Introduction
Gallstone disease is a global major health problem,
and its attendant complications and comorbidities impose a huge
financial burden on health care economy (1–4).
Gallstone disease is a disease with multiple factors, mainly caused
by the complex interaction of genetic factors and environmental
factors (5). A prerequisite for
cholesterol gallstone formation is biliary precipitation of excess
cholesterol as solid crystals (6,7). The
most important source of biliary cholesterol is preformed plasma
lipoprotein cholesterol which is mainly absorbed from the diet in
small intestine, whereas only a minor contribution of cholesterol
is generated by hepatic new synthesis or hydrolysis of cholesteryl
ester stores (8). Dietary
cholesterol is trafficked into enterocyte by Niemann-Pick C1-like 1
(NPC1L1) protein (9), and can be
reversely transported to the intestinal lumen by ATP-binding
cassette, sub-family G, member 5/8 (ABCG5/8) (10,11).
After cholesterol passes through the enterocyte membrane, it should
be esterified by acetyl-Coenzyme A acetyltransferase 2 (ACAT2);
subsequent to this, it is transported in the chylomicrons
circulating then reach the liver for biliary secretion (12,13).
Therefore, a high fat intake increases the risk of developing
gallstone. And inhibiting NPC1L1 expression suppresses intestinal
absorption of cholesterol, reduces plasma cholesterol level and
prevents the development of gallstone (14).
Osteopontin (OPN) is a soluble cytokine and a
matrix-associated protein presenting in the majority of tissues and
body fluids (15). Previous study
found that plasma OPN level had a significant correlation with
plasma cholesterol concentration in human (16). Our previous study demonstrated that
OPN was involved in the pathogenesis of gallstone disease (17,18),
and that OPN altered hepatic cholesterol metabolism thus affecting
cholesterol gallstone formation in mice (19). Because the homeostasis of
cholesterol is regulated by both absorption in intestine and
synthesis in liver (20,21), it is also important to discover the
role of OPN in intestinal cholesterol absorption. Therefore, we
investigated the mechanism of OPN in cholesterol gallstone
formation focusing on its effect on intestinal absorption of
cholesterol in this study.
Materials and methods
Animals, diets and sample
collection
WT mice were purchased from Fudan University
(Shanghai, China). OPN−/− mice in congenic
background were purchased from Jackson Laboratory (Bar Harbor, ME,
USA). Mice were housed at 22±2°C and 60±10% relative humidity in a
specific pathogen-free environment, with a 12:12 h light: dark
cycle. WT and OPN−/− male mice between 8 and 10 weeks of age were
fed a chow diet (CD) or LD (CD supplemented 15% fat, 2%
cholesterol, and 0.5% cholic acid) for up to 8 weeks. Bile was
collected, and crystal analysis was immediately performed. Blood
was collected via right ventricle heart puncture. Feces was
collected from individually housed mice for 72 h. The tissues were
harvested and then snap-frozen in liquid nitrogen for protein and
RNA isolation or fixed in 4% paraformaldehyde overnight for
histological analysis. All animals received humane care and all
experimental procedures were conducted in conformance with
principles of the National Institutes of Health guide for the care
and use of laboratory animal (NIH Publications No. 8023) and
approved by the Animal Ethics Committee of Fudan University.
Gallstone formation analysis and
biochemical detections of bile, serum and feces
Gallstone were defined as macroscopically visible
stones, whereas crystals were defined with polarizing light
microscopy (Olympus, Tokyo, Japan). The fecal lipids and bile acids
were extracted as previously described (8,22).
Cholesterol, bile acids and triglyceride levels were measured with
assay kits from KHB (Shanghai, China), and phospholipid content was
measured using an assay kit from Wako (Osaka, Japan) according to
the manufacturers' instructions. All the biochemical measures were
assayed in triplicate 3 times. Then the cholesterol saturation
index (CSI) was calculated according to Carey's critical tables
(23).
Quantitative real-time PCR
analysis
Total RNAs were collected from the proximal segment
of small intestine which was equally randomly divided into 5
segments or liver in each mouse with the RNAprep Pure Tissue kit
(TianGen, Beijing, China) according to the manufacturer's
instructions. Random primers (Takara, Shiga, Japan) were used for
reverse transcription of total RNA to complementary DNA.
Quantitative real-time PCR was performed using SYBR-Green I
chemistry (TianGen) and the ABI 7900HT Fast Real-Time PCR System
(Applied Biosystem, Shanghai, China). The gene-specific primer
sequences are shown in Table I.
Messenger RNA (mRNA) expression levels were calculated relative to
the housekeeping gene GAPDH and further normalized to the
expression levels of the respective controls following the basis of
the relative expression method (24).
 | Table I.Primer sequences for quantitative
real-time PCR in mice. |
Table I.
Primer sequences for quantitative
real-time PCR in mice.
| Gene | Sense primer
(5′→3′) | Antisense primer
(5′→3′) |
|---|
| NPC1L1 |
CTCTGCCCTCTGCAATGCTC |
GAACAGGCTGCCGAGTCTT |
| ABCG5 |
AGGGCCTCACATCAACAGAG |
GCTGACGCTGTAGGACACAT |
| ABCG8 |
TGCCCACCTTCCACATGTC |
ATGAAGCCGGCAGTAAGGTAGA |
| ACAT2 |
CTACAAGCAAGACCCAAGAG |
CATGTGGTAGATGGTTCGG |
| ABCA1 |
CGTTTCCGGGAAGTGTCCTA |
GCTAGAGATGACAAGGAGGATGGA |
| LXRα |
GATAGGGTTGGAGTCAGCA |
GGAGCGCCTGTTACACTGTT |
| PPARδ |
TCCATCGTCAACAAAGACGGG |
ACTTGGGCTCAATGATGTCAC |
| OSTα |
AGGCAGGACTCATATCAAACTTG |
TGAGGGCTATGTCCACTGGG |
| OSTβ |
AGATGCGGCTCCTTGGAATTA |
TGGCTGCTTCTTTCGATTTCTG |
| ASBT |
GTCTGTCCCCCAAATGCAACT |
CACCCCATAGAAAACATCACCA |
| ILBP |
CTTCCAGGAGACGTGATTGAAA |
CCTCCGAAGTCTGGTGATAGTTG |
| HMGCS |
GCCGTGAACTGGGTCGAA |
GCATATATAGCAATGTCTCCTGCAA |
| HMGCR |
CTTGTGGAATGCCTTGTGATTG |
AGCCGAAGCAGCACATGAT |
| SREBP2 |
GCGTTCTGGAGACCATGGA |
ACAAAGTTGCTCTGAAAACAAATCA |
| LDLR |
TGGCTGTTCCCACATCTG |
CTCGTCAATATCTTCACACCTG |
| LRP |
ACTATGGATGCCCCTAAAACTTG |
GCAATCTCTTTCACCGTCACA |
| PCSK9 |
ACCCTCATAGGCCTGGAGTT |
CTGTGATGACCTCTGGAGCA |
| GAPDH |
TGTGTCCGTCGTGGATCTGA |
CCTGCTTCACCACCTTCTTGAT |
Western blot analysis
The entire proximal segment of small intestine that
was randomly divided into 5 equal segments were lysed in ice-cold
RIPA buffer supplemented with protease inhibitors. Protein
concentration of the extracts was measured by the BCA Protein Assay
kit (Thermo, Shanghai, China). The protein was analyzed by 8%
SDS-PAGE and transferred to Poly (vinylidene fluoride) membranes.
