Introduction
Mesenchymal stem cells (MSCs) are adult stem cells
predominantly derived from bone marrow. MSCs have strong
replicative capabilities and multidirectional differentiation
potential. These stem cells can differentiate into chondrocytes,
osteoblasts, adipocytes and muscle cells (1,2).
Under normal circumstances, MSCs settle in a specialized
microenvironment termed stem-cell niche. When the body reacts to
stimuli, including trauma or tumors, MSCs leave the stem-cell niche
to achieve tissue reconstruction via directional migration,
proliferation and differentiation (3–5).
Endothelial progenitor cells (EPCs) derived from the
mesoderm have been identified as exhibiting high proliferative and
self-renewal capabilities. EPCs have also been demonstrated to
differentiate into vascular endothelial cells (6). EPCs are involved in the formation of
neovessels in sites of vascular injury, including endothelial
denudation and cardiac ischemia (7,8).
Our previous studies demonstrated that, when MSCs
were injected into the murine marrow cavity, increased adhesion
occurred between MSCs and bone marrow sinuses. The MSCs primarily
adhered to cluster of differentiation (CD)31+ vascular
EPCs resulting in the formation of cell clusters. This finding was
further confirmed by in vitro culture. In addition, our
studies revealed that MSCs and EPCs were able to promote the
proliferation of each other; however, the underlying mechanism
remains unclear (9,10).
In vitro studies have demonstrated that MSCs
can secrete insulin-like growth factor 1 (IGF-1) (11,12).
IGF-1 is the main growth factor involved in the proliferation of
numerous cell types, including myoblasts and epithelial cells
(13,14). The mitogenic action of IGF-1 on
others cells is essential and mediated by the phosphatidylinositol
3-kinase (PI3K)/protein kinase B (Akt) signaling pathway, which is
involved in cell cycle progression and cell survival (15,16).
To determine the regulatory effects of IGF-1 on the promotion of
EPC proliferation by MSCs, and the possible molecular mechanism
underlying this promotion, the present study initially investigated
whether MSCs and EPCs secrete IGF-1, and then analyzed how IGF-1
influenced EPC proliferation via the PI3K/Akt signaling
pathway.
Materials and methods
Animal preparation and cell
culture
All animals were maintained in the Animal Facility
of Shihezi University (Shihezi, China) with sawdust as nesting
material under controlled laboratory conditions (temperature, 20°C;
12 h light/12 h dark cycle with lights off at 8:00 p.m.; 55±5%
humidity), and free access to food and water. A total of 40 male
C57BL/6 J mice (wild-type; weight, 28–35 g; age, 6 weeks), were
purchased from Xinjiang Medical University (Urumqi, China), and
were used as cell sources. The same technique was used to harvest
and culture all cell types; however, different materials and
culture media were used. Third-generation cells were used in the
experiments. The present study was approved by the Medical Ethics
Committee of the First Affiliated Hospital, School of Medicine,
Shihezi University.
Isolation and culture of murine bone
marrow MSCs
Bone marrow MSCs were isolated using a technique
reported in our previous study (9,17).
Briefly, bone marrow cells were collected from 6-week-old wild-type
C57BL/6 male mice euthanized by cervical dislocation. The cells
were cultured in low-glucose Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with penicillin (100 U/ml, Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), streptomycin sulfate (100 µg/ml,
Sigma-Aldrich; Merck KGaA) and 10% lot-selected fetal bovine serum
(FBS; Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) at 37°C
in a 5% CO2 humidified incubator. Following 72 h of
adhesion, non-adherent cells were removed, whereas adherent cells
were cultured for an additional 7 days with a single media change.
The adherent cells were then harvested by trypsin digestion. The
cells were centrifuged at 4°C, 1,000 × g for 5 min, and then washed
three times with PBS containing 0.3% FBS (Hyclone; GE Healthcare
Life Sciences). Cell aliquots (1×106) were incubated for 20 min at
4°C with phycoerythrin (PE; cat. no. 108107; concentration, 0.2
mg/ml; dilution, 1:40), fluorescein isothiocyanate (FITC; cat. no.
102205; concentration, 0.5 mg/ml; dilution, 1:50), peridinin
chlorophyll protein (Per CP; cat. no. 202220; concentration, 0.2
mg/ml; dilution, 1:20) and allophycocyanin (APC; cat. no. 201809;
concentration, 0.2 mg/ml; dilution, 1:100) -conjugated antibodies
against mouse Sca-1, CD29, CD45 and CD11b, respectively (BioLegend,
Inc., San Diego, CA, USA). Acquisition was performed on a
fluorescence-activated cell sorting (FACS) device (Aria model; BD
Biosciences, Franklin Lakes, NJ, USA) and analysis was performed
using FACS DIVE software, version 6.1.3 (BD Biosciences). The
sorted CD29+, Sca-1+, CD45− and
CD11b− cells were cultured further in DMEM (containing
penicillin, streptomycin sulfate and 10% FBS) for enrichment.
Isolation and characterization of
murine bone marrow EPCs
Bone marrow EPCs were collected and cultured using
the same technique as for bone marrow MSCs. Cell aliquots were
incubated for 20 min at 4°C with the following anti-mouse
antibodies: CD11b-APC conjugated (cat. no. 201809; concentration,
0.2 mg/ml; dilution, 1:100; BioLegend, Inc.), CD31-FITC conjugated
(cat. no. 102506; concentration, 0.5 mg/ml; dilution, 1:50;
BioLegend, Inc.), CD144-Per CP conjugated (cat. no. 46-1441-82;
concentration, 0.2 mg/ml; dilution, 1:50; BioLegend, Inc.) and
CD133-PE conjugated (cat. no. 141203; concentration, 0.2 mg/ml;
dilution, 1:40; BioLegend, Inc.). Acquisition was performed using a
FACS device (Aria model; BD Biosciences) and analysis was performed
using FACS DIVE software, version 6.1.3 (BD Biosciences). The
sorted CD133+, CD31+, CD144+ and
CD11b− cells were cultured further in EBM-2 medium
(Lonza, Inc., Walkesville, MD, USA) for enrichment; EPCs were
cultured and seeded in this media for all subsequent
experiments.
