Introduction
Recurrent or residual cancer cells that persist
following treatment with anticancer drugs possess the
characteristics of cancer stem cells and an epithelial-mesenchymal
transition (EMT) phenotype (1).
Ovarian cancer is a major cause of gynecological cancer-associated
mortality worldwide (2). Although
radical surgery and platinum- and taxane-based adjuvant
chemotherapy can achieve progression-free survival in ~50% of
patients, the majority will relapse eventually (3). In ovarian cancer, tumor cells undergo
EMT, which is accompanied by invasive growth and metastasis
(4). Therefore, the inhibition of
EMT may have potential as a therapeutic strategy aiming to control
ovarian carcinogenesis and improve the outcome of anticancer
treatments.
Epidermal growth factor (EGF) -related growth
factors and their cognate receptors are widely recognized as key
autocrine regulators implicated in the control of cancer cell
proliferation, invasion and metastasis (5). EGF contributes to the post-ovulatory
proliferation of the ovarian surface epithelium, via binding to the
EGF receptor (EGFR) (6), and
aberrant EGF/EGFR expression has been reported in ovarian cancer
(7). Elevated EGFR levels have
been significantly correlated with aggressive disease
characteristics (8); however, the
molecular mechanisms underlying the actions of EGF in various types
of cancer cells and different clinical stages have yet to be
elucidated.
Cluster of differentiation (CD) 44 is the principal
transmembrane adhesion receptor for hyaluronan (HA) and serves a
central role in HA-mediated cellular migration, and cancer cell
invasion and metastasis (9).
HA-binding CD44 has been reported to decrease the expression of
E-cadherin, and increase the expression of Snail, vimentin and
N-cadherin (10). The interaction
between CD44 and EGFR has been implicated in CD44-mediated cellular
motility (11), as EGF has been
demonstrated to enhance CD44 cleavage and promote cellular
motility, through the activation of Rac (12). EGF/EGFR and CD44/HA binding are
events commonly associated with the mitogen-activated protein
kinase (MAPK)/extracellular signal-regulated kinase (ERK) signaling
pathway (13,14). Activation of the MAPK/ERK pathway
is common in malignancies and has been reported to drive the
proliferation, migration and invasion of cancer cells (15). However, the detailed effects, as
well as the interactions of EGF and CD44 during EMT regulation in
ovarian cancer have yet to be elucidated.
Sorafenib is a small molecule that inhibits the Raf
family of kinases (16), and
consequently, the MAPK/ERK pathway in cancer cells (17). The aim of the present study was to
investigate the mechanisms underlying the implication of the
EGF/EGFR and CD44/HA pathways in cellular migration and invasion
using the metastatic SK-OV-3 and the primary Caov-3 ovarian cancer
cell lines (18,19). In addition, sorafenib was used to
investigate the putative association between the EGF/EGFR and
CD44/HA signaling pathways during ovarian cancer metastasis and
invasion.
Materials and methods
Cell culture and reagents
The SK-OV-3 and Caov-3 human ovarian cancer cell
lines were obtained from American Type Culture Collection
(Manassas, VA, USA). Cells were maintained in Dulbecco's modified
Eagle's medium (DMEM; GE Healthcare Bio-Sciences, Pittsburgh, PA,
USA) supplemented with 10% heat-inactivated fetal bovine serum
(FBS; HyClone; GE Healthcare Bio-Sciences, Logan, UT, USA),
penicillin (100 U/ml) and streptomycin (100 µg/ml; GE Healthcare
Bio-Sciences), and were cultured at 37°C in a 5% CO2
atmosphere. Human recombinant EGF was purchased from BD Biosciences
(San Jose, CA, USA). HA was purchased from R&D Systems, Inc.
(Minneapolis, MN, USA). Sorafenib (BAY43-9006, Nexavar®) was
purchased from LC Laboratories (Woburn, MA, USA).
Flow cytometric analysis
For cell cycle analysis, SK-OV-3 and Caov-3 cells
were seeded in DMEM containing 10% FBS with penicillin (100 U/ml)
and streptomycin (100 µg/ml), at a density of
2×105/100-mm plate. Following overnight incubation,
cells were treated with EGF (5 µM) for 45 min and then cultured for
24 h. Cells were harvested, washed with PBS and fixed in 70%
ethanol in PBS at −20°C overnight. Cells were then resuspended in
PBS containing 40 µg/ml propidium iodide (PI; Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and 100 µg/ml RNase
(Invitrogen; Thermo Fisher Scientific, Inc.), and incubated for 30
min at 37°C in the dark. Cell cycle analysis was performed using a
FACSCalibur flow cytometer (BD Biosciences) equipped with
CellQuestpro software (version 5.1; BD Biosciences). To investigate
alterations in CD44 expression on the cell surface, Caov-3 or
SK-OV-3 cells were seeded at a density of 2×105/100-mm
plate and treated with EGF (5 µM) or EGF with sorafenib dissolved
in DMSO (5 µM) in DMEM containing 10% FBS. The cells were cultured
for 48 h at 37°C prior to analysis by flow cytometry. Cells were
then harvested and stained with an anti-CD44 fluorescein
isothiocyanate (FITC)-conjugated antibody (cat. no. #555478;
dilution, 1:50; BD Biosciences) for 30 min at 4°C in the dark.
Samples were analyzed using a FACSCalibur flow cytometer (BD
Biosciences) equipped with CellQuestpro software (version 5.1; BD
Biosciences).
Scratch wound healing assay
SK-OV-3 or Caov-3 cells were seeded into a 6-well
plate at a density of 3×105/2 ml in DMEM containing 10%
FBS. The confluent cell monolayers were wounded via scratching with
a 200-µl pipette tip and cells were incubated with EGF (5 µM) for
45 min at 37°C. After washing with PBS, EGF-treated SK-OV-3 or
Caov-3 cells were cultured in the presence or absence of sorafenib
(5 µM) for 24 h. Images of scratch wounds were captured (10x
objective) under an inverted phase contrast microscope following 24
h of culture. The number of cells that migrated into the scratched
area was analyzed using Image J v1.38 software (National Institutes
of Health, Bethesda, MD, USA). Each experiment was conducted in
triplicate.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from SK-OV-3 and Caov-3
cells, including the non-treated control, DMSO-treated,
EGF-treated, sorafenib-treated and EGF and sorafenib co-treated
groups, using TRIzol®, according to the manufacturer's protocol
(Invitrogen; Thermo Fisher Scientific, Inc.). The DMSO-treated
group was used as a control for the sorafenib-treated group.
Briefly, the RNA pellet extracted from each group was washed twice
with 1 ml 75% ethanol and then dried using a vacuum for 10 min at
room temperature. Pure RNA samples (2 µg; A260/A280 ratio >1.6)
were transcribed into cDNA using the AccuPower RT Premix (cat. no.
#K-2043; 10 mM dNTPs, 2.5 mM of each dNTP; final concentration, 0.5
mM; Bioneer Corporation, Daejeon, Korea), oligo (dT) (5 µM), RNase
inhibitor (cat. no. #2313A; 10 U/µl; Takara Bio, Inc., Otsu,
Japan), reverse transcriptase (100 U/µl; Bioneer Corporation) and
oligo-dT primers (cat. no. #N-7053; Bioneer Corporation). PCR was
performed according to the manufacturer's instructions using the
Prime Taq Premix with Prime Taq DNA Polymerase 1 unit/10 µl, 2X
reaction buffer, 4 mM MgCl2, enzyme stabilizer,
sediment, loading dye, pH 9.0 and 0.5 mM each of dATP, dCTP, dGTP,
dTTP (cat. no. #G-3002; GeNet Bio, Daejeon, Korea) with the
following primers and Takara PCR Thermal Cycler Dice (cat. no.
#TP600; Takara Bio, Inc.). Initially, the mixed RT product was
reacted at 95°C for 30 sec for denaturation and then the annealing
step was performed with specific primers: Vimentin (30 cycles at
60°C), sense GGA AGA GAA CTT TGC CGT TGA A, antisense GTG ACG AGC
CAT TTC CTC CTT; matrix metalloproteinase (MMP)-2 (30 cycles at
66°C), sense TGG CAA GTA CGG CTT CTG TC, antisense, TGG CAA GTA CGG
CTT CTG TC; MMP-9 (25 cycles at 65°C), sense TGC GCT ACC ACC TCG
AAC TT, antisense GAT GCC AT TGA CGT CGT CCT; B-Raf (30 cycles at
58°C), sense TGG GGA ACG GAA CTG ATT TTT C, antisense TTT TGT GGT
GAC TTG GGG TTG; hyaluronan synthase (HAS) 1 (30 cycles at 60°C),
sense TAC AAC CAG AAG TTC CTG GG, antisense CTG GAG GTG TAC TTG GTA
GC; HAS2 (30 cycles at 60°C), sense GTG GAT TAT GTA CAG GTT TGT GA,
antisense TCC AAC CAT GGG ATC TTC TT; HAS3 (30 cycles at 60°C),
sense GAG ATG TCC AGA TCC TCA ACA A, antisense CCC ACT AAT ACA CTG
CAC AC; and β-actin (25 cycles at 60°C), sense ATC CAC GAA ACT ACC
TTC AA and antisense ATC CAC ACG GAG TAC TTG C. Extension steps
were performed at 72°C for 1 min and the final elongation step was
at 72°C for 5 min. PCR products were analyzed by agarose gel (1%)
electrophoresis and visualized using ethidium bromide (Sigma
Aldrich; Merck KGaA, Darmstadt, Germany) under ultraviolet light
using the multiple Gel DOC system (Fujifilm Corporation, Tokyo,
Japan). Semi-quantitative analysis of PCR products was performed
using Image J v1.38 software (National Institutes of Health,
Bethesda, MD, USA). Each experiment was performed at least three
times and the results are representative of three independent
experiments.
