Introduction
Gastric cancer is one of the most common
malignancies worldwide with a mortality rate of >70%. East Asia,
Eastern Europe and South America are considered to be areas with
high incidences (1). The incidence
of gastric cancer ranks second in China among malignant cancers
(2,3). Furthermore, the overall incidence and
mortality of gastric cancer is markedly increased in rural areas
compared with urban areas, and gradually increases with age
(4,5). Although the survival of gastric
cancer patients is prolonged by effective treatment, the 5-year
survival rate remains very low (~20 to 25%) (6). Radical gastric tumor resection
combined with standard chemotherapy cannot remove the tumor
completely, which has become a major issue in current cancer
therapy (7).
Salinomycin is a type of carboxy-polyether type
compound first extracted from the white Streptomyces albus
by Japanese researchers in 1974 (8). Salinomycin is capable of neutralizing
cations within cells, and exhibits good inhibitory and destructive
effects on most gram-positive bacteria and all types of coccidian
(9–11). Gupta et al (12) in 2009 revealed that the toxicity of
salinomycin on breast cancer stem cells was 100 times that of the
chemotherapeutic drug paclitaxel. In previous years, numerous
studies have suggested that salinomycin exhibits anti-tumor
effects; therefore, it may represent a novel and effective
anticancer agent (9,13–19).
However, high doses of salinomycin has high neurotoxicity (20).
17-allylamine-17-demathoxygeldanamycin (17-AAG), an inhibitor of
heat shock protein (HSP) 90, shares an extremely similar structure
with geldanamycin. 17-AAG exhibits a more effective toxicity
profile (21,22). The anti-tumor effects of 17-AAG
have also been widely recognized (23).
In order to reduce salinomycin dose and the
associated toxicity, and to promote its use in cancer therapy, the
present study investigated the effects of salinomycin and 17-AAG
combined treatment on gastric cancer cells, which have not been
previously reported. This study focused on the inhibition of
salinomycin on proliferation of the SGC-7901 gastric cancer cell
line, and the pro-apoptotic underlying mechanism of salinolycin.
The present study aimed to provide a basis for the use of
salinomycin in gastric cancer treatment, in addition to
experimental evidence for understanding the mechanism underlying
the anti-tumor effects of salinomycin.
Materials and methods
Reagents and instruments
Salinomycin was purchased from Sigma-Aldrich
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). 17-AAG and MTT
were purchased from Sigma-Aldrich (Merck KGaA). RPMI-1640 medium
was purchased from Thermo Fisher Scientific, Inc. (Waltham, MA,
USA). Fetal bovine serum (FBS) was purchased from Zhejiang Tianhang
Biotechnology Co., Ltd. (Zhejiang, China). Propidium iodide (PI)
was purchased from Merck KGAa. Acridine orange (AO) was purchased
from Amresco, LLC (Solon, OH, USA). An Annexin-fluorescein
isothiocyanate (FITC)/PI Apoptosis kit was purchased from BD
Biosciences (Franklin Lakes, NJ, USA). A DAB chromogenic kit,
rabbit anti human nuclear factor (NF)-κB p65 polyclonal antibody
(A00224) and rabbit anti human Fas-ligand (L) polyclonal antibody
(BA0049) were purchased from GenScript Co., Ltd. (Nanjing, China),
biotinylated goat anti rabbit IgG secondary antibody and
horseradish peroxidase-labeled avidin secondary antibody were
purchased from Wuhan Boster Biological Technology, Ltd. (Wuhan,
China).
A carbon dioxide incubator was purchased from Sanyo
Electric, Co. (Moriguchi, Japan). Fluorescence and inverted
microscopes were purchased from Nikon Corporation (Tokyo, Japan). A
flow cytometer was purchased from BD Biosciences. A microplate
reader was purchased from Bio-Rad Laboratories, Inc. (Hercules, CA,
USA).
Cell culture
The SGC-7901 human gastric cancer cell line was
purchased from the Digestion Experimental Research Center of Xi'an
Jiaotong University (Xi'an, China). Cells were cultured with RPMI
1640 medium supplemented with 10% FBS and 100 U/ml penicillin and
streptomycin, and incubated in 5% CO2 at 37°C with 95%
relative humidity.
MTT assay
SCG-7901 cells in the logarithmic phase were seeded
into 96-well plates at a density of 1×105/ml with 100 µl RPMI 1640
medium per well. Cells were divided into four groups: Salinomycin
treated (2, 4, 8, 16 and 32 µmol/l); 17-AAG treated (0.625 µmol/l);
salinomycin (4, 8 and 16 µmol/l) combined with 17-AAG treated
(0.625 µmol/l); and the control (complete RPMI 1640 medium). The
total volume of each well was 100 µl and 5 duplicate wells were set
for each group. After incubation for 24, 48 or 72 h, the
supernatant was removed by centrifugation at 300 × g for 5 min at
room temperature, and 20 µl MTT was added. After a 4-h incubation,
MTT was removed and 150 µl dimethyl sulfoxide was added. The
optical density at a wavelength of 495 nm was detected using a
microplate reader. The experiment was repeated three times.
Morphology assay
SGC-7901 cell suspension was seeded into 6-well
plates at a density of 2×105 per well. After incubation for 24 h,
cells were treated with salionmycin (4, 8 or 16 µmol/l), 17-AAG
(0.625 µmol/l), or salinomycin (8 µmol/l) combined with 17-AAG
(0.625 µmol/l). Untreated cells served as the negative control.
After a 48-h incubation, cell morphology was observed under an
inverted phase contrast microscope. The above step was repeated
three times.
Apoptosis assay
SGC-7901 cells were treated as described above,
washed twice with PBS after a 48-h incubation, and then stained in
the dark using PI or AO. Cell apoptotic morphology was observed
using a fluorescent microscope.
In addition, cells were collected after trypsin
digestion, washed twice with PBS, collected in Eppendorf (EP) tubes
and stained with Annexin V-FITC and PI for 10 min. The cell
apoptotic rate of each group was detected with a flow
cytometer.
Cell cycle assay
Cells were seeded into 6-well plates and cultured
for 24 h, and then cultured in RPMI 1640 medium supplemented with
0.5% FBS for synchronization. After a continuous culture for 24 h,
cells were treated as for the morphology assay and cultured for 48
h. Cells were digested, collected, fixed in 70% alcohol for 30 min,
centrifuged at 300 × g for 5 min at room temperature to remove the
supernatant, washed with PBS and collected into an EP tube. PI (the
working solution concentration was 50 µg/ml) staining was performed
at 4°C for 30 min before a cell cycle assay was performed by flow
cytometry.
Immunocytochemistry assay
SGC-7901 cells were treated as those mentioned in
the morphology assay, washed with PBS three times after a 48-h
incubation, and fixed with 4% paraformaldehyde for 20 min. Cells
were then fixed with neutral balsam after drying, incubated with
0.3% Triton X-100 for 15 min at room temperature, and incubated
with trypsin at 37°C for 30 min and H2O2 for
20 min at room temperature after PBS washing. Subsequently, cells
were blocked with blocking reagent for 30 min and incubated with a
rabbit anti human NF-кB p65 polyclonal antibody (1:100) or a rabbit
anti human Fas-L polyclonal antibody (1:100) at 4°C overnight,
followed by PBS washing. Following this, cells were incubated with
a biotinylated goat anti rabbit secondary antibody (1:100) for 2 h,
washed with TBS, incubated with a horseradish peroxidase-labeled
avidin secondary antibody (1:100) for a further 30 min at room
temperature, and washed three times with TBS. DAB was used for
color development. Cells were re-stained with hematoxylin,
differentiated with hydrochloric acid alcohol, dehydrated with
alcohol, cleared with xylene, and mounted with neutral balsam.
