Introduction
Osteoarthritis (OA) is a chronic degenerative
disease that is characterized by articular cartilage degeneration
and subchondral osteophyte formation, and exhibits common symptoms,
including joint pain and restricted movement (1). Currently, treatment methods for OA
are limited to symptomatic approaches or surgery involving
prosthesis implantation, primarily due to the lack of targeted
treatments that are able to effectively protect against cartilage
destruction (2). Certain available
treatments, including glucosamine and chondroitin dietary
supplements, have been proven to offer moderate protection, but
long-term use is required (3).
Consequently, there is an urgent requirement to develop therapeutic
and preventive options for OA.
OA is primarily caused by an imbalance between the
degradation and the synthesis of cartilage extracellular matrix
(ECM). During the pathophysiology of OA, chondrocytes are immersed
in an inflammatory environment, which may result in loss of
cartilage matrix components. Type II collagen is a predominant and
important component of the ECM and interacts with proteoglycans,
providing the cartilage with the elasticity and capacity for
deformation. The degeneration of cartilage is accompanied by a
decrease in type II collagen. Previous studies have demonstrated
that the expression of matrix metalloproteinases (MMPs) is
significantly increased in the chondrocytes of patients with OA and
animal models (4–6). Among the MMPs, only MMP-13 has been
demonstrated to degrade the ECM directly, whereas other MMP
subtypes require the involvement of MMP-13 (5). An abnormal increase in MMP expression
is a major cause of the imbalance between synthesis and degradation
of cartilage ECM, leading to gradual erosion of articular
cartilage, ulcer formation and cartilage degradation (7,8).
Interleukin-1β (IL-1β) is one of the primary inflammatory cytokines
and stimulates IL-1 receptors and downstream signaling molecules,
which subsequently activate the transcription factor nuclear
factor-κB (NF-κB) (9–11). The NF-κB signaling pathway is
important for the regulation of MMP expression levels (12–14).
Previous studies have demonstrated that the inhibition of NF-κB
activation by curcumin leads to the inhibition of cyclooxygenase 2
expression and increased MMP-mediated degradation of cartilage,
whereas other studies have revealed that curcumin protects
cartilage cells by upregulating the expression of type II collagen
(15–18). Therefore, preventing the loss of
type II collagen and/or inhibiting the synthesis of MMPs may
provide promising options to protect against cartilage degradation
and may be beneficial for the treatment of OA.
Curcumin is a yellow pigment extracted from
Zingiberaceae and Araceae turmeric, which is widely used in foods,
cosmetics and drugs. Curcumin exhibits a broad range of properties,
including inflammatory response inhibition, antioxidative activity
and anti-rheumatoid effects. Previously, curcumin has attracted
attention for its potential to inhibit NF-κB signaling (15–17).
The aim of the present study was to investigate whether curcumin
may reverse the IL-1β-induced downregulation of type II collagen,
and whether curcumin was able to inhibit the catabolic effects of
MMP-13 by suppressing NF-κB activation and NF-κB-induced gene
expression.
Materials and methods
Reagents
Curcumin and collagenase II were obtained from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) and a 10 mM stock
solution was prepared in dimethyl sulfoxide, and diluted with cell
culture medium immediately prior to use.
Cells were maintained in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (FBS; Gibco;
ThermoFisher Scientific, Inc.) and 100 IU/ml penicillin, 100 mg/ml
streptomycin at 37°C in a humidified atmosphere of 95% air and 5%
CO2.
Recombinant human IL-1β was supplied by PeproTech,
Inc. (Rocky Hill, NJ, USA). Primary antibodies against type II
collagen (AB746) and MMP-13 (AB39012) were purchased from Abcam
(Cambridge, UK). Primary antibodies against NF-κB inhibitor α
(IκBα; 4814S), phosphorylated (p)-IκBα (9246S) and NF-κB p65/RelA
(8242S) were provided by Cell Signaling Technology, Inc. (Danvers,
MA, USA). The selective NF-κB inhibitor pyrrolidine dithiocarbamate
(PDTC), primary antibodies against GAPDH (AB8245) and lamin B1
(AB16048), and horseradish peroxidase (HRP)-conjugated secondary
antibody (AB7097) were obtained from Abcam. Cell culture reagents
were purchased from Gibco (Thermo Fisher Scientific, Inc.).
