Introduction
Triclosan (TCS), a man-made spectrum antimicrobial
agent, has been widely used in various types of personal care and
industrial products, including hand soap and shampoo, due to its
antibacterial properties and low acute toxicity (1). Until the 1990s, TCS was considered to
be safe. However, TCS has since been associated with various
pathologies and disorders, including obesity, thyroid dysfunction
and breast cell hyper-proliferation (2–5).
Humans are exposed to TCS via direct contact with household
products, as well as via exposure to water, soil and other
organisms that contain TCS, including fish (6,7).
Previous epidemiology studies have demonstrated that TCS exists in
human milk (8,9), blood plasma (10,11)
and urine (12,13). The potential toxic effects of TCS
may threaten the health of offspring if the mother is exposed
during pregnancy. Paul et al (14) identified that perinatal maternal
TCS exposure resulted in maternal and early neonatal
hypothyroxinemic rats. A previous study also demonstrated that TCS
exposure reduced thyroxine levels in pregnant and lactating rat
dams, and in directly exposed offspring (15). However, the underlying mechanisms
responsible for the observed disorders or pathologies are not well
elucidated.
Caenorhabditis elegans (C. elegans) is
a well-established animal model for the investigation of the
molecular basis of fundamental biological processes (16). The worms are easily handled and
sensitive to environmental stimuli, and highly similar to mammals
with regard to pharmacological mechanisms, with 60–80% matching
human genetic homologs having been identified (17). C. elegans has been
previously used to investigate the molecular mechanisms underlying
the initiation and development of various diseases (18–20).
Therefore, C. elegans is an excellent candidate for
investigating the mechanisms involved in toxicity following
prenatal TCS exposure.
The present study exposed adult wild-type C.
elegans N2 to TCS, collected the C. elegans filial 1
(F1) generation and performed high-throughput gene microarray
analysis to determine the gene expression levels in the offspring
in order to analyze the effect of TCS on the systematic gene
expression and investigate the potential mechanisms underlying the
toxicity of prenatal TCS exposure. The present study may help to
improve an understanding of the toxic effects of TCS and provide
evidence against the use of TCS in daily life.
Materials and methods
Chemicals and reagents
TCS was purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany) and dissolved in dimethyl sulfoxide (DMSO) at a
concentration of 50 mg/ml and stored at 20°C.
Culture conditions
C. elegans wild-type N2 was provided by the
Caenorhabditis Genetics Center of Southeast University (Nanjing,
China), C. elegans were cultured in standard nematode growth
medium (NGM) (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) with
2.0×108 living Escherichia coli OP50 bacteria (American Type
Culture Collection, Manassas, VA, USA) at 20°C (21).
C. elegans activity assay
C. elegans were seeded in 24-well plates and
treated with different doses of TCS (0, 100, 200, 300, 400 and 500
µmol/l); subsequently, an equal volume of DMSO (dissolved in M9
minimal medium, Sigma-Aldrich; Merck KGaA; cat. no. M6030) was
added to the culture medium and set as the solvent control. For
each group, 10 young adult C. elegans were seeded into the
well and exposed to TCS for 24 h at 20°C. The treated C.
elegans were observed and recorded by stereo microscope
(SZ61-SET; Olympus Corporation, Tokyo, Japan) at ×50 magnification.
The death ratio was scored by failure to move after being prodded
with a platinum wire (n=10/group).
Detection of toxicity of TCS on the
behavioral characteristics of C. elegans F1 generation
In order to detect the potential toxicity of
exposure to TCS during pregnancy, the present study observed the
locomotory behavior, brood number, generation time and fat
accumulation of the TCS-treated C. elegans F1 generation.
Generally, it is recommended to detect the toxicity of chemicals by
exposure to doses that are more than one-tenth of the
IC50 dose (i.e., the half-maximal inhibitory
concentration). Following exposure to different doses of TCS (100
nmol/l, and 1, 10 and 20 µmol/l) for 24 h at 20°C, 10 worms were
selected randomly and transferred to normal NGM plates. The
locomotory behavior, brood number, body length/width and generation
time of the C. elegans F1 generation were calculated when
the F1 generation synchronously grew to the larval stage 4 and were
subjected to RNA detection subsequently (22).
Extraction of total RNA of C.
elegans
Total RNA was extracted from 1,000 F1 generation of
the TCS-treated (20 µmol/l) C. elegans (24 h at 20°C) and
the solvent control group by using a total RNA kit (cat. no.
R6688-00, Omega Bio-Tek, Inc., Norcross, GA, USA), according to the
manufacturer's protocol. The purity and concentration of total RNA
were determined by measuring the absorbance at 260 and 280 nm
(260/280), and the integrity was checked using a NanoDrop 2000
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) (23).
Gene microarray analysis
Based on the activity assay of TCS-treated C.
elegans (F1 generation were calculated when the F1 generation
synchronously grew to the larval stage 4), the median lethal
concentration (LC50) was calculated and 1/10th the
concentration of LC50 was selected as the experimental
exposure concentration group for gene microarray analysis, and
1,000 F1 generation of TCS-treated (20 µmol/l) C. elegans
(24 h at 20°C) and the solvent control group were selected for gene
microarray analysis. Global gene expression was detected using an
Affymetrix GeneChip™ C. elegans Gene 1.0 ST Array
(Affymetrix, Inc., Santa Clara, CA, USA). Total RNA (~500 ng) was
employed in each experiment. Briefly, total RNA from each sample
was amplified using the Ambion WT Expression kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol (24).