Cells were imaged under a Nikon E600 microscope and protein
expressions were analyzed using NIS-Elements Documentation (Version
3.0.455) (both from Nikon Corporation, Tokyo, Japan). All the
antibodies were diluted with PBS. Untreated cells incubated with
PBS, instead of the primary antibodies, served as the secondary
antibody control.
Statistical analysis
SPSS software version 22.0 (IBM SPSS, Armonk, NY,
USA) was used for statistical analysis. All data are presented as
the mean ± standard deviation. Comparison between groups was
conducted by one-way analysis of variance, followed by Fisher's
least significant difference post hoc test. P<0.05 was
considered to indicate a significantly different difference.
Results
Salinomycin and 17-AAG alter SGC-7901
cell morphology
To determine the effect of salinomycin and 17-AAG on
cell morphology, cells were treated with salinomycin, 17-AAG, or
salinomycin+17-AAG, and cell morphology was observed under an
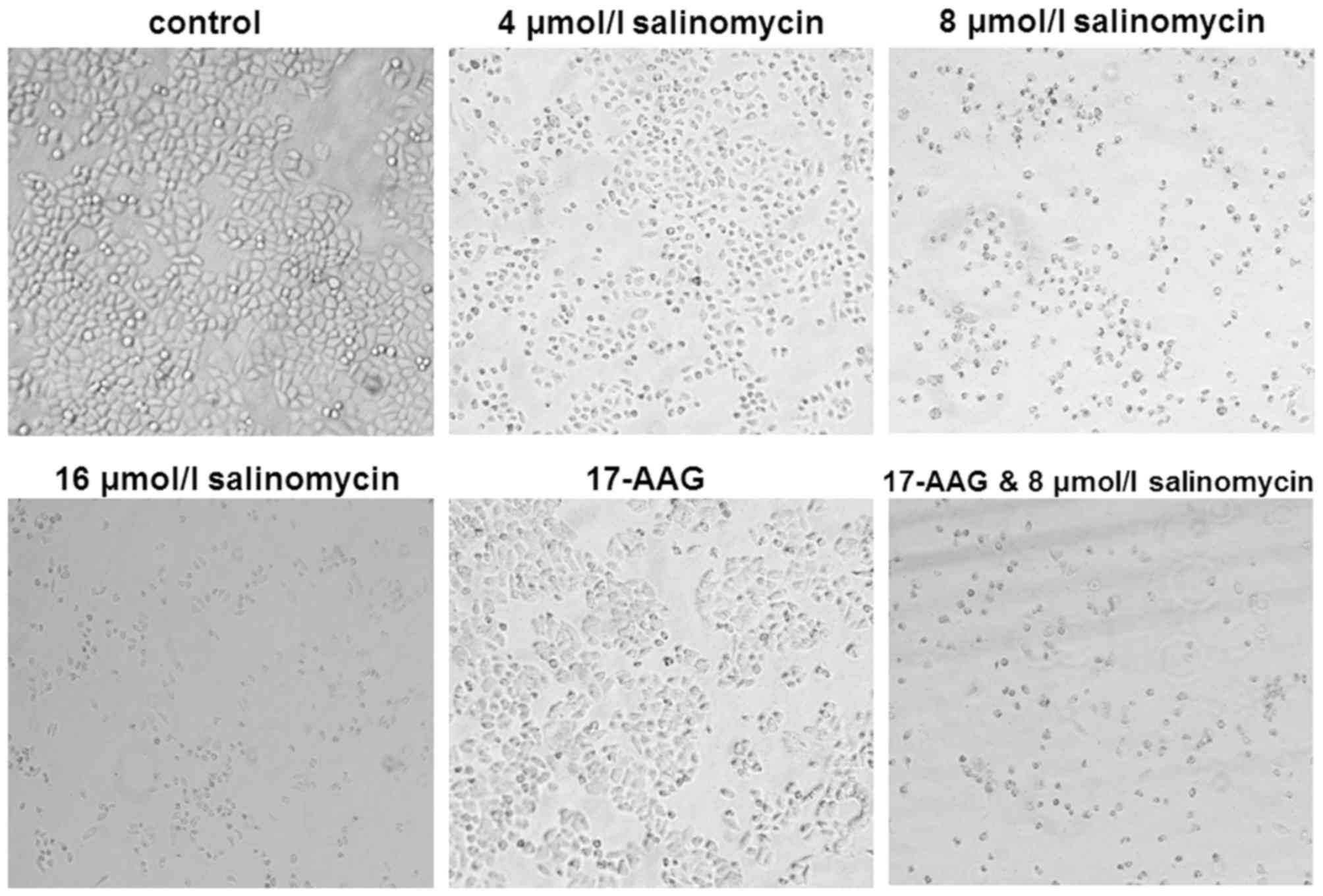
inverted microscope. As presented in Fig. 1, untreated SGC-7901 cells exhibited
adherent and tight growth with polygon or fusiform shapes, with a
plump cytoplasm and tight connections between cells. However, after
a 48-h treatment with salinomycin or 17-AAG, cell volume decreased.
The cell membrane and nuclear membrane began to break and shrink.
Cells grew slowly with increased gaps, and connections disappeared.
Furthermore, the higher the salinomycin concentration, the more
significant and severe alterations in SGC-7901 cell morphology,
with reduced cell numbers. When cells were treated with salinomycin
and 17-AAG together, the cell number and volume were further
reduced; the cell cytoplasm condensed, and nuclei were further
enriched and broken compared with groups treated with salinomycin
or 17-AAG alone. These results indicated that salinomycin may alter
SGC-7901 cell morphology when used alone or combined with
17-AAG.
Salinomycin and 17-AAG inhibits
SGC-7901 cell proliferation in vitro
To evaluate the effects of salinomycin and 17-AAG on
SGC-7902 cell proliferation, an MTT assay was conducted. The
results demonstrated salinomycin inhibited SGC-7901 cell
proliferation significantly in a time-dependent manner (24, 48 and
72 h) within a concentration range from 1 to 32 µmol/l, when
compared with the control group (Fig.
2A; P<0.01). Furthermore, the cell proliferation inhibitory
effects in cells following salinomycin treatment with the indicated
concentrations (4, 8 and 16 µmol/l) were significantly increased
compared with the control, salinomycin and 17-AAG groups when
combined with 17-AAG (0.625 µmol/l) for indicated time points (24,
48 and 72 h; Fig. 2B-F;
P<0.05). The results indicated that salinomycin may inhibit
SGC-7901 cell proliferation in vitro, and enhance cell
sensitivity to 17-AAG.