Western blot analysis
The SK-OV-3 or Caov-3 cells, including the
non-treated control, DMSO-treated, EGF-treated, sorafenib-treated
and EGF and sorafenib co-treated groups, were harvested, lysed in
NP-40 cell lysis buffer (Elpis Biotech, Inc., Daejeon, Korea), and
total protein was extracted. The DMSO-treated group was used as a
control for the sorafenib-treated group. To address phosphorylation
events, an additional set of phosphatase inhibitors (Cocktail II;
Sigma-Aldrich; Merck KGaA) was added to the NP-40 buffer. Protein
concentration was determined using a bicinchoninic assay kit
according to the manufacturer's instructions (Pierce; Thermo Fisher
Scientific, Inc.). The same volume of 2X Laemmli sample buffer
(Elpis Biotech, Inc.) was added to each lysate, and protein (10
µg/sample) was immediately boiled for 5 min at 100°C. Equal
quantities of protein (10 µg/lane) were separated using 8 to 12%
SDS-PAGE at 100 V for 1 h, then transferred to nitrocellulose
membranes (Merck Millipore; Merck KGaA) at 340 mA for 2 h.
Following blocking with 5% non-fat skim milk for 1 h at room
temperature, primary antibodies against the following proteins were
used: EGFR (cat. no. #2232; dilution, 1:1,000), MMP-2 (cat. no.
#4022; dilution, 1:1,000), MMP-9 (cat. no. #3852; dilution,
1:1,000), E-cadherin (cat. no. #3195; dilution, 1:1,000),
N-cadherin (cat. no. #13116; dilution, 1:1,000), vimentin (cat. no.
#5741; dilution, 1:1,000), p50 (cat. no. #3035; dilution,
1,000)/p65 (cat. no. #8242; dilution, 1,000) subunits of nuclear
factor (NF)-κB, phosphorylated (p)-p38 (Thr180/Tyr182; cat. no.
#9211; dilution, 1:1,000), total p38 (cat. no. #9212; dilution,
1:1,000), p-c-Jun N-terminal kinase (JNK) (Thr183/Tyr185; cat. no.
#4671; dilution, 1:1,000), total JNK (cat. no. #9258; dilution,
1:1,000) and β-actin (cat. no. #4967; dilution, 1:2,000); all
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
CD44 (cat. no. #sc-53298; dilution, 1:500), CD147 (cat. no.
#sc-13976; dilution, 1:500), phosphatidylinositol-4,5-bisphosphate
3-kinase (PI3K) (cat. no. #sc-1637; dilution, 1:200), p-Akt
(Ser473; cat. no. #sc-33437; dilution, 1:200), total Akt (cat. no.
#sc-81434; dilution, 1:200), p-MAPK/ERK kinase (MEK; Ser218/Ser222;
cat. no. #sc-7995; dilution, 1:500), total MEK (cat. no. #sc-436;
dilution, 1:500), p-ERK (Thr202/Tyr204; cat. no. #sc-7383;
dilution, 1:500), total ERK (cat. no. #sc-94; dilution, 1:500), pan
Ras (cat. no. #sc-166691; dilution, 1:200) and B-Raf (cat. no.
#sc-136263; dilution, 1:200) were all purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The membrane was probed with
primary antibodies overnight at 4°C, followed by the following
specific secondary antibodies: Goat anti-mouse-horseradish
peroxidase (HRP; cat. no. #K0211589; dilution, 1:3,000) or goat
anti-rabbit-HRP (cat. no. #K0211708; dilution, 1:3,000; both
KOMABiotech, Seoul, Korea) were incubated for 1 h at room
temperature. The protein bands were visualized using an enhanced
chemiluminescence detection kit (Advansta Inc., Menlo Park, CA,
USA) and the multiple Gel DOC system (Fujifilm Corporation).
β-actin was used as a loading control. The intensities of protein
bands were normalized to those of β-actin and semi-quantified using
Image J v1.38 software (National Institutes of Health).
Immunofluorescence and confocal
microscopy
Caov-3 cells were seeded at a density of
3×105/ml and treated with EGF (5 µM) or co-treated with
EGF (5 µM) and HA (50 µg/ml). Cells were washed with PBS, fixed in
4% methanol-free formaldehyde (pH 7.4) at 4°C for 25 min, followed
by blocking with 0.5% Tween-20 in 5% bovine serum albumin (Sigma
Aldrich; Merck KGaA) in PBS for 1 h at room temperature. The cells
were subsequently incubated with anti-N-cadherin antibody (cat. no.
#sc-7939; dilution, 1: 500; Santa Cruz Biotechnology, Inc.) for 24
h at room temperature, washed twice with PBS and incubated with
FITC-labeled secondary antibody (cat. no. #F0382; dilution, 1:80;
Sigma-Aldrich; Merck KGaA) for 1 h at room temperature. PI was used
as a nuclear stain. Confocal images were obtained using a Zeiss
LSM510 confocal microscope and analyzed with the Software Release
v2.5 Service Pack 2 for LSM 510 (Carl Zeiss AG, Oberkochen,
Germany).
Small interfering (si)RNA
transfection
Experimentally verified human CD44-siRNA duplex
(5′-AUGUCUUCAGGAUUCGUUCUU-3′) and negative control-siRNA
(5′-ACGUGACACGUUCGGAGAATT-3′) were obtained from Bioneer
Corporation. Experimentally verified human EGFR-siRNA duplex was
purchased from Santa Cruz Biotechnology, Inc. (cat. no. #sc-29301).
Cells were seeded at a density of 3×105/well in a 6-well
plate and incubated overnight prior to transfection with 200 nM
siRNA using Lipofectamine® RNAiMAX Reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Following transfection with the specific siRNA, the expression
levels of CD44 or EGFR were determined by western blot analysis.
The cells were used for the scratch wound healing assay and
immunoblotting against EGFR, CD44, Ras, B-Raf, p-MAPK/ERK and MEK
as described above, to analyze migratory activity and the signaling
pathways involved at 48 h post-transfection.
Statistical analysis
The statistical significance of the difference
between non-treated control cells and EGF or EGF with HA-treated
cells was assessed by one-way analysis of variance (ANOVA) using
SigmaPlot software (version 10.0; Systat Software, Inc., San Jose,
CA, USA). Bonferroni post hoc analysis was performed following the
ANOVA for multiple comparisons. Data are expressed as the mean ±
standard error of the mean and each value is representative of at
least two independent experiments. P<0.05 was considered to
indicate a statistically significant difference.
Results
EGF stimulates migratory activity and
mesenchymal characteristics of ovarian cancer cells
To examine the effects of EGF on ovarian cancer
cells, Caov-3 cells, derived from human ovarian primary
adenocarcinoma, and SK-OV-3 cells, derived from ovarian cancer
malignant ascites, were used (18,19).
Following EGF stimulation, the population of ovarian cancer cells
with spindle-shaped morphology was increased; however, pretreatment
with sorafenib resulted in the maintenance of epithelial cellular
characteristics following EGF activation (Fig. 1A). The effects of sorafenib on the
migratory capabilities of ovarian cancer cells were examined using
a wound healing assay following EGF treatment for 45 min, followed
by 24 h incubation in the presence of sorafenib. The motility of
cancer cells was assessed according to the distance traveled into a
scratched area. Sorafenib inhibited the migratory activity of
EGF-treated SK-OV-3 and Caov-3 cells (Fig. 1B and C). To examine whether the
effects of EGF on cancer cell proliferation differed in the two
cell types, cell cycle analysis was performed using PI staining.
Although Caov-3 cells exhibited a slight increase in the percentage
of cells in the S phase following EGF treatment, the proportion of
SK-OV-3 cells in the S and G2/M phases was markedly
increased following stimulation with EGF (Fig. 1D). These results suggested that EGF
may potentiate the invasive capabilities of ovarian cancer cells,
and may employ different signaling mechanisms in each cell
line.