Co-culture of EPCs and MSCs in a
transwell system
For the co-culture of EPCs and MSCs, a 6.5 mm,
24-well Transwell system with 0.4 µm pore polycarbonate membrane
inserts (Corning Incorporated, Corning, NY, USA) was used.
Third-passage MSCs and EPCs were seeded (5×104 cells) at a 1:1
ratio into the Transwell system. In the experimental group, the
EPCs were seeded into the lower chamber, whereas the MSCs were
seeded into the upper chamber. In the control group, only the EPCs
were seeded, no MSCs were seeded into the upper chamber, at the
same density as in the experimental group, into the lower
chamber.
5-bromo-2′-deoxyuridine (BrdU)
assay
EPCs (1.5×105 cells/dish) were seeded into a 35 mm
dish with 2 ml 0.4% fetal bovine serum and were cultured for 72 h
at 37°C in an atmosphere containing 5% CO2.
Subsequently, 40 µl BrdU solution (500 µmol; Sigma-Aldrich; Merck
KGaA) was pipetted directly onto the EPCs, which were incubated for
an additional 40 min at 37°C. The cells were washed three times in
PBS containing 0.5% inactivated fetal calf serum (IFS; heated for
30 min in a 56°C water bath prior to use; Gibco; Thermo Fisher
Scientific, Inc.), were treated with 2 mol HCl for 5 min at 37°C
and were then blocked with 0.5% IFS for 20 min at room temperature.
Subsequently, the cells were incubated in serum-free medium with a
rat anti-BrdU antibody (cat. no. ab152095; dilution, 1:50; Abcam,
Cambridge, UK) for 2 h at 37°C. A secondary rabbit anti-mouse
immunoglobulin G antibody (dilution, 1:10,000; Vector Laboratories,
Inc., Burlingame, CA, USA) was applied for a further 1 h at 37°C.
The cells were incubated with peroxidase-conjugated
streptavidin-horseradish peroxidase (Sigma-Aldrich; Merck KGaA) for
1 h at 37°C and were then stained with 0.05% 3,3′-diaminobenzidine
(DAB; Sigma-Aldrich; Merck KGaA) and hematoxylin (Sigma-Aldrich;
Merck KGaA). The BrdU-positive cells in 10 randomly selected
high-power fields were counted under a light microscope (Olympus
IX71; Olympus Corporation, Tokyo, Japan).
MTT assay
EPC proliferation was determined using MTT assay
(Sigma-Aldrich; Merck KGaA). The EPCs were incubated for 12 h at
37°C prior to treatment with IGF-1. The EPCs were seeded into
24-well plates at a density of 1.5×104 cells/well and 0.5 ml of
various concentrations of IGF-1 (20, 50, 100 and 200 ng/ml;
Peprotech, Inc., Rocky Hill, NJ, USA) were added for 72 h at 37°C.
The control group was treated with PBS only. Subsequently, 50 µl
MTT (5 mg/ml) was added to each dish prior to incubation at 37°C
for 4 h, after which, 500 µl dimethyl sulfoxide was added and the
solution was oscillated for 10 min. Absorbance was measured at 570
nm using a microplate reader (Bio-Rad Model 3550-UV; GMI Inc.,
Ramsey, MN, USA). The experiments were performed in triplicate and
repeated three times.
ELISA to determine the expression of
IGF-1 in the culture media of MSCs and EPCS
MSCs and EPCs (1×106 cells/dish) were seeded into a
60 mm dish with 4 ml factor-free medium (EBM-2 without FBS), and
were cultured for 24 h at 37°C in an atmosphere containing 5%
CO2. The culture media were then collected and
centrifuged (4°C; 5,000 × g; 10 min). The release of IGF-1 was then
determined by ELISA assay (cat. no. SEA050Mu; USCN Life Sciences,
Inc., Wuhan, China), according to the manufacturer's protocol. The
absorbance was measured at 450 nm (minus the 690 nm absorbance
background measurement) using a microplate reader (Bio-Rad Model
3550-UV; GMI Inc.).
IGF-1 small interfering (si)RNA and
IGF-1 receptor (R) siRNA transfection assays, and neutralizing the
effect of IGF-1
The MSCs and EPCs were suspended in 0.05% trypsin
and 0.02% EDTA (Gibco; Thermo Fisher Scientific, Inc.).
Subsequently, 1×106 cells in 2 ml antibiotic-free medium were
seeded into a 60 mm dish and grown for 24 h to 90% confluence. The
following transfection reagents were used: Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), Opti-MEM (Invitrogen;
Thermo Fisher Scientific, Inc.) reduced serum medium, IGF-1
receptor (IGF-1R) siRNA (cat. no. 159115; Invitrogen; Thermo Fisher
Scientific, Inc.), IGF-1 siRNA (cat. no. sc-37194; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and control siRNA (FITC
conjugate)-A (cat. no. sc-36869; Santa Cruz Biotechnology, Inc.).
The MSCs and EPCs were transfected with 13 nM IGF-1 siRNA and
IGF-1R siRNA. The siRNA control group was transfected with 13 nM
control siRNA (FITC conjugate)-A. The mock group was transfected
with Lipofectamine 2000, instead of siRNA, according to the
manufacturer's protocols, in order to visualize how effectively
siRNAs were delivered to the MSCs and EPCs. The transfection
procedure was performed in 60 mm culture dishes, which contained 2
ml medium/dish (DMEM+10% FBS; 1×106 cells/dish), as follows: In
separate RNase-/DNase-free tubes, 13 µl siRNA or 10 µl
Lipofectamine 2000 was added to 200 µl Opti-MEM reduced serum,
respectively, and the mixtures were incubated for 5 min at room
temperature. Subsequently, the mixtures were combined and incubated
at room temperature for an additional 15 min, and were then added
to the dish for 48 h transfection. Post-transfection, the mixture
was replaced with fresh complete culture medium for a further 24 h.