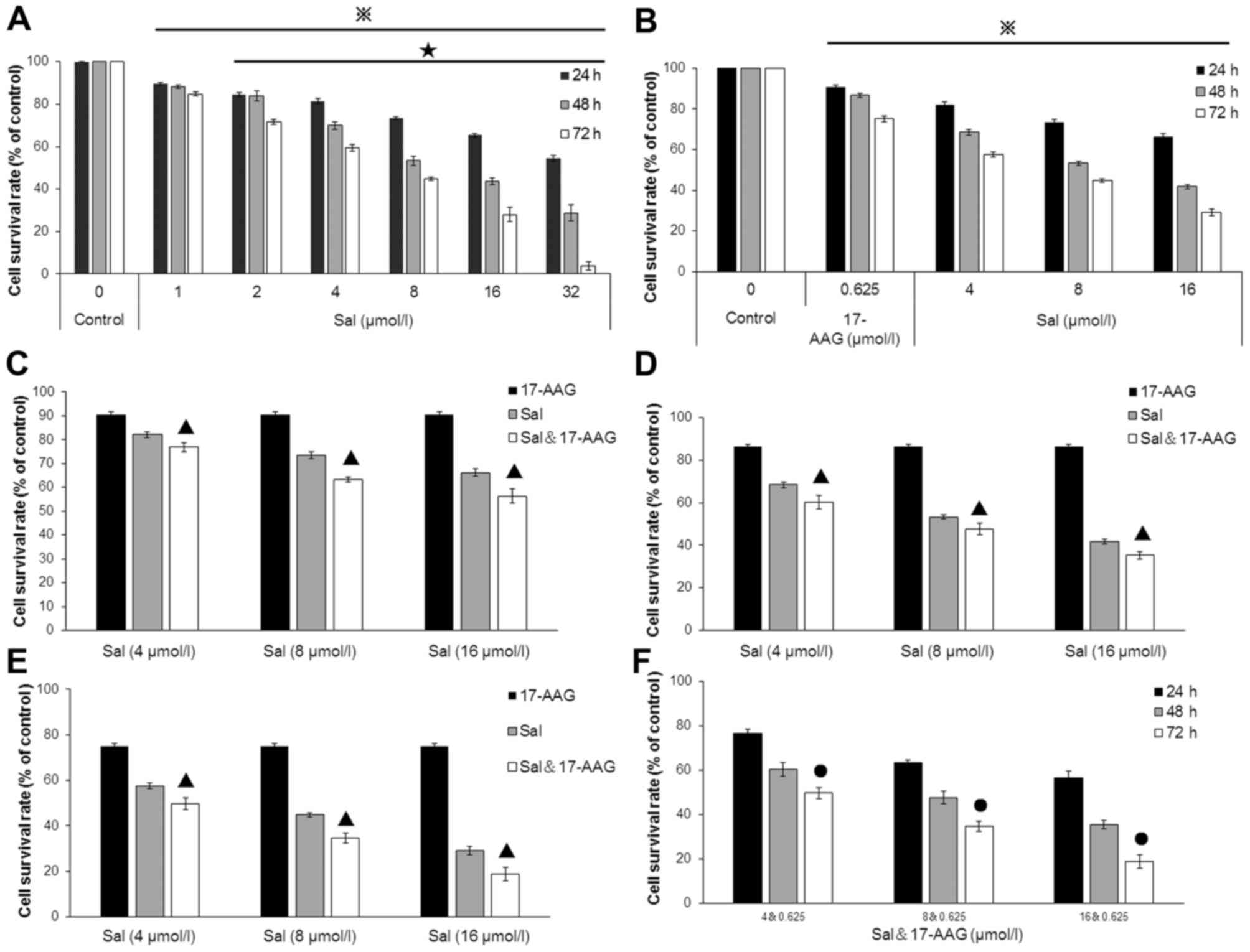 | Figure 2.Survival rate of SGC-7901 cells
treated with salinomycin and 17-AAG. To clarify the effects of
salinomycin and 17-AAG on SGC-7901 cell proliferation, MTT was
performed. Survival rates of cells treated with (A) salinomycin
only, (B) 0.625 µmol/l 17-AAG or salinomycin, (C) 0.625 µmol/l
17-AAG and salinomycin alone, or combined for 24 h, (D) 0.625
µmol/l 17-AAG and salinomycin alone, or combined for 48 h, (E)
0.625 µmol/l 17-AAG and salinomycin alone or in combination for 72
h, (F) 0.625 µmol/l 17-AAG in combination with 4, 8 and 16 µmol/l
salinomycin for 24, 48 and 72 h. Data are expressed as the mean ±
standard deviation. P<0.05 vs. control group for the same time
point. ★P<0.05 vs. previous salinomycin
concentration. ●P<0.05 vs. previous time point. ▲P<0.05 vs.
group only treated with salinomycin or 17-AAG. The experiments were
repeated in triplicate. 17-AAG,
17-allylamine-17-demathoxygeldanamycin. |
Salionomycin and 17-AAG inhibition of
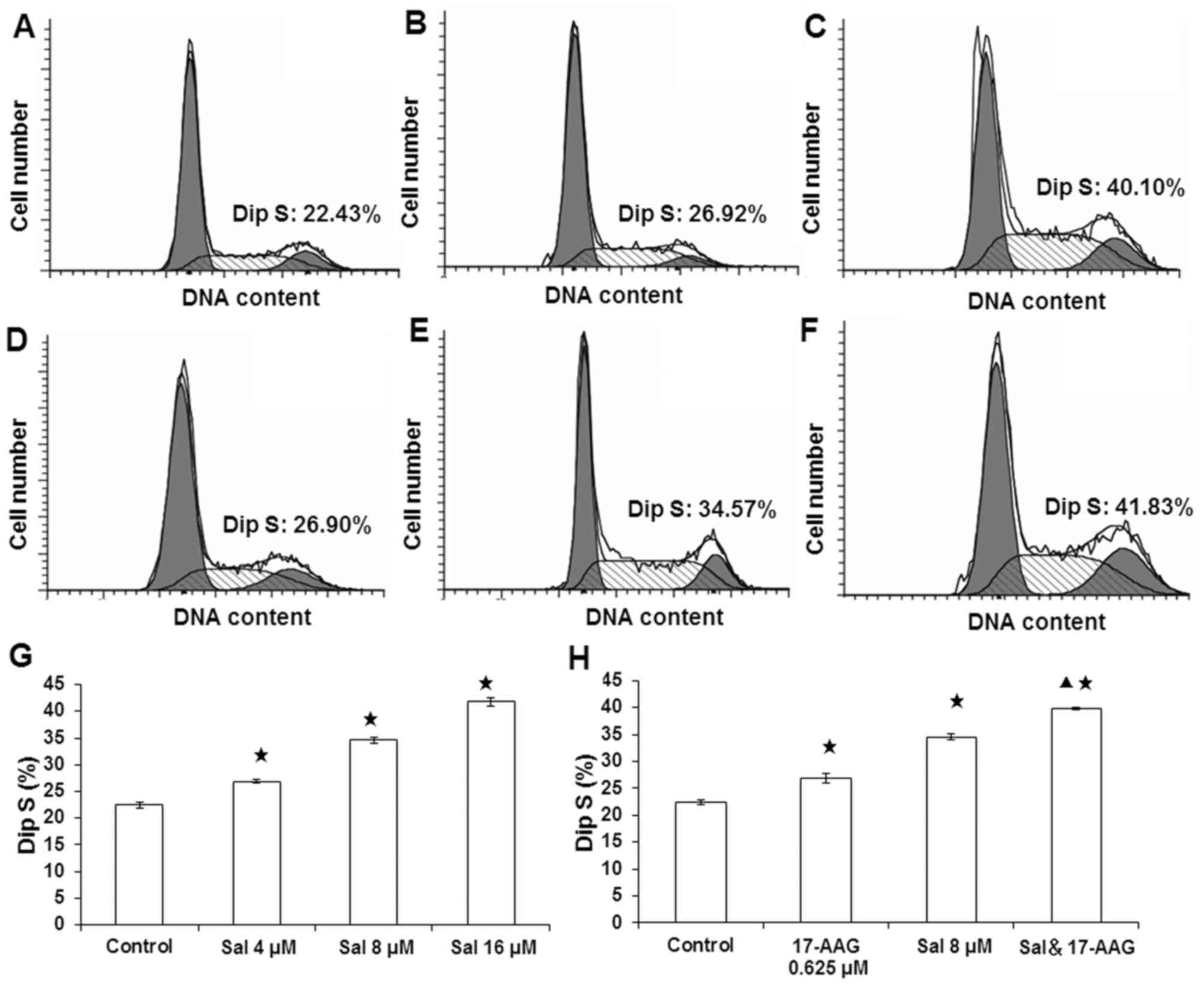
SGC-7901 cell cycle
To determine the effects of salinomycin and 17-AAG
on SGC-7901 cell cycle, flow cytometry was performed (Fig. 3). The results demonstrated that
after a 48-h treatment with salinomycin (4, 8 and 16 µmol/l) or
0.625 µmol/l 17-AAG alone (Fig. 3A and
B; D-H), numbers of cells in G0/G1 phase were significantly
decreased, whereas those in S phase were significantly increased
compared with the control group (P<0.05). Additionally, when
treated with 8 µmol/l salinomycin and 0.625 µmol/l 17-AAG, numbers
of cells in S phase were significantly increased compared with the
control, salinomycin and 17-AAG groups (Fig. 3C and H; P<0.05). These results
suggested that salinomycin and 17-AAG may arrest cells in S phase,
and that salinomycin combined with 17-AAG may enhance this effect
on the cell cycle.