Anti-NPC1L1 antibody (Santa Cruz, Shanghai, China) was used at a
1:300 dilution. A 1:10,000 dilution of rabbit anti-goat
immunoglobulin G-HRP (Santa Cruz) was used as a secondary antibody.
After probing individual antibodies, the antigen-antibody complex
was visualized by Enhanced Chemiluminescence Reagents Supersignal
(TianGen, Beijing, China). The relative average protein level was
determined by densitometry using Image J software (NIH, Maryland,
and USA).
Histopathological and
immunohistochemistry analysis
Tissues were fixed in 4% paraformaldehyde overnight
and then transferred to 70% ethanol prior to routine processing and
staining with hematoxylin and eosin. Formalin-fixed tissues were
embedded in paraffin and cut into 3 mm sections. Sample slides were
incubated with anti-NPC1L1 antibodies (Santa Cruz) with a 1:75
dilution, followed by 30 min incubation with an HRP-labeled polymer
secondary antibody (Santa Cruz). Sections were viewed with a Nikon
ECLIPSE E600 microscope (Nikon, Tokyo, Japan) using ×10 objective
lenses, and images were acquired with a SPOT INSIGHT™ digital color
camera, model 3.2.0 (Sterling, Heights, MI). Quantification of
immunoreactivity was performed on digitally captured color images
saved as TIFF files and analyzed using Image-Pro plus 6.0 software
(Media Cybernetics, Florida, USA). The blind histopathological and
immunohistochemistry analysis were conducted by a pathologist of
Fudan University.
Statistical analysis
Data are demonstrated as mean ± standard deviation
(SD) unless otherwise noted. The statistical significance of
differences between the means of the experimental groups was
evaluated with unpaired Student's t test (GraphPad, La Jolla, CA,
USA). A difference was considered to be statistically significant
at P<0.05.
Results
OPN deficiency reduced gallstone
formation in LD-fed mice
Neither WT mice (0/6) nor OPN−/− mice (0/6) showed
crystals or gallstones when fed a CD. When fed with LD for 8 weeks,
all WT mice (6/6) developed gallstones, whereas the penetrance in
OPN−/− mice was 16.7% (1/6). Compared to those of OPN−/− mice, the
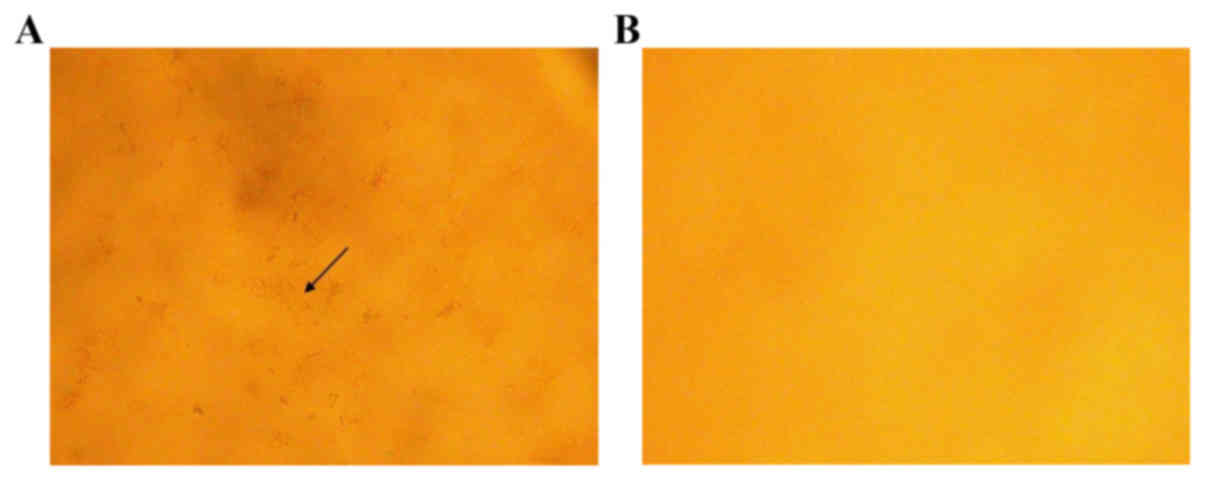
gallbladder bile of WT mice appeared turbid and full of
precipitates and stones. Microscopic examination of the gallbladder
bile revealed cholesterol crystals in WT mice, whereas those of
OPN−/− mice were largely free of cholesterol precipitates (Fig. 1).