Neutralization of IGF-1 in the MSC and EPC culture media was
performed using 1.0 µg/ml anti-IGF-1 (cat. no. ab9572; Abcam) for
48 h at 37°C. The experiments were performed in duplicate and
repeated three times.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The EPCs were cultured (1×106 cells/dish) in serum-
and factor-free medium (EBM-2 without FBS) for 24 h and then
divided into three groups: i) EPCs only; ii) EPCs treated with 100
ng/ml IGF-1 for 72 h; and iii) EPCs treated with 10 µM LY294002
(Sigma-Aldrich; Merck KGaA) for 1 h, and then 100 ng/ml IGF-1 was
added for 72 h at 37°C. The total RNA was extracted from the EPCs
using TRIzol reagent (cat. no. 15596026; Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions,
and the purity of RNA was determined using the ratio of absorbance
readings at 280 nm (A280, Nanodrop 2000; Thermo Fisher Scientific,
Inc.), with ratios within 1.8–2.0 considered appropriate for cDNA
synthesis. Total RNA (200 ng) was reverse-transcribed using the
RevertAid™ H Minus First Strand cDNA Synthesis kit (cat.
no. K1632; Fermentas, Thermo Fisher Scientific, Inc.), according to
the manufacturer's instructions. The mRNA expression levels were
determined by RT-qPCR using the SYBR®-Green PCR Master mix (cat.
no. 208054; Qiagen GmbH), according to the manufacturer's
instructions. The thermocycling conditions were as follows: 95°C
for 2 min, then 40 cycles of 95°C for 30 sec and 60°C for 20 sec.
The reaction included 10 µl SYBR® Green PCR mix, 2 µl primers, 2 µl
cDNA (500 ng) and 6 µl DNase/RNase free water to a final reaction
volume of 20 µl. The results were analyzed using Bio-Rad CFX96
Manager software version 3.1 (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). Data were collected following each annealing step.
β-actin was used as an endogenous control to correct for
differences in the amounts of total RNA in each sample. The primer
sequences and the sizes of the amplified fragments were as follows:
IGF-1R (277 bp), forward 5′-GTCGAAGAATCGCATCATCA-3′, reverse
5′-GCATCCTGCCCATCATACTC-3′; and β-actin (174 bp), forward
5′-GTGCTATGTTGCTCTAGACTTCG-3′ and reverse
5′-ATGCCACAGGATTCCATACC-3′. The experiments were performed in
triplicate and repeated three times. Results were quantified using
the 2−∆∆Cq method (18).
Western blot analysis
The EPCs were cultured (1×106 cells/dish) in
factor-free medium (EBM-2 without FBS) for 24 h and were then
divided into three groups: i) EPCs only; ii) EPCs treated with 100
ng/ml IGF-1 for 72 h; and iii) EPCs treated with 10 µM LY294002
(Sigma-Aldrich; Merck KGaA) for 1 h, and then 100 ng/ml IGF-1 was
added for 72 h at 37°C. Protein samples were extracted from the
EPCs using radioimmunoprecipitation assay buffer (Thermo Fisher
Scientific, Inc.) containing phenylmethylsulfonyl fluoride (Thermo
Fisher Scientific, Inc.) and Phosphatase Inhibitor Cocktail
(Sigma-Aldrich; Merck KGaA). Protein concentration was measured
using the bicinchoninic acid method (Thermo Fisher Scientific,
Inc.) and samples (40 µg) were run on a 10% SDS-PAGE gel. Following
transferal to a polyvinylidene fluoride membrane, the blots were
treated according to the standard procedure [electrophoresis (120
V, 90 min), transfer to 300 mA film, 3% bovine serum albumin (cat.
no. V900933; Sigma-Aldrich; Merck KGaA) treatment for 1 h at room
temperature]. The blots were initially incubated at 4°C overnight
with phosphorylated (p)-Akt (cat. no. #4060; 1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA), total (t)-Akt (cat.
no. #2920; 1:1,000; Cell Signaling Technology, Inc.) and β-actin
primary antibodies (cat. no. NB600-501; 1:5,000, Novus Biologicals,
LLC, Littleton, CO, USA). Following incubation for 2 h at room
temperature with rabbit anti-mouse (cat. no. sc-358914; dilution,
1:10,000) or goat anti-rabbit (cat. no. sc-2004; dilution, 1:7,000)
peroxidase-conjugated secondary antibodies (Santa Cruz
Biotechnology Inc.), the blots were developed using enhanced
chemiluminescence (GE Healthcare Bio-Sciences, Pittsburgh, PA,
USA). Gel-Pro Analyzer version 4.0 (Media Cybernetics, Inc.,
Rockville, MD, USA) was used to analyze the western blot results
and the integrated optical density was acquired. The experiments
were performed in triplicate and repeated three times.