Subsequently, the generated cDNA (~5.5 mg) was separated into
fragments and labeled using the GeneChip™ WT Terminal
Labeling and Controls kit (Affymetrix, Inc., cat. no. G2519F),
according to the manufacturer's protocol. Labeled cDNA target was
hybridized to the Affymetrix GeneChip™ C. elegans
Gene 1.0 ST Array using the GeneChip™ Hybridization,
Wash and Stain kit (Affymetrix, Inc.) at 45°C for 16 h;
subsequently, hybridized arrays were washed and stained on the
Affymetrix GeneChip™ Command Console (Affymetrix, Inc.),
according to the manufacturer's protocol, and scanned by a
GeneChip™ Scanner 3000 7G (Affymetrix, Inc.). The
acquired array images were analyzed by Affymetrix
GeneChip™ Operating software (version GCOS1.4;
Affymetrix, Inc.). Affymetrix Expression Console software
(Affymetrix, Inc.) was used to perform quantile normalization and
subsequent data processing. Data transformation was applied to set
all negative raw values at 1.0, followed by quantile normalization.
A filter on low gene expression was used to keep only the probes
expressed in at least one sample. Differentially regulated genes
were identified via fold change filtering.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated and reverse-transcribed into
cDNA at 42°C by using the PrimeScript RT Master Mix (Perfect Real
Time; Takara Bio, Inc., Otsu, Japan), and qPCR was performed on an
ABI 7900HT Fast Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.) using Power SYBR Green PCR Master Mix (2X;
Applied Biosystems; Thermo Fisher Scientific, Inc.) (25). Briefly, samples were incubated at
95°C for 10 min for initial denaturation, followed by 40 cycles of
amplification that were performed at 95°C for 15 sec and at 60°C
for 1 min. All data were calculated using standard relative
quantification 2−ΔΔCq methods (26) and experiments were performed in
triplicate. GAPDH was used as a normalization control. Primers for
amplification were listed in Table
I.
 | Table I.Primers for reverse
transcription-quantitative polymerase chain reaction of
differentially expressed genes. |
Table I.
Primers for reverse
transcription-quantitative polymerase chain reaction of
differentially expressed genes.
|
| Primer (5′-3′) |
|---|
|
|
|
|---|
| Gene symbol | Forward | Reverse |
|---|
| OSTR158D11_1 |
AGGCGACAACACATTTCGTC |
TCCCTCAGCCACTCTTATGC |
| F14F8.14 |
ATACGACGAAATTTGCGGCA |
CGGTCGAATATTGTGCCCTC |
| C48B6.4 |
ATCGGCTCGCAACGTATTTC |
GTCACAAACACCGAACACGA |
| skr-7 |
ATGTCGCTCCTGAGAATCGT |
TGCTCGGATGCCTCGATAAT |
| fip-1 |
TGGCTGTCTTCTGTGCTGTA |
TCGACCAGCTCCAGACAATT |
| B0563.9 |
GTTGATGCGACCCCAAGATC |
CGTCTCCAAGTCGATCCAGA |
| ent-5 |
GCAAGAGTTCCAGTGATGGC |
TGCTCCAAACGAACAGCATC |
| GAPDH |
CACCAGATGTTTCCGTCGTT |
GGCGAGGATTCCCTTCAT |
Gene ontology (GO) and pathway
analysis
GO function (www.geneontology.org) and Kyoto Encyclopedia of Genes
and Genomes pathway analysis (www.kegg.jp) for
differentially expressed genes were used to identify the
significantly enriched biological terms and pathways. The database
for annotation, visualization and integrated discovery (DAVID;
david.abcc.ncifcrf.gov) was applied to
perform GO function enrichment for differentially expressed genes.
Putative genes that were upregulated >2-fold with P<0.05
following TCS exposure were considered to be differentially
expressed and selected and submitted to DAVID Bioinformatics
Resources 6.7 (david.abcc.ncifcrf.gov). The overall functions
regulated by TCS-modulated genes were identified by functional
annotation clustering and ranked by enrichment scores.
Statistical analysis
Statistical analysis was performed by SPSS 20.0
software (IBM Corp., Armonk, NY, USA). All data are presented as
the mean ± standard deviation. Comparisons between groups were
performed by one-way analysis of variance, followed by Tukey's post
hoc test. P<0.05 was considered to indicate a statistically
significant difference. Experiments were repeated at least three
times independently.