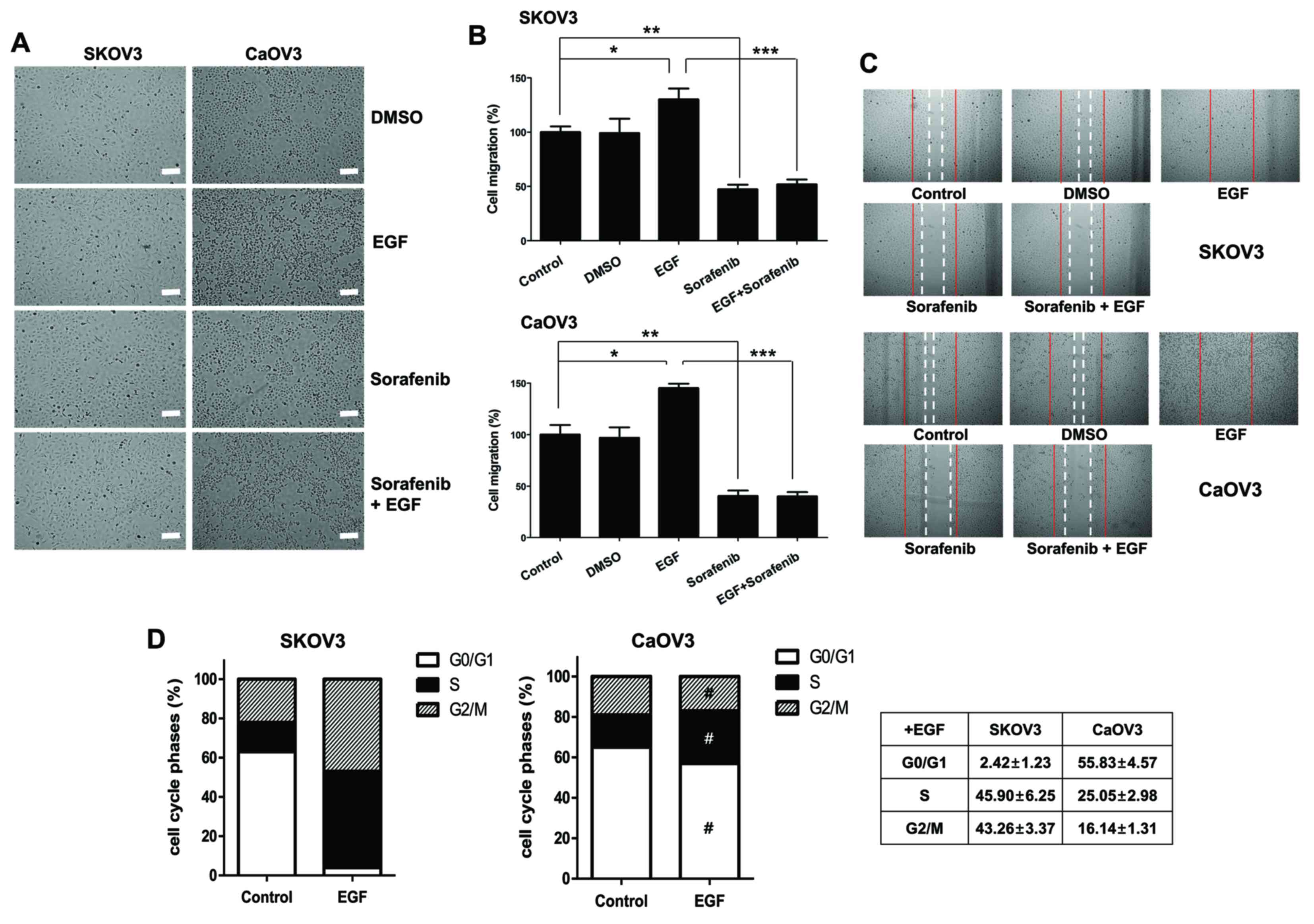 | Figure 1.Effects of EGF on cellular migration
and cell cycle distribution in SK-OV-3 and Caov-3 cells. (A)
Spindle-shaped morphological alterations were induced by EGF
treatment. Cell morphology was observed under an inverted phase
contrast microscope. Photomicrographs were captured at
magnification, ×100 using a digital camera. Scale bar, 100 µm. (B)
Cellular motility of EGF-activated cells was measured using a wound
healing assay. The migrated distance into the scratched area was
calculated and plotted as a percentage of migration. (C) SK-OV-3
and Caov-3 cells were treated with EGF, sorafenib or EGF combined
with sorafenib for 24 h. Migratory capabilities were analyzed using
a wound healing assay. The images shown are representative of three
independent experiments. (D) Effects of EGF on cell cycle
distribution, as measured using propidium iodide staining and flow
cytometric analysis. Graphs are representative of three independent
experiments. Data are expressed as the mean ± standard error of the
mean. *P<0.05, Control vs. EGF groups; **P<0.01, Control vs.
Sorafenib groups; ***P<0.001, EGF group vs. Sorafenib + EGF
groups; #P<0.01, EGF-treated SK-OV-3 cells vs.
EGF-treated Caov-3 cells. EGF, epidermal growth factor; DMSO,
dimethyl sulfoxide. |
EGF stimulation enhances the
expression of CD44 and CD147 in Caov-3 cells
The effects of EGF stimulation on the mesenchymal
properties of the two cancer cell lines were investigated. The mRNA
and protein expression levels of vimentin, MMP-2 and MMP-9 were
markedly upregulated in EGF-treated SK-OV-3 and Caov-3 cells
(Fig. 2A and B). Conversely,
sorafenib inhibited the expression of the mesenchymal marker
N-cadherin and enhanced the expression of the epithelial marker
E-cadherin (Fig. 2C). Surface EGFR
was upregulated following EGF treatment in SK-OV-3 and Caov-3
cells. Notably, EGF appeared to exert no influence on CD44 and
CD147 protein levels in SK-OV-3 cells, whereas Caov-3 cells
exhibited increased CD44 and CD147 levels following EGF treatment
(Fig. 2C). Alterations in CD44
surface expression in EGF-activated cancer cells were also examined
using flow cytometry. CD44 levels were upregulated by ~40% in
SK-OV-3 cells and by 2.4-fold in Caov-3 cells following treatment
with EGF (Fig. 2D). Sorafenib
reduced the expression of EGFR, CD44, CD147 and the p50/p65 NF-κB
subunits (Fig. 2C). These results
suggested that ovarian cancer cell motility could be differentially
controlled by EGF/EGFR signaling in a cell type-specific manner;
however, sorafenib appeared to interfere in EMT processes in both
cancer cell types.
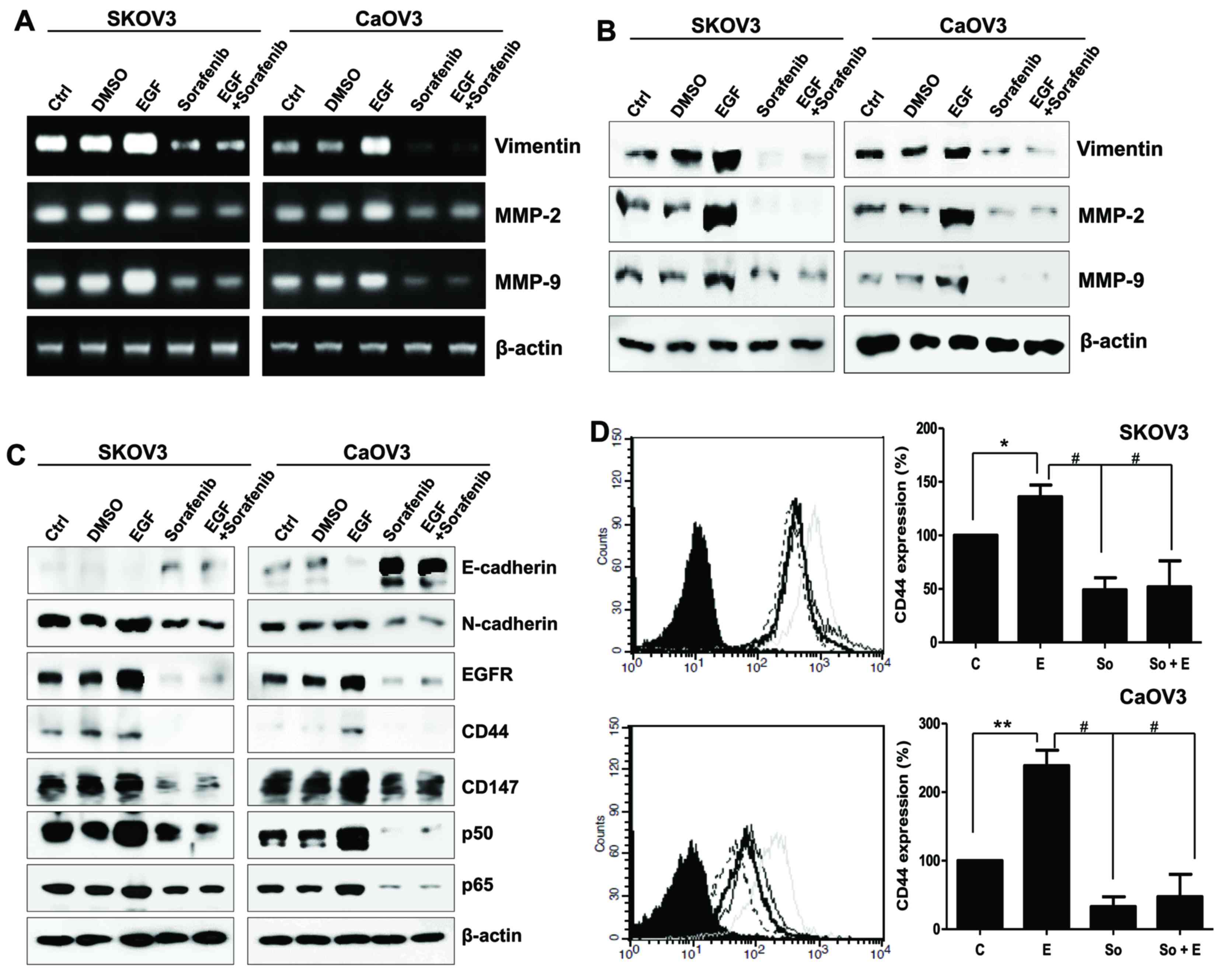 | Figure 2.Mesenchymal phenotype of SK-OV-3 and
Caov-3 cells elicited by EGF stimulation. (A) mRNA and (B) protein
expression levels of vimentin, MMP-2 and MMP-9 in EGF-treated
ovarian cancer cells. (C) E-cadherin, N-cadherin, EGFR, CD44, CD147
and p50/p65 NF-κB subunit expression was assessed using western
blot analysis in EGF-stimulated SK-OV-3 and Caov-3 cells. β-actin
served as an internal control. (D) Flow cytometric analysis
revealed the alterations in CD44 expression following treatment
with EGF or EGF and sorafenib in ovarian cancer cells. C, thin
black line; E, gray line; So, dotted-line; E + So, thick black
line. Data are expressed as the mean ± standard error of the mean.
