Introduction
Retinoblastoma (RB) is the most common childhood
primary intraocular tumor (1).
Most RB cases are diagnosed in children <5 years old (2); the incidence is ~1:15,000-20,000
newborns worldwide (3). RB is
classified into two groups: Hereditary and non-hereditary. Patients
with hereditary RB account for 30–40% of cases and usually involve
a positive family history, whereas cases with no family history are
generally classified as non-hereditary (4–6).
Previous studies have indicated that numerous risk factors
contribute to RB initiation and development, including genetic and
epigenetic alterations to oncogenes and tumor suppressor genes
(7,8). Standard therapeutic treatments
currently include enucleation and focal therapy, such as laser or
cryotherapy, chemotherapy or radiotherapy (9). However, the 5-year overall survival
rate for patients at advanced stages remains poor, mainly due to
the development of local or distant metastases (10,11).
Therefore, investigating the underlying molecular mechanism
responsible for RB carcinogenesis and progression may prove
beneficial in the exploration of novel therapeutic strategies.
MicroRNAs (miRNAs/miR) are a group of small, highly
conserved and non-protein-coding RNA molecules 18–23 nucleotides in
length, which participate in the regulation of gene expression.
Regulation occurs via semi-complementary binding to the
3′-untranslated regions (3′-UTRs) of target mRNAs, resulting in
translational repression and/or mRNA degradation (12). These regulatory effects may be
exerted over several cell types, targeting ~60% of human genes
(13). miRNAs serve critical
functions in the regulation of various oncogenic activities,
including proliferation, angiogenesis, apoptosis, cell cycle
progression, migration, invasion and metastasis (14–16).
Recently, a large number of miRNAs have been identified as
overexpressed or downregulated during the progression of human
cancer, including RB, such as miR-31 (17), miR-204 (18) and miR-21 (19). Functionally, these miRNAs may serve
either a tumor suppressing or promoting role in various types of
human cancer, depending on whether they specifically target
oncogenes or tumor suppressor genes (20). Therefore, these findings suggest
that miRNAs may serve as potential therapeutic targets for the
treatment of RB.
miR-320 has been studied in a number of human cancer
types (21–23). However, the expression pattern and
biological functions of miR-320 in RB remains unclear. The present
study explored the expression levels of miR-320 in RB, and the
results indicated that miR-320 expression levels were reduced in RB
tissues and cell lines. The effects of miR-320 on the
proliferation, migration and invasion of RB cells were also
investigated. Functional studies indicated that miR-320 may act as
a tumor suppressor, via inhibition of cell proliferation, migration
and invasion of RB cells. Finally, specificity protein 1 (SP1) was
identified as a direct target gene of miR-320 in RB. To the best of
our knowledge, the present study is the first to investigate the
expression, function and molecular mechanism of miR-320 in RB.
Materials and methods
Human tissue samples
Normal retinal tissue (n=3, 2 males, 1 female; age,
26–67 years) and RB tissue (n=7, 5 males, 2 females; age, 14–53
years) samples were collected at the Affiliated Hospital of Weifang
Medical University (Weifang, China) between August 2013 to June
2015. Tissues were obtained from patients that had undergone
enucleation and had not received any other prior therapies,
including chemotherapy, radiotherapy or local therapy. Normal
retinal tissues were collected from patients with a ruptured globe.
All tissue samples were snap-frozen in liquid nitrogen and stored
at −80°C prior to use. Patients provided written informed consent.
The present study was approved by the ethics committee of the
Affiliated Hospital of Weifang Medical University.
Cell lines, culture conditions and
transfection
Human retinoblastoma cell lines (Y79, WERI-RB-1 and
SO-RB50) and the HEK293T cell line were purchased from the American
Type Culture Collection (Manassas, VA, USA), and maintained in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 U/ml
streptomycin. Cells were incubated in a humidified atmosphere at
37°C with 5% CO2.
miR-320 mimic, negative control (NC), SP1 small
interfering RNA (siRNA) and control siRNA were obtained from
Shanghai GenePharma Co., Ltd. (Shanghai, China) with the following
sequences: miR-320 mimic, 5′-AAAAGCUGGGUUGAGAGGGCGA-3′; NC mimic,
5′-UUCUCCGAACGUGUCACGUTT-3′; SP1 siRNA,
5′-GCAACAUGGGAAUUAUGAATT-3′; control siRNA,
5′-UUCUUCCGAACGUGUCACGUTT-3′. Cells were transfected with miR-320
mimics (50 pmol/ml), NC (50 pmol/ml), SP1 siRNA (50 pmol/ml) or
control siRNA (50 pmol/ml) using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) and OPTI-MEM reduced serum medium
(Gibco; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
For miRNA and mRNA detection, total RNA from tissues
and cells was isolated using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
RNA concentration and quality was evaluated using a NanoDrop
Spectrophotometer (NanoDrop; Thermo Fisher Scientific, Inc.,
Wilmington, DE, USA). To quantify miR-320 expression, reverse
transcription was performed using a TaqMan MicroRNA Reverse
Transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.), according to the manufacturer's instructions. The relative
expression of miR-320 was measured using a TaqMan miRNA assay
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: 40 cycles of denaturation
at 95°C for 15 sec and annealing/extension at 60°C for 60 sec. The
reaction system contained 1.0 µl TaqMan miRNA assay (20X), 10.0 µl
TaqMan 2X Universal PCR Master Mix, 1.33 µl cDNA, 1 µl forward
primer and 1 µl reverse primer and 5.67 µl double distilled water.