Results
TCS exposure reduces the activity of
C. elegans
Young adult C. elegans were plated in 24-well
plates and exposed to different doses of TCS (0, 100, 200, 300, 400
and 500 µmol/l) for 24 h. An equal volume of DMSO was added to the
culture medium and used as the solvent control. The results
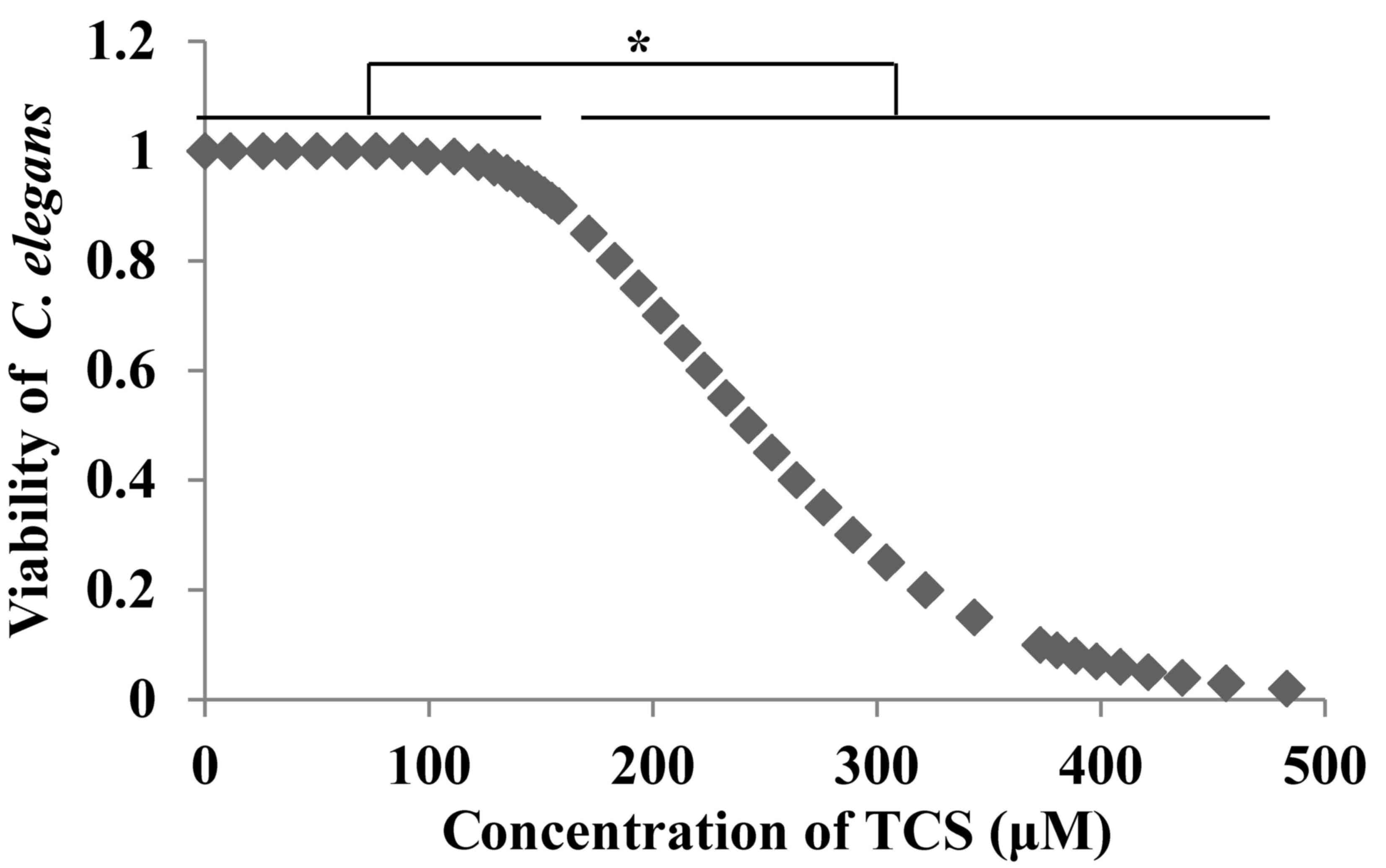
demonstrated that TCS reduced the viability of C. elegans in
a dose-dependent manner (Fig. 1).
The LC50 (LC=242.639 µmol/l) was calculated using SPSS
software and four doses of TCS were selected (100 nmol/l, and 1,
10, and 20 µmol/l), corresponding to the <1/10th LC50 for
subsequent experiments.
TCS exposure affects the behavior
characteristics of C. elegans F1 generation
In order to investigate the genetic effect of TCS
exposure on C. elegans, the present study evaluated several
behavioral characteristics of the C. elegans F1 generation,
including locomotory ability, reproduction, growth time, body
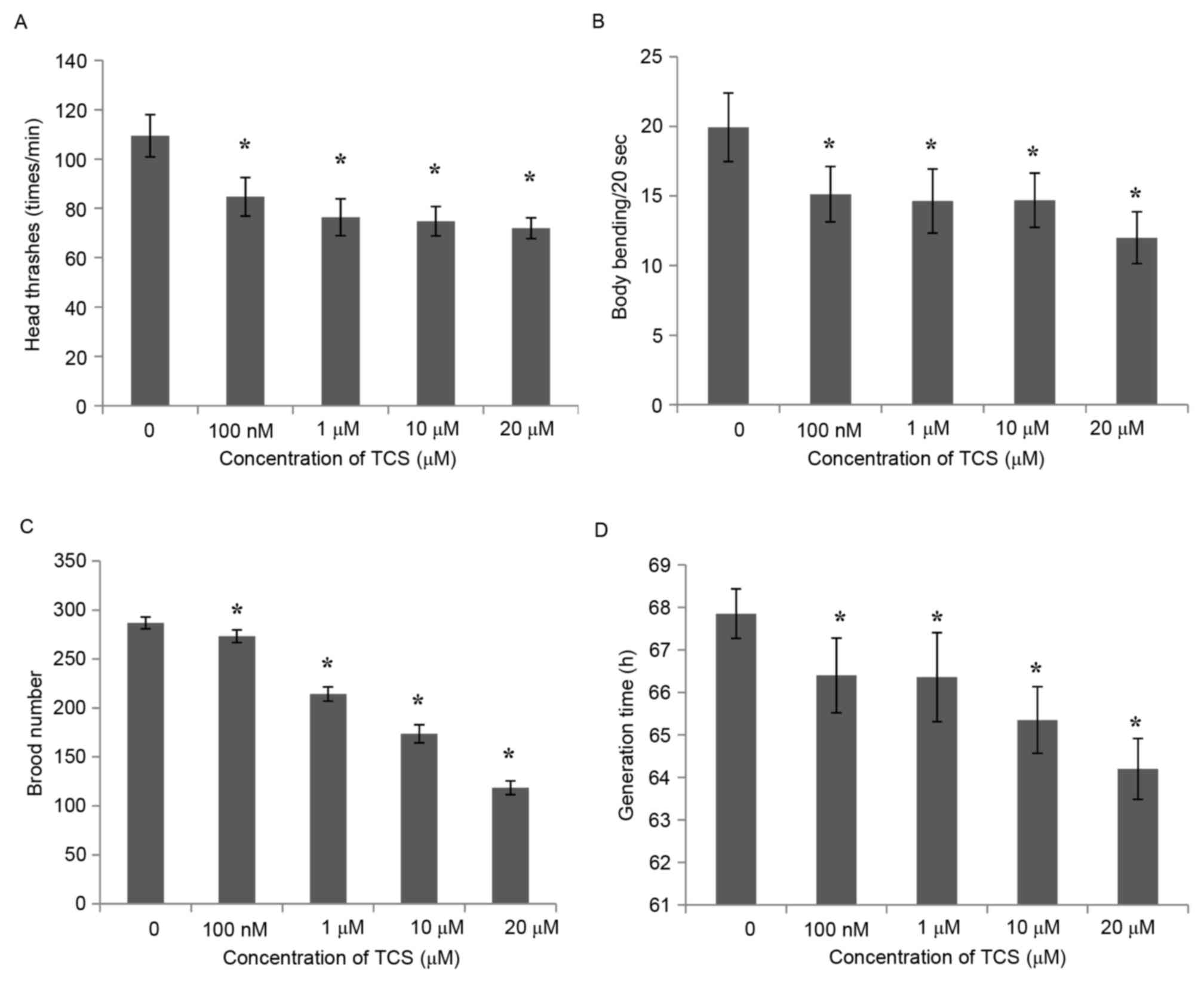
length and width. The results demonstrated that, compared with the
control group, the number of head thrashes of the F1 generation was
inhibited significantly following TCS treatment (Fig. 2A), and the same was observed for
body bending every 20 sec (Fig.