*P<0.05 and **P<0.01, EGF group vs. Control group;
#P<0.01, EGF group or EGF-treated group vs.
Sorafenib. C, control group; D, DMSO group; E, EGF-treated group;
So, sorafenib-treated group; So + E, sorafenib- and EGF-treated
group; EGF, epidermal growth factor; MMP, matrix metalloproteinase;
EGFR, EGF receptor; CD, cluster of differentiation; NF, nuclear
factor. |
Sorafenib regulates the motility of
EGF-activated Caov-3 cells through the activation of HAS2
EGF-activated Caov-3 cells exhibited enhanced CD147
expression (Fig. 2). CD147 is a
multifunctional glycoprotein located in the cell membrane, which is
highly expressed on cancer cells (20). CD147 has been reported to induce
synthesis of the large extracellular polysaccharide HA, the main
ligand for the cell-surface receptor CD44 (21). CD147-induced CD44/HA interactions
have been implicated in various signaling pathways and may
potentiate tumorigenic properties in various cancer cells (22). The present study investigated the
effects of EGF on HA production in ovarian cancer cells. The mRNA
expression levels of the hyaluronan synthases HAS1, HAS2 and HAS3
were determined using RT-PCR. HAS1 mRNA expression levels appeared
unaffected in SK-OV-3 and Caov-3 cells regardless of the treatment
applied, whereas HAS3 mRNA levels decreased following treatment
with sorafenib (Fig. 3A). Notably,
HAS2 mRNA levels were markedly upregulated following EGF
stimulation, whereas this effect was abolished by sorafenib in
Caov-3 cells (Fig. 3A). Therefore,
the present study examined whether upregulated CD44 may trigger
cell migration more effectively via ligation with HA. HA treatment
appeared to slightly increase the migratory activity of
non-EGF-activated SK-OV-3 cells; however, co-treatment with EGF and
HA had no additional effect on cellular motility (Fig. 3B). The invasive activity of Caov-3
cells was significantly enhanced following combined treatment with
EGF and HA (Fig. 3C). These
results suggested that CD44 stimulation, acting via a HA-HAS2
mediated pathway, may potentiate the EGF-induced migration and
invasion of Caov-3 cells.
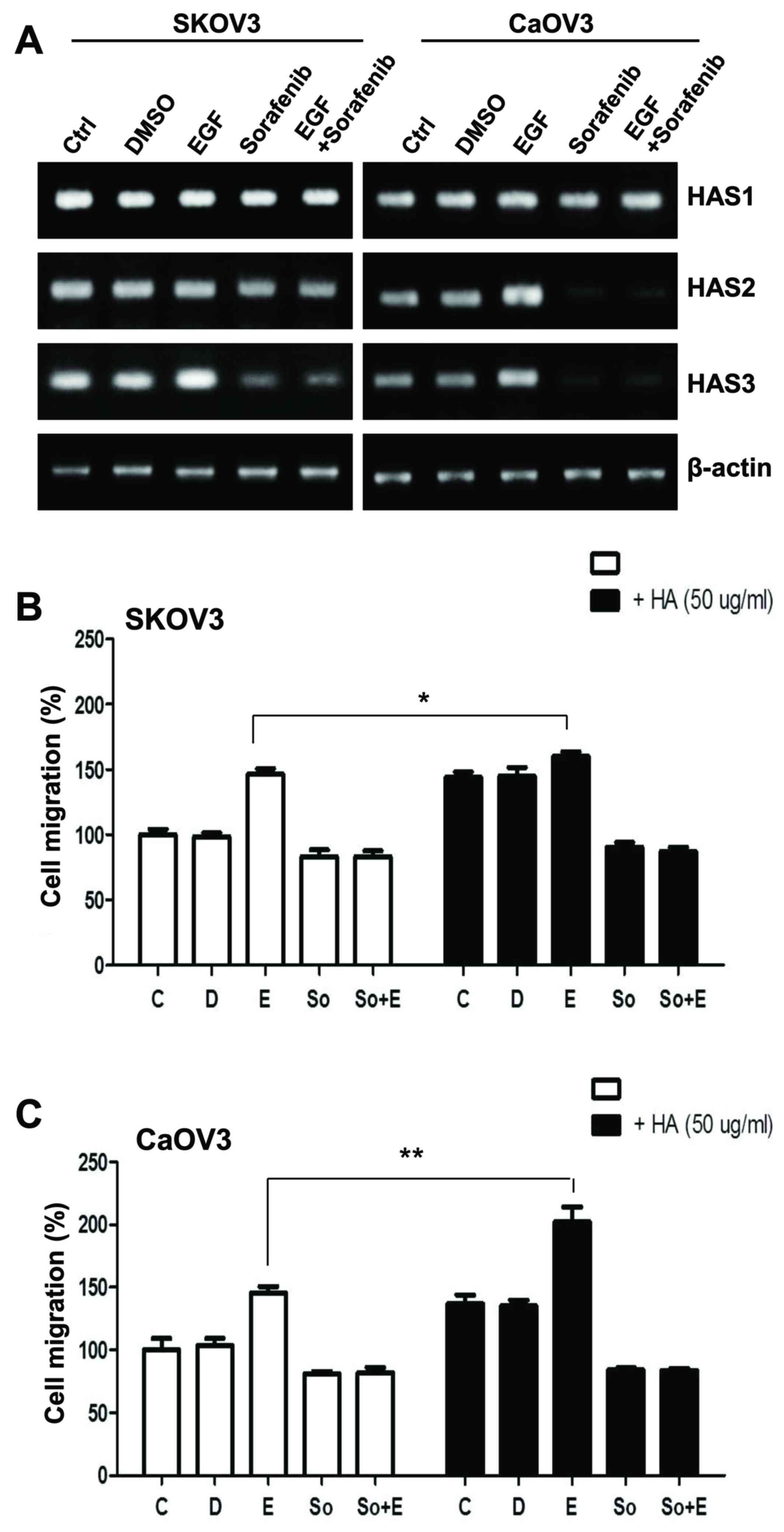 | Figure 3.Effects of HA on the migratory
activity of EGF-activated ovarian cancer cells. (A) mRNA expression
levels of HAS1, HAS2 and HAS3 in EGF-treated SK-OV-3 and Caov-3
cells were assessed using reverse transcription-polymerase chain
reaction. (B and C) Migratory capabilities of EGF-stimulated
ovarian cancer cells were calculated and plotted as percentage of
migration following treatment with HA. Data are expressed as the
mean ± standard error of the mean. *P<0.05, **P<0.01. C,
control group; D, DMSO group; E, EGF-treated group; So,
sorafenib-treated group; So + E, sorafenib- and EGF-treated group;
HA, hyaluronan; EGF, epidermal growth factor; HAS, HA synthase. |
CD44/HA-dependent MAPK/ERK pathway
controls the migration and invasion of Caov-3 cells
EGF/EGFR signaling varied between SK-OV-3 and Caov-3
cells, and involved HAS activation and HA-mediated cellular
migration, whereas sorafenib markedly inhibited the migratory
capabilities of EGF- and HA-stimulated ovarian cancer cells
(Fig. 3B and C). The molecular
mechanism underlying the actions of sorafenib on cellular migration
was investigated in SK-OV-3 and Caov-3 cells. Sorafenib has been
reported to target the Raf-1 and B-Raf kinases that form part of
the MAPK/ERK pathway (16). B-Raf
mRNA expression levels in EGF-stimulated SK-OV-3 cells appeared to
be increased, along with protein levels of the Raf-related
signaling molecules Ras and p-MEK, i.e., activated MEK (Fig. 4A and B). Furthermore, EGF treatment
suppressed the mRNA expression levels of B-Raf in Caov-3 cells
(Fig. 4A); the EGF-activated
MAPK/ERK signaling pathway also appeared to be inhibited in Caov-3
cells (Fig. 4B). In addition,
p-MAPKs, including p38, ERK and JNK, were downregulated in
EGF-stimulated Caov-3, but not SK-OV-3, cells (Fig. 4C). Although the expression of Raf
in EGF-stimulated Caov-3 cells appeared downregulated, sorafenib
effectively prevented cellular migration and the appearance of
mesenchymal phenotype.