Small nuclear U6 RNA was used as an internal control for miR-320
expression. One Step SYBR PrimeScript™ RT-PCR kit II (Takara
Biotechnology Co., Ltd., Dalian, China) was used to measure SP1
mRNA expression. The thermocycling conditions were as follows: 42°C
for 5 min, 95°C for 10 sec, then 40 cycles of 95°C for 5 sec, 55°C
for 30 sec and 70°C for 30 sec. The reaction system contained 12.5
µl 2X One Step SYBR® RT-PCR Buffer 4, 1.5 µl Takara Ex Taq™ HS Mix,
0.5 µl PrimeScript™ PLUS RTase Mix, 1 µl forward primer and 1 µl
reverse primer, 2 µl cDNA and 6.5 µl double distilled water. The
primers used in the present study were as follows: miR-302 forward,
5′-ACACTCCAGCTGGGAAAAGCTGGGTTGAGA-3′ and reverse,
5′-TGGTGTCGTGGAGTCG-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and
reverse, 5′-AACGCTTCACGAATTTGCGT-3′; SP1 forward,
5′-TGTGAATGCTGCTCAACTCTCC-3′ and reverse,
5′-CATGTATTCCATCACCACCAG-3′; GAPDH forward,
5′-ACCACAGTCCATGCCATCAC-3′ and reverse, 5′-TCCACCACCCTGTTGCTGTA-3′.
GAPDH was used as an internal control for SP1 mRNA expression. All
experiments were performed in triplicate and the results were
calculated using the 2−ΔΔCq method (24).
Cell proliferation assay
Cell proliferation of transfected cells was assessed
using the Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan). Cells (3,000 cells/well) were
seeded into 96-well plates, and transfected with miR-320 mimic, NC,
SP1 siRNA or control siRNA as aforementioned. A cell proliferation
assay was performed at various times points (24–96 h). Briefly, 10
µl CCK-8 reagent was added to the culture medium of each well, and
the plates were incubated at 37°C for 2 h, after which absorbance
was measured at 450 nm. All experiments were performed in
triplicate.
Cell migration and migration
assays
The migratory and invasive abilities of the
transfected cells were evaluated using cell migration and invasion
assays. For the cell migration assay, 1×105 transfected cells in
300 µl FBS-free medium were added into the upper chamber of a
24-well Transwell® plate (pore size, 8 µm; Corning Incorporated,
Corning, NY, USA), 500 µl culture medium containing 20% FBS was
added to the lower chamber. For the cell invasion assay, the
membranes of a Transwell® plate were first coated with Matrigel™
(BD Biosciences, Franklin Lakes, NJ, USA). Transfected cells
(1×105/300 µl) in FBS-free medium were added into the
Matrigel™-coated Transwell® chambers, 500 µl culture medium
supplemented with 20% FBS was subsequently added to the lower
chamber to act as a chemoattractant. For both assays, the
Transwell® chambers were incubated for 24 h following inoculation.
Cells remaining on the filter surface of the upper chamber were
carefully removed, and the cells that had migrated or invaded to
the lower side of the chamber were fixed and stained with 0.5%
crystal violet. The number of migrated and invaded cells were
counted under a light microscope (Olympus IX53; Olympus
Corporation, Tokyo, Japan).
Western blot analysis
SP1 and GAPDH expression levels were detected in
total cell extracts of transfected cells by western blot analysis.
A total of 72 h following transfection, cells were lysed with
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Shanghai, China). A bicinchoninic acid protein assay
kit (Pierce; Thermo Fisher Scientific, Inc.) was used to detect
protein concentration, according to the manufacturer's
instructions. Equal amounts of protein (20 µg) were separated by
10% SDS-PAGE, transferred onto a polyvinylidene difluoride membrane
(EMD Millipore, Billerica, MA, USA), and blocked with 5% non-fat
milk. The membranes were subsequently incubated with primary
antibodies: Mouse anti-human SP1 monoclonal antibody (1:1,000;
ab77441) or anti-GAPDH (1:1,000; ab9484) (both from Abcam, Tokyo,
Japan) at 4°C overnight. Following three washes with Tris-buffered
saline containing 0.05% Tween-20, the membranes were probed with
goat anti-mouse HRP-conjugated secondary antibody (1:2,000; ab6789;
Abcam). The protein blots were detected using the enhanced
chemiluminescence western blotting kit (Pierce; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions and
scanned using the GeneGnome HR Image Capture system (Syngene,
Frederick, MD, USA). Relative expression levels of SP1 were
normalized to GAPDH expression.