2B). These results indicate that TCS exposure may reduce the
exercise capacity of the F1 generation of C. elegans.
Subsequently, the brood number of the F1 generation was calculated,
and the results demonstrated that the reproductive ability of the
F1 generation was reduced in a dose-dependent manner (by 4.71,
25.60, 39.45 and 58.67% for the 100 nmol/l, and 1, 10, and 20
µmol/l treatments, respectively) compared with the control group
(Fig. 2C). In addition to
locomotory and reproductive ability, the growth time, body length
and width of the F1 generation were also detected. The results
indicated that the generation time was shortened by 2.14–5.38%
following TCS exposure, and appeared to be regulated in a
dose-dependent manner (Fig. 2D),
while the body length and width were not affected by TCS exposure
(data not shown).
Microarray analysis following TCS
treatment
To investigate the potential mechanisms of the
effect of TCS on the behavioral characteristics of C.
elegans F1 generation, gene microarray analysis was performed
to analyze the systematic gene expression pattern of the C.
elegans F1 generation following TCS exposure (data not shown).
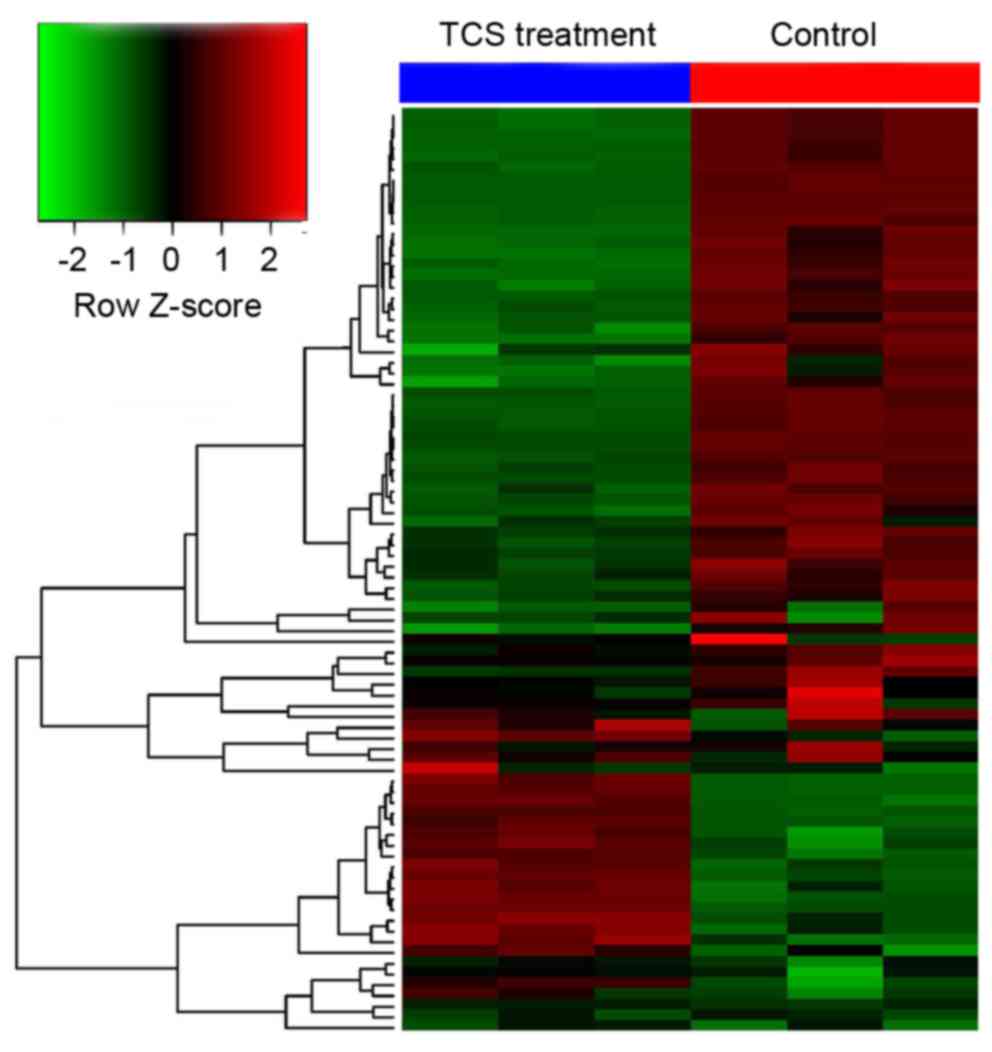
The results demonstrated that, compared with the control group, 113
genes were dysregulated following TCS treatment, including 25 that
were upregulated and 88 that were downregulated (the fold change
threshold was 2.0; P<0.05; Fig.