Chondrocyte isolation and culture
A total of eight 5-week-old male rats (300–410 g)
were purchased from Shanghai SLAC Laboratory Animal, Co., Ltd. and
kept in common rabbit cages and fed a standard diet with tap water
ad libitum. All the rat procedures were approved by the
Institutional Care and Use Committee of Shanghai Ninth People's
Hospital, Shanghai Jiao Tong University School of Medicine. The
rats were anaesthetized with pentobarbital sodium (40–45 mg/kg) and
the articular cartilage was isolated from femoral and tibial
articular joints of by aseptic dissection following air injection
into the ear vein. Following two washes with PBS, the cartilage
sections were treated with 2 mg/ml pronase (EMD Millipore,
Billerica, MA, USA) in serum-free DMEM at 37°C for 1 h in 5%
CO2, followed by overnight digestion with 0.25 mg/ml
collagenase II dissolved in serum-free DMEM. Isolated chondrocytes
were cultured in DMEM supplemented with 10% fetal bovine serum
(FBS) and 1% penicillin/streptomycin at 37°C in a humidified
atmosphere containing 5% CO2. All experiments were
performed on chondrocytes between passage 1 and 3. Fibroblasts were
used as control group, according to the manufacturer's protocol for
VECTASTAIN® ABC kit (AK-5000) and toluidine blue staining kit
(H-5000) were provided by Vector Laboratories (Southfield, MI,
USA). The chondrocytic phenotype of the cultured cells was
confirmed by toluidine blue staining of glycosaminoglycan and
immunocytochemical staining.
Experimental design
To exclude stimulation that may be caused by other
cytokines or growth factors present in the FBS, chondrocytes were
serum-starved and exposed to 10 ng/ml IL-1β for 24 h. Experiments
were designed to mimic the cellular events that occur in OA. To
acquire the optimum timepoint and concentration of curcumin for the
treatment of chondrocytes, chondrocytes were co-treated with either
of the following: i) 10 ng/ml IL-1β and 50 µM curcumin for various
time periods (0, 12, 24, 36 or 48 h); or ii) 10 ng/ml IL-1β and
various concentrations of curcumin (0, 25, 50, 75, or 100 µM) for
36 h. To investigate p65/RelA translocation and IκBα
phosphorylation, chondrocytes were pretreated with 10 ng/ml IL-1β
in the presence or absence of 50 µM curcumin for 0, 10, 20, 30, 40
or 60 min, after which nuclear and cytoplasmic extracts were
prepared using a nuclear and cytoplasmic protein extraction kit
(P0028; Beyotime Institute of Biotechnology) following the
manufacturer's protocol. The experiments were performed in
triplicate.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells
(1.2×106 cells/well) using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), and 2 µg was reverse-transcribed
into cDNA using an Advantage® RT-for-PCR kit (639506; Takara
Biotechnology Co., Ltd., Dalian, China) according to the
manufacturer's protocol. qPCR was performed using SYBR Green PCR
master mix (Takara Bio, Inc., Otsu, Japan), in accordance with the
manufacturer's protocols. The primer sequences used are presented
in Table I. The PCR conditions
were set as follows: Initial denaturation at 95°C for 3 min,
followed by 40 cycles at 95°C for 15 sec and 60°C for 30 sec with a
final extension at 72°C for 3 min. mRNA levels were normalized to
levels of the endogenous control GAPDH, and the relative expression
levels were calculated using the 2−ΔΔCq method (19). Data were obtained from three
independent experiments performed in triplicate.
