Introduction
Alzheimer's disease (AD) is the most common form of
dementia, and is a complex disease characterized by the formation
of senile plaques and neurofibrillary tangles composed of tau
amyloid fibrils. These are associated with synapse loss and
neurodegeneration, which results in memory impairment and
additional cognitive problems (1).
It has been estimated that 0.40% of the world population (26.6
million people) were living with AD in 2006, and that the
prevalence rate would triple and the absolute number of individuals
with the disease would quadruple by the year 2050 (2). Therefore, this disease is considered
to be a global public health concern and presents a challenge due
to its high prevalence and the burden of the disease (3). However, to date, there are no
available treatments that reduce the progression of AD (4). Excessive apoptosis of neurons is a
notable pathological feature of AD (5). β-amyloid (Aβ), the major component of
senile plaques, has been demonstrated to contribute to neuronal
apoptosis (6). Therefore,
anti-apoptotic therapy may be a promising treatment for AD
(7–9).
Apoptosis typically occurs via the extrinsic or
intrinsic pathway in vertebrate cells (10). It has been demonstrated that the
orphan nuclear receptor Nur77, also known as TR3 or nerve growth
factor IB, is a transcription factor expressed predominantly in
brain tissues (11) that may be
involved in the intrinsic mitochondrial-initiator pathway (12). Nur77 was reported to migrate into
the mitochondria to induce conformational alterations in B cell
lymphoma-2 (Bcl-2) and contribute to the induction of apoptosis in
multiple organs (13). However,
Nur77 nuclear export requires retinoid X receptor-α (RXRα) as a
carrier (14).
RXRα, a member of the nuclear receptor superfamily,
regulates the transcription of target genes by binding to DNA
response elements (15). It has
been demonstrated that RXRα forms heterodimers with additional
members of the steroid/thyroid/retinoid receptor family, including
Nur77 (16). Notably, RXRα may
additionally be involved in multiple biological processes,
including differentiation, inflammation and apoptosis, by
translocating from the nucleus to the cytoplasm (17–19).
In addition, a previous study revealed that retinoids were unable
to induce apoptosis in Nur77 null thymocytes, suggesting that
retinoid-induced apoptosis was dependent on Nur77 (20).
It has been demonstrated that Nur77 nuclear export
may induce apoptosis of T-cell hybridomas and immature thymocytes
(21), as well as various types of
cancer cells (22–24); however, the exact function of Nur77
requires further elucidation. In addition, the involvement of Nur77
in Aβ-induced neuronal apoptosis remains unclear. The primary aim
of the present study was to examine the potential involvement of
Nur77 in Aβ-induced neuronal apoptosis, and to evaluate the effect
of RXRα nuclear export inhibition on neuronal apoptosis.
Materials and methods
Ethical statement
All animal experimentation was performed in
accordance with the recommendations of the Guidelines for the Care
and Use of Laboratory Animals, (Ministry of Science and Technology
of the People's Republic of China, Beijing China). The present
study was approved by the Ethics Review Committee of Fujian Medical
University (permission no. FYD2013-00127).
Drugs
Aβ25–35 powder (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) was dissolved in ddH2O, and
incubated at 37°C for one week. 9-cis-retinoid acid (9-cis-RA;
Sigma-Aldrich; Merck KGaA) was diluted in dimethyl sulfoxide.
Aβ25–35 and 9-cis-RA stock solutions were stored at
−80°C for use in subsequent experiments.
Cell culture and treatment
The mouse neuroblastoma Neuro-2a (N2a) cell line was
purchased from the Cell Bank of Type Culture Collection of Chinese
Academy of Sciences (Shanghai, China). Cells were seeded onto 3
cell culture dishes (10 mm in diameter) at a density of 1.6×105
cells/dish, and were cultured in 50% Dulbecco's modified Eagle's
medium (Mediatech; Corning Incorporated, Corning, NY, USA) and 50%
Opti-minimum essential medium (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 5% fetal bovine serum (Hyclone;
GE Healthcare Life Sciences, Logan, UT, USA). N2a cells were
assigned to the following three groups: i) The Aβ25–35
treatment group, where cells were treated with 25 µmol/l
Aβ25–35 solution for 24 h; ii) the
Aβ25–35-9-cis-RA treatment group, where cells were
incubated in 0.1 µmol/l 9-cis-RA solution for 12 h, followed by
treatment with 25 µmol/l Aβ25–35 solution for 12 h; iii)
Control group, where cells were treated with the same volumes of
ddH2O. The N2a cells were then maintained at 37°C in a
humidified incubator with 5% CO2 for 24 h, before they
were harvested.
Animal grouping
A total of 15 male, 4-month-old C57/BL6 mice
(Experimental Animal Center of Fujian Medical University, Fuzhou,
China), each weighing ~20 g, were group-caged at a room temperature
of 22°C and humidity of 55±10%, with 10 mice in each cage. All mice
were kept under a 12 h light/dark cycle with free access to food
and water. Mice were randomly assigned into three equal groups of
five as follows: The Aβ25–35 treatment group, the
Aβ25–35-9-cis-RA treatment group and the sham-operation
group. The mice were anesthetized for 3 h with 10% chloral hydrate
(Tianjin Beilian Fine Chemical Products Development Co., Ltd.,
Tianjing, China) at a dose of 300 mg/kg by intraperitoneal
injection. Following excision of scalp cutaneous tissue, a bar hole
was created at 2 mm posterior to the bregma and 2.5 mm lateral from
the midline. Mice in the Aβ25–35 treatment group were
treated with 1 µl Aβ25–35 solution (Aβ25–35
powder was dissolved in physiological saline to yield a 2 µg/µl
solution and incubated at 37°C for 7 days) by inserting a
microsyringe through the bar hole to reach the hippocampus. Mice in
the Aβ25–35-9-cis-RA treatment group were treated with a
mixture (1 µl total) of Aβ25–35 (2 µg/µl) and 9-cis-RA
solutions (1 µg/µl), while mice in the sham-operation group were
treated with the same volume of physiological saline. At 24 h after
surgery, the mice were anesthetized with 10% chloral hydrate (300
mg/kg) and subjected to cardiac perfusion with 4% paraformaldehyde,
and approximately 15 to 20 mg mouse hippocampal tissues were
excised, divided into sections, lysed in radioimmunoprecipitation
(RIPA) lysis solution (Beyotime Institute of Biotechnology, Haimen,
China), heated in a microwave for 5 sec, vortexed for 30 sec (1,500
rpm), incubated on ice for 15 to 30 min and centrifuged at 4°C and
13,000 × g for 5 min. The supernatant was transferred to sterile
Eppendorf tubes for subsequent experiments. Mice that stopped
moving and developed complete muscular relaxation were considered
to be in deep anesthesia.