3).
GO and pathway analysis of
differentially expressed genes
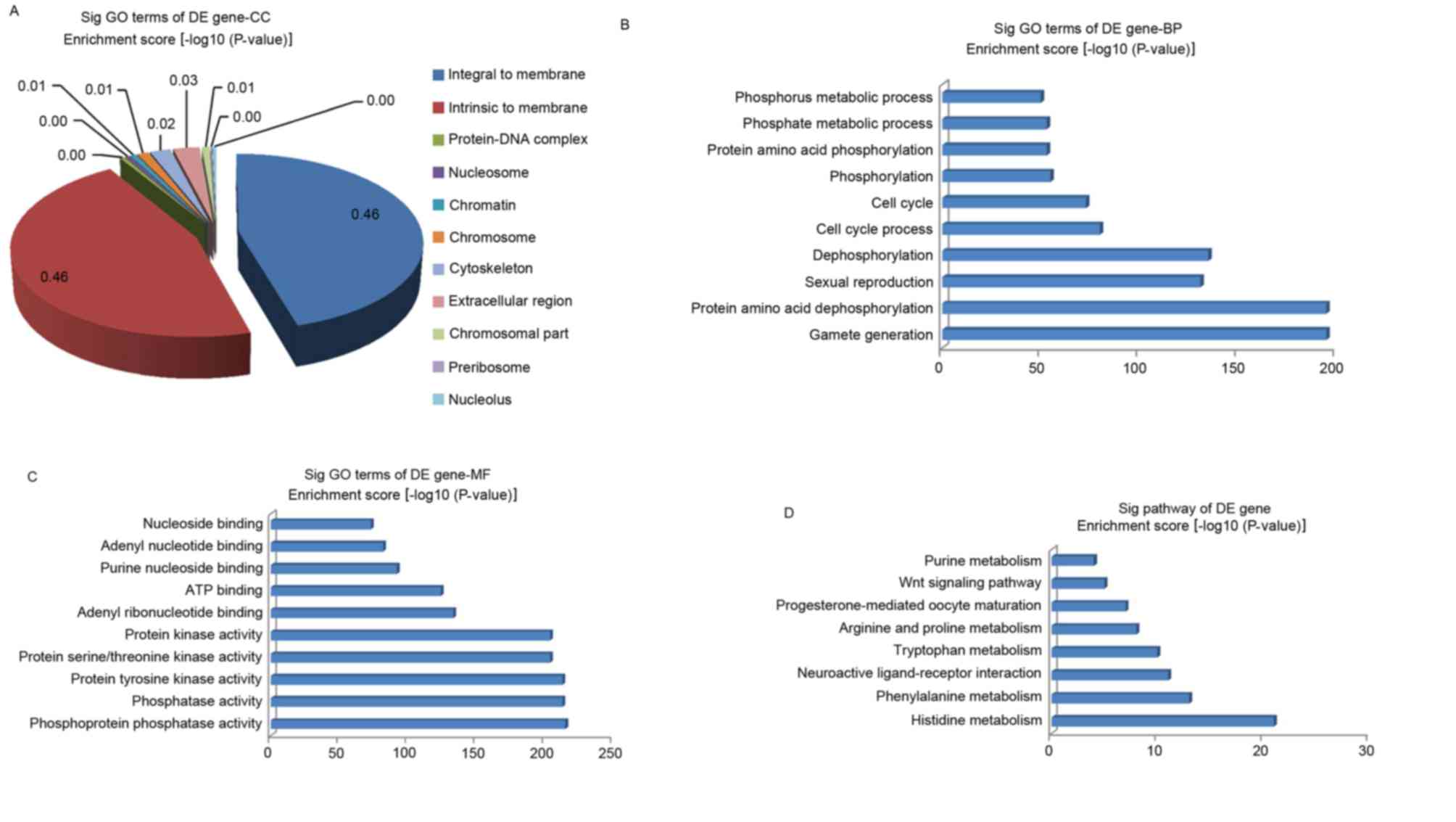
GO analysis was performed in the present study to
clarify the functions of the dysregulated genes. The GO project
(http://www.geneontology.org) primarily
covers three areas (encompassing biological processes, molecular
function, and the cellular component), and the GO-analyzed results
indicated that these gene products are primarily associated with
the cellular component terms ‘integral to membrane’, ‘intrinsic to
membrane’, ‘protein-DNA complex’, ‘nucleosome’ and ‘chromatin’
(Fig. 4A). In addition, the genes
were enriched in the biological processes of ‘phosphorus metabolic
process’, ‘phosphate metabolic process’, ‘protein amino acid
dephosphorylation’, ‘protein amino acid phosphorylation’, and
others not presented in Fig. 4B.
The molecular functions of these genes included phosphoprotein
phosphatase activity, protein tyrosine kinase activity and protein
kinase activity (Fig. 4C). At the
same time, the pathway analysis indicated that these gene products
participated in several signaling pathways, including arginine and
proline metabolism (cel00330), purine metabolism (cel00230),
progesterone-mediated oocyte maturation (cel04914), neuroactive
ligand-receptor interaction (cel04080), the Wnt signaling pathway
(cel04310), phenylalanine metabolism (cel00360), histidine
metabolism (cel00340) and tryptophan metabolism (cel00380; Fig. 4D). The P-value denotes the
significance of the GO term enrichment and the pathway associated
with the conditions: The lower the P-value, the more significant
the GO term and pathway.