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence
(5′→3′) | Expected size
(bp) |
|---|
| GAPDH | F:
CCCCAATGTATCCGTTGTG | 124 |
|
| R:
CTCAGTGTAGCCCAGGATGC |
|
| MMP-13 | F:
CGTTCAAGGAATCCAGTCTCTC | 231 |
|
| R:
TCCACATGGTTGGGAAGTTC |
|
| Type II collagen | F:
GCAAGAATCCCGCTCGCA | 158 |
|
| R:
TGGGTTGGGGTAGACGCA |
|
| IκBα | F:
GAAATACCCCTCTCCATCTTGC | 298 |
|
| R:
ACATCAGCCCCACACTTCAA |
|
| p65/RelA | F:
GACCTGGAGCAAGCCATTAG | 285 |
|
| R:
CACTTTGTCACACAGCAAGAAGA |
|
Protein extraction and western blot
analysis
Chondrocytes (1.2×106 cells/well) were
washed twice with ice-cold PBS, lysed with Radioimmunoprecipitation
Assay buffer (Beyotime Institute of Biotechnology, Haimen, China)
containing 1 mM phenylmethylsulfonyl fluoride on ice for 30 min,
and centrifuged at 16,992 × g at 4°C for 20 min. Protein
concentrations were determined using the Bicinchoninic Acid method
(Beyotime Institute of Biotechnology, Shanghai, China). Equal
amounts (60 µg) of protein were separated by 10% SDS-PAGE and
transferred onto polyvinylidene difluoride membranes. The membranes
were blocked in 1% bovine serum albumin (Gibco; Thermo Fisher
Scientific, Inc.) at room temperature for 1 h, and subsequently
incubated with the following primary antibodies: anti-type II
collagen (1:200), anti-MMP-13 (1:200), anti-IκBα (1:200),
anti-p-IκBα (1:200), anti-NF-κB p65/RelA (1:200), anti-GAPDH
(1:1,000) and anti-lamin B1 (1:1,000) at 4°C overnight. Following
three washes with Tris-buffered saline containing 0.05% Tween-20,
the membranes were incubated with goat anti-rat IgG H&L
HRP-conjugated secondary antibody (1:2,000; AB7097) at room
temperature for 1 h. The immunoblots were analyzed by Enhanced
Chemiluminescence (ECL) with an ECL Plus kit (Beyotime Institute of
Biotechnology), and the bands were quantified by ImageJ software
version 1.48 (National Institutes of Health, Bethesda, MD, USA) and
normalized to GAPDH expression. Data were obtained from three
independent experiments performed in triplicate.
Immunofluorescence microscopy
Cells (1.2×106 cells/well) were seeded
onto glass coverslips in 24-well plates, cultured for 24 h at 37°C,
pre-incubated with serum-starved DMEM for 1 h at 37°C, and
subsequently stimulated with IL-1β (10 ng/ml) alone, IL-1β (10
ng/ml) and curcumin (50 µM), or IL-1β (10 ng/ml) and PDTC (0.1
mmol/l) for 30 min at 37°C. The cells were washed three times with
PBS, fixed with 4% paraformaldehyde for 20 min at room temperature
and permeabilized with 0.1% Triton X-100/PBS for 15 min. Following
blocking in PBS containing 10% normal goat serum (Gibco; Thermo
Fisher Scientific, Inc.) for 1 h, the cells were incubated with
anti-p65/RelA (1:50) overnight at 4°C, followed by incubation with
a fluorescein isothiocyanate-conjugated goat anti-rat IgG H&L
(PE) secondary antibody (1:50; AB7010; Abcam, Cambridge, UK) for 1
h at room temperature. Finally, cells were rinsed in PBS three
times and covered with Fluoromount mountant (Gallard-Schlesinger
Industries, Garden City, NY, USA). Fluorescence signals were
examined and imaged under a Zeiss Axioskop 40 fluorescence
microscope (Carl Zeiss AG, Oberkochen, Germany) by using Image-J
software version 1.45 (National Institutes of Health).
Cell proliferation assay
Cell proliferation was evaluated using a Cell
Counting kit-8 (CCK-8; Beyotime Institute of Biotechnology) in
accordance with the manufacturer's protocol. Chondrocytes were
treated with IL-1β for 24 h prior to curcumin, and subsequently
co-treated with either curcumin or PDTC, a combination of curcumin
plus PDTC, or were untreated. Cells (6×103 cells/well)
were seeded into 96-well plates and incubated for 12, 24, 48 or 72
h at 37°C. Following this, 10 µl of CCK-8 was added to each well
(90 µl medium was mixed with 10 µl CCK-8) and the plates were
incubated at 37°C for 2 h. Following incubation, the absorbance was
measured at a wavelength of 450 nm using an automated plate reader.
The cell viability was calculated as a percentage of the viable
cells in the curcumin-treated group compared with the untreated
control. Each experiment was repeated three times
independently.
Statistical analysis
All experiments were carried out at least three
times, and the data are presented as the mean ± standard deviation.
Statistical analyses were performed using SPSS software version
17.0 (SPSS Inc., Chicago, IL, USA). Comparisons between
experimental and control data were evaluated by Student's t-test or
analysis of variance. Multiple groups were compared using analysis
of variance, the comparison between groups using LSD test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Regulation of type II collagen and
MMP-13 expression in rat chondrocytes by IL-1β
The chondrocytic phenotype of the isolated rat
chondrocytes was confirmed by toluidine blue staining of
glycosaminoglycan and immunocytochemical staining of type II
collagen (Fig. 1). RT-qPCR and
western blot analyses were performed to assess the regulation of
MMP-13 and type II collagen mRNA and protein expression by IL-1β
using chondrocytes that were cultured in the presence or absence of
IL-1β for 24 h. IL-1β treatment significantly decreased the
expression of type II collagen (Fig.