Statistical analysis
Statistical analyses were conducted using SPSS for
Windows, version 17.0 (SPSS Inc., Chicago, IL, USA). Results are
expressed as the mean ± standard deviation. Statistical
significance was assessed using Student's unpaired t-test or
two-way analysis of variance followed by Tukey and χ2 tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
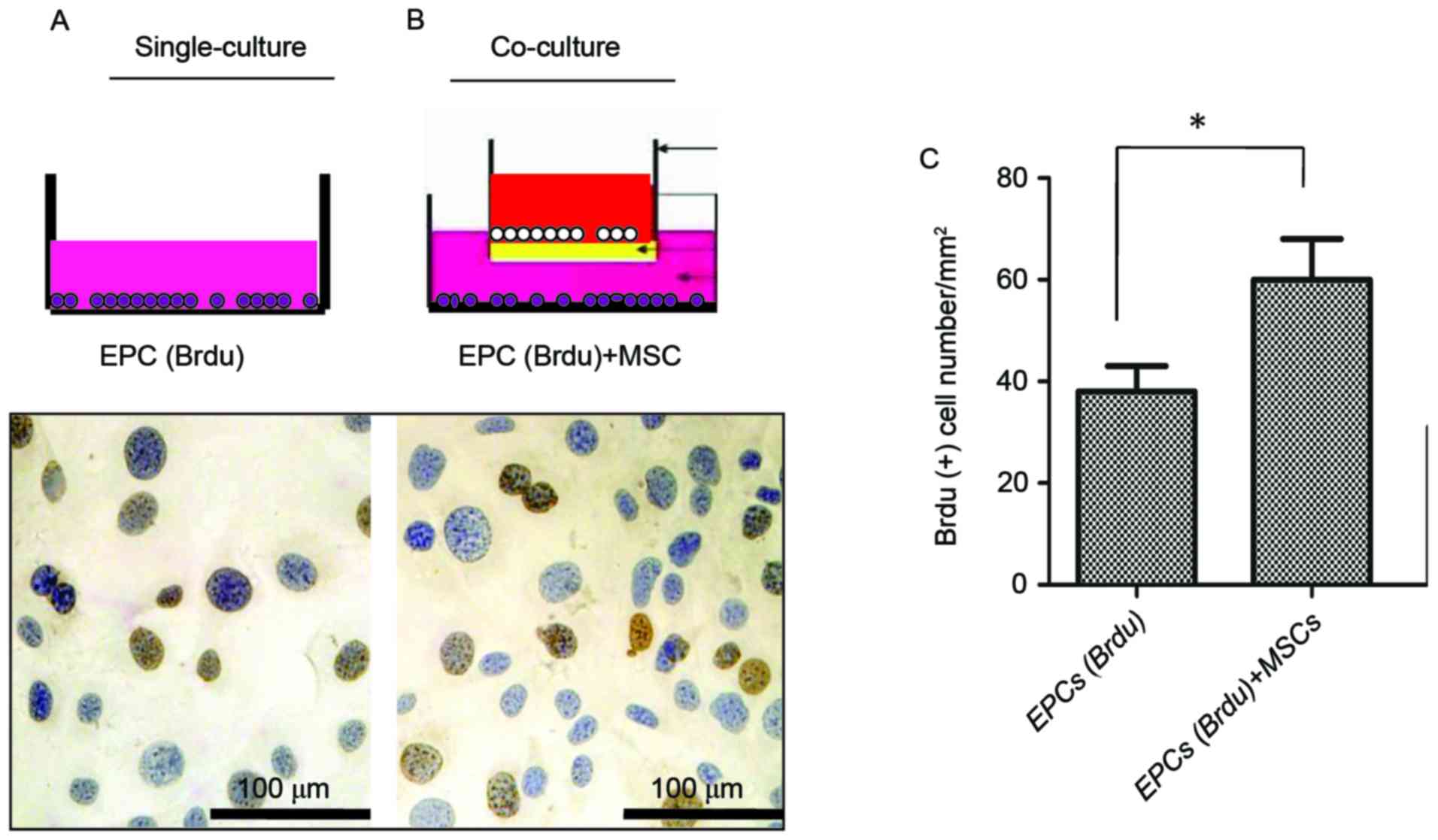
Promotion of EPC proliferation in
vitro by MSCs
As presented in Fig. 1A
and B, EPCs were identified by DAB (blue) staining, whereas
EPCs in the DNA-synthesis phase were counterstained with BrdU
(brown). As demonstrated in Fig.
1C, the total number of BrdU-positive cells in the experimental
group was significantly higher compared with in the control group
(P<0.05), significance was determined using Student's t-test.
These data indicated that MSCs may promote EPC proliferation in
vitro.
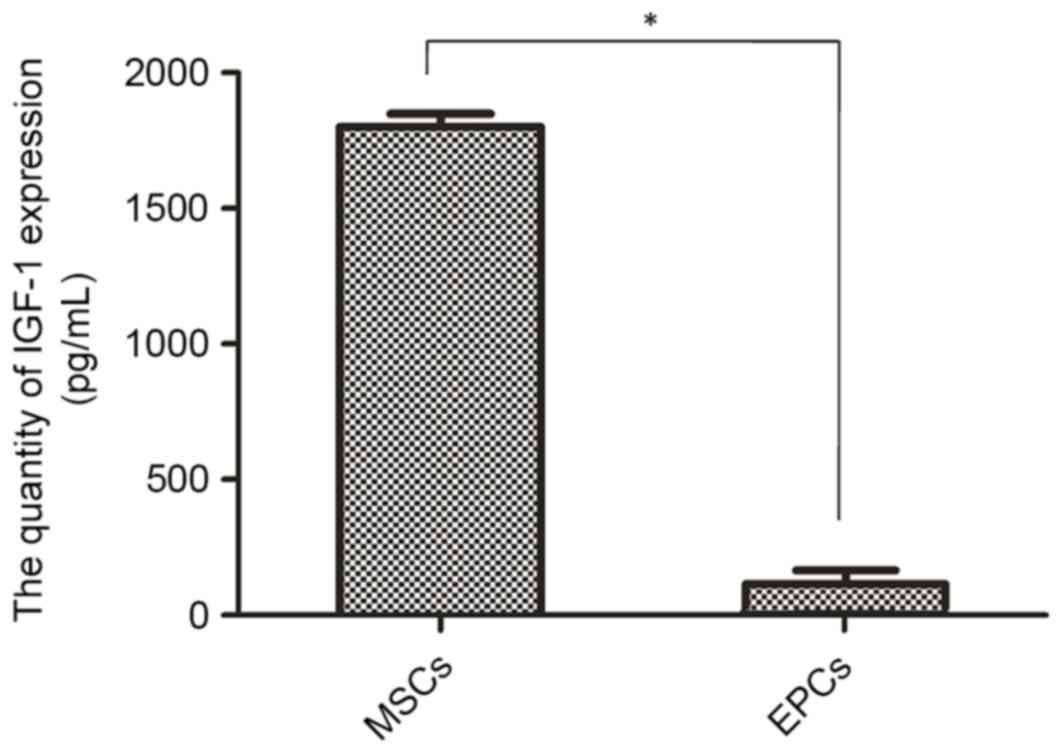
Expression levels of IGF-1 in MSCs and
EPCs
According to the literature, IGF-1 is associated
with the proliferation of other cells (19–21).