Bioinformatic analysis and luciferase
reporter miRNA target validation
The potential targets and binding sites of miR-320
were predicted using TargetScan (http://www.targetscan.org/) and miRanda (http://www.microrna.org), which are databases that use
different search algorithms.
To explore whether SP1 is a direct target gene of
miR-320, a luciferase reporter assay was performed. pMIR-Report
Luciferase vectors [pMIR-SP1-3′-UTR Wt
(5′-AGAGGAUGAGCAGACCAGCUUUG-3′) and pMIR-SP1-3′-UTR Mut (AGA GGA
UGA GCA GAC GUC GAA AG-3′)] were purchased from Shanghai GenePharma
Co., Ltd. HEK293T cells were seeded into 24-well plates at
1.5×105 cells/well and subsequently transfected with
miR-320 mimic or NC, and pMIR-SP1-3′-UTR Wt or pMIR-SP1-3′-UTR Mut,
using Lipofectamine 2000, according to manufacturer's protocol. A
total of 48 h post-transfection, Renilla and firefly
luciferase activities were determined using a Dual-Luciferase
reporter assay (Promega GmbH, Mannheim, Germany), according to the
manufacturer's protocol. The experiments were independently
performed in triplicate.
Statistical analysis
Data are presented as the mean ± standard deviation
of three repeated experiments. All data were analyzed using
two-tailed Student's t-test or one-way analysis of variance,
followed by the Student-Newman-Keuls post hoc test. Data were
analyzed using SPSS 19.0 statistical software (SPSS IBM, Armonk,
NY, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-320 expression is downregulated in
RB
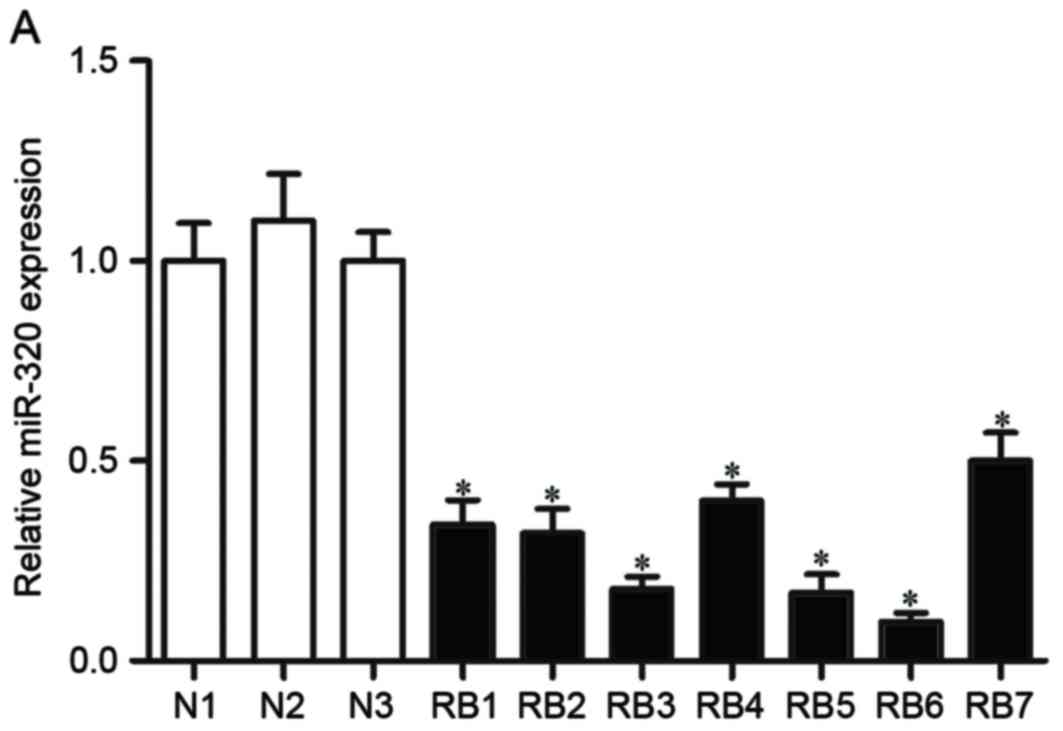
The expression levels of miR-320 were detected in RB
and normal retinal tissues. The results of the RT-qPCR indicated
that the expression levels of miR-320 were significantly decreased
in RB tissues, compared with in normal retinal tissues (P<0.05,
Fig. 1A and B). The expression of
miR-320 was also detected in RB cell lines (Y79, WERI-RB-1 and
SO-RB50). Similarly, all three RB cell lines demonstrated decreased
miR-320 expression, compared with normal retinal tissue (P<0.05,
Fig. 1C). Taken together, these
findings indicated that the expression levels of miR-320 were
markedly decreased in RB tissues and cell lines.