2A) and markedly increased the expression of MMP-13 (Fig. 2B). These results indicated that
IL-1β induces MMP-13 expression, but inhibits type II collagen
expression in rat chondrocytes.
Curcumin inhibits MMP-13 expression
and upregulates type II collagen expression levels
To determine the optimum conditions of curcumin,
serum-starved rat chondrocytes were treated with IL-1β (10 ng/ml)
for 24 h and subsequently cultured in medium containing 50 µM
curcumin. The chondrocytes were harvested after 0, 12, 24, 36 or 48
h and the expression levels of MMP-13 and type II collagen proteins
were examined by western blot analysis. As shown in Fig. 3, MMP-13 protein expression was
significantly decreased, whereas type II collagen was markedly
increased in chondrocytes following treatment with curcumin
compared with the untreated group (0 h). Moreover, the decrease in
MMP-13 expression was lowest at 36 h, and the increase in type II
collagen peaked at 48 h.
Following this, the optimum concentration of
curcumin for treatment of IL-1β-treated chondrocytes was
determined. Rat chondrocytes were treated 10 ng/ml IL-1β and
various concentrations of curcumin (0, 25, 50, 75, or 100 µM) for
36 h. As presented in Fig. 4,
curcumin markedly upregulated the expression of type II collagen,
and significantly inhibited the expression of MMP-13 in
IL-1β-pretreated chondrocytes at each concentration compared with
the untreated group (0 µM). The optimum concentration by which
curcumin had the strongest effect on type II collagen and MMP-13
expression levels was determined to be 50 µM.
Curcumin suppresses IL-1β-induced
nuclear translocation of p65/RelA
To investigate the effect of curcumin on
IL-1β-induced NF-κB activation, serum-starved chondrocytes were
treated with IL-1β in the presence or absence of curcumin for 0–60
min and then subjected to cell component fractionation. The
resultant cytoplasmic and nuclear extracts were used for western
blot analysis to examine the protein expression of p65/RelA. The
results demonstrated that IL-1β treatment led to a decrease in the
cytoplasmic expression and an increase in the nuclear translocation
of p65/RelA, which accumulated in the nucleus in a time-dependent
manner (Fig. 5A). However, upon
co-treatment with curcumin, neither the cytoplasmic nor the nuclear
expression of p65/RelA exhibited a significant difference at any of
the indicated time points compared with IL-1β-treated chondrocytes
(Fig. 5B). Protein expression
levels of p65/RelA decreased in the cytoplasm (Fig. 5C) and gradually increased in the
nucleus (Fig. 5D) over time with
IL-1β treatment.
As curcumin may stabilize p65/RelA in the cytoplasm
(20), the effect of curcumin on
the IL-1β-induced nuclear translocation of p65/RelA was further
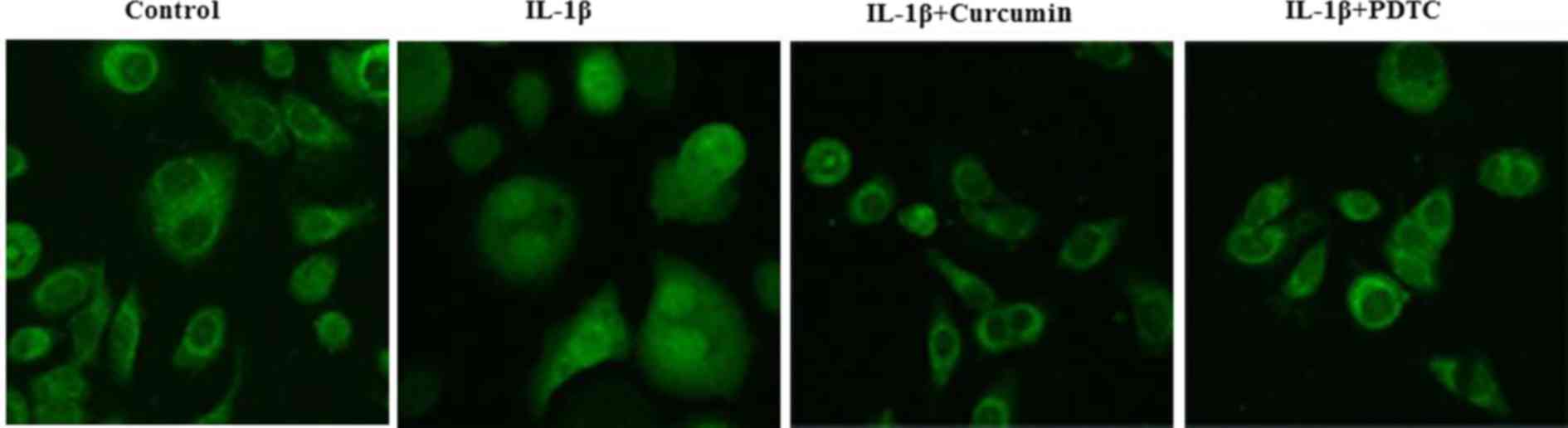
investigated by immunofluorescence staining. IL-1β-stimulated
chondrocytes exhibited clear nuclear translocation of p65/RelA from
the cytoplasm (Fig. 6), whereas
co-treatment with curcumin and PDTC notably blocked nuclear
translocation of p65/RelA. Taken together, these findings suggested
that curcumin inhibits IL-1β-induced nuclear translocation of
p65/RelA and suppresses IL-1β-induced NF-κB activation in
chondrocytes.