Therefore, it was hypothesized that IGF-1 may serve an important
function in MSC-mediated EPC proliferation. To validate this
hypothesis, the expression levels of IGF-1 in the culture media of
MSCs (cultured in factor-free medium) was detected by ELISA. To
eliminate autocrine effects on EPC proliferation the expression of
IGF-1 was also detected in the culture media of EPCs. As
demonstrated in Fig. 2, the
expression levels of IGF-1 were significantly higher in MSCs
compared with in EPCs (1,857.62±49.56 vs. 168.94±5.21 pg/ml;
P<0.01).
Effects of IGF-1 on EPC proliferation
in vitro
The effects of IGF-1 on the proliferation of EPCs
were examined, and the signaling pathway underlying the regulatory
effects was analyzed. Initially, alterations in the number and
absorbance value of EPCs following treatment with various
concentration of IGF-1 (20–200 ng/ml) for 72 h were examined. As
demonstrated in Fig. 3A and B,
IGF-1 increased the number and absorbance value of EPCs in a
concentration-dependent manner (P<0.05). As the higher
concentration of 100 ng/ml IGF-1 produced a significant effect, in
subsequent experiments, EPCs received 100 ng/ml of IGF-1.
Subsequently, the following experiments were
conducted: i) Transfection of MSCs with IGF-1 siRNA; ii)
transfection of EPCs with IGF-1R siRNA; and, iii) neutralization of
IGF-1 in the cultured media of MSCs and EPCs with anti-IGF-1. The
results demonstrated that the proliferation of EPCs was attenuated
in the IGF-1 siRNA transfection group compared with in the control
group (P<0.05; Fig. 4A). In the
EPCs transfected with IGF-1R siRNA, the expression of IGF-1R on the
surface was blocked and the proliferation of EPCs was reduced
(P<0.05; Fig. 4B). Furthermore,
neutralizing the effects of IGF-1 reduced the proliferation of EPCs
(P<0.05; Fig. 4C). These
findings indicated that IGF-1 may serve an important function in
the MSC-mediated proliferation of EPCs.
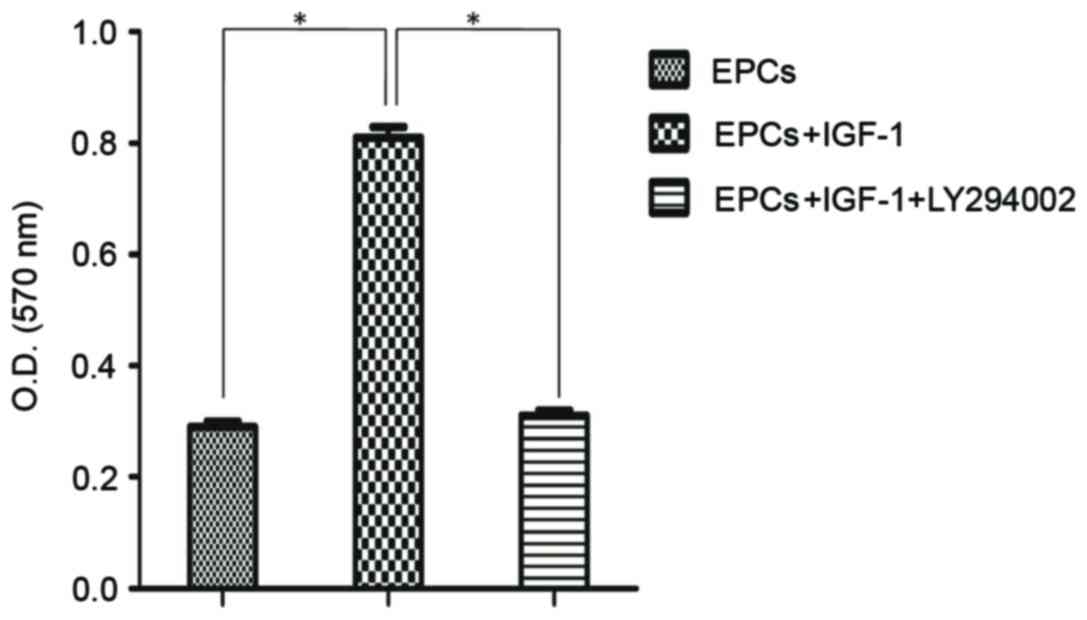
Activation of PI3K/Akt signaling
pathway by IGF-1
To reveal whether the PI3K/Akt signaling pathway was
involved in IGF-1-induced EPC proliferation, the effects of IGF-1
combined with the specific pharmacological inhibitor LY294002 were
investigated. The control group consisted of untreated EPCs. The
remaining two groups were each treated with 100 ng/ml IGF-1, with
one group treated with LY294002 prior to IGF-1 treatment. As
demonstrated in Fig. 5, the
absorbance value of EPCs was significantly decreased following
treatment with the inhibitor. Although the addition of IGF-1
enhanced EPC proliferation, treatment with the PI3K/Akt inhibitor
resulted in a significant attenuation of the IGF-1-dependent cell
proliferation (P<0.05).