Western blotting assay
Cell and hippocampal tissue lysates were prepared in
RIPA lysis buffer as described previously (25). The nuclear and cytoplasmic extracts
from N2a cells and mouse hippocampal tissues were prepared using
the Nuclear Extraction kit (Merck KGaA). Equal quantities of
protein (25 µg/per lane) were resolved on a 4–20% Tris-glycine gel,
and transferred onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were blocked for 1 h
in 0.05% TBS-Tween-20 (TBST) containing 5% skim milk at room
temperature, and subsequently incubated at 4°C overnight with the
following primary antibodies: Rabbit anti-Bcl-2 (cat. no. D160117;
1:500; Sangon Biotech Co., Ltd.; Shanghai, China), rabbit
anti-Bcl-2 associated X (Bax; cat. no. D120073; 1:500; Sangon
Biotech Co., Ltd.), rabbit anti-RXRα (cat. no. sc-553; 1:1,000;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and rabbit
anti-Nur77 (cat. no. sc-5569; 1:1,000; Santa Cruz Biotechnology,
Inc.), while rabbit anti-β-actin (13E5) antibody (cat. no. 4970;
1:3,000; Cell Signaling Technology, Inc., Danvers, MA, USA), rabbit
anti-poly (ADP-ribose) polymerase (cat. no. 9532; 1:2,000; Cell
Signaling Technology, Inc.) and rabbit anti-heat shock protein 60
(cat. no. ab46798; 1:2,000; Abcam, Cambridge, UK) served as loading
controls. After washing in TBST, the membranes were incubated with
the goat anti-rabbit horseradish peroxidase-conjugated IgG antibody
(cat. no. 7074; 1:8,000; Cell Signaling Technology, Inc.) at room
temperature for 1 h. Detection of proteins was performed by using
an EasyBlot ECL kit (Sangon Biotech Co., Ltd.) according to the
manufacturer's protocol. The band density was quantified using
ImageJ 1.49 (National Institutes of Health; Bethesda, MD, USA).
Experiments were performed in triplicate.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was prepared using TRIzol (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Single-stranded cDNA was synthesized from 1 µg total RNA using a
High Capacity cDNA Reverse Transcription kit (Thermo Fisher
Scientific, Inc.). RT-qPCR was performed using Power 2X SYBR
Real-time PCR Premixture (BioTek Instruments, Inc., Winooski, VT,
USA) according to the manufacturer's protocol. The following primer
sequences were used: RXRα, forward, 5′-TCAAGCGCAGACAAGCAGC-3′, and
reverse, 5′-GCCAGGAGAATCCCATCT-3′; Nur77 forward,
5′-GAAGCTCAGGCAGTTTGC-3′, and reverse, 5′-CGCTCTGGTCCTCATCAC-3′;
GAPH, forward, 5′-AGCCTCCTTGATGGCCTCCTTG-3′, and reverse,
5′-AGAACATCATTCCCAGCAGC-3′. PCR amplification was performed in a 25
µl reaction containing 12.5 µl of 2X Premix, 1 µl forward and 1 µl
reverse primers, 1 µl cDNA template, and 9.5 µl ddH2O
under the following conditions: 94°C for 4 min, followed by 40
cycles of 94°C for 15 sec, 60.5°C for 60 sec, and 60.5°C for 30
sec. The relative quantity of mRNA expression was calculated using
the 2−ΔΔCq method (26).
Immunohistochemistry and
immunocytochemistry
Following perfusion with 4%
paraformaldehyde/phosphate-buffered saline (PBS), mouse brains were
cryopreserved in 30% (w/v) sucrose/PBS at 4°C overnight. Brain
samples embedded in optimal cutting temperature compound (Sangon
Biotech Co., Ltd.) were divided into 20-µm coronal sections and
mounted on glass. Every tenth section was collected in sequential
order (starting at bregma-1.46 mm). The total number of sections
was 4 per animal. Brain sections were blocked at room temperature
for 1 h in a solution containing 10% normal goat serum
(Sigma-Aldrich; Merck KGaA), 0.2% Triton X-100 (Sangon Biotech Co.,
Ltd.) and 0.02% NaN3 (Kegonghua Chemical Technology Co., Beijing,
China) in Tris-buffered saline, and incubated with primary
antibodies at 4°C overnight. Brain sections were then incubated
with fluorescence-conjugated goat secondary antibodies for 1 h. To
visualize the nuclei, sections were counterstained with DAPI (2
µg/ml; Invitrogen; Thermo Fisher Scientific, Inc.) for 5 min in the
dark at room temperature. Following incubation with antibodies,
sections were washed three times with PBS containing 0.5% Tween-20
for 10 min each time. The sections were visualized using laser
confocal microscopy (Leica TCS SP5, Leica Microsystems GmbH,
Wetzlar, Germany). Acquired images were analyzed with Leica
Application Suite X software 4.2 (version 4.2; Leica Microsystems
GmbH). A total of four visual fields (magnification, ×200) of each
coronary section were randomly selected, and a total of four
sections from each animal were used for assessment.
