Introduction
Renal ischemia/reperfusion (I/R) injury is the
primary etiopathological phenomenon that leads to acute renal
failure or multiple organ failure in patients with renal transplant
or renal resection (1,2). Acute kidney injury (AKI) is an
additional consequence of renal I/R injury, and is responsible for
the high number of patients with long-term kidney dysfunction that
require intensive medical care (3). Renal I/R injury is characterised by a
pathological phenomenon associated with restriction in the blood
supply to the kidney, which leads to limited arterial blood flow
and an imbalance in the supply of metabolites thatleads to tissue
hypoxia (4–10). A reperfusion procedure is employed
to restore blood flow and reduce further tissue injury and reduce
the inflammatory response (11).
Recent advancements in treatment strategies have failed to address
the issue of morbidity and mortality in patients undergoing renal
transplant (12). Notably, higher
mortality rates have been observed in males than females (13). The pathogenesis of renal I/R injury
involves a complex combination of inflammation, oxidative stress,
autophagy, apoptosis and immunological pathways (14–16).
A cascade of inflammatory cytokines, such astumour necrosis
factor-α (TNF-α) and interleukin (IL)-6, have been demonstrated to
initiate the recruitment of leukocytes and additional antigen
presenting cells that induce a potent immune response (17,18).
In addition, renal vascular endothelial cells have been
demonstrated to serve a dominant role in recruiting immune cells
(19). However, the precise series
of events that initiates an inflammatory cascade and subsequent
renal damage remains unknown.
Autophagy is an important mechanism by which
eukaryotic cells maintain homeostasis in response to various types
of stress (20,21). Autophagy is characterised by the
presence of an autophagosome; a double-layer membrane organelle in
the cytoplasm that breaks down dysfunctional cells, proteins and
cell organelles via fusion of the autophagosome with lysosomes,
enzymatic digestion proteins or organelles (22). Niclosamide is an inhibitor of the
signal transducer and activator of transcription 3 protein that
suppresses phosphorylationof signal transducer and activator of
transcription 3at the Tyr705 site, and is an approved anthelmintic
drug (23,24). A previous study demonstrated that
niclosamide is a potent enhancer of autophagy and induces
mitochondrial fission (25). In
addition, nuclear factor-κB, reactive oxygen species, Notch,
Wnt/β-catenin and mechanistic target of rapamycin complex 1 are
additional factors targeted by niclosamide, which suggests that the
drug may be useful for the treatment of a number of disorders
(26). Previous studies have
demonstrated that inductionof autophagy in the proximal (and
associated) region of renal tubules during renal I/R injury and
acute kidney diseasemay have beneficial effects on the renal tissue
(27–29). Therefore, it is necessary to
investigate the autophagic properties of niclosamide in a model of
renal I/R injury. The present study was performed to investigate
the in vivo effects of niclosamide in a rat model of renal
I/R injury, and to examine the possible mechanisms of action.
Materials and methods
Animals
All animal procedures were performed according to
the guidelines of the Care and Use of Laboratory Animals (30), and were approved by the Animal
Ethical Care and Use Committee of the Tangshan Gongren Hospital
(Tangshan, China). A total of 40 male Sprague-Dawley (SD) rats (8
week old, weight 220–250 g) were purchased from the Chinese Academy
of Medical Sciences (Beijing, China). Rats were housed in a 12:12 h
light:dark cycle (lights on 6am-6pm) with controlled temperature
(21±2°C) and humidity (60±10%). Sterile water and food were
provided to the rats ad libitum.
Experimental design
The SD rats were randomly divided into the following
5 treatment groups (n=8 in each group): Sham group; renal I/R
injury; renal I/R injury plus 3-methyladenine (3-MA) treatment (15
mg/kg); I/R injury plus niclosamide (25 mg/kg); and I/R injury plus
rapamycin (10 mg/kg). Niclosamide, 3-MA and rapamycin were sourced
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Following drug
treatment for 24 h, biopsies of the kidney tissue were taken to
examine the level of autophagosome-associated marker proteins,
including microtubule-associated protein 1A/1B light chain 3B
(LC3-II), beclin-1, Rab7 and lysosome-associated membrane protein 2
(LAMP2) by western blotting analysis. For biochemical analysis,
blood was obtained from the inferior vena cava, and kidney function
parameters were measured at 24 h following the I/R injury
induction. For measurement of cytokine levels, kidney samples were
obtained following sacrifice.
Induction of I/R injury in rats
The rats were anaesthetised using 10% chloral
hydrate (Sigma-Aldrich; Merck KGaA), at a dose of 400 mg/kg. For
I/R injuryinduction, the flank incision method was performed
(31). Briefly, the rats were
first injected with 30 mg/kg of sodium pentobarbital
(Sigma-Aldrich; Merck KGaA), followed by a flank incision to remove
the right kidney. Using a small vascular clip, the left renal
artery and vein were clamped for 30 min prior to removal, in order
to replicate the reperfusion procedure. Using a sterile suture, the
abdomen was closed. Throughout the experiments, the rats were
maintained at 32°C and hydrated with normal saline. A blood and
kidney biopsy was conducted 24 h aftercompletion of the reperfusion
procedure. In the sham group, the identical surgical procedures
were performed, but without renal artery and vein clamping. Kidney
injury score was determined following the previous report (32).
Histological analysis
Kidney tissues were extracted from rats in all
treatment groups (24 h after treatment) following intraperitoneal
injection of chloral hydrate (300 mg/kg). The kidney tissues were
stained with Masson's trichrome to analyze alterations in the
tissue morphology (30,33). Three independent
pathologistsanalysed three different tissue sections from rats in
each treatment group. Samples were examined under aZeiss Axio
Imager A2 m microscope (Carl Zeiss AG, Oberkochen, Germany). The
evaluating pathologist was blinded to the study groups.