Curcumin inhibits IL-1β-induced IκBα
phosphorylation
The phosphorylation and subsequent degradation of
IκBα are essential for the nuclear translocation of p65/RelA and
the activation of NF-κB signaling. Therefore, the ability of
curcumin to inhibit IL-1β-induced phosphorylation and degradation
of IκBα was examined. Chondrocytes were serum-starved and then
treated with IL-1β in the presence or absence of curcumin for 0–60
min. The phosphorylation of IκBα in IL-1β-treated chondrocytes
increased markedly and peaked at 40 min (Fig. 7A). However, upon co-treatment with
IL-1β and curcumin, no significant differences in IκBα
phosphorylation were observed compared with IL-1β-treated
chondrocytes (Fig. 7B). These
findings suggested that curcumin may block IL-1β-induced IκBα
phosphorylation in rat chondrocytes.
Effect of curcumin on chondrocyte
proliferation and IκBα phosphorylation
To elucidate the potential pharmacological effects
of curcumin on OA, CCK-8 assays were performed to evaluate cell
proliferation. Proliferation of IL-1β-treated chondrocytes was
demonstrated to gradually increase following any of the three
treatments compared with the control, whereas proliferation
gradually decreased in untreated cells. Furthermore, although the
proliferation activity of each pharmacologic intervention group
increased significantly compared with IL-1β-treated chondrocytes,
there were no significant differences in the cell proliferation
activity among the groups treated with curcumin, PDTC or curcumin
plus PDTC (Fig. 8).
The effects of curcumin and PDTC on the
phosphorylation of IκBα were further examined. Consistent with the
previous data (Fig. 2B), curcumin
and PDTC inhibited the expression of p-IκBα and MMP-13 proteins,
but upregulated type II collagen protein expression levels compared
with the control (Fig. 9A); no
significant changes to IκBα expression were observed. Curcumin and
PDTC treatments also led to the downregulated expression of MMP-13
and p65/RelA mRNA, and upregulated type II collagen mRNA expression
levels compared with the control; no significant changes to IκBα
expression were observed compared with the control. (Fig. 9B). Co-treatment of IL-1β-stimulated
chondrocytes with curcumin and PDTC did not cause further
inhibition of MMP-13 expression or upregulation of type II collagen
expression beyond that caused by treatment with curcumin or PDTC
alone. Notably, no significant differences were observed in cell
proliferation or the expression levels of MMP-13 and type II
collagen in the pharmacological intervention groups, and
chondrocytes co-treated with curcumin plus PDTC did not exhibit
enhanced effects in proliferation or MMP-13 and type II collagen
expression levels compared with cells treated with curcumin or PDTC
alone, suggesting that the regulation of chondrocytes by curcumin
and PDTC may follow the same underlying molecular mechanism.
 | Figure 9.Effect of curcumin and PDTC on protein
and mRNA expression levels in interleukin-1β-treated chondrocytes.
(A) Western blot analysis of MMP-13, type II collagen, IκBα and
p-IκBα protein expression levels. GAPDH served as an internal
control. (B) Quantification of MMP-13, type II collagen, IκBα and
NF-κB subunit p65/RelA mRNA expression levels, as assessed by
reverse transcription-quantitative polymerase chain reaction
analysis. Data are presented as the mean ± standard deviation;
**P<0.01. PDTC, pyrrolidine dithiocarbamate; NF-κB, nuclear
factor-κB; IκBα, NF-κB inhibitor α; p, phosphorylated; MMP-13,
matrix metalloproteinase-13. |
Discussion
OA involves the breakdown of joint tissues in
response to a number of factors, including aging, stress and
trauma. The OA process can be simulated experimentally by
stimulating chondrocytes with IL-1β or TNF-α, which serve prominent
roles in the articular cartilage catabolism (21–23).