For immunocytochemical analysis, drug-treated N2a
cells (2×105 cells/well) were rinsed with PBS and fixed
with 4% paraformaldehyde for 20 min at room temperature. N2a cells
were permeabilized in 0.1% Triton X-100 for 25 min, and fixed N2a
cells were blocked with 10% normal goat serum in PBS for 1 h at
room temperature prior to incubation with primary antibodies at 4°C
overnight. N2a cells were then incubated with
fluorescence-conjugated secondary antibodies at room temperature
for 1 h, and counterstained with DAPI to visualize the nuclei. A
total of four visual fields (magnification, ×200) of each section
were randomly selected for visualization using laser confocal
microscopy (Leica TCS SP5; Leica Microsystems GmbH) and assessment
by using Leica Application Suite X software (version 4.2; Leica
Microsystems GmbH). For immunohistochemical and immunocytochemical
analysis, the primary antibodies used were rabbit anti-RXRα (cat.
no. sc-553; 1:200; Santa Cruz Biotechnology, Inc.) and goat
anti-Nur77 (cat. no. sc-7014; 1:200; Santa Cruz Biotechnology,
Inc.). The secondary antibodies were goat anti-rabbit IgG DyLight®
594 (cat. no. 35560; 1:200; Thermo Fisher Scientific, Inc.) and
donkey anti-goat IgG DyLight® 488 (cat. no. SA5-10086; 1:200;
Thermo Fisher Scientific, Inc.).
MTT assays
The procedures were performed by using MTT Cell
Proliferation and Cytotoxicity assay kit (Beyotime Institute of
Biotechnology), according to the manufacturer's instructions. N2a
cells were seeded onto 96-well plates at a density of 2×103
cells/well, and cells were assigned to the following 3 groups as
follows: The Aβ25–35 treatment group, where cells were
treated with 25 µmol/l Aβ25–35 solution for 24 h; the
Aβ25–35-9-cis-RA treatment group, where cells were
incubated in 0.1 µmol/l 9-cis-RA solution for 12 h, followed by
treatment with 25 µmol/l Aβ25–35 solution for 12 h; the
control group, where cells were treated with the same volume of
ddH2O. The N2a cells were maintained at 37°C in a
humidified incubator with 5% CO2 for 24 h. MTT solution
(10 µl) was then added to each well, and cells were incubated for a
further 4 h. Formazan diluent solution (100 µl) was subsequently
added to each well, and the plates were placed in a humidified
incubator with 5% CO2 at 37°C until the purple formazan
crystals were completely dissolved. Cell viability was measured
using the absorbance value at 570 nm by using a BioTek ELx808
Absorbance Microplate reader (BioTek Instruments, Inc.).
Flow cytometric assay
Cell apoptosis was determined using an Annexin
V-FITC Apoptosis kit (BioVision, Inc., Milpitas, CA, USA). N2a
cells were seeded onto 6-well plates at a density of 1×105
cells/well and were assigned to the following 3 groups: The
Aβ25–35 treatment group, where cells were treated with
25 µmol/l Aβ25–35 solution for 24 h; the
Aβ25–35-9-cis-RA treatment group, where cells were
incubated in 0.1 µmol/l 9-cis-RA solution for 12 h, followed by
treatment with 25 µmol/l Aβ25–35 solution for 12 h; the
control group, where cells were treated with the same volume of
ddH2O. The N2a cells were maintained at 37°C in a
humidified incubator with 5% CO2 for 24 h before they
were harvested. Cells were subsequently rinsed in cool PBS, and
re-suspended in 250 µl binding buffer (1X). A total of 100 µl cell
suspension (cell density of 1×105 cells/well) was transferred to a
5 ml tube, and 5 µl Annexin V-fluorescein isothiocyanate and 5 µl
propidium iodide was added, mixed and incubated in the dark at room
temperature for 15 min. A total of 400 µl binding buffer (1X) was
then added, and apoptosis of N2a cells was analyzed using an EPICS
Altra Flow Cytometer (Beckman Coulter, Inc.; Brea, CA, USA) and
CellQuest software (version 5.1; Beckman Coulter, Inc.).