Salinomycin and 17-AAG induce SGC-7901
cell apoptotic morphology
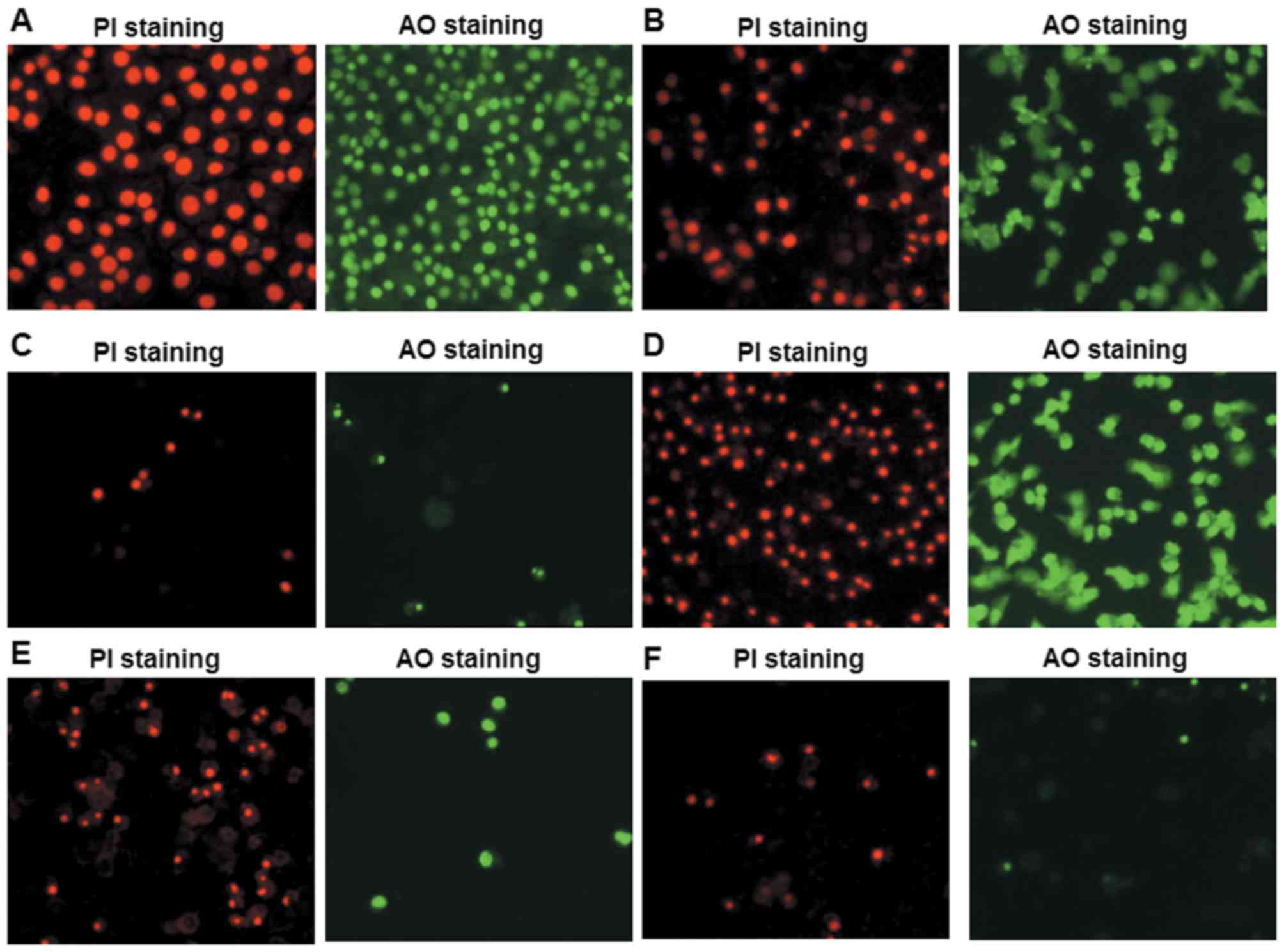
To investigate the effects of salinomycin and 17-AAG
on SGC-7901 cell apoptosis, PI and AO staining were performed and
fluorescent microscope was used for detection (Fig. 4). As presented in Fig. 4A, cells in the control group grew
well with uniform size, regular nuclei shapes, neat edges of
nuclear membranes and regular chromatin distribution. Furthermore,
cells exhibited typical red and green fluorescence signals after PI
and AO staining, respectively (Fig.
4A). When treated with 17-AAG (Fig. 4B) or salinomycin (Fig. 4D-F) alone for 48 h, cell morphology
altered significantly, including slower growth, reduced cell
numbers, nuclei condensation, DNA enrichment near the nuclear
membrane, apoptotic body formation, enhanced nuclear refraction and
dense fluorescence, which were more obvious in groups treated with
higher doses. Furthermore, when cells were treated with salinomycin
and 17-AAG together for 48 h, cell apoptotic morphology altered
more clearly compared with groups treated with salinomycin or
17-AAG alone (Fig. 4C). These
results demonstrated that salinomycin and 17-AAG may induce
SGC-7901 cell apoptotic morphology, and that the apoptosis was more
severe when salinomycin and 17-AAG were used together.