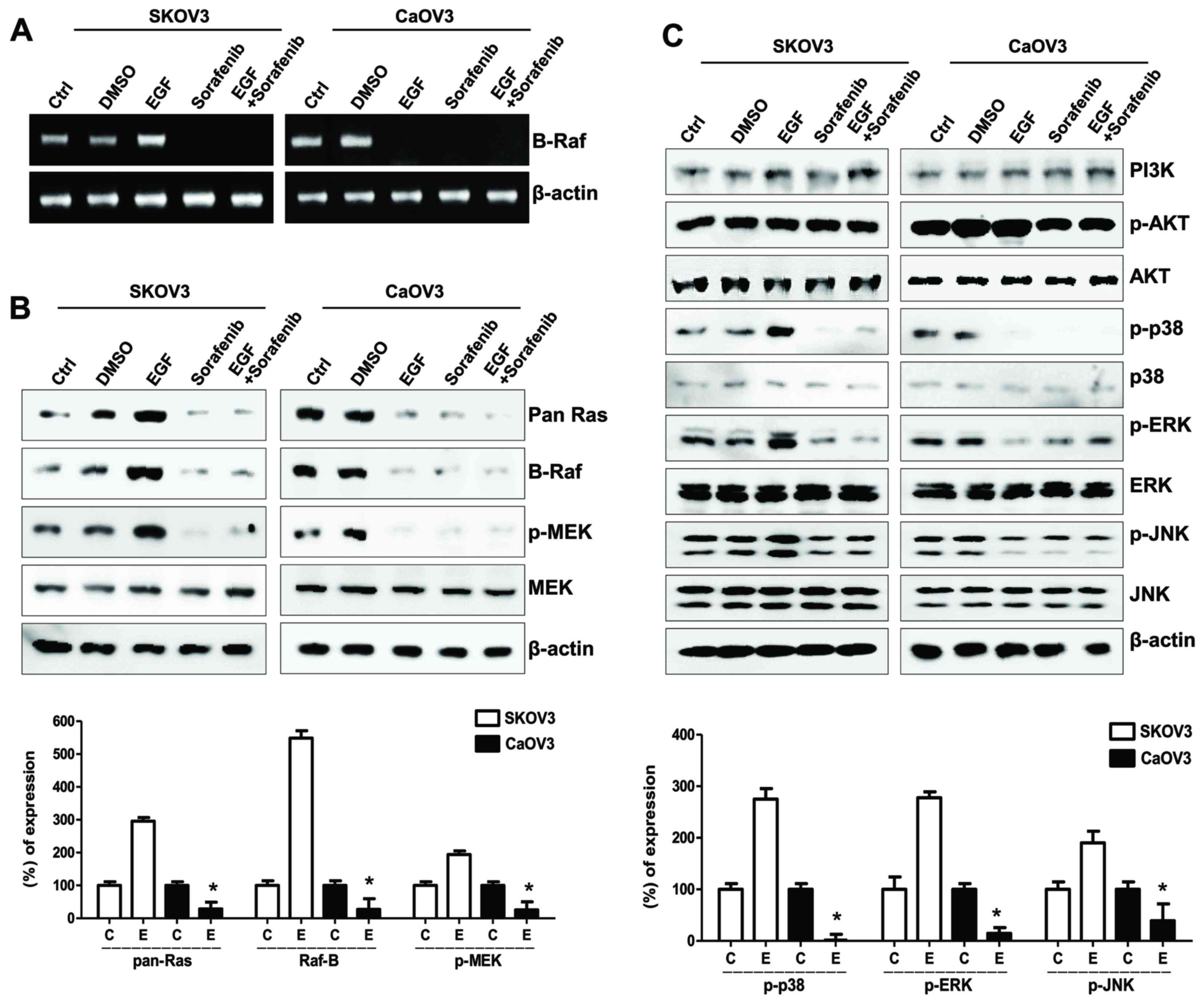 | Figure 4.Effects of sorafenib on the MAPK/ERK
signaling pathway in ovarian cancer cells following EGF
stimulation. (A) Reverse transcription-polymerase chain reaction
analysis of B-Raf mRNA expression levels in EGF-treated ovarian
cancer cells. (B) Western blot analysis of Ras, Raf, p- and total
MEK protein expression levels in EGF-treated ovarian cancer cells.
β-actin served as the loading control. (C) Western blot analysis of
EGF-related signaling molecules, including PI3K, p-Akt, total Akt,
p-p38, total p38, p-ERK, total ERK, p-JNK and total JNK protein
expression levels. Densitometry values of each MAPK and MEK protein
were normalized to the total MAPK and MEK protein in the same
sample. Densitometry values of Ras and Raf proteins were normalized
to β-actin in the same sample. Data are expressed as the mean ±
standard error of the mean. *P<0.05 EGF-stimulated Caov-3 vs.
SK-OV-3 cells. C, control group; D, DMSO group; E, EGF-treated
group; So, sorafenib-treated group; So + E, sorafenib- and
EGF-treated group; MAPK, mitogen-activated protein kinase; ERK,
extracellular signal-regulated kinase; EGF, epidermal growth
factor; p-, phosphorylated; MEK, MAPK/ERK kinase; PI3K,
phosphatidylinositol-4,5-bisphosphate 3-kinase; JNK, c-Jun
N-terminal kinase. |
To identify the mechanism underlying Caov-3
migration following treatment with EGF, the effects of HA treatment
on the MAPK/ERK signaling pathway were examined in EGF-activated
Caov-3 cells. The combined treatment of SK-OV-3 cells with EGF and
HA exerted no significant effect on the MAPK/ERK signaling pathway,
compared with in cells treated with EGF alone (Fig. 5A). Conversely, the EGF/HA combined
treatment considerably enhanced the expression of MAPK/ERK kinases
in Caov-3 cells compared with in cells treated with EGF alone. In
addition, treatment of EGF/HA-co-stimulated Caov-3 cells with
sorafenib attenuated activation of the MAPK/ERK signaling pathway
(Fig. 5B). These results suggested
that CD44 stimulation by HA may be associated with the MAPK/ERK
signaling pathway in EGF-stimulated Caov-3 cells.
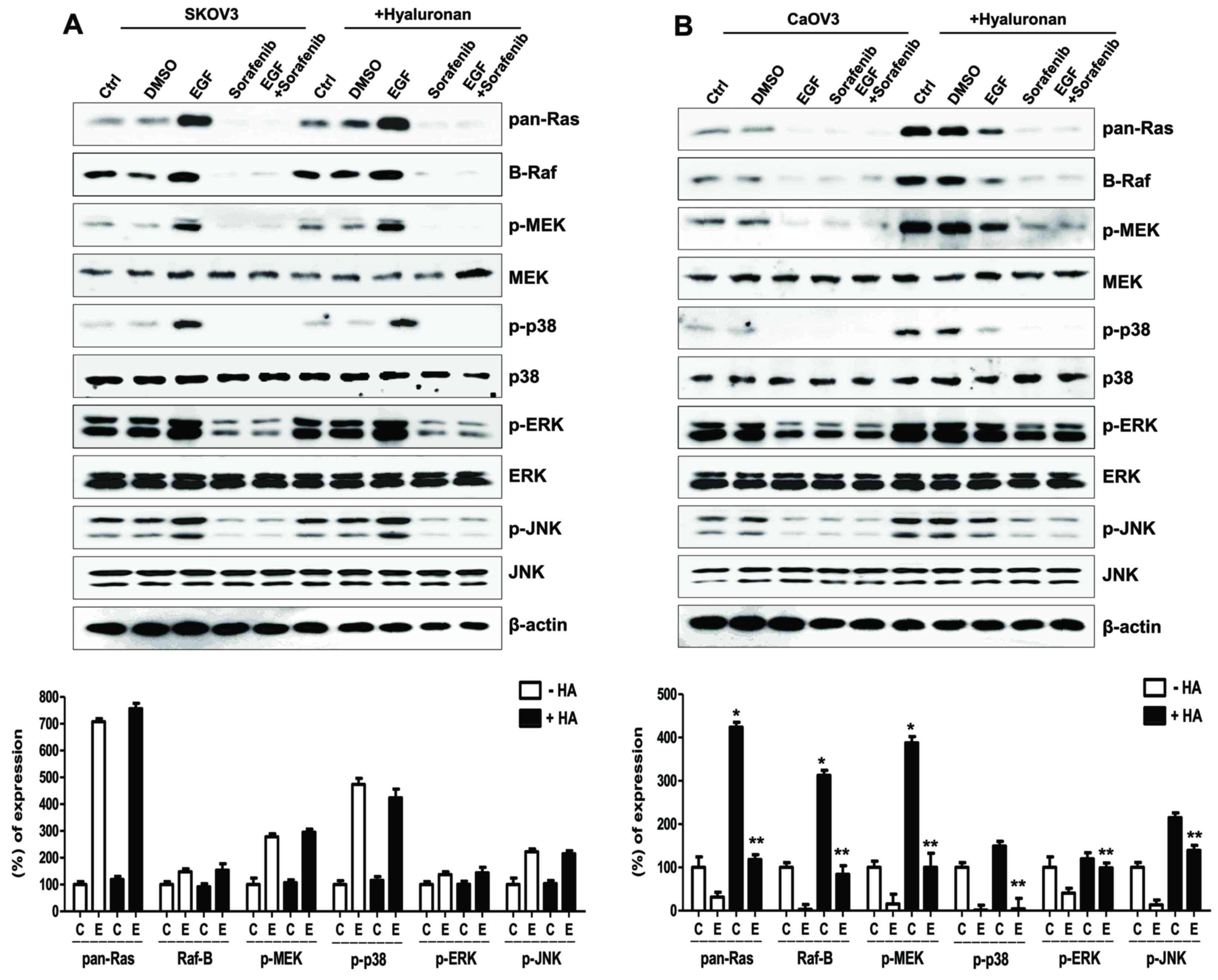 | Figure 5.Effects of HA on the MAPK/ERK
signaling pathway in EGF-activated ovarian cancer cells. Expression
of Ras/Raf/MEK signaling molecules and sequential activation of
MAPK following treatment with HA in EGF-activated (A) SK-OV-3 and
(B) Caov-3 cells. Densitometry values of MAPK and MEK proteins were
normalized to the total MAPK and MEK protein in the same sample.