miR-320 overexpression inhibits the
proliferation, migration and invasion of RB cells
The Y79 and WERI-RB-1 cell lines demonstrated lower
miR-320 expression compared with the SO-RB50 cell line; therefore,
Y79 and WERI-RB-1 cells were selected for further studies. To
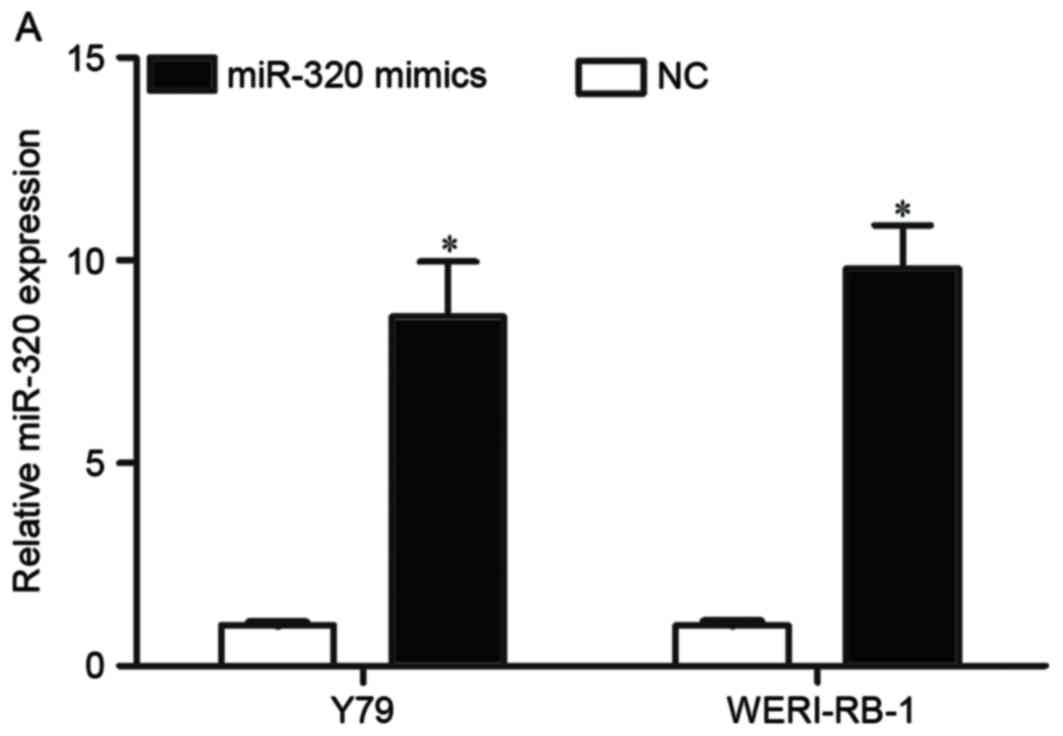
explore the role of miR-320 in RB, Y79 and WERI-RB-1 cells were
transfected with miR-320 mimic or NC, and miR-320 expression was
validated; miR-320 expression was increased in both miR-320
mimic-transfected cell lines, compared with NC-transfected cells
(P<0.05, Fig. 2A). Cell
proliferation, migration and invasion assays were performed to
investigate the role of miR-320 in the growth and metastasis of RB
cells. Analysis of cell proliferation indicated that miR-320
overexpression significantly inhibited the growth of Y79 and
WERI-RB-1 cells, compared with the NC-transfected cells (P<0.05,
Fig. 2B). Furthermore, miR-320
significantly decreased cell migration and invasion in Y79 and
WERI-RB-1 cells, compared with NC-transfected cells (P<0.05,
Fig. 2C). These results indicated
that miR-320 may serve as a tumor suppressor in RB.