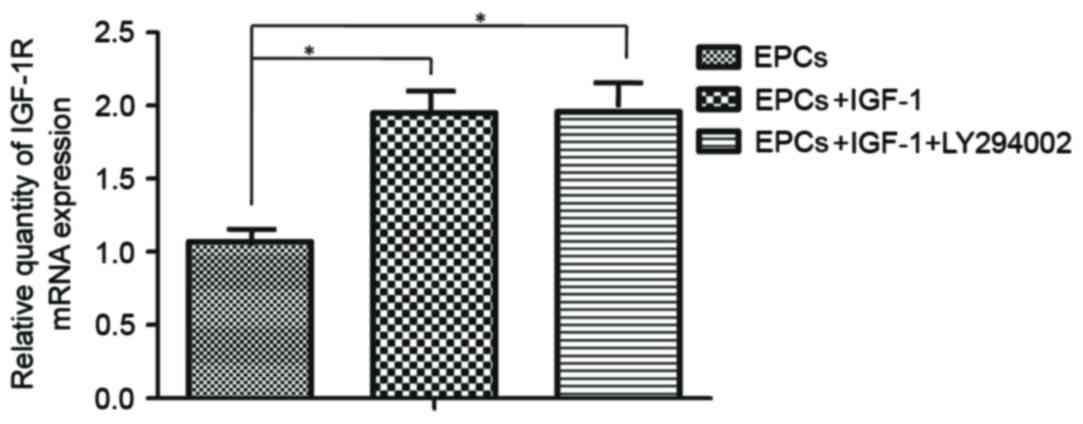
In mammals, the combination of IGF-1 and IGF-1R
initiates a downstream signal transduction pathway, activating a
transcription factor by transducing the extracellular signals into
the nucleus (21). Therefore,
RT-qPCR was used to detect the mRNA expression levels of IGF-1R;
the results demonstrated that IGF-1R mRNA expression was increased
in response to IGF-1 treatment and the specific inhibitor of PI3K,
LY294002, did not inhibit the expression of IGF-1R mRNA. (Fig. 6).
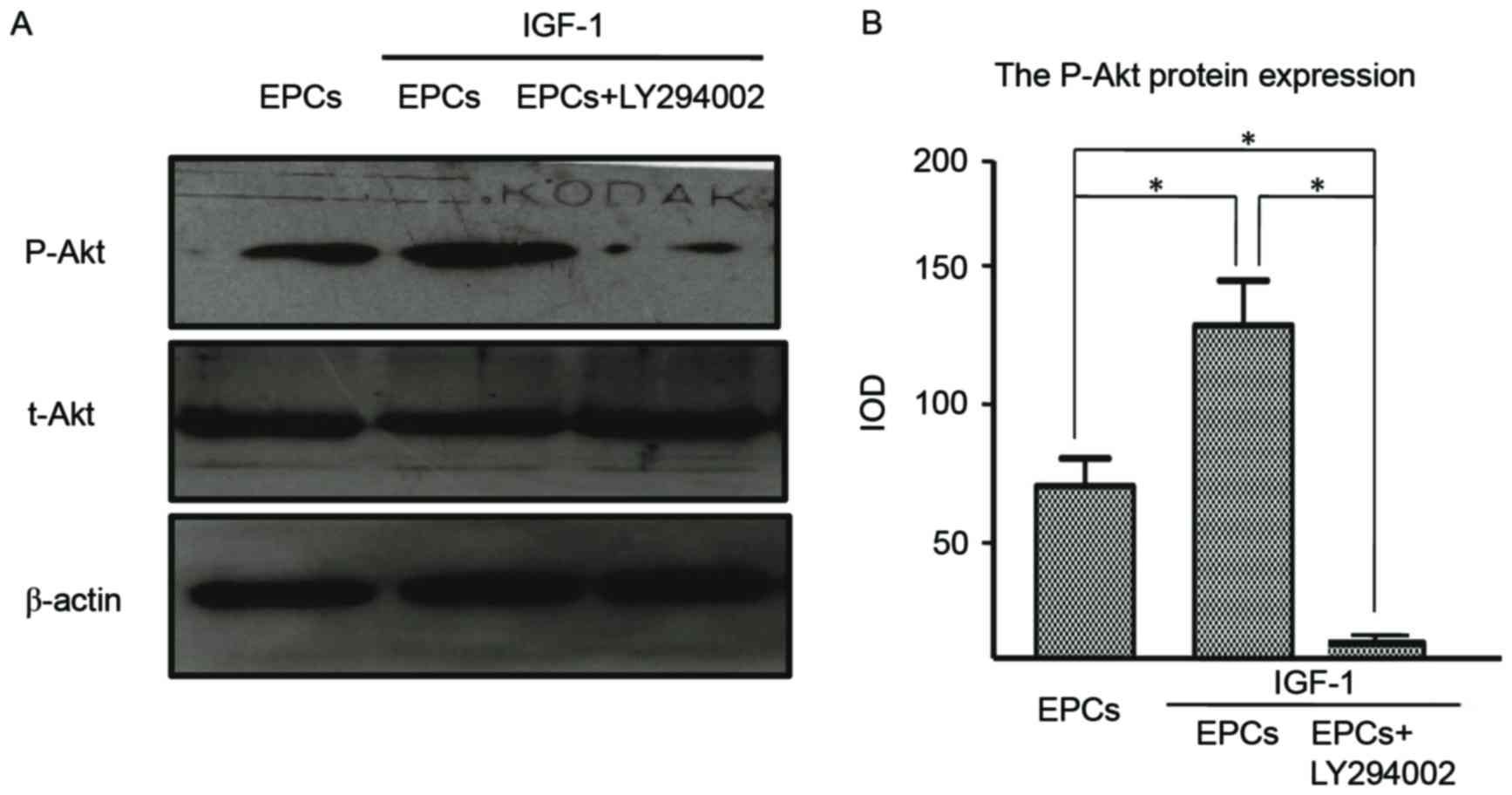
The expression levels of p-Akt and t-Akt protein
were measured by western blot analysis, with the expression of
β-actin used as the control. To test the role of PI3K/Akt in IGF-1
mediated EPCs proliferation, the specific inhibitor of PI3K,
LY294002, was used to reveal the inhibitory effects. The results
demonstrated that Akt phosphorylation (Thr-308) was increased in
EPCs following treatment with IGF-1, whereas the presence of
LY294002 offset this effect (Fig.