Assessment of renal function
Following treatment of rats for 24 h, blood samples
were obtained from rats in all experimental groups. Serum
creatinine and blood urea nitrogen (BUN) levels were evaluated
using the Samsung LABGEOPT10 clinical chemistry analyzer (Samsung,
Seoul, South Korea) according to the manufacturer's protocol.
Western blot analysis of LC3-II,
beclin-1, Rab7 and LAMP2 expression
Proteins from the renal issue were extracted using
M-PER Mammalian Protein Extraction Reagent (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and quantified using a Protein
Assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA) according
to the manufacturer's protocol. Proteins (10 µg) were separated by
12% SDS-PAGE and subsequently transferred to polyvinylidene
fluoride membranes. Blots were blocked in 5% skimmed milk for 2 h
at room temperature and then incubated withthe following primary
antibodies: Beclin-1 (1:1,000; no. 3738; Cell Signaling Technology,
Inc., Danvers, MA, USA,), LC3-I/II (1:1,000; no. ABC929; EMD
Millipore, Billerica, MA, USA), RAB7 (1:1,000; ab137029; Abcam,
Cambridge, UK), LAMP-2 (1:500; ab203224; Abcam), and beta-actin
(1:1,000; ab8227; Abcam) overnight at 4°C. The membranes were
washed in PBS 4–5 times and incubated withhorseradish
peroxidase-conjugated goat anti-rabbit IgG secondary antibody
(1:2,000; no. ab6721; Abcam) for 2 h at room temperature. The
Amersham ECL Western Blotting Detection kit (GE Healthcare Life
Sciences, Chalfont, UK) was used todetect protein expression.
Measurement of TNF-α, high mobility
group box 1 (HMGB1), IL-6 and IL-10 cytokine levels
Using a tissue homogenizer (Thomas Scientific,
Swedesboro, NJ, USA), a tissue lysate was prepared and cytokine
levels were estimated using commercially available ELISA kits from
R&D Systems, Inc. (Minneapolis, MN, USA) for rat TNF-α (no.
SRTA00), IL-6 (no. PR6000B), IL-10 (no. DY522) or the Cloud-Clone
Corp. (Houston, TX, USA) for rat HMGB1 (no. SEA399Ra). Experiments
were performed and measured according to the manufacturer's
protocol.
Immunofluorescence detection of
beclin-1-labelled autophagosomes in renal tissues
Kidney samples were obtained from the following five
treatment groups: Control group (sham I/R injury); I/R group; the
3-MA treatment group; the niclosamide treatment group; and the
rapamycin treatment group. Tissue samples were processed for
immunofluorescence staining for beclin-1 expression using the
standard procedure (34). Briefly,
kidney tissueswere fixed in 4% paraformaldehyde overnight and
embedded in paraffin. From the paraffin blocks, 5-µm sections were
cut using a steel microtome. The paraffin sections were then
deparaffinised and incubated overnight with a polyclonal
anti-beclin-1 antibody (1:200; ab217179; Abcam) overnight at 4°C.
Sections weresubsequently labeled with anti-rabbit Alexa
Fluor-488-labeled secondary antibody (no. A32723; Thermo Fisher
Scientific, Inc.) at room temperature for 2 h. Photomicrographs
were obtained using the Zeiss Axio Imager A2 m microscope with a
florescence filter (magnification, ×40; Carl Zeiss AG).
Ultrastructural studies
Kidney tissue samples (size, 1 mm3), consisting of a
section of renal cortex and outer medulla, were obtained from the
following treatment groups: Control group (sham I/R injury); I/R
group; the 3-MA treatment group; the niclosamide treatment group;
and the rapamycin treatment group. They were subsequently fixed in
4% paraformaldehyde and 1% glutaraldehyde in a 0.1 M phosphate
buffer (pH 7.3) for 12 h at 4°C, before they were washed in sodium
cacodylate buffer. The tissues were post-fixed in 1% aqueousosmium
tetroxide (OsO4) solution for 2 h at 4°C. Tissues were
then dehydrated using a graded ethanol series, before they were
embedded in standard Spurr resin (Sigma-Aldrich; Merck KGaA).
Finally, 60–90-nm thick sections were stained using uranyl acetate
and lead citrate, and were examined under a Hitachi H-7500
Transmission Electron Microscope equipped with a Gatan 780
dual-view CCD camera (Hitachi, Ltd., Tokyo, Japan). Samples were
visualised under ×1,000 and ×10,000 magnifications. The autophagic
vacuoles/100 µm of cytoplasm were evaluated using AxioVision 4
software (v 4.8; Zeiss GmbH, Jena, Germany).
Statistical analysis
Statistical analysis was performed using the SPSS
software (version 15.0; SPSS Inc., Chicago, IL, USA). To compare
the variation of data among groups, one-way analysis of variance
was performed, followed by Fisher's least significant difference
test for multiple comparisons. P<0.05 was considered to indicate
a statistically significant difference.