Salinomycin and 17-AAG promotes
SGC-7901 cell apoptosis
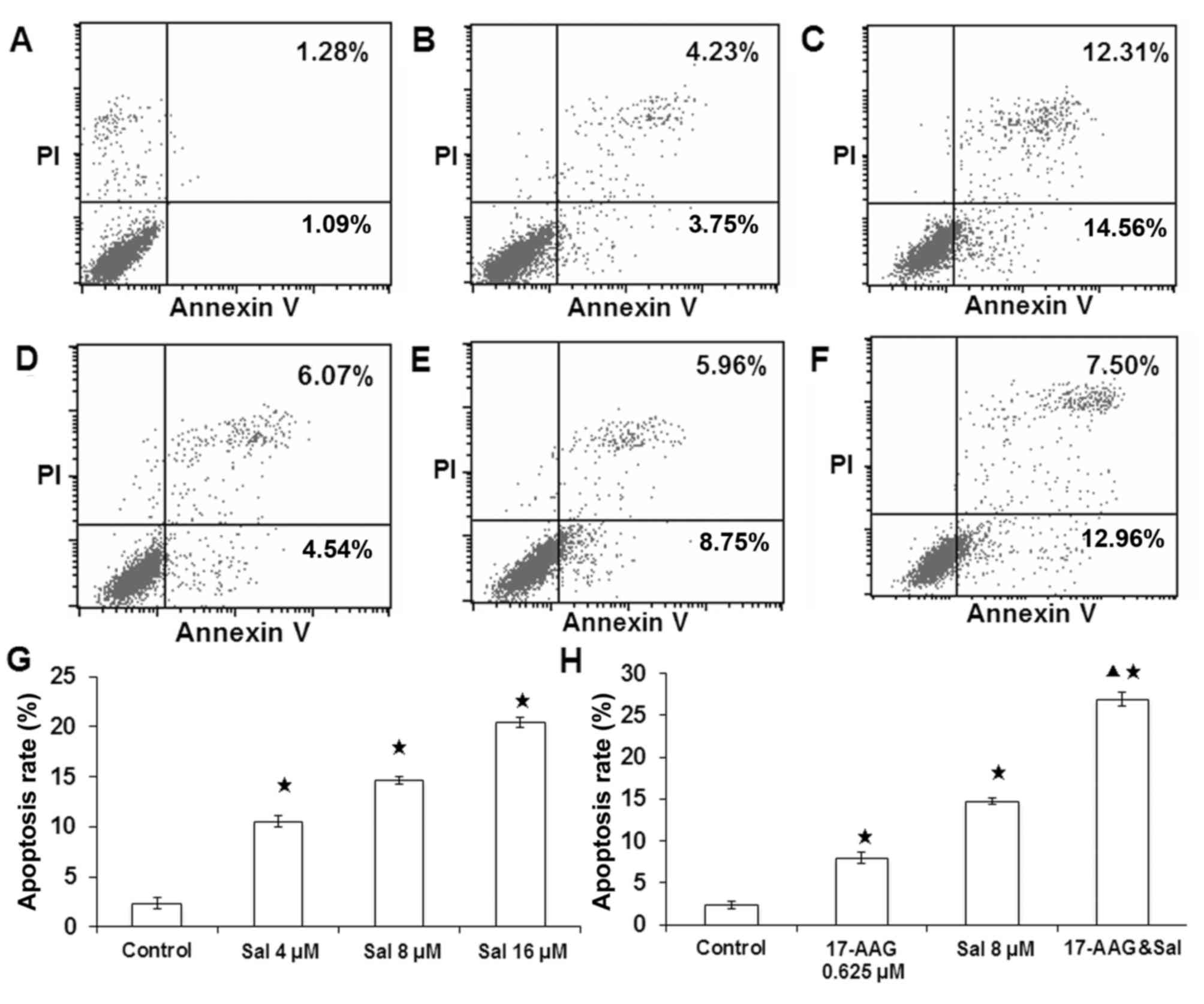
To identify whether salinomycin and 17-AAG could
promote cell apoptosis in SGC-7901 cells, flow cytometry was
performed. As presented in Fig. 5,
compared with the control group (Fig.
5A), 48-h treatment with 0.625 µmol/l 17-AAG (Fig. 5B) or 4, 8, 16 µmol/l salinomycin
(Fig. 5D-G) significantly
increased SGC-7901 cell apoptosis (P<0.05). Furthermore, the 48
h co-treatment of 0.625 µmol/l 17-AAG and 8 µmol/l salinomycin
significantly promoted cell apoptosis compared with the control and
drug treatment alone groups (Fig. 5C
and H; P<0.05). These results implied that both salinomycin
and 17-AAG could promote cell apoptosis with a synergistic
effect.
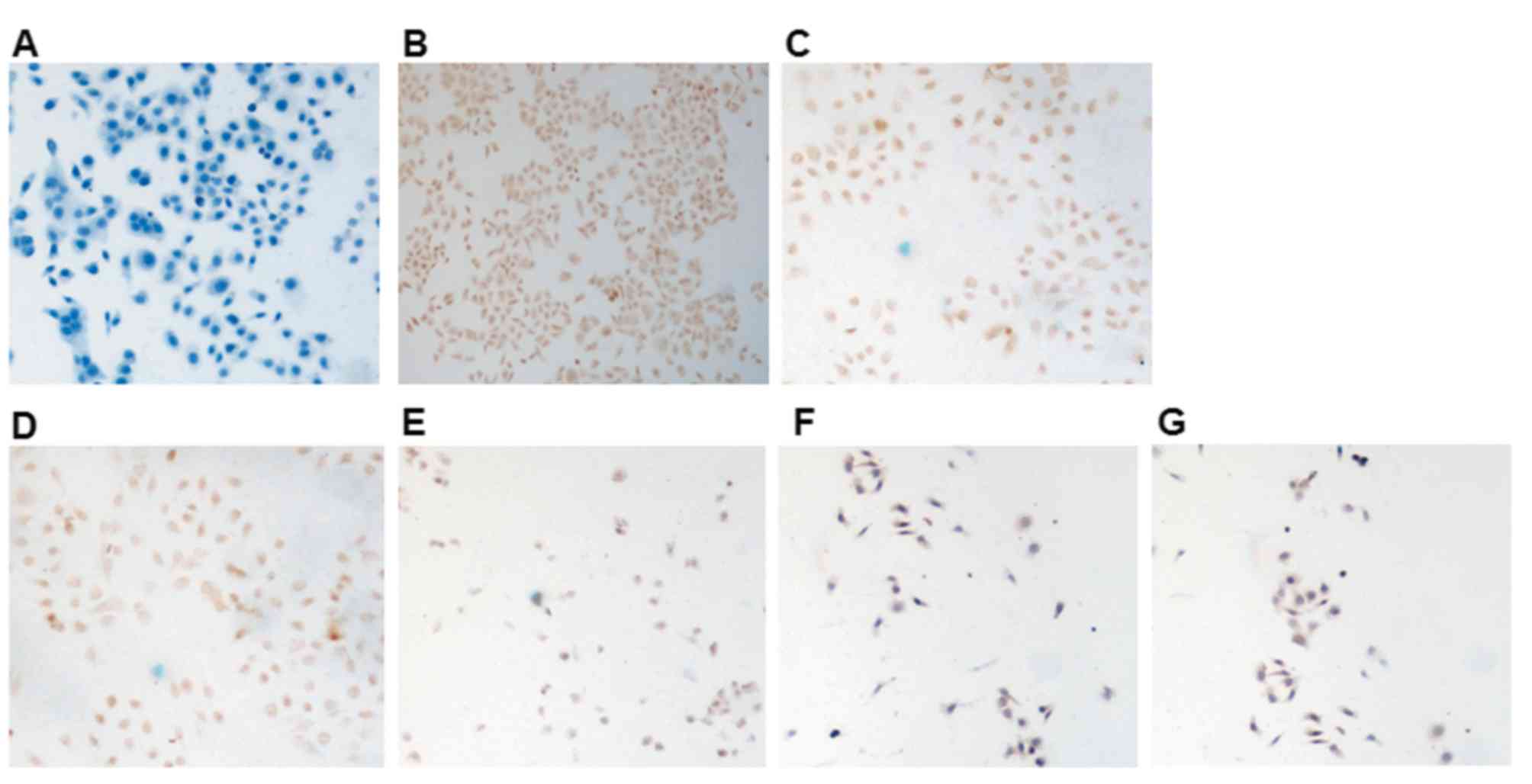
Salinomycin and 17-AAG activates the
Fas/Fas-L signaling pathway while inhibiting the NF-κB pathway
To further elucidate the pro-apoptotic mechanism of
salinomycin and 17-AAG, immunocytochemistry was conducted to detect
the protein expression levels of NF-κB and Fas-L in SGC-7901 cells
of each group; factors which serve important roles in tumorigenesis
and pro-apoptosis, respectively.