Type II collagen is a predominant and important component of the
cartilage matrix, and a decrease in type II collagen expression is
one of the hallmarks of cartilage degeneration. Type II collagen
occupies the vast majority of space in healthy cartilage tissue.
During the process of cartilage tissue degeneration, type II
collagen proteins and polysaccharides are primarily destroyed by
decomposition, and protein contents become clearly decreased. In
the present study, the effects of curcumin on the expression of
type II collagen in IL-1β-stimulated rat chondrocytes were
investigated. The results revealed that the IL-1β-inhibited
expression of type II collagen was markedly reversed by curcumin,
suggesting that curcumin may have a protective effect on
IL-1β-induced cartilage degeneration.
IL-1β induces inflammatory conditions and increases
the production of protein-degrading enzymes including MMPs,
particularly collagenase-2 (24).
Remodeling and breakdown processes of the cartilage matrix are
primarily regulated by MMPs, which lead to cleavage of the ECM
components. MMP-13 has a higher affinity for cleaving the ECM than
other MMPs, and is regarded as a crucial enzyme for cartilage
degradation during the progression of OA (25,26).
Inhibition of MMPs may prevent the loss of cartilage ECM and
cartilage degradation. Therefore, to investigate the preventive
effect of curcumin on cartilage degradation, IL-1β was used to
stimulate chondrocytes. As expected, IL-1β treatment significantly
induced MMP-13 expression, leading to degradation of the ECM
produced by the chondrocytes corresponding to the protein contents
of type II collagen. Curcumin suppressed the expression of MMP-13
in IL-1β-stimulated chondrocytes. Thus, curcumin may inhibit
cartilage degradation during inflammatory factor-mediated joint
degeneration.
During the treatment of OA, it is important to
maintain healthy cartilage matrix metabolism and reduce cartilage
degradation. In the future, the most effective treatment for OA
will be one that both blocks inflammatory factor-mediated cartilage
destruction and improves the stability of the cartilage matrix. The
results of the present study indicated that curcumin had a
protective effect on cartilage by reversing the IL-1β-induced
decrease in type II collagen and inhibiting IL-1β-induced increase
in MMP-13 expression. Taken together, as a small-molecule
chemopreventive agent, curcumin may have a role in the treatment of
OA.
Curcumin suppresses NF-κB activation via direct
inhibition of IκBα phosphorylation, which induces the retention of
NF-κB in the cytoplasm and thus interferes with NF-κB binding to
DNA to regulate the transcription of target genes (17,18,27).
In the inactive state, the p65/RelA subunit of NF-κB is retained in
the cytoplasm, whereas activated p65/RelA is immediately
translocated into the nucleus where it binds to DNA and regulates
the transcription of its target genes (28). In the present study, IL-1β-induced
nuclear translocation of p65/RelA in rat chondrocytes was clearly
observed by cell component fractionation analyses and
immunofluorescence staining. Furthermore, curcumin blocked
IL-1β-induced nuclear translocation of p65/RelA, presumably by
inhibiting IL-1β-induced IκBα phosphorylation. The present study
further demonstrated that NF-κB signaling was involved in the
regulation of type II collagen and MMP-13 expression levels in
IL-1β-stimulated chondrocytes by using an inhibitor of NF-κB
activation. Clutterbuck et al (29,30)
reported that treatment with curcumin at concentrations >100 µM
for 48 h or 5 days led to the death of chondrocytes, and that the
release of glycosaminoglycan by tissues cultured in vitro
was suppressed by curcumin at high concentrations, which
subsequently inhibited the proliferation of chondrocytes. However,
no significant effects of 50 µM curcumin treatment on survival and
migration of chondrocytes were identified in the present study
(data not shown), thereby indicating the safety of the curcumin
concentration employed.
A previous study demonstrated that multiple
signaling pathways are involved in the regulation of MMPs,
including the p38, extracellular signal-regulated kinase, c-Jun
N-terminal kinase, activator protein-1 and NF-κB signaling pathways
(31). However, it remains unclear
whether a single or numerous signaling pathways are involved in
this process. PDTC is an antioxidant that inhibits the degradation
of IκBα by blocking the de novo phosphorylation of IκBα,
thereby preventing NF-κB activation. The present study primarily
focused on the effects of curcumin on the NF-κB signaling pathway.