Results
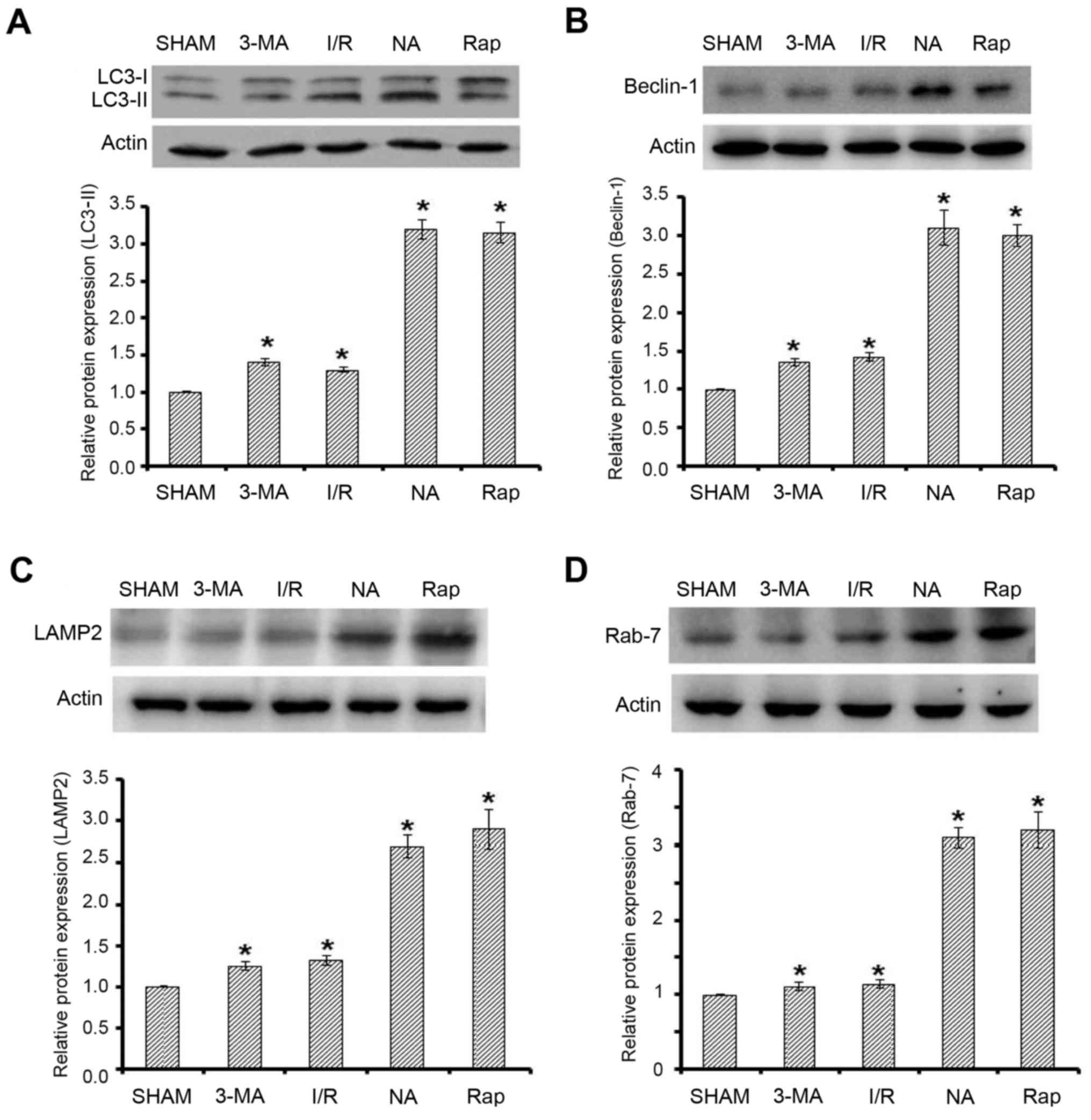
Expression of autophagy-associated
protein in the renal tissues of rats with renal I/R injury
To evaluate kidney function following I/R injury and
to establish a possible association with enhanced autophagy,
westudied the expression of autophagy-associated proteins, beclin-1
and LC3-II. Rapamycin was used as positive control, as it is a
known activator of autophagy, whereas 3-MA was used as inhibitor of
autophagy. An increase in the protein expression levels of
beclin-1, LC3-II, LAMP2 and Rab7 in the kidney tissuesfrom rats
with renal I/R injury was observed. Treatment with niclosamide
resulted in an increase in the expression of beclin-1, LC3-II and
lysosome-associated proteins LAMP2 and Rab7, when compared with the
sham group (P<0.05; Fig. 1).
After 24 h of treatment with niclosamide, the increase in the
expression of all autophagy-associated markers was comparable with
rapamycin treatment (Fig. 1). As
expected, a significant reduction in the expression of beclin-1 and
LC3-II was observed following treatment with 3-MA compared with the
niclosamide- or rapamycin-treated groups. In addition, treatment of
rats with I/R injury with 3-MA demonstrated a reduced expression of
lysosome-associated proteins LAMP2 and Rab7 compared with the
niclosamide- or rapamycin-treated groups, which correlated with the
results of subsequent histopathological and electron microscope
studies.
Effect of niclosamide on histological
alterations and cytokine expression in rats with renal I/R
injury
In order to determine the association between renal
function with inflammation-mediated autophagy, the levels of
pro-inflammatory cytokines TNF-α, IL-6, HMGB1 and the
anti-inflammatory cytokine IL-10 were measured. Treatment with
niclosamide and rapamycin was associated with a reduction in the
levels of pro-inflammatory cytokines and an increase in the levels
of anti-inflammatory cytokines (P<0.05, Fig. 2A-D). As expected, an increase in
the levels of pro-inflammatory cytokines and a decrease in the
levels of anti-inflammatory cytokines were observed in the renal
tissues of rats treated with the autophagy inhibitor 3-MA compared
with the sham group (P<0.05, Fig.
2A-D). These results suggest that induction of autophagy may be
associated with increased levels of IL-10 and decreased levels of
pro-inflammatory cytokines.