Untreated cells incubated with PBS, in place of the
primary antibody, served as the secondary antibody control
(Fig. 6A). In untreated negative
control cells, NF-κB expression was primarily identified in the
nucleus and cytoplasm in control cells (Fig. 6B). In cells treated with 0.625
µmol/l 17-AAG (Fig. 6C) or
salinomycin (4, 8 and 16 µmol/l; Fig.
6D-F, respectively), NF-κB expression was significantly
decreased in both in the nucleus and cytoplasm. Furthermore, in
cells treated with both 0.625 µmol/l 17-AAG and 8 µmol/l
salinomycin (Fig. 6G), the
decrease of NF-κB signal was more marked compared with the control,
salinomycin and 17-AAG groups.
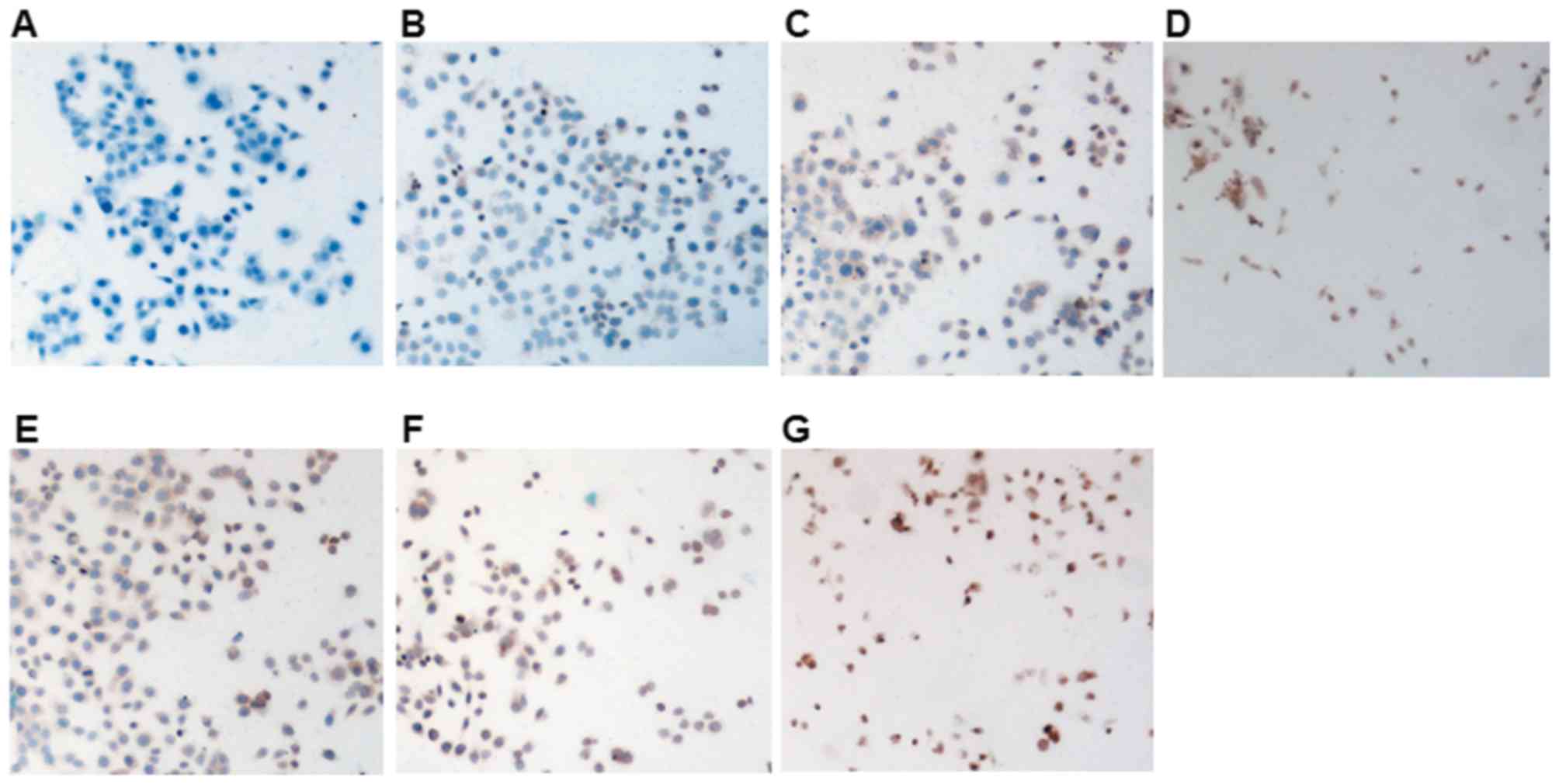
Fas-L expression was also detected by
immunocytochemistry. No Fas-L expression was observed in the
untreated secondary antibody control cells (Fig. 7A). Fas-L was expressed in the
cytoplasm and membrane of untreated negative control cells
(Fig. 7B). Fas-L expression was
increased in the cytoplasm and membrane of cells treated with
17-AAG compared with control cells (Fig. 7C). In cells treated with both
17-AAG and salinomycin, increased Fas-L signals were observed both
in the cytoplasm and nucleus (Fig.
7D). In cells treated with 4, 8 and 16 µmol/l salinomycin
(Fig. 7E-G, respectively), Fas-L
expression in the cytoplasm was increased, particularly in the 16
µmol/l salinomycin group (Fig.
7G), in which nuclear Fas-L signals were observed as well.
These results indicated that the pro-apoptotic
effects of salinomycin and 17-AAG on SGC-7901 cells may be
associated with activation of the Fas/Fas-l signaling pathway, and
inhibition of the NF-κB signaling pathway.
Discussion
Gastric cancer is one of the most common types of
malignant tumor (24). As an
ionophore type antibiotic, salinomycin serves an important role in
inhibiting tumor cell proliferation (9,13–19).
However, the strong neurotoxic effects of salinomycin have been
previously reported (20,25). 17-AAG is an inhibitor of HSP; its
anti-tumor effect has already been widely accepted (26,27),
and it has fewer side effects (21,22).
The present study demonstrated that the combination of salinomycin
and 17-AAG exhibited improved anti-tumor effects compared with
treatment of one alone.