Statistical analysis
Data are expressed as the mean ± standard deviation,
and statistical analyses were performed using GraphPad Prism 5
(GraphPad Software, Inc., La Jolla, CA, USA). Differences between
the means of groups were tested for statistical significance using
the one-way analysis of variance followed by Tukey's multiple
comparisons test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Aβ25–35 treatment
demonstrates no significant effect on RXRα and Nur77
expression
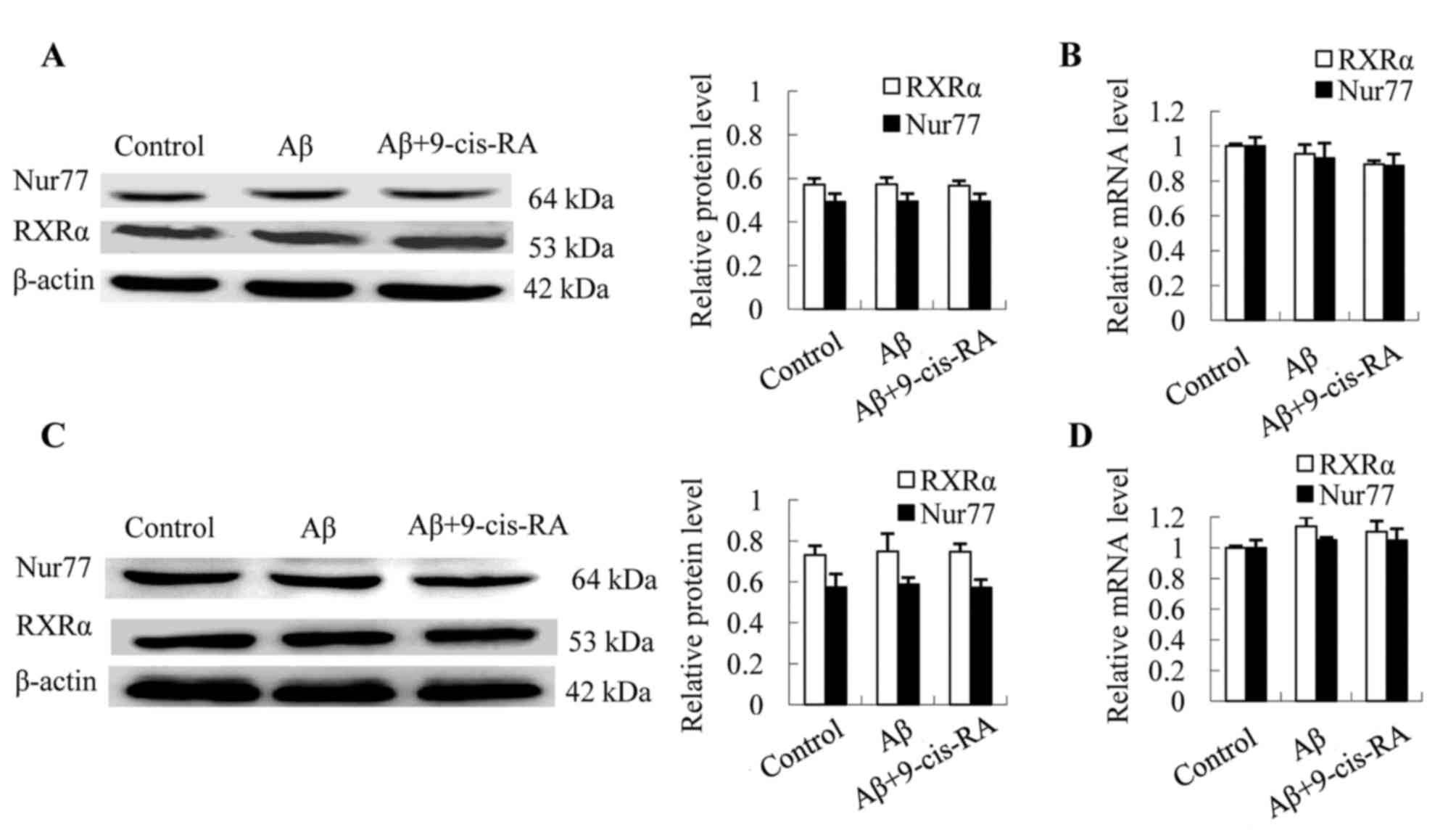
In order to evaluate the effect of
Aβ25–35 treatment on the expression of RXRα and Nur77,
N2a cells were treated with 25 µmol/l Aβ25–35 alone or
in combination with 0.1 µmol/l 9-cis-RA, while untreated cells
served as controls. Western blotting and RT-qPCR analyses revealed
comparative protein and mRNA expression levels of RXRα and Nur77
relative to the controls (Fig. 1).
These results indicated that treatment with Aβ25–35
alone or in combination with 9-cis-RA demonstrated no significant
effect on the expression levels of RXRα and Nur77, at the
translational (Fig. 1A) or
transcriptional levels (Fig. 1B).
In addition, mouse hippocampi were injected with 2 µg
Aβ25–35 or 2 µg Aβ25–35 combined with 1 µg
9-cis-RA, while untreated mice served as controls. No significant
difference in the expression of RXRα and Nur77 among the three
experimental groups at the translational (Fig. 1C) or transcriptional (Fig. 1D) levels were observed, indicating
that treatment with Aβ25–35 alone or
Aβ25–35-9-cis-RA combination demonstrated no significant
effects on RXRα and Nur77 mRNA and protein expression levels.
Nuclear-cytoplasmic translocation of
RXRα is required for cytoplasmic targeting of Nur77 in N2a cells
and the mouse hippocampus
RXRα and Nur77 are primarily located in the nuclei
of untreated N2a cells, and the cytoplasmic protein ratios of RXRα
and Nur77 were 5.26 and 4.85%, respectively (Fig. 2A). Treatment of N2a cells with 25
µmol/l Aβ25–35 for 24 h resulted in significantly
reduced RXRα and Nur77 expression in the nucleus (Fig. 2A) and significantly elevated
expression in the cytoplasm when compared with the controls (RXRα,
8.41-fold, P<0.001; Nur77, 7.33-fold, P<0.001; Fig. 2A). In order to investigate whether
RXRα nuclear export is required for Nur77 cytoplasmic targeting in
neurons, N2a cells were pretreated with 0.1 µmol/l 9-cis-RA or
control medium for 12 h, followed by treatment with 25 µmol/l
Aβ25–35 for 24 h. Western blot analysis demonstrated
that the cytoplasmic protein ratios of RXRα and Nur77 were
significantly reduced in the Aβ25–35-9-cis-RA group
compared with Aβ25–35-treated N2a cells [RXRα,
Aβ25–35 (42.22%) vs. Aβ25–35-9-cis-RA
(6.67%), P<0.001; Nur77, Aβ25–35 (35.49%) vs.
Aβ25–35-9-cis-RA (5.44%), P<0.001; Fig. 2A]. Confocal microscopy revealed
that Nur77 and RXRα proteins were predominantly localized in the
nuclei of untreated N2a cells, while RXRα and Nur77 were
predominantly co-localized in the cytoplasm of
Aβ25–35-treated N2a cells (Fig. 2B). In addition, the distribution of
RXRα and Nur77 overlapped, suggesting an interaction between RXRα
and Nur77 in the cytoplasm (Fig.
2B). However, the nuclear export of RXRα and Nur77 was visibly
inhibited in N2a cells treated with Aβ25–35 plus
9-cis-RA (Fig. 2B).
