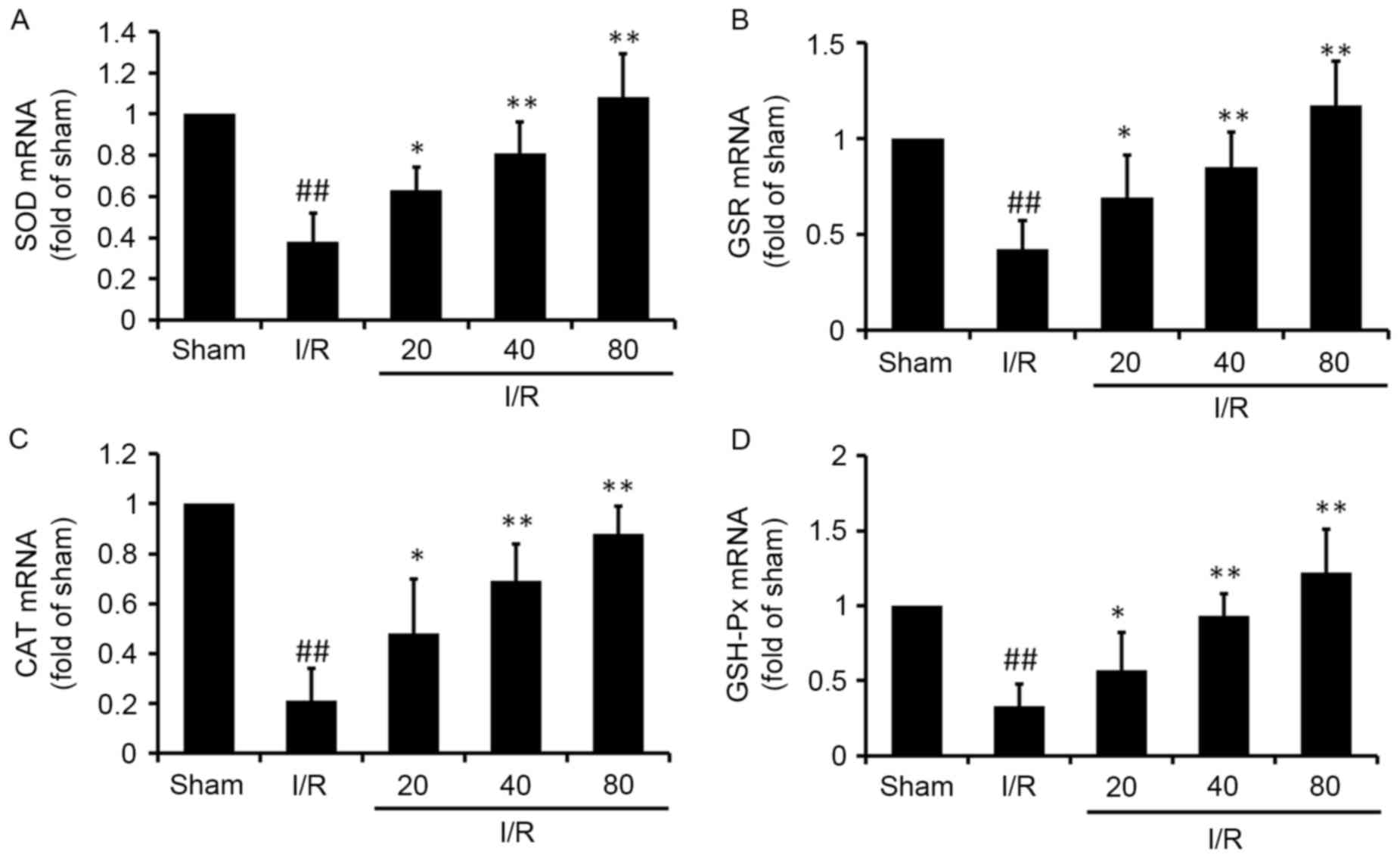 | Figure 4.XJP regulates the mRNA expression of
SOD, GSR, CAT and GSH-Px in rat hearts subjected to I/R. Reverse
transcription-quantitative polymerase chain reaction analysis was
used to assess the relative mRNA levels of cardiac (A) SOD, (B)
GSR, (C) CAT and (D) GSH-Px. Results were normalized to GAPDH. Data
are expressed as the mean ± standard deviation (n=6/group).