OPN deficiency altered the cholesterol
content and CSI in LD-fed mice
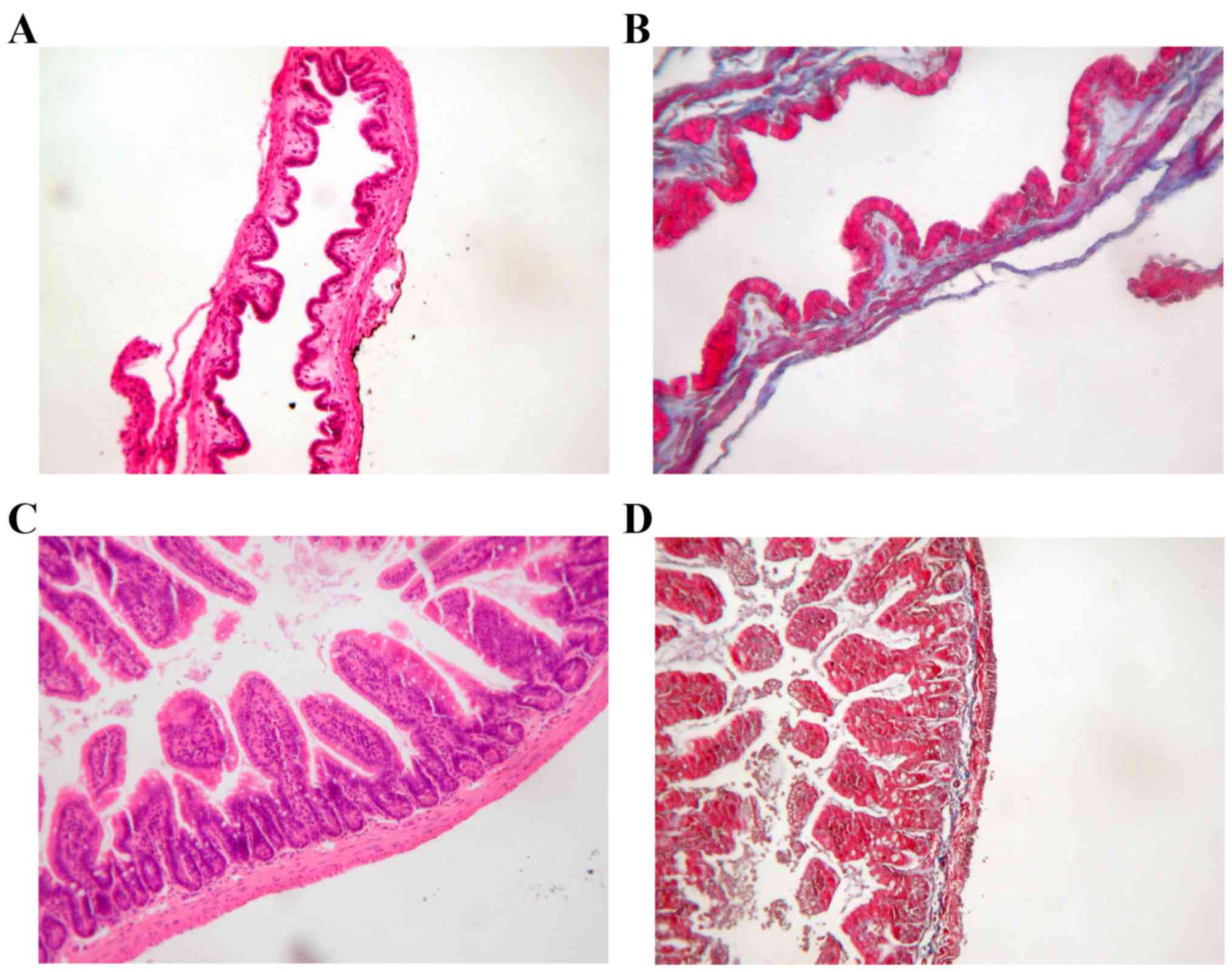
The histology examinations of gallbladder and ileum
were similar in WT mice and OPN−/− mice (Fig. 2). And the body weight and
gallbladder volume between two strains showed no difference
(Table II). The cholesterol
content of two genotypes mice were also similar when fed CD. After
being fed with LD for 8 weeks, OPN−/− mice exhibited reduced in
biliary cholesterol content and serum cholesterol profile compared
to WT mice. But the fecal cholesterol content showed no
statistically difference in two LD-fed strains (Table II). There was no difference in
serum phospholipid, triglyceride and bile acids profiles between
two LD-fed genotypes (Table
III). And the fecal bile acids content showed no statistically
difference in two strains. However, the LD-fed OPN−/− mice
increased in biliary bile acids and biliary phospholipid content
compared to LD-fed WT mice (Table
III). This combined effect of biliary biochemical alterations
led to a decreased CSI in LD-fed OPN−/− mice (Table II), providing a biochemical
mechanism for the phenotype that protecting OPN−/− mice from
cholesterol gallstone formation.
 | Table II.The body weight, gallbladder volume
and cholesterol content in WT and OPN−/− mice. |
Table II.
The body weight, gallbladder volume
and cholesterol content in WT and OPN−/− mice.
|
| CD | LD for 8 weeks |
|---|
|
|
|
|
|---|
| Parameters | WT |
OPN−/− | WT |
OPN−/− |
|---|
| Body weight
(g) | 20.1±1.06 | 20.5±1.06 | 20.3±0.75 | 22.8±2.38 |
| Gallbladder volume
(µl) | 23.2±7.57 | 25.6±11.5 | 27.7±9.14 | 23.5±6.51 |
| Biliary cholesterol
(mmol/l) | 2.58±0.67 | 2.88±0.59 | 8.81±1.14 |
5.34±0.90a |
| Serum cholesterol
(mmol/l) | 1.85±0.15 | 1.96±0.33 | 3.10±0.56 |
2.31±0.25a |
| Fecal cholesterol
output (mg/g feces/day) | 3.83±0.84 | 3.77±0.73 | 15.29±1.22 | 17.08±2.11 |
| CSI | 0.48±0.12 | 0.49±0.05 | 1.19±0.13 |
0.59±0.07b |
 | Table III.Lipids and bile acids profiles in WT
and OPN−/− mice. |
Table III.