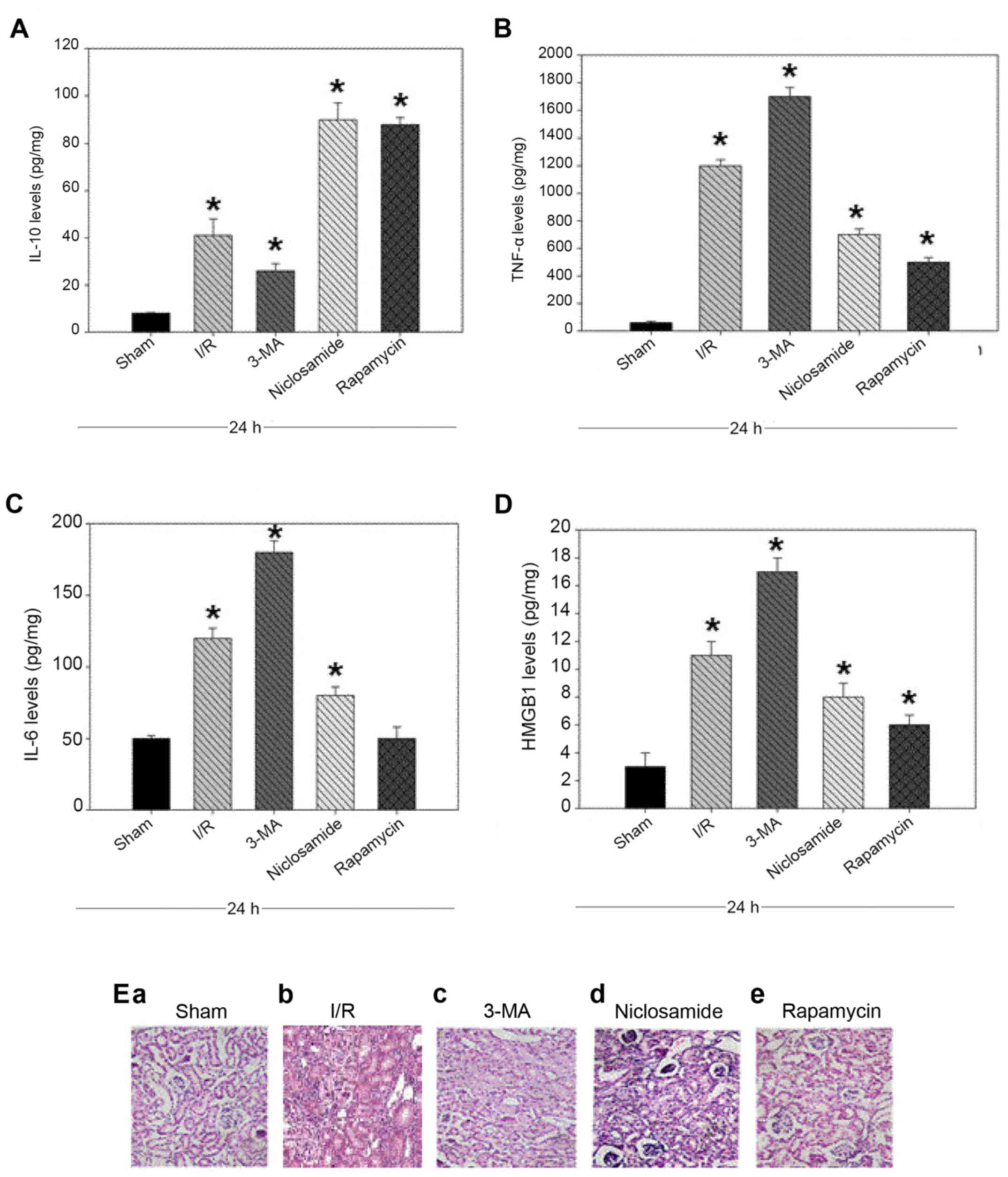 | Figure 2.Levels of (A) IL-10, (B) TNF-α, (C)
IL-6 and (D) HMGB1 in the renal tissues of rats, following renal
I/R injury and treatment with niclosamide (25 mg/kg), 3-MA (15
mg/kg) or rapamycin (10 mg/kg). (E) Alterations in the histological
profile of kidney tissues, following treatment with niclosamide,
3-MA or rapamycin. Values are presented as the mean ± standard
deviation (n=8/group); *P<0.05 vs. sham. IL, interleukin; TNF-α,
tumour necrosis factor-α; HMGB1, high mobility group box 1; I/R
injury, ischemia/perfusion injury; 3-MA, 3-methyladenine. |
Alterations in the histological profile of kidney
samples following treatment with niclosamide, 3-MA and rapamycin
were subsequently examined. There was a protuberance in epithelial
cells, corticullary and medullary regions, loss of nuclei and
degeneration of vacuoles in the kidney tissue of the 3-MA,
niclosamide and rapamycin-treated animals with I/R injury (Fig. 2E). In contrast, cells of the
untreated group appeared to present normal histological profile.
The kidney injury score was higher in the treatment groups when
compared with the sham group at 24 h following I/R injury (Fig. 2E and data not shown).