##P<0.01, vs. Sham group; *P<0.05 and **P<0.01,
vs. I/R group. XJP, Xinji pill; I/R, ischemia/reperfusion; SOD,
superoxide dismutase; GSR, glutathione reductase; CAT, catalase;
GSH-Px, glutathione peroxidase. |
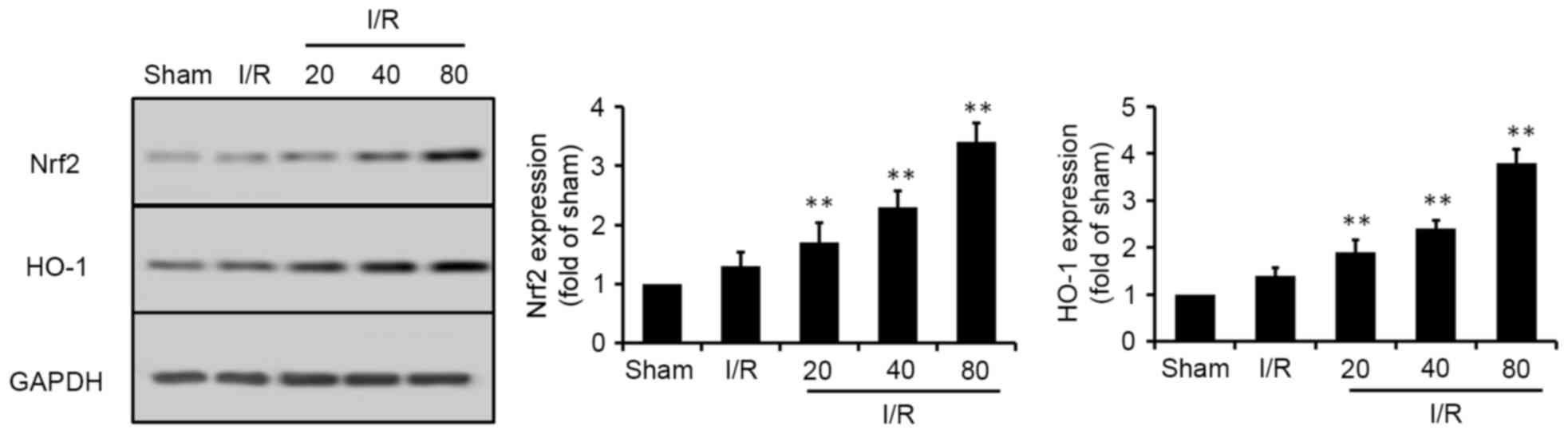
XJP increases the phosphorylation of
Akt and the expression of Nrf2
Nrf2, a crucial regulator against oxidative stress,
can regulate the expression of antioxidant proteins. Therefore, the
present study examined the effects of XJP on the Nrf2 signaling
pathway. HO-1, a cytoprotective downstream target protein of Nrf2,
is a typical antioxidant. To determine the potential effects of XJP
on the Nrf2 signaling pathway in vivo, the expression of
Nrf2 and HO-1 were analyzed. As shown in Fig. 5, I/R induced a marginal increase in
the expression levels of Nrf2 and HO-1, compared with those in the
Sham group. The expression levels of Nrf2 and HO-1 were further
increased by XJP pretreatment in a dose-dependent manner.
Akt pathway mediates the activation of
Nrf2 by XJP
It has been shown that activation of the Akt pathway
can protect the heart from I/R injury (22). To further investigate upstream of
Nrf2 and the mechanisms underlying the XJP-induced cardioprotective
effects, the activation of Akt was measured using western blot
analysis for rats subjected to MI/R (Fig. 6A). The results showed that the
phosphorylation of Akt in the heart was significantly decreased by
I/R treatment, compared with that in the sham group (P<0.01).