Lipids and bile acids profiles in WT
and OPN−/− mice.
|
| CD | LD for 8 weeks |
|---|
|
|
|
|
|---|
| Parameters | WT |
OPN−/− | WT |
OPN−/− |
|---|
| Biliary bile acids
(mmol/l) | 56.65±6.08 | 63.59±7.66 | 79.79±11.0 |
97.34±15.8a |
| Biliary
phospholipid (mmol/l) | 20.53±1.55 | 21.99±2.82 | 24.68±2.45 |
30.48±4.80a |
| Serum phospholipid
(mg/dl) | 157.4±18.1 | 167.0±31.0 | 182.6±61.0 | 190.7±22.5 |
| Serum bile acids
(µmol/l) | 4.30±5.15 | 4.17±3.13 | 10.3±8.03 | 13.5±5.25 |
| Serum triglyceride
(mmol/l) | 0.32±0.19 | 0.39±0.18 | 0.46±0.73 | 0.30±0.03 |
| Fecal bile acids
output (µmol/g feces/day) | 2.82±0.25 | 3.17±0.75 | 66.80±5.21 | 71.3±11.80 |
OPN deficiency suppressed the
expression of intestinal NPC1L1 in LD-fed mice
To understand the mechanisms responsible for the
changes in biochemical contents, we profiled the expression of
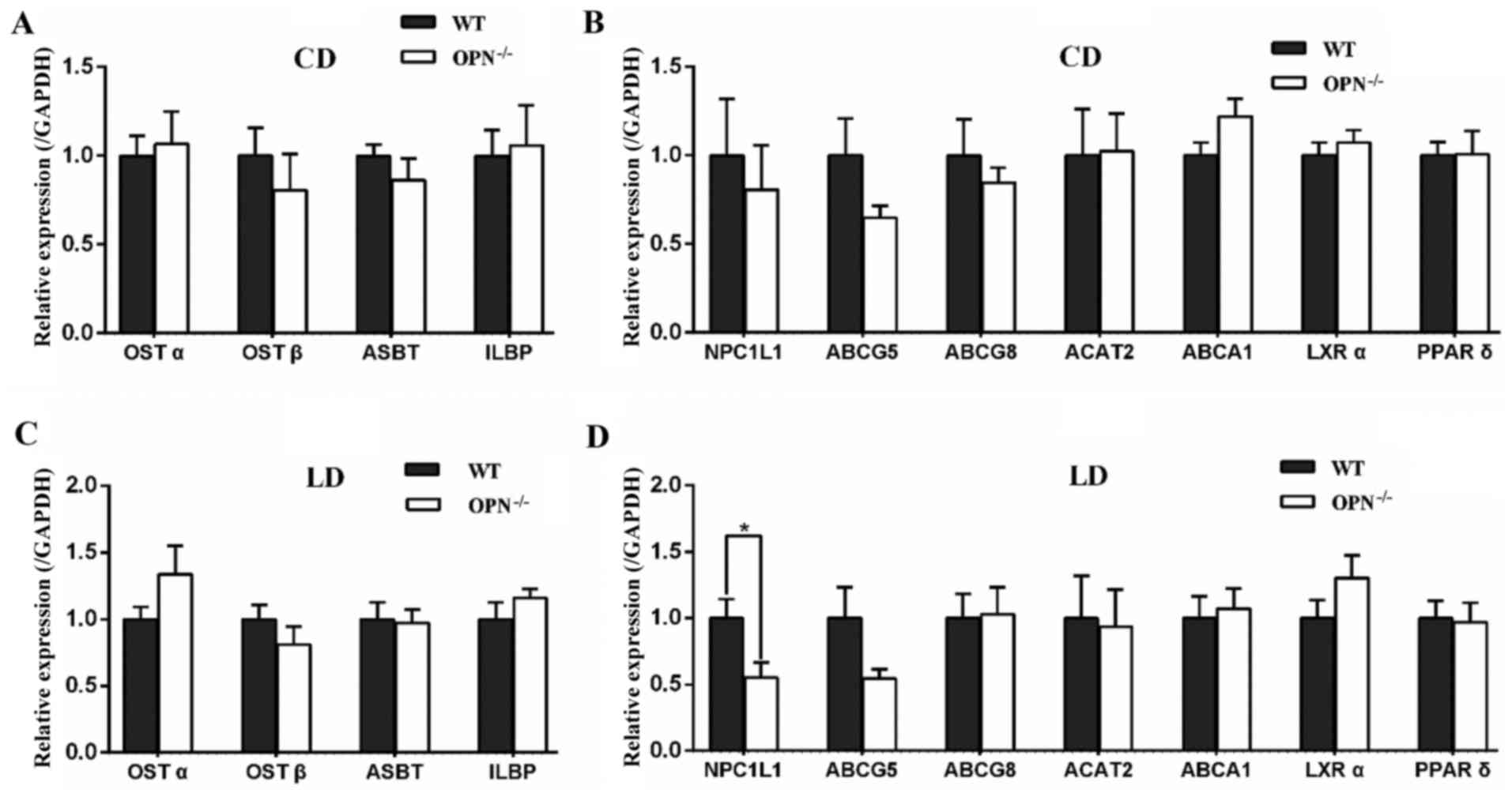
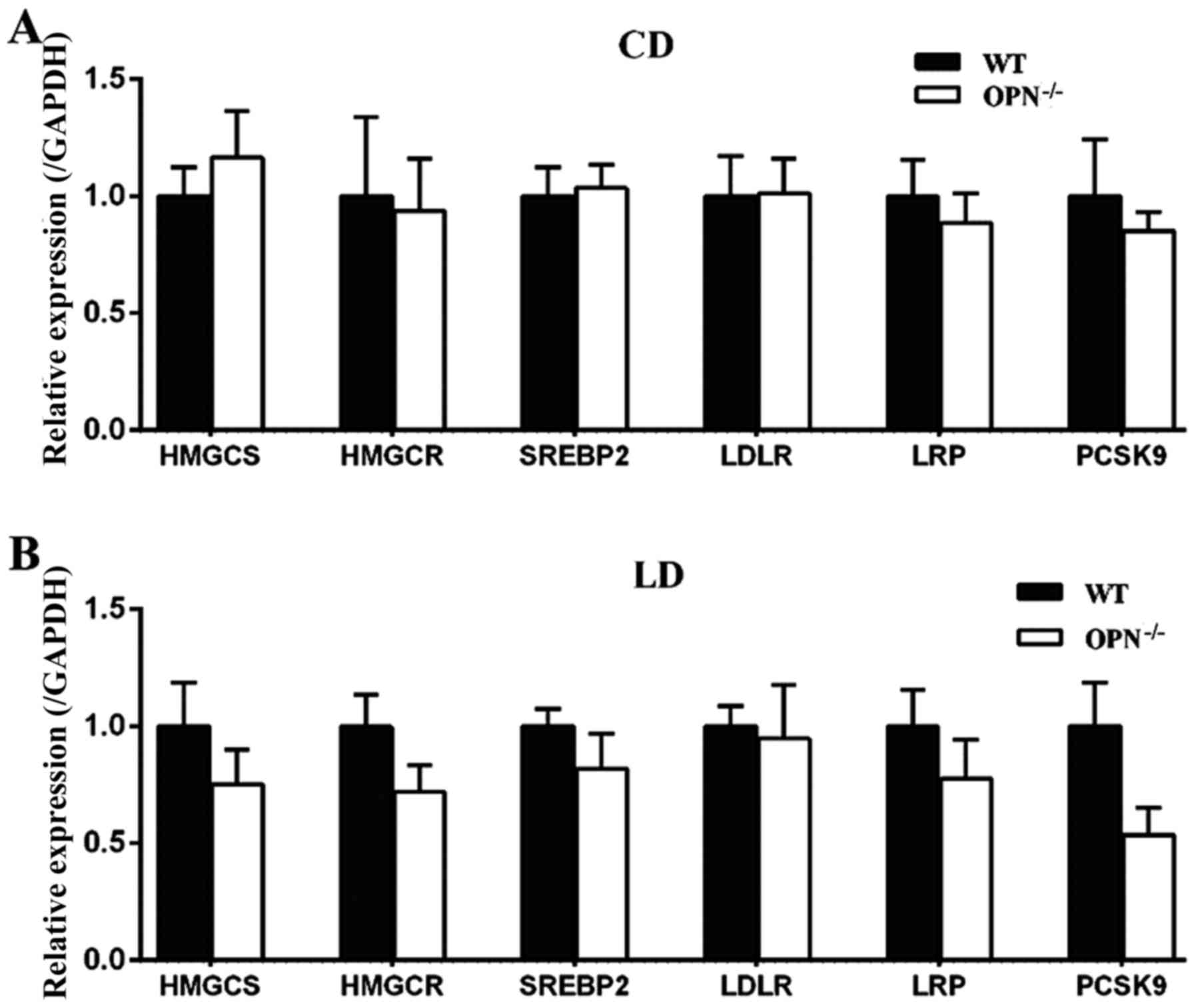
intestinal related genes. There was no significant difference in
genes expression between the two genotypes on CD (Fig. 3A and B). When challenged with the
LD for 8 weeks, OPN−/− mice showed no difference in the expression
of apical sodium-dependent bile acid transporter (ASBT), ileal
lipid binding protein (ILBP) and organic solute transporters α/β