Discovery of TCS toxicity-associated
genes
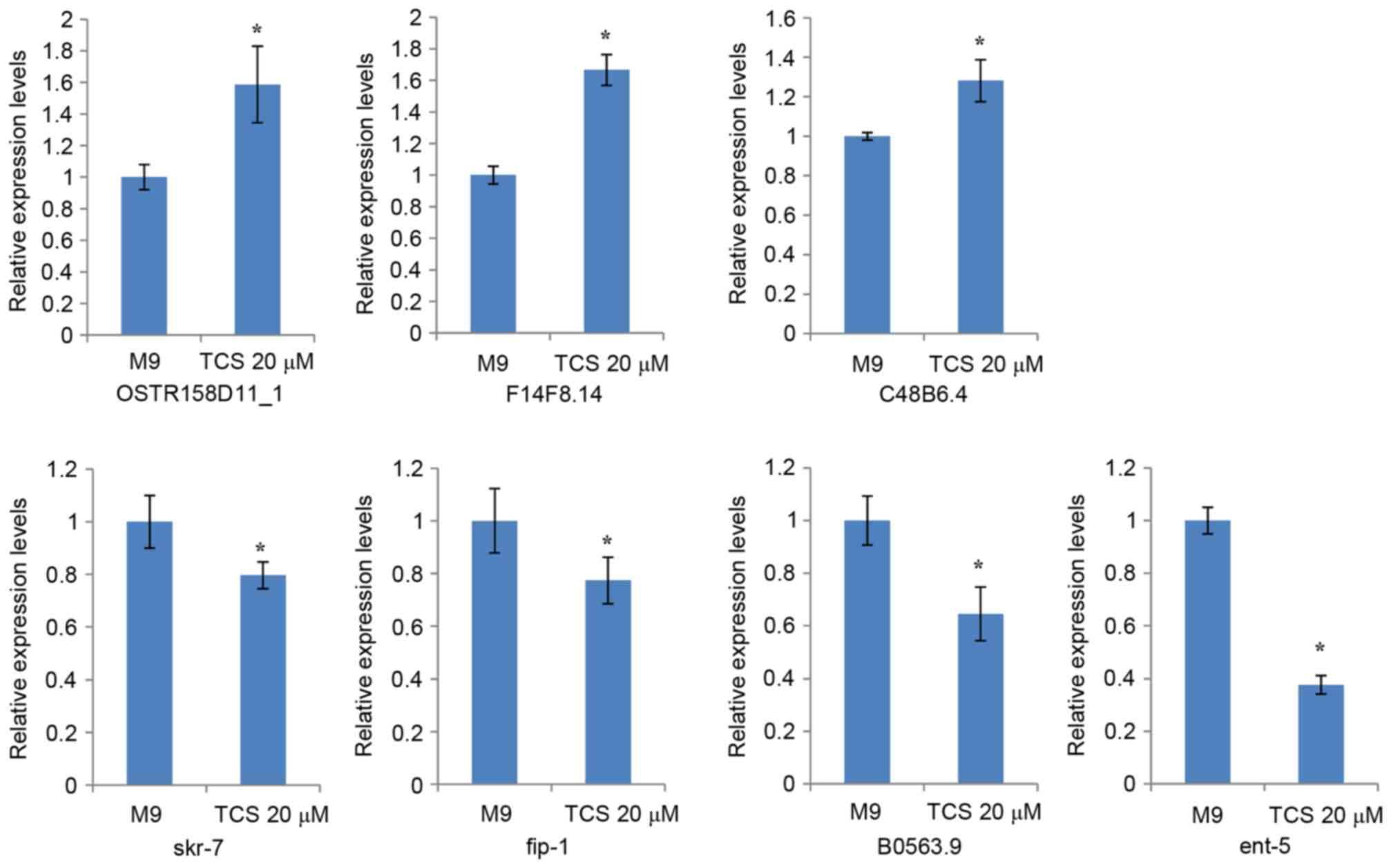
The present study validated the expression levels of
seven dysregulated genes, which were associated with motor nerves
and selected according to the following criteria: Fold-change
filtering, absolute fold-change >2.0; standard Student t-test,
P<0.05; multiple hypotheses testing, false discovery rate
<0.05; and at least 1 out of 2 groups have flags in Present or
Marginal. The RT-qPCR results demonstrated that 7 genes
(OSTR158D11_1, F14F8.14, C48B6.4, skr-7, fip-1, B0563.9, and ent-5)
had differential expression levels in the TCS-treated F1 generation
compared with the controls (Fig.
5).
Discussion
TCS has been widely used in the environment around
human beings and has been identified to be associated with various
types of diseases, including thyroid dysfunction and obesity
(3,4). The mechanism that is responsible for
TCS-induced disorders remains unclear, and TCS exposure may
threaten the health of offspring if exposed during pregnancy. The
current study aimed to investigate the potential mechanisms
underlying the effect of TCS on offspring by using C.
elegans.
In order to exclude the effect of TCS exposure on
C. elegans viability, the toxicity of TCS on C.
elegans was investigated. The results demonstrated that TCS
reduced the viability of C. elegans at doses >100 µmol/l
in a dose-dependent manner (Fig.
1) and the LC50 was calculated to be 242.639 µmol/l.
Subsequently, four doses of TCS were selected for further
investigation.
In addition, the toxic effect of TCS on offspring
(F1 generation) during pregnancy exposure was investigated by
comparing the behavioral characteristics of the TCS-treated F1
generation with those of the control group. The results
demonstrated that, compared with the control group, the head
thrashes, body bending, brood number and generation time of the F1
generation were inhibited significantly by treatment with TCS.