However, pretreatment with XJP for 14 days significantly increased
the phosphorylation of Akt in the heart. These results suggested
that Akt kinase may have be involved in the XJP-stimulated
activation of Nrf2.
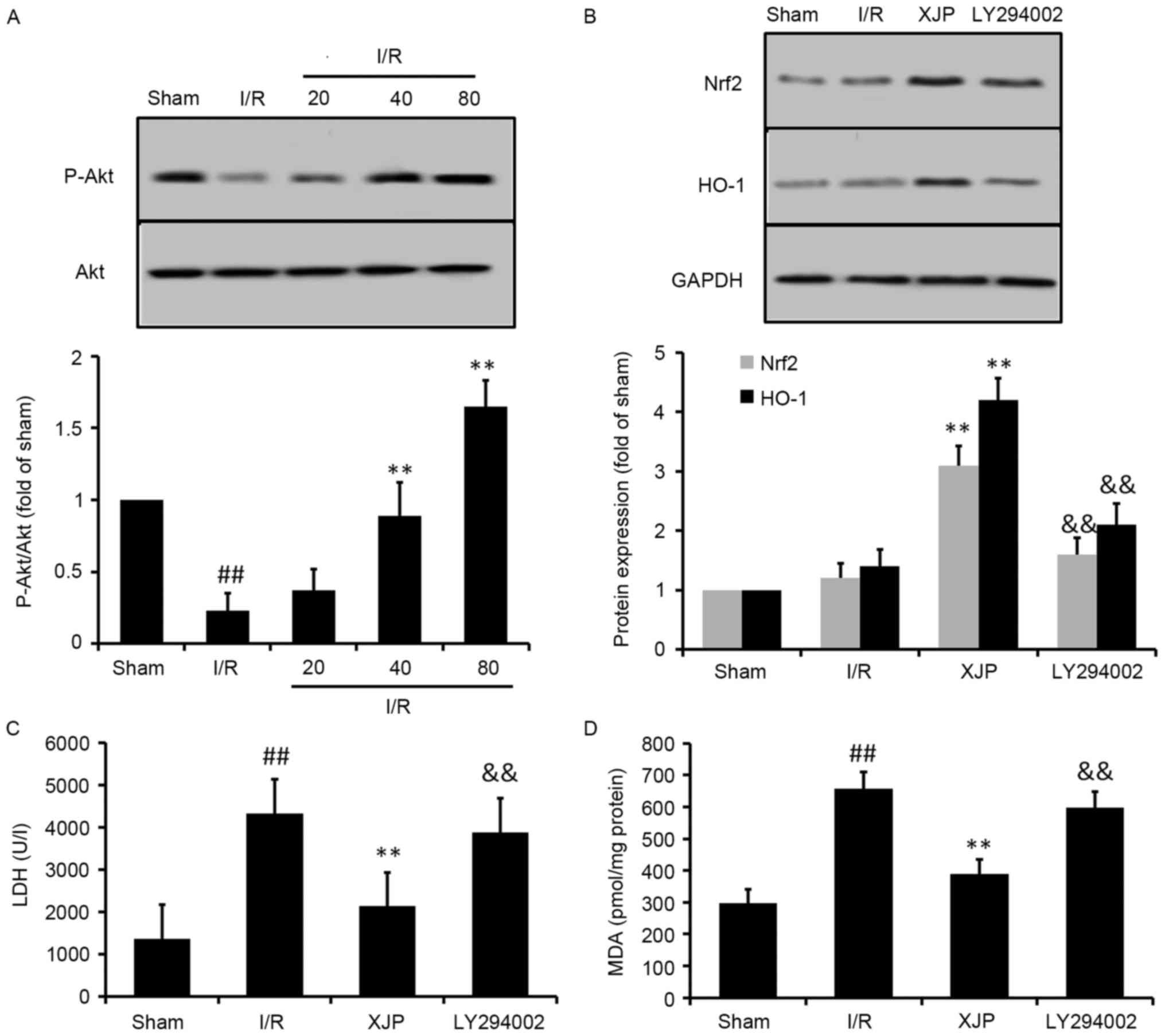 | Figure 6.Role of Akt on the expression of Nrf2
by XJP in MI/R rats. (A) Phosphorylation of Akt induced by XJP. (B)
Effects of inhibition of Akt in XJP-induced expression of Nrf2 and
HO-1. Experiments aimed to determine the role of the Akt pathway in
the protective effect of XJP against I/R-induced injury in rats,
demonstrated by levels of (C) LDH and (D) MDA. Data are expressed
as the mean ± standard deviation (n=6/group).