7A). By examining the effects of blocking the PI3K/Akt
signaling pathway on protein expression, it was confirmed that the
IGF-1-induced expression of p-Akt was considerably attenuated by
pretreatment with the inhibitor (Fig.
7B). These results confirmed the involvement of the PI3K/Akt
signaling pathway in IGF-1-mediated EPC proliferation.
Discussion
IGF-1 can promote mammalian cell proliferation and
is conducive to the growth of organisms (20,22,23).
In mammals, the combination of IGF-1 and IGF-1R initiates a
downstream signal transduction pathway, activating a transcription
factor by transducing the extracellular signals into the nucleus.
Therefore, IGF-1 is considered to serve an important role in
various biological effects, including cell proliferation, promotion
and apoptotic inhibition (21,24,25).
Li et al (19) reported
that the age-associated decrease in IGF-1 levels resulted in
dysfunctional EPCs, whereas IGF-1 was able to enhance EPC
proliferation. Recently, considerable progress has been made in
understanding the specific IGF-1 downstream signaling pathways
mediating protein synthesis (26).
Our previous study (9) demonstrated that MSCs had an extensive
and close association with EPCs; MSCs were able to promote EPC
proliferation and EPCs enhanced MSC self-renewal. Fedorovich et
al (27) demonstrated that
EPCs derived from peripheral blood contributed to the osteogenic
differentiation of MSCs in vitro, and MSCs supported EPC
proliferation and stabilized the formed cellular networks (27). The present study indicated that
MSCs could promote EPC proliferation; however, the mechanisms
underlying the MSC-induced activation of EPC proliferation remain
poorly understood. To reveal the molecular mechanisms regulating
this process, the effects of IGF-1, which is secreted by MSCs, on
EPC proliferation via the PI3K/Akt signaling pathway were
examined.
In the present study, EPCs were treated with various
doses of IGF-1 and the proliferative capabilities of treated EPCs
were detected by MTT assay. The results demonstrated that IGF-1
could significantly promote the proliferation of EPCs in
vitro. To further explore the signaling pathways involved in
EPC proliferation, the small molecule inhibitor LY294002 was used
to block the PI3K/Akt pathway. Subsequently, the IGF-1-mediated
alterations in EPC proliferation were detected. The results
indicated that the use of LY294002 had a significant inhibitory
effect on EPC proliferation despite treatment with IGF-1.
Furthermore, IGF-1 was able to increase the mRNA expression levels
of IGF-1R in EPCs, thus indicating that the PI3K/Akt signaling
pathway was involved in IGF-1 and IGF-1R-induced EPC
proliferation.
The primary signaling pathway hypothesized to be
associated with IGF-1 is PI3K activation, which is involved in
various cellular processes, including protection from apoptosis and
promotion of proliferation via Akt activation. Inhibition of PI3K
signaling prevents cell cycle completion in cultured satellite
cells by inducing cell cycle arrest in G1 phase, thus
reducing cell proliferation. The PI3K signaling pathway has been
suggested to be important under certain conditions for the
continuance of cell proliferation, but not for cell differentiation
(28). In addition, IGFs can
activate the target of rapamycin signaling pathway by inducing the
PI3K/Akt signaling pathway (29).
In conclusion, the results of the present study
demonstrated that the phosphorylation of Akt was significantly
increased in the presence of IGF-1 compared with in the control
group, whereas treatment with the inhibitor offset the effects of
IGF-1 on EPCs. These findings indicated that IGF-1 may exert
proliferative effects on EPCs via the PI3K/Akt signaling pathway.
Activation of the PI3K/Akt pathway leads to the altered
transcription of genes involved in the cell cycle, thus
accelerating cell cycle progression upon stimulation by IGF-1.
These results suggested that IGF-1-induced EPC proliferation occurs
via the PI3K/Akt signaling pathway. Clarifying the effect of IGF-1
on EPCs proliferation via the PI3K/Akt signaling pathway, may
provide a novel strategy to treat vascular diseases in cell
transplantation.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 31271458),
the Science and Technology Program of Xinjiang Production and
Construction Corps (grant no. 2014AB047), the Scientific Research
Foundation for returned overseas Chinese scholars, Ministry of
Human Resources and Social Security of the People's Republic of
China (grant no. RSLX201201) and Shihezi University youth science
and technology research and development program, basis and
application research project (grant no. 20142RKXYQ20).
References
|
1
|
Friedenstein AJ, Chailakhyan RK and
Gerasimov UV: Bone marrow osteogenic stem cells: In vitro
cultivation and transplantation in diffusion chambers. Cell Tissue
Kinet. 20:263–272. 1987.PubMed/NCBI
|
|
2
|
Bruder SP, Jaiswal N and Haynesworth SE:
Growth kinetics, self-renewal, and the osteogenic potential of
purified human mesenchymal stem cells during extensive
subcultivation and following cryopreservation. J Cell Biochem.
64:278–294. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang Y, Jahagirdar BN, Reinhardt RL,
Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund
T, Blackstad M, et al: Pluripotency of mesenchymal stem cells
derived from adult marrow. Nature. 418:41–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bianco P, Robey PG and Simmons PJ:
Mesenchymal stem cells: Revisiting history, concepts, and assays.