These results indicated that TCS clearly induced genetic toxicity
in the F1 generation. Previously, Jung et al (27) demonstrated that TCS was able to
decrease total serum triiodothyronine and thyroxine in pregnant
rats, and decrease sex ratio and pup body weights, and delayed
vaginal opening in offspring, which also demonstrated the toxicity
of TCS exposure on the next generation.
To investigate the potential mechanisms of the
effect of TCS on the behavioral characteristics of the C.
elegans F1 generation, gene microarray analysis was performed
to analyze the systematic gene expression pattern of the C.
elegans F1 generation following TCS exposure. Compared with the
control group, 113 genes were dysregulated after TCS treatment,
including 25 that were up-regulated, and 88 that were
down-regulated (the fold change threshold was 2.0; P<0.05). In
order to elucidate the basic function of these dysregulated genes,
GO analysis was performed. The GO project (http://www.geneontology.org) primarily covers three
areas (encompassing biological processes, molecular function, and
the cellular component), and the GO-analyzed results indicated that
these gene products are primarily associated with the cellular
component terms ‘integral to membrane’, ‘intrinsic to membrane’,
‘protein-DNA complex’, ‘nucleosome’ and ‘chromatin’. In addition,
the genes were primarily enriched in the biological processes of
‘phosphorus metabolic process’, ‘phosphate metabolic process’,
‘protein amino acid dephosphorylation’ and others not mentioned in
Fig. 4B. The molecular functions
of these genes included phosphoprotein phosphatase activity,
protein tyrosine kinase activity, protein kinase activity, pattern
binding, and polysaccharide binding and chitin binding. At the same
time, the pathway analysis indicated that these gene products
participated in several signaling pathways, including arginine and
proline metabolism (cel00330), purine metabolism (cel00230),
progesterone-mediated oocyte maturation (cel04914), neuroactive
ligand-receptor interaction (cel04080), the Wnt signaling pathway
(cel04310), phenylalanine metabolism (cel00360), histidine
metabolism (cel00340) and tryptophan metabolism (cel00380).
A previous report demonstrated that neuroactive
ligand-receptors are associated with neuron development (28). In the present study, it was
observed that TCS decreased the ability of head thrashes and body
bending in C. elegans, meaning that TCS may possibly affect
neuronal development in C. elegans through regulation of
neuroactive ligand-receptor interaction. In an additional study,
Tribulo et al (29)
demonstrated that the Wnt signaling pathway was involved in the
regulation of embryonic development, through which TCS may decrease
the brood number of C. elegans. The bioinformatic analysis
results from the present study demonstrated that TCS was able to
regulate the behaviors of C. elegans through the
dysregulation of certain genes. In order to investigate the
underlying mechanism of the toxic effect of TCS, motor nerve and
reproduction-associated genes were selected for examination. The
results of the present study demonstrated that 7 genes
(OSTR158D11_1, F14F8.14, C48B6.4, skr-7, fip-1, B0563.9, and ent-5)
were differentially expressed in the TCS-treated F1 generation
compared with the controls. These 7 genes may be important in
explaining the effect of TCS exposure during pregnancy.
Coincidentally, Nayak et al (30) demonstrated that the SKp1-related
gene family in C. elegans may have critical roles in
regulating cell proliferation, meiosis and morphogenesis. This
finding may explain the toxicity of TCS exposure observed on brood
number and generation time of C. elegans progeny in the
present study.
In conclusion, the present study demonstrated that
locomotory behavior and reproductive capacity of C. elegans
offspring was severely affected by prenatal exposure to different
concentrations of TCS, and 7 genes were confirmed to be associated
with toxicity induced by TCS exposure, which merits further
investigation from an environmental health perspective.
Acknowledgements
The present study was financially supported by the
National Key Basic Research Program of China (grant no.
2013CB530604), the Key Project of the National Natural Science
Foundation of China (grant no. 81330067), the Key Project of
Medical Science and Technology Development Foundation, Nanjing
Department of Health (grant no. JQX13012) and the Program for
Innovative Research Teams of Jiangsu Province (grant no.
LJ201108).
References
|
1
|
Dhillon GS, Kaur S, Pulicharla R, Brar SK,
Cledón M, Verma M and Surampalli RY: Triclosan: Current status,
occurrence, environmental risks and bioaccumulation potential. Int
J Environ Res Public Health. 12:5657–5684. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jung EM, An BS, Choi KC and Jeung EB:
Potential estrogenic activity of triclosan in the uterus of
immature rats and rat pituitary GH3 cells. Toxicol Lett.
208:142–148. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Geens T, Dirtu AC, Dirinck E, Malarvannan
G, van Gaal L, Jorens PG and Covaci A: Daily intake of bisphenol A
and triclosan and their association with anthropometric data,
thyroid hormones and weight loss in overweight and obese
individuals. Environ Int. 76:98–105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xue J, Wu Q, Sakthivel S, Pavithran PV,
Vasukutty JR and Kannan K: Urinary levels of endocrine-disrupting
chemicals, including bisphenols, bisphenol A diglycidyl ethers,
benzophenones, parabens, and triclosan in obese and non-obese
Indian children. Environ Res. 137:120–128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee HR, Hwang KA, Nam KH, Kim HC and Choi
KC: Progression of breast cancer cells was enhanced by
endocrine-disrupting chemicals, triclosan and octylphenol, via an
estrogen receptor-dependent signaling pathway in cellular and mouse
xenograft models. Chem Res Toxicol. 27:834–842. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Symsaris EC, Fotidis IA, Stasinakis AS and
Angelidaki I: Effects of triclosan, diclofenac, and nonylphenol on
mesophilic and thermophilic methanogenic activity and on the
methanogenic communities. J Hazard Mater. 291:45–51. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang YS, Kwon JT, Shim I, Kim HM, Kim P,
Kim JC and Lee K: Evaluation of toxicity to triclosan in rats
following 28 days of exposure to aerosol inhalation. Regul Toxicol
Pharmacol. 71:259–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Adolfsson-Erici M, Pettersson M, Parkkonen
J and Sturve J: Triclosan, a commonly used bactericide found in
human milk and in the aquatic environment in Sweden. Chemosphere.