##P<0.01, vs. Sham group; **P<0.01, vs. I/R group;
&&P<0.01, vs. I/R+XJP group. XJP, Xinji pill;
I/R, ischemia/reperfusion; Nfr2, NFE2-related factor 2; HO-1, heme
oxygense-1; MDA, malondialdehyde; LDH, lactase dehydrogenase p-Akt,
phosphorylated Akt. |
To further confirm these findings, an inhibitor of
phosphoinositide 3-kinase (PI3K)/Akt signaling, LY294002 was used
subsequent experiments. As shown in Fig. 6B, the XJP-induced expression of
Nrf2 and HO-1 were effectively inhibited in the presence of
LY294002. It was also observed that LY294002 eliminated the
cardioprotective effects of XJP, which was shown by the changes in
the levels of LDH (Fig. 6C) and
MDA (Fig. 6D).
Discussion
The present study is the first, to the best of our
knowledge, to examine the cardioprotective effects of XJP against
MI/R injury in rats. It was found that pretreatment with XJP
significantly improved cardiac function and reduced infarct size in
the I/R rat hearts in vivo. These beneficial effects were
demonstrated by the preservation of left ventricular function, as
reflected by a significant increase in the indices of contraction
(+dP/dt max) and relaxation (−dP/dt max), and the increase in
preload (LVSP). The results demonstrated that XJP reduced
I/R-induced myocardial injury, increased the expression levels of
Nrf2 and Akt, increased the levels of antioxidant proteins
following MI/R, and inhibited necrosis in the I/R myocardium.
CK and LDH are used clinically as biomarkers of
myocardial damage. They are expressed constitutively in endochylema
and, in the normal physiological state, cannot transit through
cytoplasmic membrane. LDH and CK are released from cells when the
cell is damaged or dead, therefore, they are appropriate for the
assessment of cellular injury (23–25).
The activities of LDH and CK in the serum can represent the extent
of myocardial injury induced by I/R. The results of the present
study indicated that I/R significantly increased the serum levels
of LDH and CK, whereas pretreatment with XJP significantly
decreased these changes. These results showed that XJP had
cardioprotective effects against I/R injury.
There are several reports that oxidative stress
contributes to the pathogenesis of MI/R injury (26,27).
In normal physiological conditions, ROS are usually scavenged by
antioxidants. In disease states, including sudden hypoxia, the
overproduction of ROS and overconsumption of antioxidants results
in oxidative cellular damage (28,29).
ROS can oxidize nucleic acids, proteins and lipids, and affect
critical signal transduction pathways (30,31).
Finally ROS-induced abnormalities result in alterations of cardiac
function, cardiac stunning, arrhythmias, cellular injury and death
(32,33). Cellular antioxidants can scavenge
ROS and minimize injuries caused by the oxidative stress (34,35).
In the present study, it was found that I/R caused a rapid and
significant increase in ROS generation and levels of MDA in the
heart. Pretreatment with XJP eliminated these increases to a
certain extent. These results suggested that the attenuation of
oxidative stress was involved in the cardioprotective effect of
XJP.