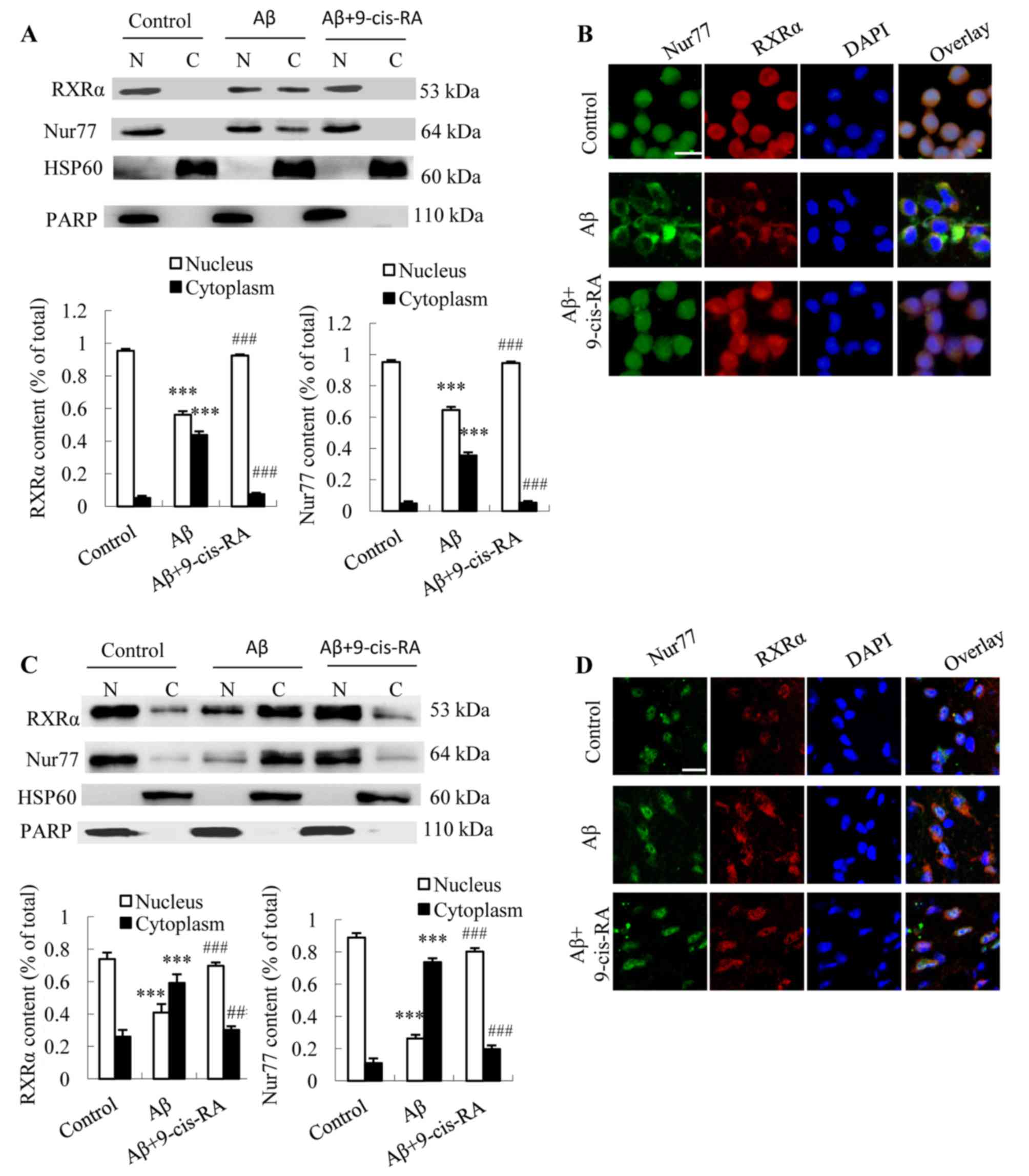 | Figure 2.Effect of Aβ25–35
treatment or Aβ25–35 plus 9-cis-RA treatment on the
cellular location of RXRα and Nur77 in N2a cells and the mouse
hippocampus. (A) RXRα and Nur77 nuclear and cytoplasmic protein
expression in N2a cells, normalized to the expression of
cytoplasmic protein HSP60 and nuclear protein PARP (n=3). (B)
Representative fluorescence microscope images showing
immunocytochemical staining of RXRα and Nur77 expression in N2a
cells (scale bar, 10 µm). (C) RXRα and Nur77 nuclear and
cytoplasmic protein expression in mouse hippocampus tissue was
normalized to the expression of cytoplasmic protein HSP60 and
nuclear protein PARP (n=5). (D) Representative fluorescence
microscope images showing immunohistochemical staining of RXRα and
Nur77 in the CA1 region of C57BL/6 mouse hippocampi (scale bar, 10
µm). Values are presented as the mean ± standard deviation of ≥3
independent experiments. *P<0.05, **P<0.01 and ***P<0.001
vs. control group; #P<0.05, ##P<0.01
and ###P<0.001 vs. Aβ group. Aβ, β-amyloid; 9-cis-RA,
9-cis-retinoid acid; RXRα, retinoid X receptor-α; N2a, Neuro-2a;
HSP60, heat shock protein 60; PARP, poly(ADP-ribose)
polymerase. |
The dependence of the cytoplasmic localization of
Nur77 on RXRα localization in vivo was then evaluated. The
hippocampi of C57BL/6 mice were injected with 2 µg
Aβ25–35 or 2 µg Aβ25–35 plus 1 µg 9-cis-RA
for 24 h, while sham-operated mice served as controls. Western
blotting analysis revealed that Aβ25–35 treatment was
associated with a marked increase in the translocation of RXRα and
Nur77 from the nucleus to the cytoplasm of hippocampus cells, and
RXRα and Nur77 protein expression increased from 26.1 and 11.1% to
59.2 and 73.7%, respectively, in the cytoplasm relative to the
controls (P<0.001 and P<0.001, respectively; Fig. 2C). In addition, the cytoplasmic
protein ratios of RXRα and Nur77 decreased from 59.2 and 73.7% in
the hippocampal tissues of Aβ25–35-treated mice to 30.2
and 19.8% in Aβ25–35 plus 9-cis-RA co-treated mice,
respectively, when compared with mice treated with
Aβ25–35 alone (P<0.01 and P<0.001, respectively;
Fig. 2C). Confocal microscopy
revealed that RXRα and Nur77 were primarily localized to the
nucleus and the nuclear periphery of untreated mice, while an
increase in RXRα and Nur77 fluorescence in the cytoplasm of
Aβ25–35-treated mice was observed (Fig. 2D). In the mice treated with 2 µg
Aβ25–35 plus 1 µg 9-cis-RA, RXRα and Nur77 were observed
to reside in the nucleus and nuclear periphery when compared with
the Aβ25–35 treatment group (Fig. 2D).