Densitometry values of Ras and Raf proteins were normalized to
β-actin in the same sample. Data are expressed as the mean ±
standard error of the mean. ***P<0.001 vs. non-HA-treated C
group; #P<0.05 vs. non-HA-treated E group. C, control
group; D, DMSO group; E, EGF-treated group; So, sorafenib-treated
group; So + E, sorafenib- and EGF-treated group; HA, hyaluronan;
MAPK, mitogen-activated protein kinase; ERK, extracellular
signal-regulated kinase; EGF, epidermal growth factor; MEK,
MAPK/ERK kinase; JNK, c-Jun N-terminal kinase; p-,
phosphorylated. |
EGF/HA combined treatment potentiates
the mesenchymal properties of ovarian cancer cells
The effects of HA treatment on the expression of
mesenchymal properties in EGF-stimulated SK-OV-3 and Caov-3 cells
were examined. Stimulation with HA enhanced the expression of the
mesenchymal markers N-cadherin and vimentin, and of the p50/p65
NF-κB subunits, whereas it inhibited the expression of the
epithelial marker E-cadherin in EGF-activated SK-OV-3 and Caov-3
cells (Fig. 6A). There were no
significant differences in the expression of signaling molecules
between EGF-treated, and EGF and HA-treated SK-OV-3 cells (Fig. 5A). In addition, EGF treatment
combined with HA activated the MAPK/ERK signaling pathway in Caov-3
cells when compared with the group stimulated with EGF alone
(Fig. 5B). Therefore, the present
study examined whether combined treatment of EGF with HA enhanced
the expression of mesenchymal markers in Caov-3. Immunofluorescence
was used to observe the increased N-cadherin levels in EGF-treated
Caov-3 cells following HA treatment, compared with cells treated
with EGF alone. Conversely, the expression of N-cadherin was
downregulated following treatment with sorafenib in HA-treated and
non-treated EGF-activated SK-OV-3 and Caov-3 cells (Fig. 6A and B). These results suggested
that CD44/HA activation may be implicated in EMT induction in
EGF-activated ovarian cancer cells.
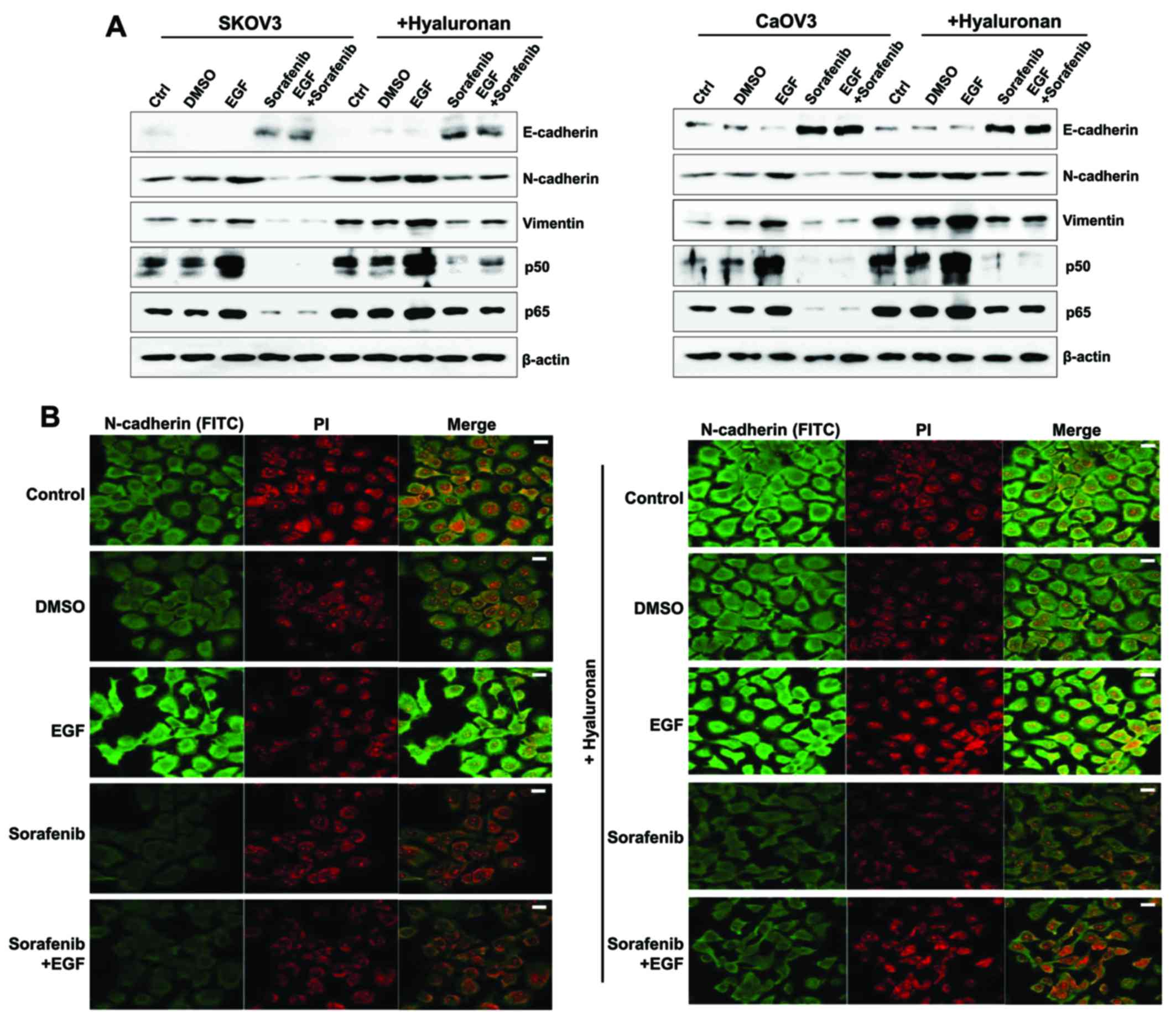 | Figure 6.Mesenchymal phenotype enhancement in
EGF-treated ovarian cancer cells following HA stimulation. (A)
Western blot analysis was used to assess protein expression levels
of E-cadherin, N-cadherin, vimentin and the p50/p65 NF-κB subunits.
β-actin served as the loading control. (B) Immunofluorescence was
used to visualize Caov-3 cells. Photomicrographs were captured
using a confocal microscope under ×200 magnification. The treated
cells were incubated with an anti-N-cadherin antibody for 24 h,
which was followed by incubation with a FITC-labeled secondary
antibody (shown in green). The nucleus was stained with PI and is
presented in red. Scale bars, 20 µm. The results are representative
of three independent experiments. C, control group; D, DMSO group;
E, EGF-treated group; So, sorafenib-treated group; So + E,
sorafenib- and EGF-treated group; EGF, epidermal growth factor; HA,
hyaluronan; NF, nuclear factor; FITC, fluorescein isothiocyanate;
PI, propidium iodide. |
EGFR in SK-OV-3 and CD44 in Caov-3
cells promote EMT through activation of the MAPK/ERK pathway
The roles of EGF in SK-OV-3 and of CD44 in Caov-3
cells in activation of the MAPK/ERK signaling pathway were
investigated during EMT. EGFR silencing via RNA interference
resulted in a decrease in the migratory activity of SK-OV-3 cells
compared with EGFR-expressing cells following treatment with EGF
(Fig. 7A). In addition, EGFR
silencing abolished the expression of MAPK/ERK kinases in SK-OV-3
cells (Fig. 7B). Treatment with a
combination of EGF and HA increased the migratory activity of
Caov-3 cells compared with in cells treated with EGF alone
(Fig. 7C). Furthermore, CD44
silencing in Caov-3 cells resulted in reduced migratory
capabilities compared with in control cells following EGF
stimulation (Fig. 7C). Treatment
with HA or HA combined with EGF upregulated the expression of CD44
and MAPK/ERK kinases in Caov-3 cells; however, CD44 silencing
inhibited the expression of MAPK/ERK kinases in Caov-3 cells
following co-stimulation with EGF and HA (Fig. 7D). These results suggested that
MAPK/ERK signaling may be initiated by various molecules in a cell
type-dependent manner.
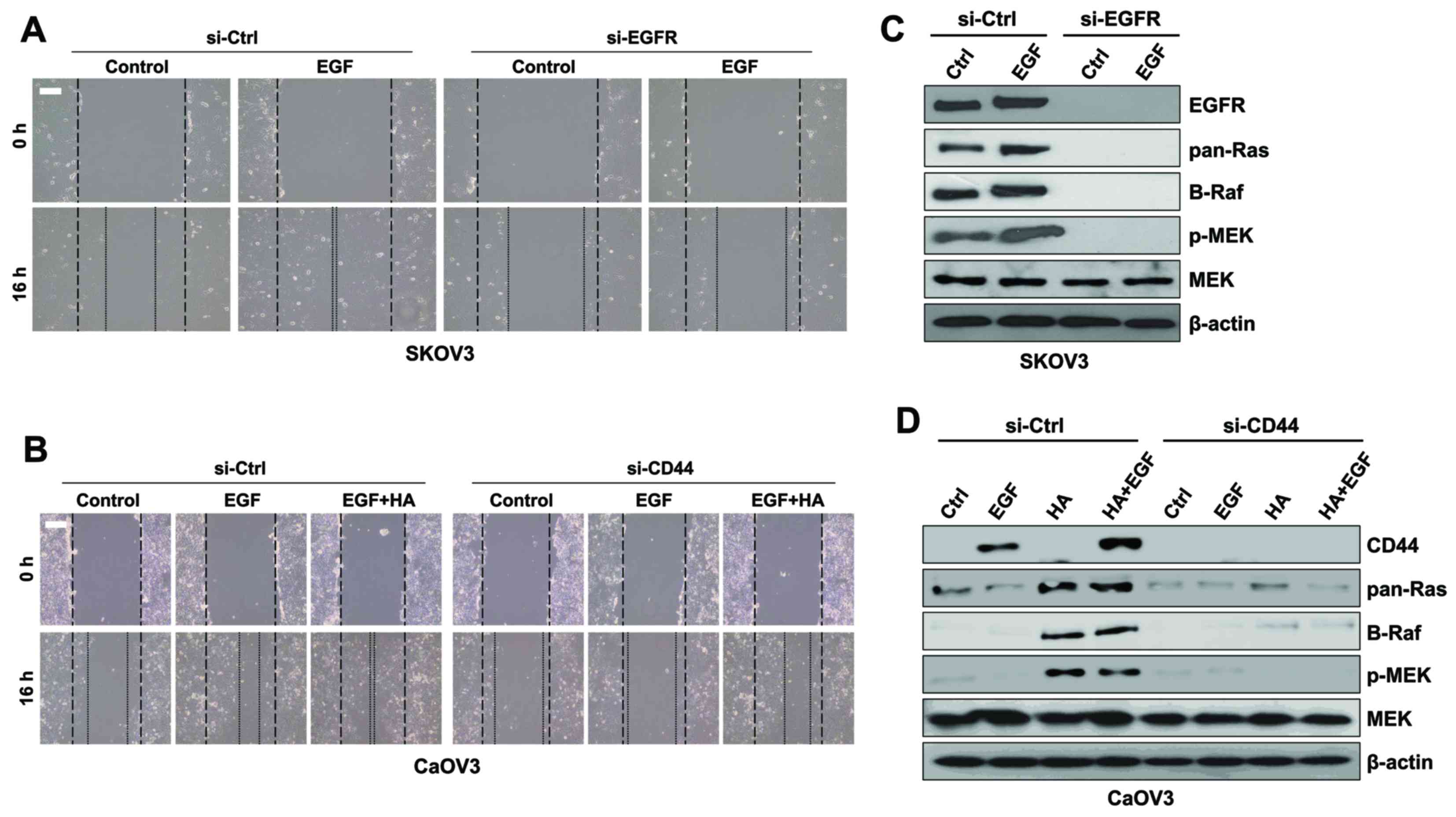 | Figure 7.Downregulation of EGFR and CD44 with
siRNA inhibited the migration of ovarian cancer cells. (A) SK-OV-3
cells were treated with si-EGFR or si-Ctrl and EGF for 16 h.