The removal of excess ROS requires several
antioxidant enzymes. Among these, SOD can transform intracellular
superoxide anions to H2O2, which can be
scavenged by CAT and GSH-Px through enzymatic reactions. CAT, an
enzyme located in the peroxisome, promotes the conversion of
H2O2 to H2O and O2
(36). GSH-Px acts in conjunction
with the GSH tripeptide, which is present in cells in high
(micromolar) concentrations. GSH-Px decomposes peroxides to water
and simultaneously oxidizes GSH (37). To further support the findings of
the present study, the gene expression levels of SOD, GSR, CAT and
GSH-Px were determined using RT-qPCR analysis. In the cardiac
muscle tissue exposed to I/R, it was found that the expression
levels of SOD, GSR, CAT and GSH-Px were significantly enhanced
following treatment with XJP. Thus, the protective effect of XJP
pretreatment may be achieved through upregulation in the gene
expression levels of SOD, GSR, CAT and GSH-Px, and the subsequent
inhibition of oxidative stress.
Nrf2, a redox-sensitive transcription factor,
predominantly mediates the transcriptional regulation of
antioxidant genes, including SOD, GSR, CAT and GSH-Px. It is
expressed in multiple tissues, but is only activated in response to
certain electrophilic agents and oxidative stress, including ROS,
specific antioxidants and certain disease processes. Upon
activation, the interaction between Nrf2 with the
antioxidant-response element (ARE) mediates the induction of a
series of cytoprotective proteins, including phase II enzymes SOD,
GSH and CAT (38,39). These findings suggest that
activation of the Nrf2/ARE pathway may be involved in the gene
expression and activities of SOD, CAT, GSH and GPx induced by XJP.
In order to confirm this, the present study examined the effects of
XJP on the protein expression levels of Nrf2 and HO-1. The data
showed that XJP upregulated the expression of Nrf2 in the hearts
subjected to I/R for the first time, demonstrating the activation
of a cytoprotective pathway. Further investigation showed that XJP
caused an increase in the expression of HO-1, which is a gene known
to be upregulated by the activation of Nrf2.
A number of studies have identified that Akt is
involved in the activation of Nrf2/ARE and its associated gene
expression, and several studies have shown that several
phytochemicals from herbal medicines, including butin and 3
a,4′-didemethylnobiletin, protect against oxidative stress-induced
cell injury via the PI3K/Akt/Nrf2-dependent pathway (40). The present study investigated
whether this pathway contributed to the protective effects of XJP
against I/R-induced oxidative stress. Of note, the phosphorylation
of Akt was significantly increased in the XJP-treated hearts in a
dose-dependent manner, and the inhibition of Akt signaling by the
Akt inhibitor, LY294002, completely inhibited the XJP-induced
protein expression of Nrf2 and HO-1. Subsequent experiments showed
that LY294002 eliminated the ability of XJP to control the levels
of LDH and MDA, which were significantly increased by I/R. These
results indicated that Akt/Nrf2 signaling was involved in the
cytoprotective effects of XJP.
In conclusion, the present study demonstrated that
XJP protected myocardial function and damage in rats exposed to
MI/R injury. It significantly decreased infarct volume, improved
hemodynamics and alleviated myocardial damage. The cardioprotective
effects of XJP against I/R injury may be attributed to the
increasing activities and protein expression levels of certain
antioxidative enzymes, including SOD, CAT, GSR and GSH-Px. These
may occur through a mechanism involving the activation of Akt and
the upregulted expression of Nrf2 and its downstream antioxidant
genes. These findings provide insight into the protective potential
of XJP in MI/R injury.
Acknowledgements
The present study was supported by the Key Project
of Natural Science Foundation Research of Shaanxi province (grant
no. 2014JZ2-006).
References
|
1
|
Zhong X, Li X, Qian L, Xu Y, Lu Y, Zhang
J, Li N, Zhu X, Ben J, Yang Q and Chen Q: Glycine attenuates
myocardial ischemia-reperfusion injury by inhibiting myocardial
apoptosis in rats. J Biomed Res. 26:346–354. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Piper HM, García-Dorado D and Ovize M: A
fresh look at reperfusion injury. Cardiovasc Res. 38:291–300. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jennings RB, Sommers HM, Smyth GA, Flack
HA and Linn H: Myocardial necrosis induced by temporary occlusion
of a coronary artery in the dog. Arch Pathol. 70:68–78.