(OSTα/β), which are involved in intestinal reabsorption of bile
acids (Fig. 3C). Among the
intestinal cholesterol transporters, the expression of NPC1L1 had
an almost 50% reduction in LD-fed OPN−/− mice, whereas the
expression of ABCG5/8, ATP-binding cassette, sub-family A, member 1
(ABCA1), and ACAT2 was not affected (Fig. 3D). Then we detected the gene
expression of liver X receptor α (LXRα) and peroxisome proliferator
activated receptor δ (PPARδ), and found it was similar between two
mice strains (Fig. 3D). Then we
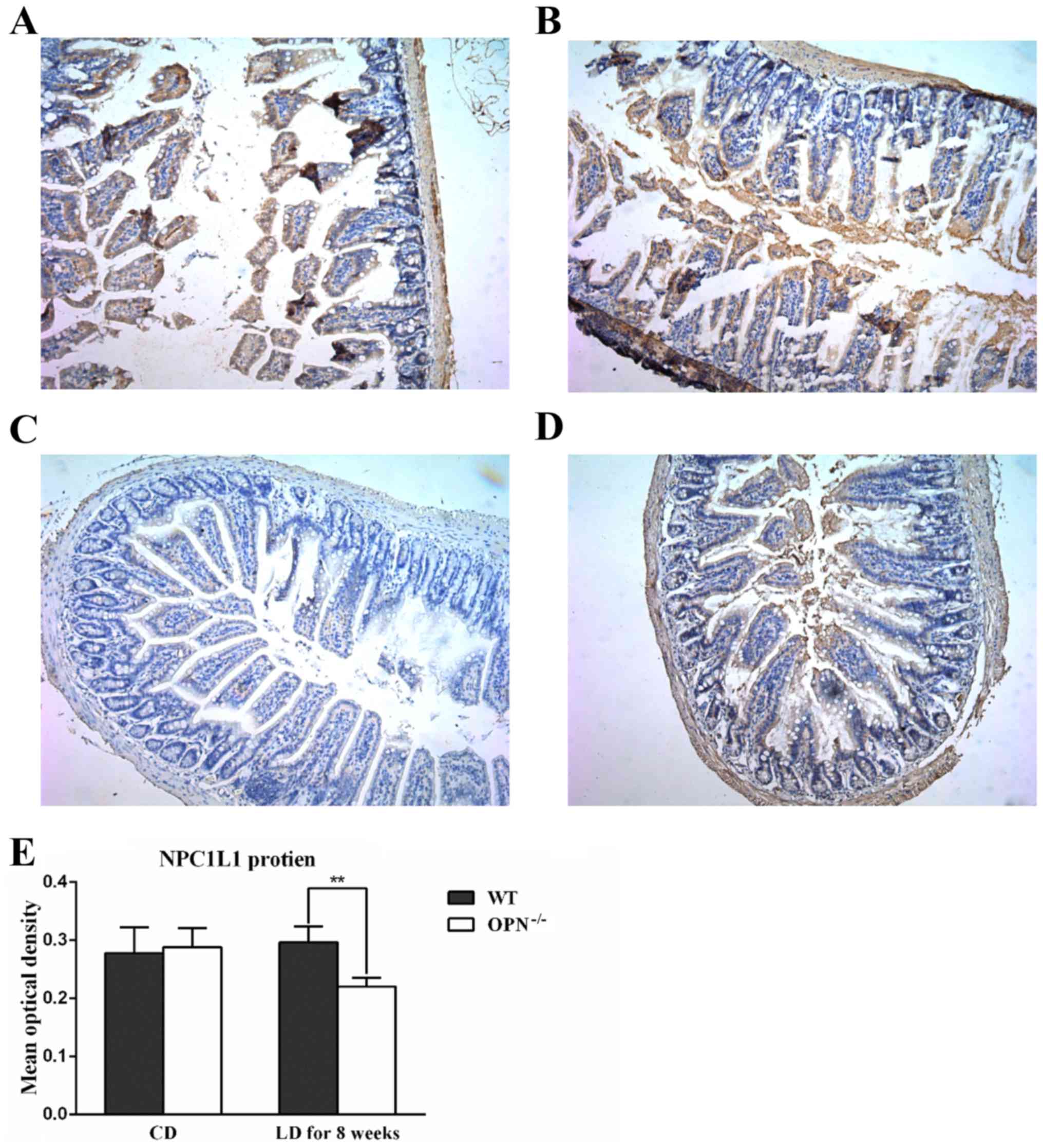
challenged the protein expression of intestinal NPC1L1 with
immunohistochemistry, and found that the protein expression of
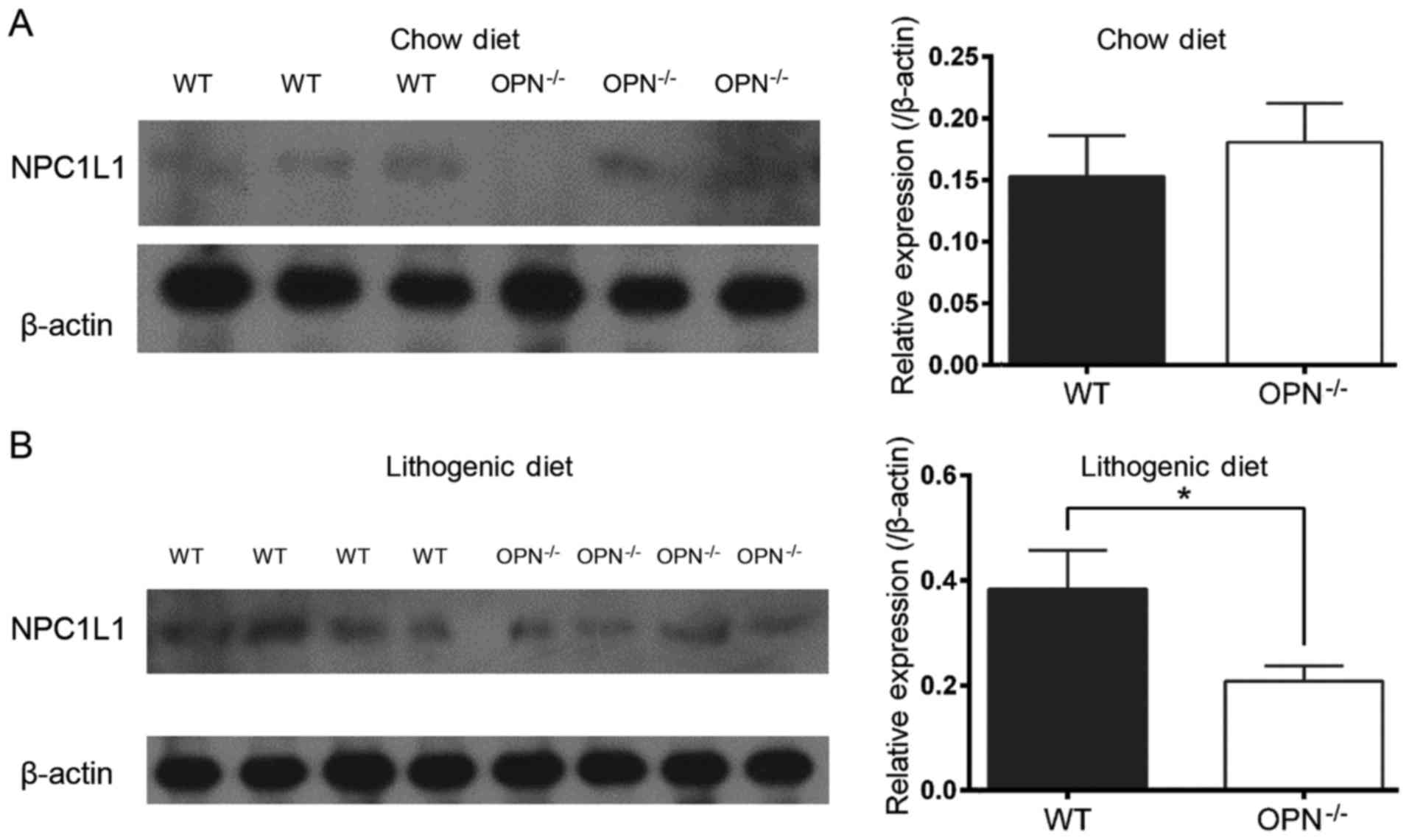
NPC1L1 was similar in two CD-fed strains but decreased in LD-fed
OPN−/− mice (Fig. 4). And the
changed expression of NPC1L1 protein was also confirmed by western
blot analysis (Fig. 5).
Because the homeostasis of cholesterol is regulated
by both absorption in intestine and synthesis in liver, we also
measured the expression of hepatic related genes. There were no
significant differences in those genes between the two genotypes
fed a CD (Fig. 6A). When
challenged with the LD for 8 weeks the expression of proprotein
convertase subtilisin/kexin type 9 (PCSK9) tended to be decreased
in OPN−/− mice, but the change was not statistically
significant. The expression of other hepatic related genes also
showed no difference between two mice strains fed a LD (Fig. 6B).
Discussion
In this study, we found that OPN deficiency could
attenuate the absorption of cholesterol by reducing intestinal
expression of NPC1L1, thus protecting mice from diet-induced
cholesterol gallstone formation.
OPN−/− mice was less susceptible to
diet-induced gallstone than WT mice, which is consistent with our
previous work (19). The body
weight and gallbladder volume did not differ between the
OPN−/− mice and WT mice fed with CD nor LD for 8 weeks.
In addition, gallbladder histology was also similar in two strains.
These findings indicate that the abnormalities of gallbladder are
unlikely to be primarily responsible for the protection of LD-fed
OPN−/− mice from gallstone formation. As the
prerequisite for cholesterol gallstone formation is the
precipitation of excess cholesterol in bile as solid crystals, this
protection of LD-fed OPN−/− mice from gallstone
formation may be due to the remarkably lower biliary cholesterol
concentration, with greater levels of bile acids and phospholipid
and consequently a lower CSI.