Migratory capabilities were analyzed using a wound healing assay.
(B) Protein expression levels of Ras, Raf, p-MEK and total MEK were
assessed using western blot analysis in SK-OV-3 cells. (C) Caov-3
cells were treated with si-CD44 or si-Ctrl and EGF or EGF combined
with HA for 16 h. Migratory capabilities were analyzed using a
wound healing assay. (D) Protein expression levels of Ras, Raf,
p-MEK and total MEK were assessed using western blot analysis in
Caov-3 cells. β-actin served as the loading control.
Photomicrographs were captured at ×100 magnification using a
digital camera under an inverted phase-contrast microscope. Scale
bars, 100 µm. Results are representative of three independent
experiments. EGF, epidermal growth factor; EGFR, EGF receptor; CD,
cluster of differentiation; si, small interfering; Ctrl, control;
p-, phosphorylated; MEK, mitogen-activated protein
kinase/extracellular signal-regulated kinase kinase; HA,
hyaluronan. |
Discussion
EMT is characterized by the loss of epithelial cell
polarity and the acquisition of migratory mesenchymal properties
(1).
CD44high/EGFRlow cell subpopulations in head
and neck squamous cell carcinoma have been reported to exhibit a
spindle-shaped EMT-like morphology and were resistant to anticancer
therapy (23). In addition, it has
been reported that CD44 promoted the MMP-dependent activation of
EGFR and the EGFR-dependent migration of fibroblasts (24). Tumor-specific HA accumulation has
been observed in several types of human cancer, including colon
(25) and breast (26). HA has been demonstrated to interact
with CD44 to regulate cellular proliferation and motility (27) and to induce MMP-9 secretion
(28). Although CD44/HA signaling
has been implicated in the promotion of cellular motility, the
mechanisms underlying its involvement in EGF-stimulated ovarian
cancer have yet to be elucidated.
In the present study, EGF/EGFR signaling was
revealed to induce mesenchymal morphology and potentiate the
migratory activity of ovarian cancer cells. Conversely, treatment
with sorafenib prevented the migration of EGF-treated SK-OV-3
cells, through inhibition of the MAPK/ERK signaling pathway. Raf
expression in EGF-treated Caov-3 cells appeared to be reduced;
however, sorafenib efficiently blocked cancer cell motility
following EGF stimulation. CD44 and HAS levels in EGF-stimulated
Caov-3 cells were also downregulated following treatment with
sorafenib. Treatment with HA was demonstrated to activate the
MAPK/ERK pathway in Caov-3 cells. The present results suggested
that the activation of EMT processes involved in ovarian cancer
metastasis may be associated with various mechanisms and may depend
on cell type. Furthermore, EGF stimulation may contribute to the
induction of mesenchymal phenotypes in primary ovarian cancer,
through the regulation of HA production and CD44/HA-mediated
MAPK/ERK signaling.
EGF requires EGFR and CD44 to exert its effects on
cellular proliferation and motility (12). CD44/HA stimulation has been
reported to decrease the expression of E-cadherin, and to increase
the expression of Snail, vimentin and N-cadherin (10). Previous studies have demonstrated
that CD147 is expressed in invasive areas and proliferative regions
and has pleiotropic functions, including inducing MMP synthesis in
the stroma and tumor, contributing to drug resistance, and
promoting the migration and invasion of tumor cells (29,30).
CD147 has also been reported to promote the synthesis of the large
extracellular polysaccharide HA and potentiate EGFR/Ras/ERK
signaling in a CD44/HA-dependent manner (13). Increased HA and CD44 levels have
been correlated with enhanced malignancy in various types of cancer
cells in vitro and in vivo (31,32).
Increased ERK activation has also been associated with local
aggressiveness in vitro and in vivo, and enhanced
CD44 transcription (14). The
inhibition of MEK, upstream of ERK1/2, has been revealed to
decrease CD44 expression and promoter activity, and to reduce
cellular migration and invasion (14), whereas ERK1/2 has been demonstrated
to promote metastasis via inducing Slug, Snail and EMT (33). The present study examined the
relationship between EGF and CD44 signaling in the regulation of
cellular migration and invasion using sorafenib. Treatment of
SK-OV-3 cells with sorafenib suppressed EGF-mediated CD44
expression and MAPK/ERK signaling. In addition, sorafenib
suppressed the mesenchymal phenotype and the invasive capabilities
of EGF-stimulated Caov-3 cells; however, EGF stimulation abolished
the expression of Raf mRNA and Ras/Raf/MEK proteins. These results
suggested that EGF stimulation may trigger various signaling
pathways to promote ovarian cancer cell migration in a cell
type-specific manner.
Previous studies have reported that the expression
of HAS1, HAS2, and HAS3 increased during embryonic development and
malignant progression (34),
whereas epithelial HAS2 overexpression induced the transition of
epithelial cells to fibroblastic and migratory phenotypes (35). However, the role of EGF in HA
synthesis and the implication of different HAS isoforms in ovarian
cancer cell migration have yet to be elucidated. The present study
demonstrated that treatment with EGF resulted in HAS2 activation;
HA treatment exerted a more pronounced effect on the migratory
capabilities of EGF-activated Caov-3 cells compared with of
EGF-activated SK-OV-3 cells.
Notably, high cell surface HA levels are associated
with a less aggressive phenotype of ovarian cancer (36). In addition, increased HA levels
(>50 µg/ml) prior to chemotherapy have been associated with poor
prognosis and drug resistance (37). In the present study, MAPK/ERK
kinases were upregulated in HA-treated Caov-3 cells. Furthermore,
treatment with a combination of EGF and HA potentiated the invasive
capabilities and induced expression of MAPK/ERK kinases in Caov-3
cells. Conversely, silencing the expression of CD44 abolished
activation of the MAPK/ERK pathway. Therefore, it may be
hypothesized that EGF can collaborate with HA to regulate the
migration and invasion of primary ovarian cancer cells, through the
regulation of MAPK/ERK-mediated signaling pathways.
HA binding to CD44 has been demonstrated to activate
NF-κB through Ras (38), whereas
treatment with sorafenib suppressed tumor growth via inhibiting the
activation of NF-κB (39). In the
present study, treatment with sorafenib prevented the activation of
NF-κB in EGF-stimulated and EGF/HA-co-stimulated ovarian cancer
cells. It has previously been reported that the MAPK/ERK pathway
was activated via Ras and B-Raf, predominantly in ovarian tumors
with low malignant potential (40). Since sorafenib can inhibit the
c-Raf and B-Raf kinases that participate in the MAPK/ERK pathway,
it is currently being used in combination with platinum and
taxane-based chemotherapy or as a single agent for the treatment of
patients with ovarian cancer (41).
In conclusion, the present results suggested that HA
binding to CD44 may activate the MAPK/ERK signaling pathway during
EGF stimulation, whereas sorafenib, in combination with a standard
chemotherapeutic agent, may hold potential as a therapeutic
strategy for the prevention of CD44/HA-dependent metastasis of
primary ovarian cancer.
Acknowledgements
The present study was supported by the Basic Science
Research Program of the Ministry of Education (grant no.
NRF-2015R1D1A1A01056672) and Ministry of Science, ICT & Future
Planning (grant no. NRF-2015R1C1A2A01053732) through the National
Research Foundation (NRF) of the Republic of Korea.
References
|
1
|
Heppner G, Yamashina K, Miller B and
Miller F: Tumor heterogeneity in metastasis. Prog Clin Biol Res.