miR-320 directly targets SP1 3′-UTR in
RB
To investigate the molecular mechanisms underlying
the potential tumor suppressive abilities of miR-320, bioinformatic
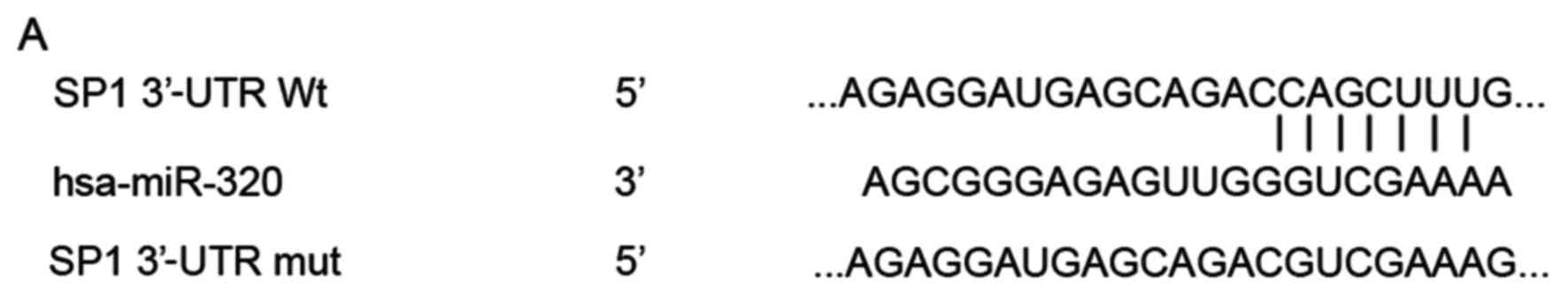
analysis was used to search for potential targets of miR-320. The
analysis indicated that the 3′-UTR of SP1 contains a complementary
site for the seed region of miR-320 (Fig. 3A). To explore whether the SP1
3′-UTR is a direct target of miR-320, a luciferase reporter assay
was performed. The results demonstrated that the relative
luciferase activities of pMIR-SP1-3′-UTR Wt were significantly
inhibited following transfection with the miR-320 mimic, compared
with the NC-transfected cells. However, transfection with
pMIR-SP1-3′-UTR Mut abolished this suppression, demonstrating that
miR-320 directly binds to the 3′-UTR of SP1 (Fig. 3B, P<0.05). Furthermore, the
expression levels of SP1 were detected in Y79 and WERI-RB-1 cells
following transfection with the miR-320 mimic. As indicated in
Fig. 3C and D, miR-320
overexpression significantly decreased SP1 expression at the mRNA
and the protein level, in Y79 and WERI-RB-1 cells (P<0.05).
Collectively, these results indicated that SP1 may be a direct
target gene of miR-320 in RB.
Effects of SP1 silencing on
proliferation, migration and invasion of RB cells
To investigate the role of SP1 in RB cells,
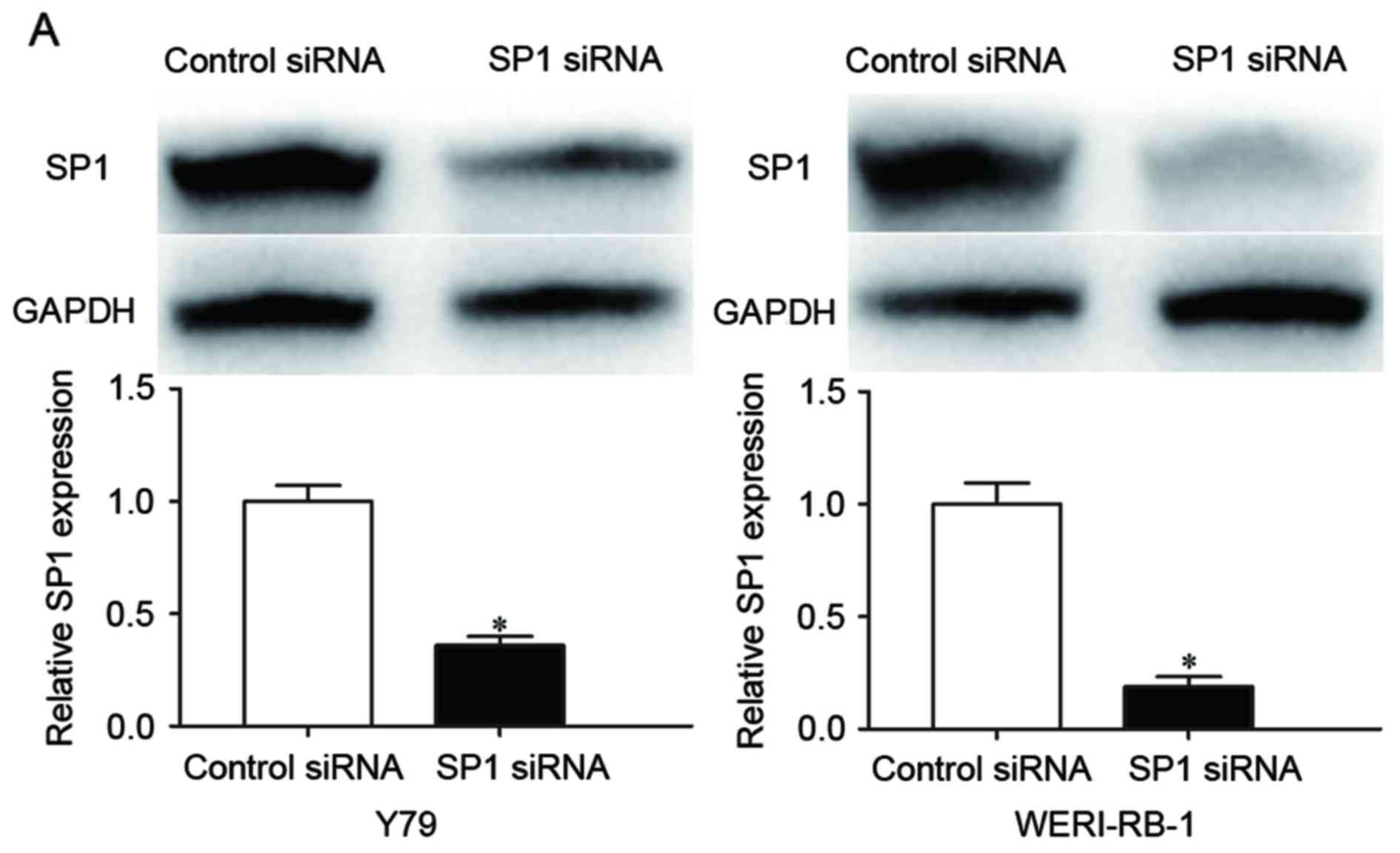
loss-of-function studies were performed using SP1 siRNA. Western
blot analysis confirmed the knockdown of SP1; the results indicated
that transfection with SP1 siRNA resulted in decreased SP1 protein
expression in Y79 and WERI-RB1 cells (P<0.05, Fig. 4A), compared with control
siRNA-transfected cells. To investigate the role of SP1, cell
growth, migration and invasion were evaluated. Cell growth was
significantly inhibited following transfection with SP1 siRNA,
compared with cells transfected with control siRNA (P<0.05,
Fig. 4B). Furthermore, cell
migration and invasion assays indicated that these functions were
decreased by SP1 siRNA, compared with control siRNA-transfected
cells (P<0.05, Fig. 4C). These
results revealed that the effects of SP1 silencing on
proliferation, migration and invasion of RB cells were similar to
those induced by miR-320 overexpression, supporting the hypothesis
that SP1 is a direct functional target of miR-320 in RB.
Discussion
Research has demonstrated that understanding the
expression and functions of miRNAs may provide unique insights into
the molecular mechanisms that underlie the carcinogenesis, and
progression, of human cancer. Previous studies have detected
abnormal expression profiles of miRNAs in RB carcinogenesis and
development. Montoya et al reported that miR-31 and miR-200a
regulate proliferation in RB (17). In addition, miR-204 has been
reported to be downregulated in RB cells, and exhibited an ability
to inhibit invasion and proliferation (18), whereas, Lei et al revealed