Then we observed that the content of serum
cholesterol in LD-fed OPN−/− mice was lower than that of
LD-fed WT mice. And the fecal cholesterol output in LD-fed
OPN−/− was increased in value, although the difference
is not statistically significant. Additionally, the mRNA and
protein expressions of NPC1L1, which is localized to the
brush-border membrane of enterocytes +(25) and plays an important role in
intestinal cholesterol absorption (9), were both reduced in LD-fed
OPN−/− mice. It is known that cholesterol derived from
the intestine provides the first major source for cholesterol pool
and influences biliary cholesterol secretion (26–28),
and that high absorption of cholesterol enhances gallstone
formation through this pathway (29). Therefore the participation of the
NPC1L1 protein is very important, for it can determine the amount
of cholesterol circulating to the liver and allow it to be excreted
into bile. The increased level of intestinal NPC1L1 protein in
gallbladder gallstone patients produces a high absorption of
cholesterol (30). But inhibiting
intestinal NPC1L1 expression reduces the intestinal absorption of
cholesterol and prevents the formation of gallstone (31–34).
Ezetimibe, which is a drug that blocks the expression of NPC1L1,
could prevent cholesterol gallstone formation by effectively
reducing intestinal cholesterol absorption and biliary cholesterol
secretion (34,35). Although we did not measure the
cholesterol absorption rates during the mice experiments, these
findings suggest that the protection of LD-fed OPN−/−
mice from gallstone formation may be partially resulted from the
reduced intestinal absorption of cholesterol, which is caused by
the decreased expression of intestinal NPC1L1. However, it is a
limitation that we have not performed NPC1L1 overexpression assays
in mice to confirm the function of NPC1L1.
The expression of intestinal NPC1L1 is mainly
regulated by LXRα (36) and PPARδ
(37). Unfortunately, we found no
difference in the expression of LXRα nor PPARδ between two mice
strains. It suggests that the effect of OPN deficiency on
intestinal NPC1L1 expression may not function through the LXRα or
PPARδ pathway. Further studies are necessary in order to fully
address the mechanism by which OPN deficiency leads to intestinal
NPC1L1 suppression.
For the increased biliary bile acids content in
LD-fed OPN−/− mice, we did not find the expression of
intestinal genes involved in reabsorption of bile acids differ
between two strains. The reason might be that OPN could influence
the expression of the cytochrome P450, family 7, subfamily a,
polypeptide 1, the rate limiting enzyme of bile acids synthesis, as
we found in our previous study (19).
In conclusion, we have described a previously
unknown function of OPN, that it can affect intestinal absorption
of cholesterol through NPC1L1, thus impacting the formation of
gallstone in mice.
Acknowledgements
The present study was supported by the National
Science Foundation of China (no. 81270536).
Glossary
Abbreviations
Abbreviations:
|
OPN
|
osteopontin
|
|
OPN−/−
|
OPN gene knockout
|
|
WT
|
wild-type
|
|
CD
|
chow diet
|
|
LD
|
lithogenic diet
|
|
NPC1L1
|
Niemann-Pick C1-like L1
|
|
ABCG5/8
|
ATP-binding cassette, sub-family G,
member 5/8
|
|
ACAT2
|
acetyl-Coenzyme A acetyltransferase
2
|
|
ASBT
|
apical sodium-dependent bile acid
transporter
|
|
ILBP
|
ileal lipid binding protein
|
|
OSTα/β
|
organic solute transporter α/β
|
|
ABCA1
|
ATP-binding cassette, sub-family A,
member 1
|
|
LXRα
|
liver X receptor α
|
|
PPARδ
|
peroxisome proliferator activated
receptor δ
|
|
CSI
|
cholesterol saturation index
|
|
SD
|
standard deviation
|
References
|
1
|
Sandler RS, Everhart JE, Donowitz M, Adams
E, Cronin K, Goodman C, Gemmen E, Shah S, Avdic A and Rubin R: The
burden of selected digestive diseases in the United States.
Gastroenterology. 122:1500–1511. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Everhart JE and Ruhl CE: Burden of
digestive diseases in the United States Part III: Liver, biliary
tract, and pancreas. Gastroenterology. 136:1134–1144. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaechele V, Wabitsch M, Thiere D, Kessler
AL, Haenle MM, Mayer H and Kratzer W: Prevalence of gallbladder
stone disease in obese children and adolescents: Influence of the
degree of obesity, sex, and pubertal development. J Pediatr
Gastroenterol Nutr. 42:66–70. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shaffer EA: Gallstone disease:
Epidemiology of gallbladder stone disease. Best Pract Res Clin
Gastroenterol. 20:981–996. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Portincasa P, Moschetta A and Palasciano
G: Cholesterol gallstone disease. Lancet. 368:230–239. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hofmann AF, Amelsberg A and VanSonnenberg
E: Pathogenesis and treatment of gallstones. N Engl J Med.
328:1854–1855. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lamont JT and Carey MC: Cholesterol
gallstone formation. 2. Pathobiology and pathomechanics. Prog Liver
Dis. 10:165–191. 1992.PubMed/NCBI
|
|
8
|
Turley SD, Daggy BP and Dietschy JM:
Effect of feeding psyllium and cholestyramine in combination on low
density lipoprotein metabolism and fecal bile acid excretion in
hamsters with dietary-induced hypercholesterolemia. J Cardiovasc
Pharmacol. 27:71–79. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Altmann SW, Davis HJ Jr, Zhu LJ, Yao X,
Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, et al:
Niemann-Pick C1 like 1 protein is critical for intestinal
cholesterol absorption. Science. 303:1201–1204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Graf GA, Yu L, Li WP, Gerard R, Tuma PL,
Cohen JC and Hobbs HH: ABCG5 and ABCG8 are obligate heterodimers
for protein trafficking and biliary cholesterol excretion. J Biol
Chem. 278:48275–48282. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu L, Li-Hawkins J, Hammer RE, Berge KE,
Horton JD, Cohen JC and Hobbs HH: Overexpression of ABCG5 and ABCG8
promotes biliary cholesterol secretion and reduces fractional
absorption of dietary cholesterol. J Clin Invest. 110:671–680.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grenier E, Garofalo C, Delvin E and Levy
E: Modulatory role of PYY in transport and metabolism of
cholesterol in intestinal epithelial cells. PLoS One. 7:e409922012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anderson RA, Joyce C, Davis M, Reagan JW,
Clark M, Shelness GS and Rudel LL: Identification of a form of
acyl-CoA: Cholesterol acyltransferase specific to liver and
intestine in nonhuman primates. J Biol Chem. 273:26747–26754. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang W, Jia L, Ma Y, Xie P, Haywood J,
Dawson PA, Li J and Yu L: Ezetimibe restores biliary cholesterol
excretion in mice expressing Niemann-Pick C1-like 1 only in liver.