1960.PubMed/NCBI
|
|
4
|
Armstrong SC: Protein kinase activation
and myocardial ischemia/reperfusion injury. Cardiovasc Res.
61:427–436. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Go AS, Mozaffarian D, Roger VL, Benjamin
EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al:
Heart disease and stroke statistics-2013 update: A report from the
American Heart Association. Circulation. 127:e6–e245. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murphy E and Steenbergen C: Mechanisms
underlying acute protection from cardiac ischemia-reperfusion
injury. Physiol Rev. 88:581–609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Becker LB: New concepts in reactive oxygen
species and cardiovascular reperfusion physiology. Cardiovasc Res.
61:461–470. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Frangogiannis NG, Smith CW and Entman ML:
The inflammatory response in myocardial infarction. Cardiovasc Res.
53:31–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Laskowski A, Woodman OL, Cao AH, Drummond
GR, Marshall T, Kaye DM and Ritchie RH: Antioxidant actions
contribute to the antihypertrophic effects of atrial natriuretic
peptide in neonatal rat cardiomyocytes. Cardiovas Resear.
72:112–123. 2006. View Article : Google Scholar
|
|
10
|
Moens AL, Claeys MJ, Timmermans JP and
Vrints CJ: Myocardial ischemia/reperfusion-injury, a clinical view
on a complex pathophysiological process. Int J Cardiol.
100:179–190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rajak S, Banerjee SK, Sood S, Dinda AK,
Gupta YK, Gupta SK and Maulik SK: Emblica officinalis causes
myocardial adaptation and protects against oxidative stress in
ischemic-reperfusion injury in rats. Phytother Res. 18:54–60. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Ichikawa T, Villacorta L, Janicki
JS, Brower GL, Yamamoto M and Cui T: Nrf2 protects against
maladaptive cardiac responses to hemodynamic stress. Arterioscler
Thromb Vasc Biol. 29:1843–1850. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McMahon M, Itoh K, Yamamoto M and Hayes
JD: Keap1-dependent proteasomal degradation of transcription factor
Nrf2 contributes to the negative regulation of antioxidant response
element-driven gene expression. J Biol Chem. 278:21592–21600. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boran AD and Iyengar R: Systems approaches
to polypharmacology and drug discovery. Curr Opin Drug Discov
Devel. 13:297–309. 2010.PubMed/NCBI
|
|
15
|
Zhang A and Hui A: Inhibition of coxsackie
virus and effect on the treatment of viral myocarditis of Xinji
Pill in mice. Shanxi Tradit Chin Med. 12:563–564. 1998.(In
Chinese).
|
|
16
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th.
National Academies Press (US); Washington, DC: pp. 1072–1073.
2011
|
|
17
|
China's food and drug administration, .
Good laboratory practice. Standards Press of China; Beijing: pp.
54–59. 2003
|
|
18
|
China's food and drug administration: Good
manufacturing practice. Standards Press of China; Beijing: pp.
106–109. 2015
|
|
19
|
Tao L, Gao E, Bryan NS, Qu Y, Liu HR, Hu
A, Christopher TA, Lopez BL, Yodoi J, Koch WJ, et al:
Cardioprotective effects of thioredoxin in myocardial ischemia and
reperfusion: Role of S-nitrosation (corrected). Proc Natl Acad Sci
USA. 101:11471–11476. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao Z and Li Y: Protecting against
peroxynitrite-mediated cytotoxicity in vascular smooth muscle cells
via upregulating endogenous glutathione biosynthesis by
3H-1,2-dithiole-3-thione. Cardiovasc Toxicol. 4:339–353. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu W, Zhang P, Gu J, Yu Q and Zhang D:
NEDD4-1 protects against ischaemia/reperfusion-induced
cardiomyocyte apoptosis via the PI3K/Akt pathway. Apoptosis.
22:437–448. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cho MH, Niles A, Huang R, Inglese J,
Austin CP, Riss T and Xia M: A bioluminescent cytotoxicity assay
for assessment of membrane integrity using a proteolytic biomarker.