that miR-101 may act as a tumor suppressor in RB (25).
The present study investigated the expression and
function of miR-320 in RB tissues and cell lines. Analysis of
miR-320 expression demonstrated significant miR-320 downregulation
in RB, suggesting that miR-320 may serve a role in RB progression.
The biological functions of miR-320 were explored in the RB cell
lines Y79 and WERI-RB-1, through analysis of cell proliferation,
migration and invasion, post-transfection with miR-320 mimic or NC.
The results of these analyses indicated that overexpression of
miR-320 may inhibit the proliferation, migration and invasion of RB
cells, thus suggesting that miR-320 may act as a tumor suppressor
in the carcinogenesis and progression of RB.
Previous research regarding miR-320 has focused on
glioma (21), colon cancer
(22), osteosarcoma (23), oral cancer (26) and prostate cancer (27). Hsieh et al reported that
miR-320 was downregulated in prostate cancer, and overexpression of
miR-320 inhibited prostate cancer carcinogenesis in vitro
and in vivo (27). In oral
cancer, miR-320 was observed to be downregulated in tumor tissues
and cell lines. Furthermore, ectopic miR-320 expression inhibited
the migration, adhesion and tube formation of vascular endothelial
cells in oral cancer, via the targeting of neuropilin-1 (NRP1)
(26). Cheng et al
demonstrated that miR-320 expression was reduced in osteosarcoma
tissues; conversely, upregulation of miR-320 suppressed
osteosarcoma cell proliferation via blockade of fatty acid synthase
(FAS) (23). Sun et al
reported that miR-320 was significantly reduced in glioma tissues,
and miR-320 overexpression inhibited the proliferation and
metastasis of glioma cells (21).
Furthermore, miR-320 enhanced the sensitivity of human colon cancer
cells to chemoradiotherapy, via modulation of forkhead box M1
(FOXM1) (22). These findings
suggested that miR-320 serves a role in tumor suppression; further
research should investigate the possibility of therapeutically
targeting this miRNA in these specific types of cancer.
miRNAs have demonstrated an ability to regulate
several physiological and pathological processes, via modulation of
the expression of various target genes (28). Previous studies have indicated that
miR-320 may target genes including E2F transcription factor 1
(21), FOXM1 (22), FAS (23), NRP1 (26) and cluster of differentiation 71
(29). The present study
demonstrated that SP1 is a direct functional target of miR-320 in
RB, and this was supported through several lines of evidence.
Firstly, bioinformatic analysis demonstrated that SP1 contains a
complementary site for the seed region of miR-320. Furthermore,
upregulation of miR-320 decreased SP1 3′-UTR luciferase activity,
an effect that was abolished by mutation of the miR-320 seed
region. Secondly, miR-320 overexpression suppressed SP1 at mRNA and
protein levels. Collectively, these results indicated that SP1 is a
direct target gene of miR-320 in RB.