Biochim Biophys Acta. 1811:549–555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang KX and Denhardt DT: Osteopontin: Role
in immune regulation and stress responses. Cytokine Growth Factor
Rev. 19:333–345. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takemoto M, Tada K, Nakatsuka K, Moriyama
Y, Kazui H, Yokote K, Matsumoto T, Saito Y and Mori S: Effects of
aging and hyperlipidemia on plasma osteopontin level. Nihon Ronen
Igakkai Zasshi. 36:799–802. 1999.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang L, Chen JH, Cai D, Wang LY and Zha
XL: Osteopontin and integrin are involved in cholesterol gallstone
formation. Med Sci Monit. 18:BR16–BR23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang L, Chen JH, Cai D, Wang LY and Zha
XL: Osteopontin plays an anti-nucleation role in cholesterol
gallstone formation. Hepatol Res. 41:437–445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin J, Shao WQ, Chen ZY, Zhu WW, Lu L, Cai
D, Qin LX, Jia HL, Lu M and Chen JH: Osteopontin deficiency alters
biliary homeostasis and protects against gallstone formation. Sci
Rep. 6:302152016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dietschy JM and Siperstein MD: Effect of
cholesterol feeding and fasting on sterol synthesis in seventeen
tissues of the rat. J Lipid Res. 8:97–104. 1967.PubMed/NCBI
|
|
21
|
Spady DK and Dietschy JM: Sterol synthesis
in vivo in 18 tissues of the squirrel monkey, guinea pig, rabbit,
hamster, and rat. J Lipid Res. 24:303–315. 1983.PubMed/NCBI
|
|
22
|
Nervi F, Marinović I, Rigotti A and Ulloa
N: Regulation of biliary cholesterol secretion. Functional
relationship between the canalicular and sinusoidal cholesterol
secretory pathways in the rat. J Clin Invest. 82:1818–1825. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carey MC: Critical tables for calculating
the cholesterol saturation of native bile. J Lipid Res. 19:945–955.
1978.PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Davis HJ Jr, Zhu LJ, Hoos LM, Tetzloff G,
Maguire M, Liu J, Yao X, Iyer SP, Lam MH, Lund EG, et al:
Niemann-Pick C1 like 1 (NPC1L1) is the intestinal phytosterol and
cholesterol transporter and a key modulator of whole-body
cholesterol homeostasis. J Biol Chem. 279:33586–33592. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Buhman KK, Accad M, Novak S, Choi RS, Wong
JS, Hamilton RL, Turley S and Farese RV Jr: Resistance to
diet-induced hypercholesterolemia and gallstone formation in
ACAT2-deficient mice. Nat Med. 6:1341–1347. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang HH and Wang DQ: Reduced
susceptibility to cholesterol gallstone formation in mice that do
not produce apolipoprotein B48 in the intestine. Hepatology.
42:894–904. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Amigo L, Quinones V, Mardones P, Zanlungo
S, Miquel JF, Nervi F and Rigotti A: Impaired biliary cholesterol
secretion and decreased gallstone formation in apolipoprotein
E-deficient mice fed a high-cholesterol diet. Gastroenterology.
118:772–779. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang DQ, Zhang L and Wang HH: High
cholesterol absorption efficiency and rapid biliary secretion of
chylomicron remnant cholesterol enhance cholelithogenesis in
gallstone-susceptible mice. Biochim Biophys Acta. 1733:90–99. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang ZY, Jiang CY, Wang L, Wang JC, Zhang
SD, Einarsson C, Eriksson M, Han TQ, Parini P and Eggertsen G:
Increased NPC1L1 and ACAT2 expression in the jejunal mucosa from
Chinese gallstone patients. Biochem Biophys Res Commun. 379:49–54.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Y, Li M, Wu S and Tian Y: Combination
of curcumin and piperine prevents formation of gallstones in C57BL6
mice fed on lithogenic diet: Whether NPC1L1/SREBP2 participates in
this process? Lipids Health Dis. 14:1002015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Betters JL and Yu L: NPC1L1 and
cholesterol transport. Febs Lett. 584:2740–2747. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Valasek MA, Repa JJ, Quan G, Dietschy JM
and Turley SD: Inhibiting intestinal NPC1L1 activity prevents
diet-induced increase in biliary cholesterol in Golden Syrian
hamsters. Am J Physiol Gastrointest Liver Physiol. 295:G813–G822.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang HH, Portincasa P, Mendez-Sanchez N,
Uribe M and Wang DQ: Effect of ezetimibe on the prevention and
dissolution of cholesterol gallstones. Gastroenterology.
134:2101–2110. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zúñiga S, Molina H, Azocar L, Amigo L,
Nervi F, Pimentel F, Jarufe N, Arrese M, Lammert F and Miquel JF:
Ezetimibe prevents cholesterol gallstone formation in mice. Liver
Int. 28:935–947. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Duval C, Touche V, Tailleux A, Fruchart
JC, Fievet C, Clavey V, Staels B and Lestavel S: Niemann-Pick C1
like 1 gene expression is down-regulated by LXR activators in the
intestine. Biochem Biophys Res Commun. 340:1259–1263. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
van der Veen JN, Kruit JK, Havinga R,
Baller JF, Chimini G, Lestavel S, Staels B, Groot PH, Groen AK and
Kuipers F: Reduced cholesterol absorption upon PPARdelta activation
coincides with decreased intestinal expression of NPC1L1. J Lipid
Res. 46:526–534. 2005. View Article : Google Scholar : PubMed/NCBI
|