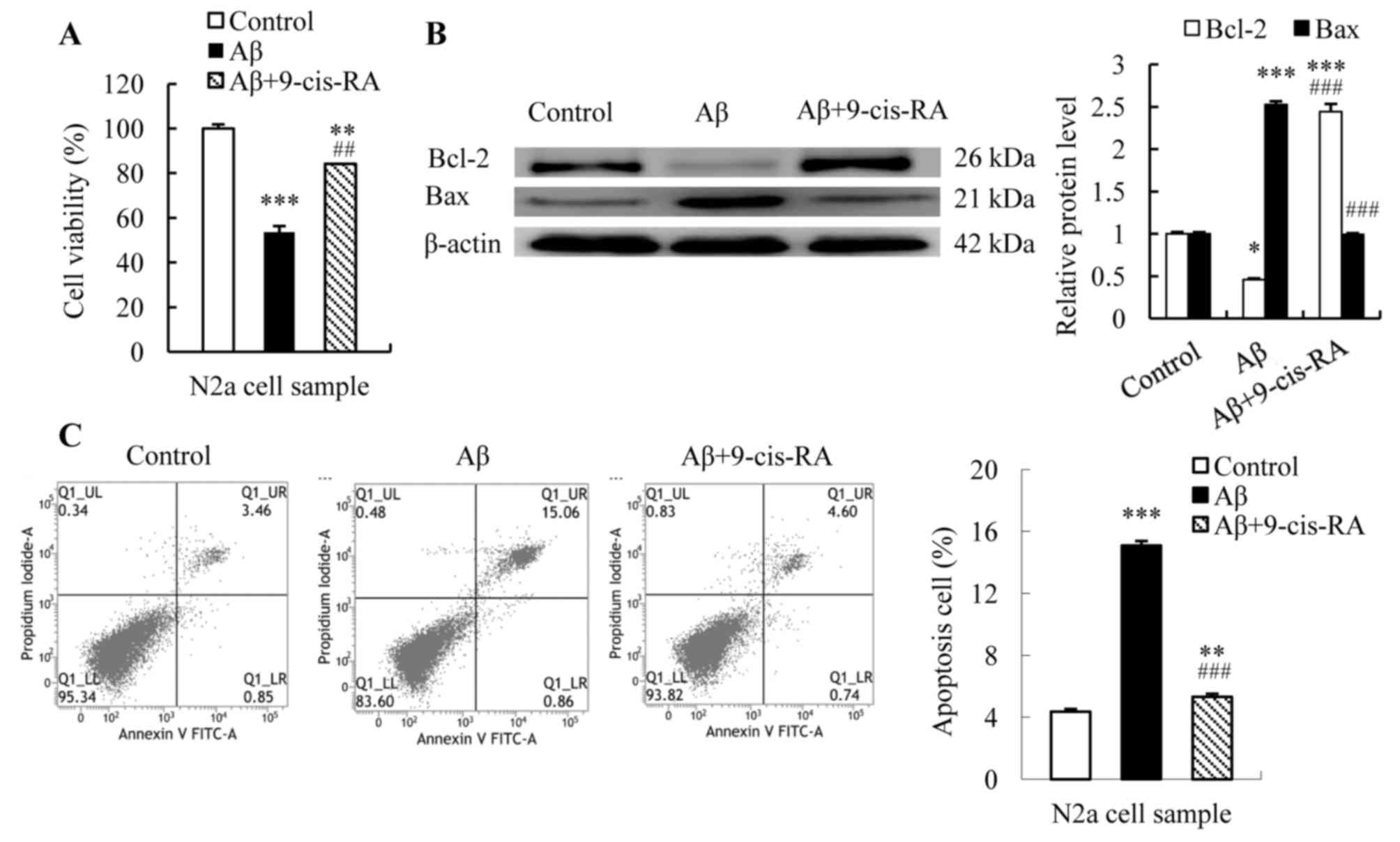
9-cis-RA improves cell viability and
inhibits apoptosis of N2a cells in the presence of
Aβ25–35
The results of the MTT assay revealed a significant
reduction in the viability of N2a cells following treatment with
Aβ25–35 for 24 h when compared with the controls (from
100 to 53.65% cell viability; P<0.001; Fig. 3A). By contrast, the viability of
N2a cells treated with Aβ25–35 plus 9-cis-RA
significantly increased from 53.65 to 84.10% (P<0.01 vs.
Aβ25–35-treated cells; Fig.
3A).
Mitochondrial outer membrane permeabilization, which
is involved in the induction of apoptosis, is controlled by pro-
and anti-apoptotic factors (27).