To further investigate the role of SP1 in RB,
loss-of-function studies were performed using SP1 siRNA. The
results demonstrated that SP1 knockdown suppressed proliferation,
migration and invasion of RB cells, a response that was similar to
the phenotype induced by miR-320 overexpression in RB. Taken
together, these results suggested that the suppressive functions of
miR-320 in RB may be partly mediated by suppression of SP1
expression. SP1 is a sequence-specific DNA-binding protein that
encodes a protein of 785 amino acids (30). Functional studies have demonstrated
that SP1 modulates numerous biological functions, including cell
proliferation, differentiation, migration, metastasis and invasion
(31–33). Notably, there are several
SP1-targeting therapeutic compounds currently employed in the
clinical management of cancer, with further compounds in
development (34–37). The present study indicated that
miR-320 targets SP1 to inhibit proliferation, migration and
invasion of RB cells. Therefore, miR-320/SP1-based targeted therapy
may represent a novel therapeutic strategy for the treatment of
patients with RB.
In conclusion, the present study demonstrated the
tumor suppressive role of miR-320 in human RB. miR-320 was
significantly downregulated in RB tissues and cell lines, and
restoration of miR-320 expression suppressed proliferation,
migration and invasion of RB cells. Furthermore, SP1 was
demonstrated to be a direct target of miR-320; miR-320-induced
tumor suppressive roles may therefore be achieved via suppression
of SP1, and miR-320 may represent a potential therapeutic target
for the future treatment of RB.
References
|
1
|
Balmer A, Zografos L and Munier F:
Diagnosis and current management of retinoblastoma. Oncogene.
25:5341–5349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alonso J, Garcia-Miguel P, Abelairas J,
Mendiola M and Pestaña A: A microsatellite fluorescent method for
linkage analysis in familial retinoblastoma and deletion detection
at the RB1 locus in retinoblastoma and osteosarcoma. Diagn Mol
Pathol. 10:9–14. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dimaras H, Kimani K, Dimba EA, Gronsdahl
P, White A, Chan HS and Gallie BL: Retinoblastoma. Lancet.
379:1436–1446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kleinerman RA, Schonfeld SJ and Tucker MA:
Sarcomas in hereditary retinoblastoma. Clin Sarcoma Res. 2:152012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marees T, Moll AC, Imhof SM, de Boer MR,
Ringens PJ and van Leeuwen FE: Risk of second malignancies in
survivors of retinoblastoma: More than 40 years of follow-up. J
Natl Cancer Inst. 100:1771–1779. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moll AC, Imhof SM, Bouter LM and Tan KE:
Second primary tumors in patients with retinoblastoma. A review of
the literature. Ophthalmic Genet. 18:27–34. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beta M, Venkatesan N, Vasudevan M,
Vetrivel U, Khetan V and Krishnakumar S: Identification and
insilico analysis of retinoblastoma serum microRNA profile and gene
targets towards prediction of novel serum biomarkers. Bioinform
Biol Insights. 7:21–34. 2013.PubMed/NCBI
|
|
8
|
Wang J, Wang X, Wu G, Hou D and Hu Q:
MiR-365b-3p, down-regulated in retinoblastoma, regulates cell cycle
progression and apoptosis of human retinoblastoma cells by
targeting PAX6. FEBS Lett. 587:1779–1786. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Friedman DL, Himelstein B, Shields CL,
Shields JA, Needle M, Miller D, Bunin GR and Meadows AT:
Chemoreduction and local ophthalmic therapy for intraocular
retinoblastoma. J Clin Oncol. 18:12–17. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shields CL and Shields JA: Diagnosis and
management of retinoblastoma. Cancer Control. 11:317–327.
2004.PubMed/NCBI
|
|
11
|
Xu X, Jia R, Zhou Y, Song X, Wang J, Qian
G, Ge S and Fan X: Microarray-based analysis: Identification of
hypoxia-regulated microRNAs in retinoblastoma cells. Int J Oncol.
38:1385–1393. 2011.PubMed/NCBI
|
|
12
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: Diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 4:143–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thieu W, Tilki D, White RW deVere and
Evans CP: The role of microRNA in castration-resistant prostate
cancer. Urol Oncol. 32:517–523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reardon DA, Rich JN, Friedman HS and
Bigner DD: Recent advances in the treatment of malignant
astrocytoma. J Clin Oncol. 24:1253–1265. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer. 96
Suppl:R40–R44. 2007.PubMed/NCBI
|
|
16
|
Calin GA, Ferracin M, Cimmino A, Di Leva
G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M, et
al: A MicroRNA signature associated with prognosis and progression
in chronic lymphocytic leukemia. N Engl J Med. 353:1793–1801. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Montoya V, Fan H, Bryar PJ, Weinstein JL,
Mets MB, Feng G, Martin J, Martin A, Jiang H and Laurie NA: Novel
miRNA-31 and miRNA-200a-mediated regulation of retinoblastoma
proliferation. PLoS One. 10:e01383662015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu X, Zeng Y, Wu S, Zhong J, Wang Y and Xu
J: MiR-204, down-regulated in retinoblastoma, regulates
proliferation and invasion of human retinoblastoma cells by
targeting CyclinD2 and MMP-9. FEBS Lett. 589:645–650. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen F, Mo MH, Chen L, An S, Tan X, Fu Y,
Rezaei K, Wang Z, Zhang L and Fu SW: MicroRNA-21 Down-regulates Rb1
expression by targeting PDCD4 in retinoblastoma. J Cancer.