An et al (28) reported that salinomycin inhibits
mammary stem cell proliferation via an apoptosis-independent
pathway. Zhi et al (29)
additionally demonstrated that salinomycin could selectively
inhibit gastric cancer cells. The present study revealed that
salinomycin inhibits SGC-7901 gastric cancer cell proliferation in
a dose- and time-dependent manner within a certain concentration
range, consistent with the previously mentioned studies. These
results imply that salinomycin may induce apoptosis of gastric
cancer cells. The present study additionally demonstrated that
combination with 17-AAG could enhance the cell proliferation
inhibitory effects of salinomycin significantly, and the
sensitivity of SGC-7901 cells to 17-AAG. Notably, single and
combined treatment of salinomycin and 17-AAG altered SGC-7901 cell
morphology, particularly combined treatment. PI and AO fluorescent
staining and flow cytometry results indicated that the apoptotic
rate of cells treated with both salinomycin and 17-AAG together was
significantly increased compared with cells treated with
salinomycin or 17-AAG alone, implying that combination of these two
agents could induce gastric cancer cell apoptosis synergistically.
These results were consistent with a study by Liu et al
(30), where salinomycin was
identified to promote Jurkat cell apoptosis when used alone or
combined with Vincristin. According to flow cytometry detection,
salinomycin alone altered the cell cycle and prolonged S phase, and
this effect was stronger when salinomycin was used together with
17-AAG. The potential mechanism may be the interference of DNA
synthesis and replication. However, Zhang et al (31) demonstrated that salinomycin
arrested the cell cycle of nasopharyngeal carcinoma cells at G2/M
phase, while Parajuli et al (32) revealed that salinomycin could
arrest cisplatin-resistant ovarian cancer cells at G1 phase.
Therefore, the effect of salinomycin on cancer cell cycle requires
further investigation.
The present study further investigated the mechanism
underlying the pro-apoptotic effect of salinomycin and 17-AAG.
NF-кB is an important nuclear transcription factor with numerous
biological activities, including inflammation, viral infection,
tumorigenesis and cancer progression (33,34).
Fas/Fas-L are cell membrane molecules, and serve as pro-apoptotic
factors in the death receptor signaling pathway (35,36).
The specific binding of Fas/Fas-L may initiate pro-apoptitic
signaling (37,38). Due to the important roles of NF-κB
and Fas in apoptosis (39–43), it was hypothesized that the
anti-tumor effects of salinomyin may be associated with these
proteins. Parajuli et al (44) demonstrated that salinomycin could
inhibit nuclear transport of NF-κB. Consistently,
immunocytochemistry results of the present study revealed that
salinomycin downregulated NF-κB protein expression, whereas
upregulated Fas-L protein expression in SGC-7901 cells. Therefore,
the pro-apoptotic effects of salinomycin and 17-AAG may be
associated with inhibition of the NF-κB signaling pathway, and
activation of the Fas/Fas-L signaling pathway.
In conclusion, the individual use of salinomycin and
combined use with 17-AAG may significantly inhibit SGC-7901 gastric
cancer cell proliferation and induce cell apoptosis. The potential
mechanisms may be associated with upregulation of Fas-L and
downregulation of NF-κB. These results provide a basis for the
potential use of salinomycin in gastric cancer treatment.
Acknowledgements
The present study was supported by the National
Natural Science Fund Grant (grant no. 81470140) and the Science and
Technology Project of Education Department of Shanxi Province
(grant no. 2013JK0783).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen WQ, Zeng HM, Zheng RS, Zhang SW and
He J: Cancer incidence and mortality in China, 2007. Chin J Cancer
Res. 24:1–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zhang R, Zhang S, Zhao P, Li G, Wu
L and He J: Report of incidence and mortality in China cancer
registries, 2009. Chin J Cancer Res. 25:10–21. 2013.PubMed/NCBI
|
|
4
|
Chen WQ, Zheng RS, Zeng HM, Zhang SW, Zhao
P and Hao J: Trend analysis and projection of cancer incidence in
China between 1989 and 2008. Zhonghua Zhong Liu Za Zhi. 34:517–524.
2012.(In Chinese). PubMed/NCBI
|
|
5
|
Sjödahl K, Lu Y, Nilsen TI, Ye W, Hveem K,
Vatten L and Lagergren J: Smoking and alcohol drinking in relation
to risk of grastric cancer: A population-based, prospective cohort
study. Int J Cancer. 120:128–132. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Niccolai E, Taddei A, Prisco D and Amedei
A: Gastric cancer and the epoch of immunotherapy approaches. World
J Gastroenterol. 21:5778–5793. 2015.PubMed/NCBI
|
|
7
|
Xu W, Yang Z and Lu N: Molecular targeted
therapy for the treatment of gastric cancer. J Exp Clin Cancer Res.
35:12016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miyazaki Y, Shibuya M, Sugawara H,
Kawaguchi O and Hirsoe C: Salinomycin, a new polyether antibiotic.
J Antibiot (Tokyo). 27:814–821. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Daugschies A, Gässlein U and Rommel M:
Comparative efficacy of anticoccidials under the conditions of
commercial broiler production and in battery trials. Vet Parasitol.
76:163–171. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Danforth HD, Ruff MD, Reid WM and Miller
RL: Anticoccidial activity of salinomycin in battery raised broiler
chickens. Poult Sci. 56:926–932. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mahmoudi N, de Julián-Ortiz JV, Ciceron L,
Gálvez J, Mazier D, Danis M, Derouin F and García-Domenech R:
Identification of new antimalarial drugs by linear discriminant
analysis and topological virtual screening. J Antimicrob Chemother.
57:489–497. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gupta PB, Onder TT, Jiang G, Tao K,
Kuperwasser C, Weinberg RA and Lander ES: Identification of
selective inhibitors of cancer stem cells by high-throughput
screening. Cell. 138:645–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Naujokat C, Fuchs D and Opelz G:
Salinomycin in cancer: A new mission for an old agent. Mol Med
Report. 3:555–559. 2010. View Article : Google Scholar
|
|
14
|
Kopp F, Hermawan A, Oak PS, Ulaganathan
VK, Herrmann A, Elnikhely N, Thakur C, Xiao Z, Knyazev P, Ataseven
B, et al: Sequential salinomycin treatment results in resistance
formation through clonal selection of epithelial-like tumor cells.
Transl Oncol. 7:702–711. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim JH, Chae M, Kim WK, Kim YJ, Kang HS,
Kim HS and Yoon S: Salinomycin sensitizes cancer cells to the
effects of doxorubicin and etoposide treatment by increasing DNA
damage and reducing p21 protein. Br J Pharmacol. 162:773–784. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao Z, Sperl B, Ullrich A and Knyazev P:
Metformin and salinomycin as the best combination for the
eradication of NSCLC monolayer cells and their alveospheres (cancer
stem cells) irrespective of EGFR, KRAS, EML4/ALK and LKB1 status.
Oncotarget. 5:12877–12890. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Antoszczak M, Popiel K, Stefańska J,
Wietrzyk J, Maj E, Janczak J, Michalska G, Brzezinski B and
Huczyński A: Synthesis, cytotoxicity and antibacterial activity of
new esters of polyether antibiotic-salinomycin. Eur J Med Chem.