The results revealed that curcumin, in addition to PDTC, inhibited
IL-1β-induced IκBα phosphorylation and subsequent nuclear
translocation of p65/RelA, supporting the hypothesis that curcumin
may be an effective treatment for OA through the inhibition of
NF-κB signaling. The present results further revealed that curcumin
had similar effects as PDTC in decreasing the expression of MMP-13
and increasing the expression of type II collagen in
IL-1β-stimulated chondrocytes. Furthermore, co-treatment with
curcumin and PDTC did not cause further inhibition of MMP-13 or
upregulation of type II collagen compared with either treatment
alone, suggesting that the NF-κB signaling pathway may be the
primary molecular pathway for the effects of curcumin on
IL-1β-stimulated chondrocytes.
Acknowledgements
The present study was supported by the Science and
Technology Development Fund of BaoShan District (grant no. 13-E-5),
and the Medicine and Technology Cooperation Fund of Shanghai Jiao
Tong University (grant no. YG2014MS23).
References
|
1
|
Qin J, Shang L, Ping AS, Li J, Li XJ, Yu
H, Magdalou J, Chen LB and Wang H: TNF/TNFR signal transduction
pathway-mediated anti-apoptosis and anti-inflammatory effects of
sodium ferulate on IL-1β-induced rat osteoarthritis chondrocytes in
vitro. Arthritis Res Ther. 14:R2422012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shi D, Dai J, Xu Z, Chen D and Jiang Q:
Update on basic and clinical aspects of osteoarthritis. Ann Transl
Med. 3:1422015.PubMed/NCBI
|
|
3
|
Lugo JP, Saiyed ZM and Lane NE: Efficacy
and tolerability of an undenatured type II collagen supplement in
modulating knee osteoarthritis symptoms: A multicenter randomized,
double-blind, placebo-controlled study. Nutr J. 15:142016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kaneva MK, Kerrigan MJ, Grieco P, Curley
GP, Locke IC and Getting SJ: Chondroprotective and
anti-inflammatory role of melanocortin peptides in TNF-α activated
human C-20/A4 chondrocytes. Br J Pharmacol. 167:67–79. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mitchell PG, Magna HA, Reeves LM,
Lopresti-Morrow LL, Yocum SA, Rosner PJ, Geoghegan KF and Hambor
JE: Cloning, expression and type II collagenolytic activity of
matrix metalloproteinase-13 from human osteoarthritic cartilage. J
Clin Invest. 97:761–768. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Borden P, Solymar D, Sucharczuk A, Lindman
B, Cannon P and Heller RA: Cytokine control of interstitial
collagenase and collagenase-3 gene expression in human
chondrocytes. J Biol Chem. 271:23577–23581. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vermeij EA, Koenders MI, Blom AB, Arntz
OJ, Bennink MB, van den Berg WB, van Lent PL and van de Loo FA: In
vivo molecular imaging of cathepsin and matrix metalloproteinase
activity discriminates between arthritic and osteoarthritic
processes in mice. Mol Imaging. 13:1–10. 2014.PubMed/NCBI
|
|
8
|
Garcia S, Forteza J, López-Otin C,
Gómez-Reino JJ, González A and Conde C: Matrix metalloproteinase-8
deficiency increases joint inflammation and bone erosion in the
K/BxN serum-transfer arthritis model. Arthritis Res Ther.
12:R2242010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shakibaei M, Allaway D, Nebrich S and
Mobasheri A: Botanical extracts from rosehip (rosa canina), willow
bark (salix alba) and nettle leaf (urtica dioica) suppress
IL-1β-induced NF-κB activation in canine articular chondrocytes.
Evid Based Complement Alternat Med. 2012:5093832012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kong D, Zheng T, Zhang M, Wang D, Du S, Li
X, Fang J and Cao X: Static mechanical stress induces apoptosis in
rat endplate chondrocytes through MAPK and mitochondria-dependent
caspase activation signaling pathways. PLoS One. 8:e694032013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawai T and Akira S: Toll-like receptor
downstream signaling. Arthritis Res Ther. 7:12–19. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan C and Boyd DD: Regulation of matrix
metalloproteinase gene expression. J Cell Physiol. 211:19–26. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rowan AD and Young DA: Collagenase gene
regulation by pro-inflammatory cytokines in cartilage. Front
Biosci. 12:536–550. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Otero M and Goldring MB: Cells of the
synovium in rheumatoid arthritis. Chondrocytes. Arthritis Res Ther.