Therefore, the expression of mitochondrial membrane
permeabilization factors Bcl-2 and Bax was examined in the present
study. N2a cells were treated with 25 µmol/l Aβ25–35 for
24 h alone or in combination with 0.1 µmol/l 9-cis-RA for 12 h,
while untreated cells served as controls. Bcl-2 and Bax protein
expression levels were subsequently quantified. Western blotting
analysis revealed elevated Bax expression and reduced Bcl-2
expression (Bax, 2.52-fold, P<0.001; Bcl-2, 0.46-fold,
P<0.05; Fig. 3B) in
Aβ25–35-treated N2a cells when compared with controls.
Combined treatment with Aβ25–35 and 9-cis-RA recovered
Bcl-2 expression from 0.46-fold in Aβ25–35-treated cells
to 2.44-fold relative to controls (P<0.001; Fig. 3B), and decreased Bax expression
from 2.52-fold in Aβ25–35-treated cells to 0.99-fold
relative to controls (P<0.001; Fig.
3B).
Flow cytometry analysis revealed that the apoptotic
rate of untreated N2a cells was 4.36%, while an apoptotic rate of
15.1% was detected in cells cultured in the presence of 25 µmol/l
Aβ25–35 for 24 h (P<0.001; Fig. 3C). In addition, combined treatment
with 25 µmol/l Aβ25–35 plus 0.1 µmol/l 9-cis-RA resulted
in a ~5.31% apoptotic rate, which was a significant reduction when
compared with cells treated with Aβ25–35 alone
(P<0.001; Fig. 3C).
Discussion
Aβ accumulation is generally accepted to be critical
for the development of AD dementia (28,29).
Aβ has been confirmed to exhibit neurotoxic effects on neurons
(30). In the present study, an
increased apoptotic rate of Aβ25–35-treated N2a cells
was observed, and cell viability decreased when compared with
untreated controls. These results are consistent with the notion
that Aβ is toxic to neurons. However, these results were obtained
from cell and animal experiments, and further human studies are
therefore required to validate this conclusion.
Modulation of RXRα levels has been previously
reported to directly affect Aβ generation (25). Given the involvement of RXRα/Nur77
in the regulation of cell death, the effect of Aβ25–35
treatment on RXRα and Nur77 expression at translational and
transcriptional levels was investigated in the present study.
Western blotting and RT-qPCR analysis revealed no significant
difference in the protein and mRNA levels of RXRα and Nur77 between
the Aβ25–35-treated and untreated control groups. This
is inconsistent with the report demonstrating that higher levels of
RXRα gene expression occur in AD (19). It is possible that a single
injection was insufficient to alter RXRα expression
significantly.
RXRα has been demonstrated to regulate
Nur77-dependent apoptosis by modulating Nur77 nuclear export
(8). However, the involvement of
RXRα in the regulation of neuronal apoptosis is unclear. In the
present study, this potential function of RXRα was confirmed using
in vivo and in vitro experimental systems, which
mimic the pathologic condition of AD. In response to
Aβ25–35 stimulation RXRα/Nur77 were co-transported into
the cytoplasm in N2a cells and hippocampal neurons, as the rate of
apoptosis increased. Further studies to validate the
co-localization of RXRα/Nur77 in mitochondria and investigate the
mechanisms underlying the association between RXRα/Nur77 nuclear
export and apoptosis are required.
In the present study, the role of RXRα nuclear
export in mediating Nur77 nuclear export was investigated. In the
presence of 9-cis-RA, Aβ25–35-induced RXRα/Nur77 nuclear
export was inhibited both in vivo and in vitro. The
results of the present study provide some evidence to suggest that
Nur77 may be transported to the cytoplasm via its interaction with
RXRα, which is consistent to a previous report (14). The pro-apoptotic Bax and
anti-apoptotic Bcl-2 protein expression levels were then analyzed.
Aβ25–35 treatment was demonstrated to result in elevated
Bax expression and reduced Bcl-2 expression, accompanied by
RXRα/Nur77 nuclear export and an increased apoptotic rate in N2a
cells. The expression of Bax and Bcl-2 was consistent with previous
observations that Bcl-2 inhibits apoptosis and Bax promotes
apoptosis (31). However,
treatment with 9-cis-RA plus Aβ25–35 resulted in a
decreased apoptotic rate of N2a cells, and inhibition of RXRα/Nur77
nuclear export coincided with the decreased Bax and increased Bcl-2
expression. These results suggested that Aβ-induced RXRα and Nur77
co-translocation was associated with the alterations in Bcl-2 and
Bax expression, which may have initiated apoptosis in neurons.
In conclusion, the results of the present study
demonstrated that Aβ-mediated neuronal death may be dependent on
Nur77, and RXRα and its ligands may be involved in regulating
Nur77-dependent apoptosis in neurons. Elucidation of the mechanisms
underlying the inhibition of RXRα translocation may be of
significance for the development of anti-AD agents that suppress
neuronal apoptosis.
Acknowledgements
The present study was supported by the grants from
the Special Funds of the National Natural Science Foundation of
China (grant no. 81241017), the Science and Technology Project of
Fujian Provincial Department of Education (grant no. JA11109), and
the Academic Development Funds for the Professors in Fujian Medical
University (grant no. JS/4003).
References
|
1
|
Querfurth HW and LaFerla FM: Alzheimer's
disease. N Engl J Med. 362:329–344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brookmeyer R, Johnson E, Ziegler-Graham K
and Arrighi HM: Forecasting the global burden of Alzheimer's
disease. Alzheimers Dement. 3:186–191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ballard C, Gauthier S, Corbett A, Brayne
C, Aarsland D and Jones E: Alzheimer's disease. Lancet.