76:435–444. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kopp F, Hermawan A, Oak PS, Herrmann A,
Wagner E and Roidl A: Salinomycin treatment reduces metastatic
tumor burden by hampering cancer cell migration. Mol Cancer.
13:162014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huczynski A: Salinomycin: A new cancer
drug candidate. Chem Biol Drug Des. 79:235–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boehmerle W, Muenzfeld H, Springer A,
Huehnchen P and Endres M: Specific targeting of neurotoxic side
effects and pharmacological profile of the novel cancer stem cell
drug salinomycin in mice. J Mol Med (Berl). 92:889–900. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schulte TW and Neckers LM: The
benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin binds
to Hsp90 and shares important biologic activities with
geldanamycin. Cancer Chemother Pharmacol. 42:273–279. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schnur RC, Corman ML, Gallaschun RJ,
Cooper BA, Dee MF, Doty JL, Muzzi ML, Moyer JD, DiOrio CI, Barbacci
EG, et al: Inhibition of the oncogene product p185erbB-2 in vitro
and in vivo by geldanamycin and dihydrogeldanamycin derivatives. J
Med Chem. 38:3806–3812. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen S, Chen X, Li Y, Yang S, Mo X, Zhang
F, Mo K and Ding Y: Inhibitory effect of 17-AAG combined with
paclitaxel on proliferation of esophageal squamous cell carcinoma
Eca-109 cells in vitro. Nan Fang Yi Ke Da Xue Xue Bao. 35:844–847.
2015.(In Chinese). PubMed/NCBI
|
|
24
|
Wu HH, Lin WC and Tsai KW: Advances in
molecular biomarkers for gastric cancer: miRNAs as emerging novel
cancer markers. Expert Rev Mol Med. 16:e12014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song SP, Zhang XG, Wang M, et al: Changes
and significance of serum creatine kinase and isoenzymes in
salinomycin poisoning patients. Zhong Guo Wei Sheng Jian Yan Za
Zhi. 21:28–29. 2011.(In Chinese).
|
|
26
|
Kim SH, Kang JG, Kim CS, Ihm SH, Choi MG,
Yoo HJ and Lee SJ: 17-Allylamino-17-demethoxygeldanamycin and
Herbimycin A induce cell death by modulating β-catenin and PI3K/AKT
signaling in FRO anaplastic thyroid carcinoma cells. Anticancer
Res. 35:5453–5460. 2015.PubMed/NCBI
|
|
27
|
Xu Y, Zhu Q, Chen D, Shen Z, Wang W, Ning
G and Zhu Y: The HSP90 inhibitor 17-AAG exhibits potent antitumor
activity for pheochromocytoma in a xenograft model. Tumour Biol.
36:5103–5108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
An H, Kim JY, Lee N, Cho Y, Oh E and Seo
JH: Salinomycin possesses anti-tumor activity and inhibits breast
cancer stem-like cells via an apoptosis-independent pathway.
Biochem Biophys Res Commun. 466:696–703. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhi QM, Chen XH, Ji J, Zhang JN, Li JF,
Cai Q, Liu BY, Gu QL, Zhu ZG and Yu YY: Salinomycin can effectively
kill ALDH(high) stem-like cells on gastric cancer. Biomed
Pharmacother. 65:509–515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu PP, Zhu JC, Liu GX, Shui CX and Li XM:
Salinomycin enhances the apoptosis of T-cell acute lymphoblastic
leukemia cell line jurkat cells induced by vincristine. Zhongguo
Shi Yan Xue Ye Xue Za Zhi. 23:653–657. 2015.(In Chinese).
PubMed/NCBI
|
|
31
|
Zhang Y, Zuo Y, Guan Z, Lu W, Xu Z, Zhang
H, Yang Y, Yang M, Zhu H and Chen X: Salinomycin radiosensitizes
human nasopharyngeal carcinoma cell line CNE-2 to radiation. Tumor
Biol. 37:305–311. 2016. View Article : Google Scholar
|
|
32
|
Parajuli B, Lee HG, Kwon SH, Cha SD, Shin
SJ, Lee GH, Bae I and Cho CH: Salinomycin inhibits Akt/NF-κB and
induces apoptosis in cisplatin resistant ovarian cancer cells.
Cancer Epidemiol. 37:512–517. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cai Z, Tchou-Wong KM and Rom WN: NF-kappaB
in lung tumorigenesis. Cancers (Basel). 3:4258–4268. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu GZ, Pan CX, Jiang D, Zhang Q, Li Y and
Zheng SY: Clinicopathological significance of Fas and Fas ligand
expressions in esophageal cancer. Am J Cancer Res. 5:2865–2871.
2015.PubMed/NCBI
|
|
36
|
Liu W, Xu C, Zhao H, Xia P, Song R, Gu J,
Liu X, Bian J, Yuan Y and Liu Z: Osteoprotegerin induces apoptosis
of osteoclasts and osteoclast precursor cells via the fas/fas
ligand pathway. PLoS One. 10:e01425192015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Deng Y, Feng P, Chen D, et al: Effects of
Fas and Fas-L in cell apoptosis. Guo Wai Yi Xue Mian Yi Xue Fen Ce.
4:213–216. 1997.(In Chinese).
|
|
38
|
Wang Y, Wang C, Jiang C, Zeng H and He X:
Novel mechanism of harmaline on inducing G2/M cell cycle arrest and
apoptosis by up-regulating Fas/FasL in SGC-7901 cells. Sci Rep.
5:186132015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kong FC, Zhang JQ, Zeng C, Chen WL, Ren
WX, Yan GX, Wang HX, Li QB and Chen ZC: Inhibitory effects of
parthenolide on the activity of NF-κB in multiple myeloma via
targeting TRAF6. J Huazhong Univ Sci Technolog Med Sci. 35:343–349.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Diab S, Fidanzi C, Léger DY, Ghezali L,
Millot M, Martin F, Azar R, Esseily F, Saab A, Sol V, et al:
Berberis libanotica extract targets NF-κB/COX-2, PI3K/Akt and
mitochondrial/caspase signalling to induce human erythroleukemia
cell apoptosis. Int J Oncol. 47:220–230. 2015.PubMed/NCBI
|
|
41
|
Gmeiner WH, Jennings-Gee J, Stuart CH and
Pardee TS: Thymineless death in F10-treated AML cells occurs via
lipid raft depletion and Fas/FasL co-localization in the plasma
membrane with activation of the extrinsic apoptotic pathway. Leuk
Res. 39:229–235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu W, Lin YT, Yan XL, Ding YL, Wu YL,
Chen WN and Lin X: Hepatitis B virus core protein inhibits
Fas-mediated apoptosis of hepatoma cells via regulation of
mFas/FasL and sFas expression. FASEB J. 29:1113–1123. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li L, Yao YC, Fang SH, Ma CQ, Cen Y, Xu
ZM, Dai ZY, Li C, Li S, Zhang T, et al: Pigment epithelial-derived
factor (PEDF)-triggered lung cancer cell apoptosis relies on p53
protein-driven Fas ligand (Fas-L) up-regulation and Fas protein
cell surface translocation. J Biol Chem. 289:30785–30799. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Parajuli B, Shin SJ, Kwon SH, Cha SD,
Chung R, Park WJ, Lee HG and Cho CH: Salinomycin induces apoptosis
via death receptor-5 up-regulation in cisplatin-resistant ovarian
cancer cells. Anticancer Res. 33:1457–1462. 2013.PubMed/NCBI
|