212:45–59. 1986.PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gadducci A, Sartori E, Maggino T, Zola P,
Landoni F, Fanucchi A, Palai N, Alessi C, Ferrero AM, Cosio S and
Cristofani R: Analysis of failures after negative second-look in
patients with advanced ovarian cancer: An Italian multicenter
study. Gynecol Oncol. 68:150–155. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bagnato A and Rosanò L:
Epithelial-mesenchymal transition in ovarian cancer progression: A
crucial role for the endothelin axis. Cells Tissues Organs.
185:85–94. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salomon DS, Brandt R, Ciardiello F and
Normanno N: Epidermal growth factor-related peptides and their
receptors in human malignancies. Crit Rev Oncol Hematol.
19:183–232. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Auersperg N, Wong AS, Choi KC, Kang SK and
Leung PC: Ovarian surface epithelium: Biology, endocrinology and
pathology. Endocr Rev. 22:255–288. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lafky JM, Wilken JA, Baron AT and Maihle
NJ: Clinical implications of the ErbB/epidermal growth factor (EGF)
receptor family and its ligands in ovarian cancer. Biochim Biophys
Acta. 1785:232–265. 2008.PubMed/NCBI
|
|
8
|
Lassus H, Sihto H, Leminen A, Joensuu H,
Isola J, Nupponen NN and Butzow R: Gene amplification, mutation,
and protein expression of EGFR and mutations of ERBB2 in serous
ovarian carcinoma. J Mol Med (Berl). 84:671–681. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagano O and Saya H: Mechanism and
biological significance of CD44 cleavage. Cancer Sci. 95:930–935.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim Y, Lee YS, Choe J, Lee H, Kim YM and
Jeoung D: CD44-epidermal growth factor receptor interaction
mediates hyaluronic acid-promoted cell motility by activating
protein kinase C signaling involving Akt, Rac1, Phox, reactive
oxygen species, focal adhesion kinase and MMP-2. J Biol Chem.
283:22513–22528. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Murai T, Miyauchi T, Yanagida T and Sako
Y: Epidermal growth factor-regulated activation of Rac GTPase
enhances CD44 cleavage by metalloproteinase disintegrin ADAM10.
Biochem J. 39:65–71. 2006. View Article : Google Scholar
|
|
12
|
Ellis IR, Schor AM and Schor SL: EGF AND
TGF-alpha motogenic activities are mediated by the EGF receptor via
distinct matrix-dependent mechanisms. Exp Cell Res. 313:732–741.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grass GD, Tolliver LB, Bratoeva M and
Toole BP: CD147, CD44, and the epidermal growth factor receptor
(EGFR) signaling pathway cooperate to regulate breast epithelial
cell invasiveness. J Biol Chem. 288:26089–26104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Judd NP, Winkler AE, Murillo-Sauca O,
Brotman JJ, Law JH, Lewis JS Jr, Dunn GP, Bui JD, Sunwoo JB and
Uppaluri R: ERK1/2 regulation of CD44 modulates oral cancer
aggressiveness. Cancer Res. 72:365–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Frémin C and Meloche S: From basic
research to clinical development of MEK1/2 inhibitors for cancer
therapy. J Hematol Oncol. 3:82010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wilhelm SM, Carter C, Tang L, Wilkie D,
McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al:
BAY 43–9006 exhibits broad spectrum oral antitumor activity and
targets the RAF/MEK/ERK pathway and receptor tyrosine kinases
involved in tumor progression and angiogenesis. Cancer Res.
64:7099–7109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J, Chen YL, Ji G, Fang W, Gao Z, Liu
Y, Wang J, Ding X and Gao F: Sorafenib inhibits
epithelial-mesenchymal transition through an epigenetic-based
mechanism in human lung epithelial cells. PLoS One. 8:e649542013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu D, Wolf JK, Scanlon M, Price JE and
Hung MC: Enhanced c-erbB-2/neu expression in human ovarian cancer
cells correlates with more severe malignancy that can be suppressed
by E1A. Cancer Res. 53:891–898. 1993.PubMed/NCBI
|
|
19
|
Buick RN, Pullano R and Trent JM:
Comparative properties of five human ovarian adenocarcinoma cell
lines. Cancer Res. 45:3668–3676. 1985.PubMed/NCBI
|
|
20
|
Yan L, Zucker S and Toole BP: Roles of the
multifunctional glycoprotein, emmprin (basigin; CD147), in tumour
progression. Thromb Haemost. 93:199–204. 2005.PubMed/NCBI
|
|
21
|
Marieb EA, Zoltan-Jones A, Li R, Misra S,
Ghatak S, Cao J, Zucker S and Toole BP: Emmprin promotes
anchorage-independent growth in human mammary carcinoma cells by
stimulating hyaluronan production. Cancer Res. 64:1229–1232. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Toole BP and Slomiany MG: Hyaluronan, CD44
and Emmprin: Partners in cancer cell chemoresistance. Drug Resist
Updat. 11:110–121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
La Fleur L, Johansson AC and Roberg K: A
CD44high/EGFRlow subpopulation within head and neck cancer cell
lines shows an epithelial-mesenchymal transition phenotype and
resistance to treatment. PLoS One. 7:e440712012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grandis JR and Sok JC: Signaling through
the epidermal growth factor receptor during the development of
malignancy. Pharmacol Ther. 102:37–46. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim HR, Wheeler MA, Wilson CM, Iida J, Eng
D, Simpson MA, McCarthy JB and Bullard KM: Hyaluronan facilitates
invasion of colon carcinoma cells in vitro via interaction with
CD44. Cancer Res. 64:4569–4576. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cook AC, Chambers AF, Turley EA and Tuck
AB: Osteopontin induction of hyaluronan synthase 2 expression
promotes breast cancer malignancy. J Biol Chem. 281:24381–24389.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nedvetzki S, Gonen E, Assayag N, Reich R,
Williams RO, Thurmond RL, Huang JF, Neudecker BA, Wang FS, Turley
EA and Naor D: RHAMM, a receptor for hyaluronan-mediated motility,
compensates for CD44 in inflamed CD44-knockout mice: A different
interpretation of redundancy. Proc Natl Acad Sci USA.
101:18081–18086. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim MS, Park MJ, Kim SJ, Lee CH, Yoo H,
Shin SH, Song ES and Lee SH: Emodin suppresses hyaluronic
acid-induced MMP-9 secretion and invasion of glioma cells. Int J
Oncol. 27:839–846. 2005.PubMed/NCBI
|
|
29
|
Caudroy S, Polette M, Tournier JM, Burlet
H, Toole B, Zucker S and Birembaut P: Expression of the
extracellular matrix metalloproteinase inducer (EMMPRIN) and the
matrix metalloproteinase-2 in bronchopulmonary and breast lesions.
J Histochem Cytochem. 47:1575–1580. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weidle UH, Scheuer W, Eggle D, Klostermann
S and Stockinger H: Cancer-related issues of CD147. Cancer Genomics
Proteomics. 7:157–169. 2010.PubMed/NCBI
|
|
31
|
Toole BP: Hyaluronan-CD44 interactions in
cancer: Paradoxes and possibilities. Clin Cancer Res. 15:7462–7468.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jacobson A, Rahmanian M, Rubin K and
Heldin P: Expression of hyaluronan synthase 2 or hyaluronidase 1
differentially affect the growth rate of transplantable colon
carcinoma cell tumors. Int J Cancer. 102:212–219. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen H, Zhu G, Li Y, Padia RN, Dong Z, Pan
ZK, Liu K and Huang S: Extracellular signal-regulated kinase
signaling pathway regulates breast cancer cell migration by
maintaining slug expression. Cancer Res. 69:9228–9235. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Boregowda RK, Appaiah HN, Siddaiah M,
Kumarswamy SB, Sunila S, Thimmaiah KN, Mortha K, Toole B and
Banerjee Sd: Expression of hyaluronan in human tumor progression. J
Carcinog. 5:22006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y and Heldin P: Hyaluronan production
increases the malignant properties of mesothelioma cells. Br J
Cancer. 85:600–607. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tamada Y, Takeuchi H, Suzuki N, Aoki D and
Irimura T: Cell surface expression of hyaluronan on human ovarian
cancer cells inversely correlates with their adhesion to peritoneal
mesothelial cells. Tumour Biol. 33:1215–1222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ricciardelli C, Ween MP, Lokman NA, Tan
IA, Pyragius CE and Oehler MK: Chemotherapy-induced hyaluronan
production: A novel chemoresistance mechanism in ovarian cancer.
BMC Cancer. 13:4762013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fitzgerald KA, Bowie AG, Skeffington BS
and O'Neill LA: Ras, protein kinase C zeta, and I kappa B kinases 1
and 2 are downstream effectors of CD44 during the activation of
NF-kappa B by hyaluronic acid fragments in T-24 carcinoma cells. J
Immunol. 164:2053–2063. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dudgeon C, Peng R, Wang P, Sebastiani A,
Yu J and Zhang L: Inhibiting oncogenic signaling by sorafenib
activates PUMA via GSK3β and NF-κB to suppress tumor cell growth.
Oncogene. 31:4848–4858. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kurman RJ, Visvanathan K, Roden R, Wu TC
and Shih IeM: Early detection and treatment of ovarian cancer:
Shifting from early stage to minimal volume of disease based on a
new model of carcinogenesis. Am J Obstet Gynecol. 198:351–356.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gemignani ML, Schlaerth AC, Bogomolniy F,
Barakat RR, Lin O, Soslow R, Venkatraman E and Boyd J: Role of KRAS
and BRAF gene mutations in mucinous ovarian carcinoma. Gynecol
Oncol. 90:378–381. 2003. View Article : Google Scholar : PubMed/NCBI
|





