46:1485–1489. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dayan AD: Risk assessment of triclosan
[Irgasan] in human breast milk. Food Chem Toxicol. 45:125–129.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hovander L, Malmberg T, Athanasiadou M,
Athanassiadis I, Rahm S, Bergman A and Wehler EK: Identification of
hydroxylated PCB metabolites and other phenolic halogenated
pollutants in human blood plasma. Arch Environ Contam Toxicol.
42:105–117. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Allmyr M, McLachlan MS, Sandborgh-Englund
G and Adolfsson-Erici M: Determination of triclosan as its
pentafluorobenzoyl ester in human plasma and milk using electron
capture negative ionization mass spectrometry. Anal Chem.
78:6542–6546. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Calafat AM, Ye X, Wong LY, Reidy JA and
Needham LL: Urinary concentrations of triclosan in the U.S.
population: 2003–2004. Environ Health Perspect. 116:303–307. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li X, Ying GG, Zhao JL, Chen ZF, Lai HJ
and Su HC: 4-Nonylphenol, bisphenol-A and triclosan levels in human
urine of children and students in China, and the effects of
drinking these bottled materials on the levels. Environ Int.
52:81–86. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Paul KB, Hedge JM, Devito MJ and Crofton
KM: Developmental triclosan exposure decreases maternal and
neonatal thyroxine in rats. Environ Toxicol Chem. 29:2840–2844.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Axelstad M, Boberg J, Vinggaard AM,
Christiansen S and Hass U: Triclosan exposure reduces thyroxine
levels in pregnant and lactating rat dams and in directly exposed
offspring. Food Chem Toxicol. 59:534–540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chalasani SH, Chronis N, Tsunozaki M, Gray
JM, Ramot D, Goodman MB and Bargmann CI: Corrigendum: Dissecting a
circuit for olfactory behaviour in Caenorhabditis elegans. Nature.
533:1302016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chatterjee N, Yang JS, Park K, Oh SM, Park
J and Choi J: Screening of toxic potential of graphene family
nanomaterials using in vitro and alternative in vivo toxicity
testing systems. Environ Health Toxicol. 30:e20150072015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tedesco P, Visone M, Parrilli E, Tutino
ML, Perrin E, Maida I, Fani R, Ballestriero F, Santos R, Pinilla C,
et al: Investigating the role of the host multidrug resistance
associated protein transporter family in burkholderia cepacia
complex pathogenicity using a Caenorhabditis elegans infection
model. PLoS One. 10:e01428832015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao Y, Yu X, Jia R, Yang R, Rui Q and
Wang D: Lactic acid bacteria protects Caenorhabditis elegans from
toxicity of graphene oxide by maintaining normal intestinal
permeability under different genetic backgrounds. Sci Rep.
5:172332015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Srivastava S, Pant A, Trivedi S and Pandey
R: Curcumin and β-caryophellene attenuate cadmium quantum dots
induced oxidative stress and lethality in Caenorhabditis elegans
model system. Environ Toxicol Pharmacol. 42:55–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Warnhoff K and Kornfeld K: New links
between protein N-terminal acetylation, dauer diapause and the
insulin/IGF-1 signaling pathway in Caenorhabditis elegans. Worm.
4:e10234982015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roussel N, Sprenger J, Tappan SJ and
Glaser JR: Robust tracking and quantification of C. elegans body
shape and locomotion through coiling, entanglement, and omega
bends. Worm. 3:e9824372015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lv J, Xia K, Xu P, Sun E, Ma J, Gao S,
Zhou Q, Zhang M, Wang F, Chen F, et al: miRNA expression patterns
in chemoresistant breast cancer tissues. Biomed Pharmacother.
68:935–942. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lv J, Fu Z, Shi M, Xia K, Ji C, Xu P, Lv
M, Pan B, Dai L and Xie H: Systematic analysis of gene expression
pattern in has-miR-760 overexpressed resistance of the MCF-7 human
breast cancer cell to doxorubicin. Biomed Pharmacother. 69:162–169.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang W, Wang F, Xu P, Miao C, Zeng X, Cui
X, Lu C, Xie H, Yin H, Chen F, et al: Perfluorooctanoic acid
stimulates breast cancer cells invasion and up-regulates matrix
metalloproteinase-2/−9 expression mediated by activating NF-κB.
Toxicol Lett. 229:118–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jung EM, An BS, Choi KC and Jeung EB:
Potential estrogenic activity of triclosan in the uterus of
immature rats and rat pituitary GH3 cells. Toxicol Lett.
208:142–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiao J, Vemula SR, Xue Y, Khan MM,
Kuruvilla KP, Marquez-Lona EM, Cobb MR and LeDoux MS: Motor
phenotypes and molecular networks associated with germline
deficiency of Ciz1. Exp Neurol. 283:110–120. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tribulo P, Moss JI, Ozawa M, Jiang Z, Tian
XC and Hansen PJ: WNT regulation of embryonic development likely
involves pathways independent of nuclear CTNNB1. Reproduction.
153:405–419. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nayak S, Santiago FE, Jin H, Lin D, Schedl
T and Kipreos ET: The Caenorhabditis elegans Skp1-related gene
family: Diverse functions in cell proliferation, morphogenesis, and
meiosis. Curr Biol. 12:277–287. 2002. View Article : Google Scholar : PubMed/NCBI
|