377:1019–1031. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gandy S and DeKosky ST: Toward the
treatment and prevention of Alzheimer's disease: Rational
strategies and recent progress. Annu Rev Med. 64:367–383. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mattson MP: Apoptosis in neurodegenerative
disorders. Nat Rev Mol Cell Biol. 1:120–129. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morishima Y, Gotoh Y, Zieg J, Barrett T,
Takano H, Flavell R, Davis RJ, Shirasaki Y and Greenberg ME:
Beta-amyloid induces neuronal apoptosis via a mechanism that
involves the c-Jun N-terminal kinase pathway and the induction of
Fas ligand. J Neurosci. 21:7551–7560. 2001.PubMed/NCBI
|
|
7
|
Favaloro B, Allocati N, Graziano V, Di
Ilio C and De Laurenzi V: Role of apoptosis in disease. Aging
(Albany NY). 4:330–349. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Muradian K and Schachtschabel DO: The role
of apoptosis in aging and age-related disease: Update. Z Gerontol
Geriatr. 34:441–446. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He H, Dong W and Huang F:
Anti-amyloidogenic and anti-apoptotic role of melatonin in
Alzheimer disease. Curr Neuropharmacol. 8:211–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tait SW and Green DR: Mitochondria and
cell death: Outer membrane permeabilization and beyond. Nat Rev Mol
Cell Biol. 11:621–632. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dai Y, Zhang W, Sun Q, Zhang X, Zhou X, Hu
Y and Shi J: Nuclear receptor nur77 promotes cerebral cell
apoptosis and induces early brain injury after experimental
subarachnoid hemorrhage in rats. J Neurosci Res. 92:1110–1121.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moll UM, Marchenko N and Zhang XK: p53 and
Nur77/TR3-transcription factors that directly target mitochondria
for cell death induction. Oncogene. 25:4725–4743. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin B, Kolluri SK, Lin F, Liu W, Han YH,
Cao X, Dawson MI, Reed JC and Zhang XK: Conversion of Bcl-2 from
protector to killer by interaction with nuclear orphan receptor
Nur77/TR3. Cell. 116:527–540. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin XF, Zhao BX, Chen HZ, Ye XF, Yang CY,
Zhou HY, Zhang MQ, Lin SC and Wu Q: RXR alpha acts as a carrier for
TR3 nuclear export in a 9-cis retinoic acid-dependent manner in
gastric cancer cells. J Cell Sci. 117:5609–5621. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang XK, Su Y, Chen L, Chen F, Liu J and
Zhou H: Regulation of the nongenomic actions of retinoid X
receptor-α by targeting the coregulator-binding sites. Acta
Pharmacol Sin. 36:102–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Salerno AJ, He Z, Goos-Nilsson A, Ahola H
and Mak P: Differential transcriptional regulation of the apoAI
gene by retinoic acid receptor homo- and heterodimers in yeast.
Nucleic Acids Res. 24:566–572. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lefebvre P, Benomar Y and Staels B:
Retinoid X receptors: Common heterodimerization partners with
distinct functions. Trends Endocrinol Metab. 21:676–683. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao X, Liu W, Lin F, Li H, Kolluri SK, Lin
B, Han YH, Dawson MI and Zhang XK: Retinoid X receptor regulates
Nur77/TR3-dependent apoptosis [corrected] by modulating its nuclear
export and mitochondrial targeting. Mol Cell Biol. 24:9705–9725.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Akram A, Schmeidler J, Katsel P, Hof PR
and Haroutunian V: Increased expression of RXRα in dementia: A
nearly harbinger for the cholesterol dyshomeostasis? Mol
Neurodegener. 5:362010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kiss B, Tóth K, Sarang Z, Garabuczi É and
Szondy Z: Retinoids induce Nur77-dependent apoptosis in mouse
thymocytes. Biochim Biophys Acta. 1853:660–670. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cunningham NR, Artim SC, Fornadel CM,
Sellars MC, Edmonson SG, Scott G, Albino F, Mathur A and Punt JA:
Immature CD4+CD8+ thymocytes and mature T
cells regulate Nur77 distinctly in response to TCR stimulation. J
Immunol. 177:6660–6666. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu Q, Liu S, Ye XF, Huang ZW and Su WJ:
Dual roles of Nur77 in selective regulation of apoptosis and cell
cycle by TPA and ATRA in gastric cancer cells. Carcinogenesis.
23:1583–1592. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu H, Kumar SM, Fang D, Acs G and Xu X:
Nuclear orphan receptor TR3/Nur77 mediates melanoma cell apoptosis.
Cancer Biol Ther. 6:405–412. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suzuki S, Suzuki N, Mirtsos C, Horacek T,
Lye E, Noh SK, Ho A, Bouchard D, Mak TW and Yeh WC: Nur77 as a
survival factor in tumor necrosis factor signaling. Proc Natl Acad
Sci USA. 100:8276–8280. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
You X, Zhang YW, Chen Y, Huang X, Xu R,
Cao X, Chen J, Liu Y, Zhang X and Xu H: Retinoid X receptor-alpha
mediates (R)-flurbiprofen's effect on the levels of Alzheimer's
beta-amyloid. J Neurochem. 111:142–149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gillies LA and Kuwana T: Apoptosis
regulation at the mitochondrial outer membrane. J Cell Biochem.
115:632–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Haass C and Selkoe DJ: Soluble protein
oligomers in neurodegeneration: Lessons from the Alzheimer's
amyloid beta-peptide. Nat Rev Mol Cell Biol. 8:101–112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Watson D, Castaño E, Kokjohn TA, Kuo YM,
Lyubchenko Y, Pinsky D, Connolly ES Jr, Esh C, Luehrs DC, Stine WB,
et al: Physicochemical characteristics of soluble oligomeric Abeta
and their pathologic role in Alzheimer's disease. Neurol Res.
27:869–881. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mucke L and Selkoe DL: Neurotoxicity of
amyloid β-protein: synaptic and network dysfunction. Cold Spring
Harb Perspect Med. 2:a0063382012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Basu A and Haldar S: The relationship
between BcI2, Bax and p53: Consequences for cell cycle progression
and cell death. Mol Hum Reprod. 4:1099–1109. 1998. View Article : Google Scholar : PubMed/NCBI
|