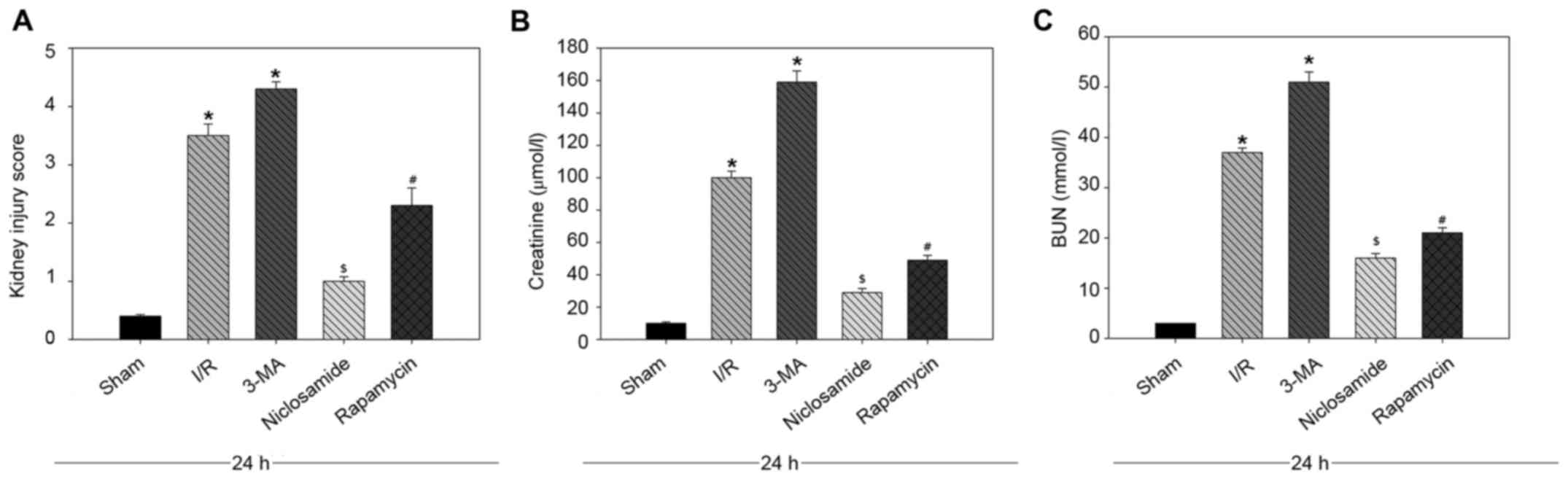
Effect of niclosamide on renal
function in rats with I/R injury
The level of kidney function was evaluated by
measuring the levels of serum creatinine and BUN. Treatment with
niclosamide and rapamycin and was associated with improvedkidney
function, as reflected in the decreased kidney injury scorewhen
compared with the I/R group (Fig.
3A). In addition, a decrease in the level of creatinine and BUN
in niclosamide and rapamycin treatment groups was observed compared
with the I/R group (P<0.05; Fig. 3B
and C). By contrast, treatment with the autophagy inhibitor
3-MA, was associated with an increased kidney injury score
(Fig. 3A). When compared with the
I/R injury group, creatinine and BUN levels were increased in the
3-MA treatment group (Fig. 3B and
C).
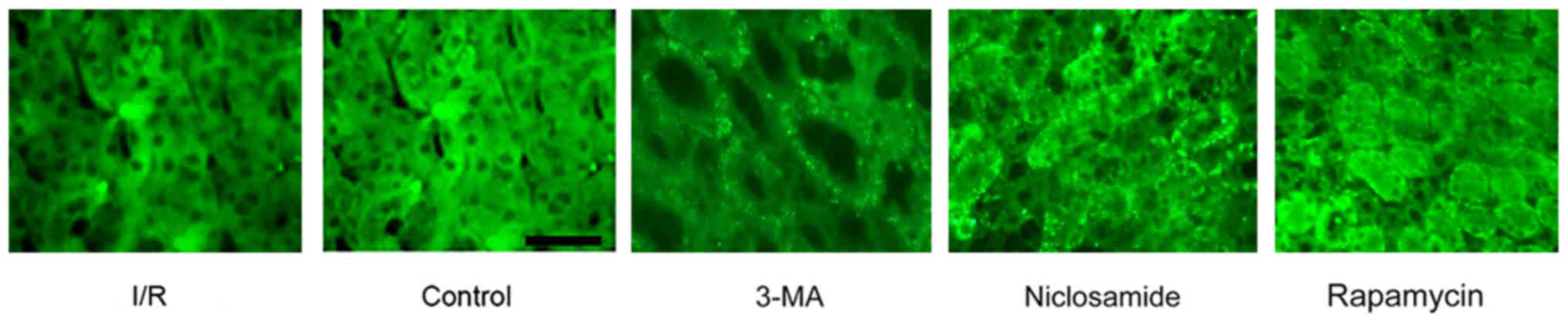
In vivo expression of beclin-1
following I/R injury
As shown in Fig. 4,
administration of niclosamide (25 mg/kg) induced a marked increase
in the level of beclin-1 when compared with the sham group, and the
expression of beclin-1 was comparable to that observed in the
rapamycin (10 mg/kg) treatment group. However, the expression was
limited following treatment with the autophagy inhibitor 3-MA (15
mg/kg). Immunostaining of renal tissues with the beclin-1 antibody
indicated clear regions of beclin-1 expression, which is a
characteristic indicator of autophagosomes. The expression of
beclin-1 around the renal cortical and outer medulla regions was
higher in tissues from rats with I/R injury when compared with the
sham group. Furthermore, beclin-1 expression was higher and more
dispersed in the niclosamide group compared withthe I/R group.
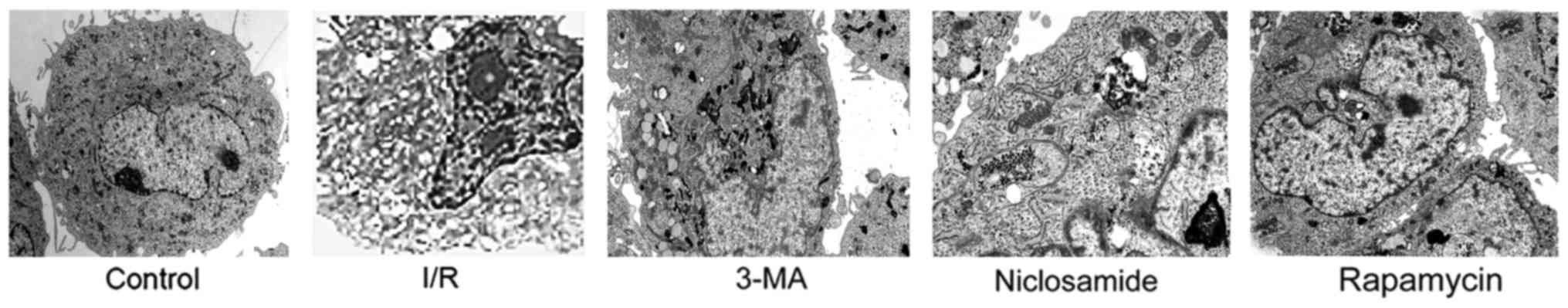
Ultrastructural studies
Autophagy levels in renal tissueswereexamined by
electron microscopy by examining the number of autophagosomes and
additional autophagic vacuoles. No consistent autophagic vacuoles
were observed in the shamgroup, however, numerous autophagic
vacuoles were observed in the proximal tubular cellsin the treated
(I/R) groups (Fig. 5). Under high
magnification (x10,000), the autophagic vacuoles possessed a
double-membrane structure in the cytoplasm, which is a
characteristic of autophagosomes (Fig.