5:804–812. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Porkka KP, Pfeiffer MJ, Waltering KK,
Vessella RL, Tammela TL and Visakorpi T: MicroRNA expression
profiling in prostate cancer. Cancer Res. 67:6130–6135. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun JY, Xiao WZ, Wang F, Wang YQ, Zhu YH,
Wu YF, Miao ZL and Lin YC: MicroRNA-320 inhibits cell proliferation
in glioma by targeting E2F1. Mol Med Rep. 12:2355–2359.
2015.PubMed/NCBI
|
|
22
|
Wan LY, Deng J, Xiang XJ, Zhang L, Yu F,
Chen J, Sun Z, Feng M and Xiong JP: miR-320 enhances the
sensitivity of human colon cancer cells to chemoradiotherapy in
vitro by targeting FOXM1. Biochem Biophys Res Commun. 457:125–132.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng C, Chen ZQ and Shi XT: MicroRNA-320
inhibits osteosarcoma cells proliferation by directly targeting
fatty acid synthase. Tumour Biol. 35:4177–4183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lei Q, Shen F, Wu J, Zhang W, Wang J and
Zhang L: MiR-101, downregulated in retinoblastoma, functions as a
tumor suppressor in human retinoblastoma cells by targeting EZH2.
Oncol Rep. 32:261–269. 2014.PubMed/NCBI
|
|
26
|
Wu YY, Chen YL, Jao YC, Hsieh IS, Chang KC
and Hong TM: miR-320 regulates tumor angiogenesis driven by
vascular endothelial cells in oral cancer by silencing neuropilin
1. Angiogenesis. 17:247–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hsieh IS, Chang KC, Tsai YT, Ke JY, Lu PJ,
Lee KH, Yeh SD, Hong TM and Chen YL: MicroRNA-320 suppresses the
stem cell-like characteristics of prostate cancer cells by
downregulating the Wnt/beta-catenin signaling pathway.
Carcinogenesis. 34:530–538. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li M, Marin-Muller C, Bharadwaj U, Chow
KH, Yao Q and Chen C: MicroRNAs: Control and loss of control in
human physiology and disease. World J Surg. 33:667–684. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schaar DG, Medina DJ, Moore DF, Strair RK
and Ting Y: miR-320 targets transferrin receptor 1 (CD71) and
inhibits cell proliferation. Exp Hematol. 37:245–255. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang WC and Hung JJ: Functional role of
post-translational modifications of Sp1 in tumorigenesis. J Biomed
Sci. 19:942012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Black AR, Black JD and Azizkhan-Clifford
J: Sp1 and kruppel-like factor family of transcription factors in
cell growth regulation and cancer. J Cell Physiol. 188:143–160.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li L, He S, Sun JM and Davie JR: Gene
regulation by Sp1 and Sp3. Biochem Cell Biol. 82:460–471. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mukhopadhyay D and Datta K: Multiple
regulatory pathways of vascular permeability factor/vascular
endothelial growth factor (VPF/VEGF) expression in tumors. Semin
Cancer Biol. 14:123–130. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu S, Liu Z, Xie Z, Pang J, Yu J, Lehmann
E, Huynh L, Vukosavljevic T, Takeki M, Klisovic RB, et al:
Bortezomib induces DNA hypomethylation and silenced gene
transcription by interfering with Sp1/NF-kappaB-dependent DNA
methyltransferase activity in acute myeloid leukemia. Blood.
111:2364–2373. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chadalapaka G, Jutooru I, Chintharlapalli
S, Papineni S, Smith R III, Li X and Safe S: Curcumin decreases
specificity protein expression in bladder cancer cells. Cancer Res.
68:5345–5354. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pathi SS, Jutooru I, Chadalapaka G,
Sreevalsan S, Anand S, Thatcher GR and Safe S: GT-094, a NO-NSAID,
inhibits colon cancer cell growth by activation of a reactive
oxygen species-microRNA-27a: ZBTB10-specificity protein pathway.
Mol Cancer Res. 9:195–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hsu TI, Wang MC, Chen SY, Huang ST, Yeh
YM, Su WC, Chang WC and Hung JJ: Betulinic acid decreases
specificity protein 1 (Sp1) level via increasing the sumoylation of
sp1 to inhibit lung cancer growth. Mol Pharmacol. 82:1115–1128.
2012. View Article : Google Scholar : PubMed/NCBI
|