9:2202007. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dickinson DA, Moellering DR, Iles KE,
Patel RP, Levonen AL, Wigley A, Darley-Usmar VM and Forman HJ:
Cytoprotection against oxidative stress and the regulation of
glutathione synthesis. Biol Chem. 384:527–537. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Okada K, Wangpoengtrakul C, Tanaka T,
Toyokuni S, Uchida K and Osawa T: Curcumin and especially
tetrahydrocurcumin ameliorate oxidative stress-induced renal injury
in mice. J Nutr. 131:2090–2095. 2001.PubMed/NCBI
|
|
17
|
Brennan P and O'Neill LA: Inhibition of
nuclear factor kappaB by direct modification in whole
cells-mechanism of action of nordihydroguaiaritic acid, curcumin
and thiol modifiers. Biochem Pharmacol. 55:965–973. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shishodia S, Potdar P, Gairola CG and
Aggarwal BB: Curcumin (diferuloylmethane) down-regulates cigarette
smoke-induced NF-kappaB activation through inhibition of
IkappaBalpha kinase in human lung epithelial cells: Correlation
with suppression of COX-2, MMP-9 and cyclin D1. Carcinogenesis.
24:1269–1279. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Collett GP and Campbell FC: Overexpression
of p65/RelA potentiates curcumin-induced apoptosis in HCT116 human
colon cancer cells. Carcinogenesis. 27:1285–1291. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wollenhaupt J, Alten R, Burkhardt H,
Edelmann E, Gromnica-Ihle E, Krause A, Krüger K, Manger B, Lorenz
H, Müller-Ladner U, et al: Current therapeutic strategy for
rheumatoid arthritis. MMW Fortschr Med. 148:38–42; quiz 43.
2006.(In German). PubMed/NCBI
|
|
22
|
Schmidt MB, Chen EH and Lynch SE: A review
of the effects of insulin-like growth factor and platelet derived
growth factor on in vivo cartilage healing and repair.
Osteoarthritis Cartilage. 14:403–412. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brennan FM and McInnes IB: Evidence that
cytokines play a role in rheumatoid arthritis. J Clin Invest.
118:3537–3545. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang SN, Xie GP, Qin CH, Chen YR, Zhang
KR, Li X, Wu Q, Dong WQ, Yang J and Yu B: Aucubin prevents
interleukin-1 beta induced inflammation and cartilage matrix
degradation via inhibition of NF-κB signaling pathway in rat
articular chondrocytes. Int Immunopharmacol. 24:408–415. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tardif G, Reboul P, Pelletier JP and
Martel-Pelletier J: Ten years in the life of an enzyme: The story
of the human MMP-13 (collagenase-3). Mod Rheumatol. 14:197–204.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aigner T, Söder S, Gebhard PM, McAlinden A
and Haag J: Mechanisms of disease: Role of chondrocytes in the
pathogenesis of osteoarthritis-structure, chaos and senescence. Nat
Clin Pract Rheumatol. 3:391–399. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bharti AC, Donato N, Singh S and Aggarwal
BB: Curcumin (diferuloylmethane) down-regulates the constitutive
activation of nuclear factor-kappa B and IkappaBalpha kinase in
human multiple myeloma cells, leading to suppression of
proliferation and induction of apoptosis. Blood. 101:1053–1062.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scherer DC, Brockman JA, Chen Z, Maniatis
T and Ballard DW: Signal-induced degradation of I kappa B alpha
requires site-specific ubiquitination. Proc Natl Acad Sci USA.
92:11259–11263. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Clutterbuck AL, Allaway D, Harris P and
Mobasheri A: Curcumin reduces prostaglandin E2, matrix
metalloproteinase-3 and proteoglycan release in the secretome of
interleukin 1β-treated articular cartilage. F1000Res.
2:1472013.PubMed/NCBI
|
|
30
|
Clutterbuck AL, Mobasheri A, Shakibaei M,
Allaway D and Harris P: Interleukin-1beta-induced extracellular
matrix degradation and glycosaminoglycan release is inhibited by
curcumin in an explant model of cartilage inflammation. Ann N Y
Acad Sci. 1171:428–435. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liacini A, Sylvester J, Li WQ, Huang W,
Dehnade F, Ahmad M and Zafarullah M: Induction of matrix
metalloproteinase-13 gene expression by TNF-alpha is mediated by
MAP kinases, AP-1, and NF-kappaB transcription factors in articular
chondrocytes. Exp Cell Res. 288:208–217. 2003. View Article : Google Scholar : PubMed/NCBI
|