5). In addition, the double-membrane structure in the cytoplasm
was observed to contain mitochondria and additional cytoplasmic
components, includingfragments of the endoplasmic reticulum.
Morphometric analysis revealed 1.48 autophagic vacuoles/100 µm
cytoplasmin the sham group, 1.2 in the 3-MA group, 2.1 in the I/R
group, 5.23 in the niclosamideand 5.9 in the rapamycin treatment
groups. Therefore, there was an increased number of autophagosomes
in the niclosamide and rapamycin treatment groups.
Discussion
I/R is a major underlying cause of AKI development
in native and allograft kidney transplants (34). The incidence of AKIin hospitalized
patients has increased, due to the lack of preventive and curative
measures (35). The complex
pathogenesis of AKI is the result of I/R injury-induced effects on
vascular endothelial cells, tubular epithelial cells, and immune
cells (36). The effects are
characterised by an accumulation of waste products, such as urea
and nitrogen, in the cells, alterations in extracellular fluid
volume, electrolyte and acid-base balance (35), damage of endothelial cells,
necrosis and apoptosis of tubular cells and inflammation (37). In the present study, the effects of
niclosamide on renal I/R injury was investigated to determine
whether it may serve a protective role by regulating the level of
inflammatory cytokines and apoptosis via activation of autophagy in
a rat model of renal I/R injury.
Autophagy, a self-destructive response of cells to
stress, leads to degradation of endogenous cellular protein
aggregates and damaged organelles by lysosomes (38). Previous reports have identified
increased levels of autophagy-associated proteins, including LC3-II
and beclin-1, in the renal tubules of rats with I/R injury
(26,27). LC3 is converted into LC3-I,
following proteolytic cleavage at the C-terminal region by
autophagy-related protein-4. LC3-I is then transformed by the lipid
phosphatidyl ethanolamine to LC3-II. LC3-II is deployed and
attaches to the membrane of an autophagosome. LC3-II remains
attached to the membrane, until the autophagosome fuses with a
lysosome (39,40). Beclin-1 serves a pivotal role in
the formation of autophagosome, and has been reported to improve
the fusion of the autophagosome and lysosome (41,42).
The results of the present study are consistent with earlier
reports regarding the activation of autophagy-associated proteins,
namely LC3-II and beclin-1, in the renal tubules of rats with I/R
injury (26,27). Autophagy is mediated by LC3-II and
beclin-1 and a series of regulatory proteins, which serve an
important role during the progression of autophagy through
different steps; namely the formation of autophagosome, followed by
its fusion with lysosome and the final release of degraded products
(38). In a rat model of kidney
I/R injury, LC3- and LAMP2-positive vacuoles were observed to
aggregate in cells, thereby indicating fusion between
autophagosomes and lysosomes (39). Therefore, LAMP2 may be required for
effective fusion (43,44). Additionally, Rab7 has been reported
to be required for the fusion of autophagosomes and lysosomes, and
for the full development of autophagosomes (45). In the present study, LC3-II,
beclin-1, LAMP2 and Rab7 expression levels were increased following
induction of renal I/R injury in rats, when compared with the sham
group. In the current study, the effects of niclosamide, rapamycin
(an activator of autophagy) and 3-MA (an inhibitor of autophagy)
were evaluated in a rat model of renal I/R injury. Compared with
the sham group, niclosamide and rapamycin treatment increased the
expression of LC3-II, beclin-1, LAMP2 and Rab7. In the present
study, rats in the I/R injury group demonstrated an increase in
kidney injury scores, as well as the levels of creatinine and BUN
at 24 h, when compared with the sham group. AKI was associated with
the increased expression of autophagy-associated proteins,
including LC3-II, beclin-1, LAMP2 and Rab7 at 24 h following
induction of renal I/R injury. Therefore, the results demonstrating
then iclosamide-induced increase in the expression of
autophagy-associated proteins, decreased kidney injury scores and
reduced levels of creatinine and BUN, suggest that it may serve a
protective role against renal I/R injury in rats, potentially via
the induction of autophagy.
Renal I/R injury has been reported to induce an
inflammatory response by triggering the innate and adaptive immune
systems, followed by infiltration of leukocytes to the site of
inflammation and activation tubular epithelial cells (46,47).
The infiltration of leukocytes, including neutrophils and
macrophages, in the damaged renal tissue promotes the secretion of
pro-inflammatory cytokines (48).
The infiltrated neutrophils and macrophages reduce blood flow in
kidney, resulting dysfunction of the microcirculation (49,50).
The levels of pro-inflammatory cytokines, including IL-1β, IL-6,
TNF-α and HMGB1, have been reported to significantly increase
following renal I/R injury in rats, which demonstrates that
cytokines may be used as an indicator of severity of kidney damage
(51). In the present study, rats
in the renal I/R injury group demonstrated an increase in the
levels of pro-inflammatory cytokines TNF-α, IL-6 and HMGB, and a
reduction in the levels of the anti-inflammatory cytokine IL-10 at
24h post-renal I/R injury, when compared with the sham group. In
the treated renal I/R injury groups, niclosamide and rapamycin
decreased the levels of TNF-α, HMGB1 and IL-6 and promoted the
release of IL-10 when compared with the untreated I/R group. By
contrast, 3-MA treatment was associated with the opposite effect on
the expression of these cytokines when compared with the untreated
I/R group. Therefore, niclosamide reduces the renal I/R
injury-induced inflammatory response in AKI.