Toxicol In Vitro. 22:1099–1106. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Corey MJ, Kinders RJ, Brown LG and
Vessella RL: A very sensitive coupled luminescent assay for
cytotoxicity and complement-mediated lysis. J Immunol Methods.
207:43–51. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim H, Yoon SC, Lee TY and Jeong D:
Discriminative cytotoxicity assessment based on various cellular
damages. Toxicol Lett. 184:13–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu Y and Yuan BX: Effects of Qiangxin
capsules on myocardial reperfusion arrhythmias in rats. Northwest
Pharmac J. 16:23–24. 2001.
|
|
27
|
Fu J, Huang H, Liu J, Pi R, Chen J and Liu
P: Tanshinone IIA protects cardiac myocytes against oxidative
stress-triggered damage and apoptosis. Eur J Pharmac. 568:213–221.
2007. View Article : Google Scholar
|
|
28
|
Halliwell B and Gutteridge JMC: Free
radicals in biology and medicine. 3rd. Oxford: Clarendon Press; pp.
246–350. 1999
|
|
29
|
Maxwell SR and Lip GY: Reperfusion injury:
A review of the pathophysiology, clinical manifestations and
therapeutic options. Int J Cardiol. 58:95–117. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Duranteau J, Chandel NS, Kulisz A, Shao Z
and Schumacker PT: Intracellular signaling by reactive oxygen
species during hypoxia in cardiomyocytes. J Biol Chem.
273:11619–11624. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hensley K, Robinson KA, Gabbita SP,
Salsman S and Floyd RA: Reactive oxygen species, cell signaling,
and cell injury. Free Radic Biol Med. 28:1456–1462. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Das DK and Maulik N: Antioxidant
effectiveness in ischemia reperfusion tissue injury. Methods
Enzymol. 233:601–610. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lefer DJ and Granger DN: Oxidative stress
and cardiac disease. Am J Med. 109:315–323. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ferrari R, Ceconi C, Curello S, Cargnoni
A, Alfieri O, Pardini A, Marzollo P and Visioli O: Oxygen free
radicals and myocardial damage: Protective role of thiolcontaining
agents. Am J Med. 91 Suppl:95–105. 1991. View Article : Google Scholar
|
|
35
|
Frei B: On the role of vitamin C and other
antioxidants in atherogenesis and vascular dysfunction. Proc Soc
Exp Biol Med. 222:196–204. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Valko M, Rhodes CJ, Moncol J, Izakovic M
and Mazur M: Free radicals, metals and antioxidants in oxidative
stress-induced cancer. Chem Biol Interact. 160:1–40. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tengattini S, Reiter RJ, Tan DX, Terron
MP, Rodella LF and Rezzani R: Cardiovascular diseases: Protective
effects of melatonin. J Pineal Res. 44:16–25. 2008.PubMed/NCBI
|
|
38
|
Ma Q, Kinneer K, Bi Y, Chan JY and Kan YW:
Induction of murine NAD(P)H:quinine oxidoreductase by
2,3,7,8-tetrachlorodibenzo-p-dioxin requires the CNC (cap ‘n’
collar) basic leucine zipper transcription factor Nrf2 (nuclear
factor erythroid 2-related factor 2): Cross-interaction between AhR
(aryl hydrocarbon receptor) and Nrf2 signal transduction. Biochem
J. 377:205–213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
He X, Chen MG, Lin GX and Ma Q: Arsenic
induces NAD(P)H-quinone oxidoreductase I by disrupting the Nrf2 ×
Keap1 x·Cul3 complex and recruiting Nrf2 x·Maf to the antioxidant
response element enhancer. J Biol Chem. 281:23620–23631. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Su JD, Yen JH, Li S, Weng CY, Lin MH, Ho
CT and Wu MJ: 3′,4′-didemethylnobiletin induces phase II
detoxification gene expression and modulates PI3K/Akt signaling in
PC12 cells. Free Radic Biol Med. 52:126–141. 2012. View Article : Google Scholar : PubMed/NCBI
|