Cell Stem Cell. 2:313–319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moore KA and Elmendorf SC: Propagule vs.
niche limitation: Untangling the mechanisms behind plant species'
distributions. Ecol Lett. 9:797–804. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Timmermans F, Plum J, Yöder MC, Ingram DA,
Vandekerckhove B and Case J: Endothelial progenitor cells: Identity
defined? J Cell Mol Med. 13:87–102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Werner N, Junk S, Laufs U, Link A, Walenta
K, Bohm M and Nickenig G: Intravenous transfusion of endothelial
progenitor cells reduces neointima formation following vascular
injury. Circ Res. 93:e17–e24. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Atluri P, Miller JS, Emery RJ, Hung G,
Trubelja A, Cohen JE, Lloyd K, Han J, Gaffey AC, MacArthur JW, et
al: Tissue engineered, hydrogel-based endothelial progenitor cell
therapy robustly revascularizes ischemic myocardium and preserves
ventricular function. J Thorac Cardiovasc Surg. 148:1090–1098.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang H, Xian L, Lin Z, Yang C, Zhang M,
Feng W, Peng X, Chen X and Wu X: Endothelial progenitor cells as a
possible component of stem cell niche to promote self-renewal of
mesenchymal stem cells. Mol Cell Biochem. 397:235–243. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao X, Wu X, Frassica D, Yu B, Pang L,
Xian L, Wan M, Lei W, Armour M, Tryggestad E, et al: Irradiation
induces bone injury by damaging bone marrow microenvironment for
stem cells. Proc Natl Acad Sci. 108:1609–1614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kinnaird T, Stabile E, Burnett MS, Lee CW,
Barr S, Fuchs S and Epstein SE: Marrow-derived stromal cells
express genes encoding a broad spectrum of arteriogenic cytokines
and promote in vitro and in vivo arteriogenesis through paracrine
mechanisms. Circ Res. 94:678–685. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nagaya N, Kangawa K, Itoh T, Iwase T,
Murakami S, Miyahara Y, Fujii T, Uematsu M, Ohgushi H, Yamagishi M,
et al: Transplantation of mesenchymal stem cells improves cardiac
function in a rat model of dilated cardiomyopathy. Circulation.
112:1128–1135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Noguchi S: The biological function of
insulin-like growth factor-I in myogenesis and its therapeutic
effect on muscular dystrophy. Acta Myol. 24:115–118.
2005.PubMed/NCBI
|
|
14
|
hen Z, Seyfert HM, Löhrke B, Schneider F,
Zitnan R, Chudy A, Kuhla S, Hammon HM, Blum JW, Martens H, et al:
An energy-rich diet causes rumen papillae proliferation associated
with more IGF type 1 receptors and increased plasma IGF-1
concentrations in young goats. J Nutr. 134:11–17. 2004.PubMed/NCBI
|
|
15
|
Sandri M, Barberi L, Bijlsma AY, Blaauw B,
Dyar KA, Milan G, Mammucari C, Meskers CG, Pallafacchina G, Paoli
A, et al: Signalling pathways regulating muscle mass in ageing
skeletal muscle: The role of the IGF1-Akt-mTOR-FoxO pathway.
Biogerontology. 14:303–323. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu M and Zhang S: Amphioxus IGF-like
peptide induces mouse muscle cell development via binding to IGF
receptors and activating MAPK and PI3K/Akt signalling pathways. Mol
Cell Endocrinol. 343:45–54. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu X, Pang L, Lei W, Lu W, Li J, Li Z,
Frassica FJ, Chen X, Wan M and Cao X: Inhibition of Sca-1-positve
skeletal stem cell recruitment by alendronate blunts the anabolic
effects of parathyroid hormone on bone remodeling. Cell Stem Cell.
7:571–580. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li W, Yang SY, Hu ZF, Winslet MC, Wang W
and Seifalian AM: Growth factors enhance endothelial progenitor
cell proliferation under high-glucose conditions. Med Sci Monit.
15:BR357–BR363. 2009.PubMed/NCBI
|
|
20
|
Shavlakadze T, Chai J, Maley K, Cozens G,
Grounds G, Winn N, Rosenthal N and Grounds MD: A growth stimulus is
needed for IGF-1 to induce skeletal muscle hypertrophy in vivo. J
Cell Sci. 123:960–971. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bibollet-Bahena O and Almazan G:
IGF-1-stimulated protein synthesis in oligodendrocyte progenitors
requires PI3K/mTOR/Akt and MEK/ERK Pathways. J Neurochem.
109:1440–1451. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Blum JW and Baumrucker CR: Colostral and
milk insulin-like growth factors and related substances: Mammary
gland and neonatal (intestinal and systemic) targets. Domest Anim
Endocrinol. 23:101–110. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Zhu X, Li X, Wang W, Wang X, Liu
L, Deng Q, Bai G, Wang J, Feng H, et al: Effects of copper on
proliferation and autocrine secretion of insulin-like growth
factor-1 (IGF-1) and IGF-binding protein-3 (IGFBP-3) in
chondrocytes from newborn pigs in vitro. Biol Trace Elem Res.
144:588–596. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu Y, Mu J, Fan Z, Lei G, Yan M, Wang S,
Tang C, Wang Z, Yu J and Zhang G: Insulin-like growth factor 1
enhances the proliferation and osteogenic differentiation of human
periodontal ligament stem cells via ERK and JNK MAPK pathways.
Histochem Cell Biol. 137:513–525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thum T, Hoeber S, Froese S, Klink I,
Stichtenoth DO, Galuppo P, Jakob M, Tsikas D, Anker SD,
Poole-Wilson PA, et al: Age-dependent impairment of endothelial
progenitor cells is corrected by growth hormone mediated increase
of insulin-like growth factor-1. Circ Res. 100:434–443. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Glass DJ: Skeletal muscle hypertrophy and
atrophy signalling pathways. Int J Biochem Cell Biol. 37:1974–1978.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fedorovich NE, Haverslag RT, Dhert WJ and
Alblas J: The role of endothelial progenitor cells in
prevascularized bone tissue engineering: Development of
heterogeneous constructs. Tissue Eng Part A. 16:2355–2367. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chakravarthy MV, Abraha TW, Schwartz RJ,
Fiorotto ML and Booth FW: Insulin-like growth factor-I extends in
vitro replicative life span of skeletal muscle satellite cells by
enhancing G1/S cell cycle progression via the activation of
phosphatidylinositol 3′-kinase/Akt signaling pathway. J Biol Chem.
275:35942–35952. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Glass DJ: PI3 kinase regulation of
skeletal muscle hypertrophy and atrophy. Curr Top Microbiol
Immunol. 346:267–278. 2010.PubMed/NCBI
|