In conclusion, in a rat model of renal I/R injury,
niclosamide was observed to induce autophagy and an inflammatory
response, as well as decrease kidney injury scores and the levels
of creatinine and BUN. This agent may thereforeserve a protective
role during AKI by regulating the levels of pro-inflammatory and
anti-inflammatory cytokines, potentially via I/R injury-induced
autophagy. An exploratory clinical trial involving the
incorporation of niclosamide in the treatment protocol will be
required to investigate these findings further.
References
|
1
|
Kunzendorf U, Haase M, Rolver L and
Haase-Fielitz A: Novel aspects of pharmacological therapies for
acute renal failure. Drugs. 70:1099–1114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mangano CM, Diamondstone LS, Ramsay JG,
Aggarwal A, Herskowitz A and Mangano DT: Renal dysfunction after
myocardial revascularization: Risk factors, adverse outcomes and
hospital resource utilization. The Multicenter Study of
Perioperative Ischemia Research Group. Ann Intern Med. 128:194–203.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malek M and Nematbakhsh M: Renal
ischemia/reperfusion injury; from pathophysiology to treatment. J
Renal Inj Prev. 4:20–27. 2015.PubMed/NCBI
|
|
4
|
Aydin Z, van Zonneveld AJ, de Fijter JW
and Rabelink TJ: New horizons in prevention and treatment of
ischaemic injury to kidney transplants. Nephrol Dial Transplant.
22:342–346. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chertow GM, Burdick E, Honour M, Bonventre
JV and Bates DW: Acute kidney injury, mortality, length of stay,
and costs in hospitalized patients. J Am Soc Nephrol. 16:3365–3370.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kazmers A, Jacobs L and Perkins A: The
impact of complications after vascular surgery in veterans affairs
medical centers. J Surg Res. 67:62–66. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Levy EM, Viscoli CM and Horwitz RI: The
effect of acute renal failure on mortality. A cohort analysis.
JAMA. 275:1489–1494. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang J, Li JH, Wang L, Han M, Xiao F, Lan
XQ, Li YQ, Xu G and Yao Y: Glucocorticoid receptor agonist
dexamethasone attenuates renal ischemia/reperfusion injury by
up-regulating eNOS/iNOS. J Huazhong Univ Sci Technolog Med Sci.
34:516–520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bonventre JV: Daily hemodialysis-will
treatment each day improve the outcome in patients with acute renal
failure? N Engl J Med. 346:362–364. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion-from mechanism to translation. Nat Med. 17:1391–1401.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gabay C and Kushner I: Acute-phase
proteins and other systemic responses to inflammation. N Engl J
Med. 340:448–454. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McCaughan JA, Patterson CC, Maxwell AP and
Courtney AE: Factors influencing survival after kidney transplant
failure. Transplant Res. 3:182014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grams ME and Rabb H: The distant organ
effects of acute kidney injury. Kidney Int. 81:942–948. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hotta O, Yusa N, Ooyama M, Unno K, Furuta
T and Taguma Y: Detection of urinary macrophages expressing the
CD16 (Fc gamma RIII) molecule: A novel marker of acute inflammatory
glomerular injury. Kidney Int. 55:1927–1934. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akcay A, Nguyen Q and Edelstein CL:
Mediators of inflammation in acute kidney injury. Mediators
Inflamm. 2009:1370722009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee DW, Faubel S and Edelstein CL:
Cytokines in acute kidney injury (AKI). Clin Nephrol. 76:165–173.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Molitoris BA and Sutton TA: Endothelial
injury and dysfunction: Role in the extension phase of acute renal
failure. Kidney Int. 66:496–499. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Umehara H, Goda S, Imai T, Nagano Y,
Minami Y, Tanaka Y, Okazaki T, Bloom ET and Domae N: Fractalkine, a
CX3C-chemokine, functions predominantly as an adhesion molecule in
monocytic cell line THP-1. Immunol Cell Biol. 79:298–302. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gottlieb RA and Mentzer RM: Autophagy
during cardiac stress: Joys and frustrations of autophagy. Annu Rev
Physiol. 72:45–59. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Glick D, Barth S and Macleod KF:
Autophagy: Cellular and molecular mechanisms. J Pathol. 221:3–12.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yorimitsu T and Klionsky DJ: Autophagy:
Molecular machinery for self-eating. Cell Death Differ. 12 Suppl
2:S1542–S1552. 2005. View Article : Google Scholar
|
|
22
|
Chien CT, Shyue SK and Lai MK: Bcl-xL
augmentation potentially reduces ischemia/reperfusion induced
proximal and distal tubular apoptosis and autophagy.
Transplantation. 84:1183–1190. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu HH, Hsiao TY, Chien CT and Lai MK:
Ischemic conditioning by short periods of reperfusion attenuates
renal ischemia/reperfusion induced apoptosis and autophagy in the
rat. J Biomed Sci. 16:192009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suzuki C, Isaka Y, Takabatake Y, Tanaka H,
Koike M, Shibata M, Uchiyama Y, Takahara S and Imai E:
Participation of autophagy in renal ischemia/reperfusion injury.
Biochem Biophys Res Commun. 368:100–106. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang M, Liu K, Luo J and Dong Z:
Autophagy is a renoprotective mechanism during in vitro hypoxia and
in vivo ischemia-reperfusion injury. Am J Pathol. 176:1181–1192.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lo S, Yuan SS, Hsu C, Cheng YJ, Chang YF,
Hsueh HW, Lee PH and Hsieh YC: Lc3 over-expression improves
survival and attenuates lung injury through increasing
autophagosomal clearance in septic mice. Ann Surg. 257:352–363.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hsieh CH, Pai PY, Hsueh HW, Yuan SS and
Hsieh YC: Complete induction of autophagy is essential for
cardioprotection in sepsis. Ann Surg. 253:1190–1200. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takahashi W, Watanabe E, Fujimura L,
Watanabe-Takano H, Yoshidome H, Swanson PE, Tokuhisa T, Oda S and
Hatano M: Kinetics and protective role of autophagy in a mouse
cecal ligation and puncture-induced sepsis. Crit Care. 17:R1602013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu CT, Sheu ML, Tsai KS, Chiang CK and Liu
SH: Salubrinal, an eIF2a dephosphorylation inhibitor, enhances
cisplatin-induced oxidative stress and nephrotoxicity in a mouse
model. Free Radic Biol Med. 51:671–680. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
NRC [National Research Council]: Guide for
the Care and Use of Laboratory Animals. 7th. Washington DC:
National Academy Press; 1996
|
|
31
|
Wei Q and Dong Z: Mouse model of ischemic
acute kidney injury: Technical notes and tricks. Am J Physiol Renal
Physiol. 303:F1487–F1494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Khalid U, Pino-Chavez G, Nesargikar P,
Jenkins RH, Bowen T, Fraser DJ and Chavez R: Kidney ischaemia
reperfusion injury in the rat: The EGTI scoring system as a valid
and reliable tool for histological assessment. J Histol
Histopathol. 3:12016. View Article : Google Scholar
|
|
33
|
Bellomo R, Kellum JA and Ronco C: Acute
kidney injury. Lancet. 380:756–766. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang X, Du Z, Li L, Shi M and Yu Y: Beclin
1 and p62 expression in non-small cell lung cancer: Relation with
malignant behaviors and clinical outcome. Int J Clin Exp Pathol.
8:10644–10652. 2015.PubMed/NCBI
|
|
35
|
Huber TB, Edelstein CL, Hartleben B, Inoki
K, Jiang M, Koya D, Kume S, Lieberthal W, Pallet N, Quiroga A, et
al: Emerging role of autophagy in kidney function, diseases and
aging. Autophagy. 8:1009–1031. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Basile DP, Friedrich JL, Spahic J, Knipe
N, Mang H, Leonard EC, Changizi-Ashtiyani S, Bacallao RL, Molitoris
BA and Sutton TA: Impaired endothelial proliferation and
mesenchymal transition contribute to vascular rarefaction following
acute kidney injury. Am J Physiol Renal Physiol. 300:F721–F733.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Linkermann A, Bräsen JH, Himmerkus N, Liu
S, Huber TB, Kunzendorf U and Krautwald S: Rip1
(receptor-interacting protein kinase 1) mediates necroptosis and
contributes to renal ischemia/reperfusion injury. Kidney Int.
81:751–761. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Livingston MJ and Dong Z: Autophagy in
acute kidney injurySeminars in nephrology. 34. WB Saunders; pp.
17–26. 2014, View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hsieh YC, Athar M and Chaudry IH: When
apoptosis meets autophagy: Deciding cell fate after trauma and
sepsis. Trends Mol Med. 15:129–138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mizushima N: Autophagy: Process and
function. Genes Dev. 21:2861–2873. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhong Y, Wang QJ, Li X, Yan Y, Backer JM,
Chait BT, Heintz N and Yue Z: Distinct regulation of autophagic
activity by Atg14L and Rubicon associated with Beclin
1-phosphatidylinositol-3-kinase complex. Nat Cell Biol. 11:468–476.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
Saftig P and Eskelinen EL: Live longer
with LAMP-2. Nat Med. 14:909–910. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang YL, Cao YJ, Zhang X, Liu HH, Tong T,
Xiao GD, Yang YP and Liu CF: The autophagy-lysosome pathway: A
novel mechanism involved in the processing of oxidized LDL in human
vascular endothelial cells. Biochem Biophys Res Commun.
394:377–382. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gutierrez MG, Munafó DB, Berón W and
Colombo MI: Rab7 is required for the normal progression of the
autophagic pathway in mammalian cells. J Cell Sci. 117:2687–2697.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Land WG: The role of postischemic
reperfusion injury and other nonantigen-dependent inflammatory
pathways in transplantation. Transplantation. 79:505–514. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Serteser M, Koken T, Kahraman A, Yilmaz K,
Akbulut G and Dilek ON: Changes in hepatic TNF-alpha levels,
antioxidant status and oxidation products after renal
ischemia/reperfusion injury in mice. J Surg Res. 107:234–240. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ysebaert DK, de Greef KE, Vercauteren SR,
Ghielli M, Verpooten GA, Eyskens EJ and De Broe ME: Identification
and kinetics of leukocytes after severe ischaemia/reperfusion renal
injury. Nephrol Dial Transplant. 15:1562–1574. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bolisetty S and Agarwal A: Neutrophils in
acute kidney injury: Not neutral any more. Kidney Int. 75:674–676.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jing XX, Wang ZG, Ran HT, Li L, Wu X, Li
XD, Peng XQ, Yang CJ, Li XS and Zhang QX: Evaluation of renal
ischemia-reperfusion injury in rabbits using microbubbles targeted
to activated neutrophils. Clin Imaging. 32:178–182. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dessing MC, Pulskens WP, Teske GJ, Butter
LM, van der Poll T, Yang H, Tracey KJ, Nawroth PP, Bierhaus A,
Florquin S and Leemans JC: RAGE does not contribute to renal injury
and damage upon ischemia/reperfusion-induced injury. J Innate
Immun. 4:80–85. 2012. View Article : Google Scholar : PubMed/NCBI
|