Introduction
Endometriosis, a disease in which endometrium-like
tissue grows outside of the uterine cavity, has been estimated to
affect 2 to 10% of reproductive-age women (1). The chief complaint of women with
endometriosis is pain, which negatively affects quality of life.
However, the pathogenesis of endometriosis-related pain, in
particularly, chronic pain remains elusive and needs further
investigation.
It is universally accepted that peritoneal
inflammation serves an important role in pain production (2). In previous years, the concept of
neuropathic pain has been recognized and given much concern and
study (3,4). Numerous studies have indicated the
presence of nerve fibers with neurotransmitters immunoreactive
cells in ovarian and deep infiltrating endometriosis, such as CGRP
and SP (5–7). Therefore, the research is crucially
needed to explore how the nerve fibers in endometriosis lesions
influence dorsal root neurons and brain to evoke pain. The
nociceptive sensors in DRG neurons are the first station in the
transmission of pain and are, thereby, a research hotspot for pain
study (3,8). An increasing number of studies
revealed that transient receptor potential vanilloid type 1 (TRPV1)
serves an important role in initiating neurogenic inflammation and
pain sensitization (9,10). TRPV1 is a member of non-selective
cation channels, which mediates responses to pain-inducing stimuli,
such as acid (pH<5.9), heat (>43°C), inflammatory mediators
and chemical irritants (11–13).
A previous study has revealed that the expression level of TRPV1
was higher in the ectopic endometrium than the normal endometrium.
This provides evidence that TRPV1 may be involved in dysmenorrhea
of adenomyosis (14). However, the
expression of TRPV1 in the dorsal root ganglia (DRG) and its role
in endometriosis related pain has not yet been further studied.
The present study was designed to investigate the
expression of TRPV1, calcitonin gene-related peptide (CGRP) and
substance P (SP) in the DRG of a rat endometriosis model. Moreover,
the authors use
N-(4-tertiarybutylphenyl)-4-(3-cholorphyridin-2-yl)-tetrahydro
pryazine-1(2H)-carbox-amide (BCTC), the selective antagonist of
TRPV1, to further confirm whether TRPV1 acts as an important
mediator in endometriosis-related pain.
Materials and methods
Animals
A total of 36 female Sprague-Dawley rats, (~8 weeks
old), were purchased from Shanghai Xipuer-Bikai Laboratory Animal
Science (Shanghai, China).
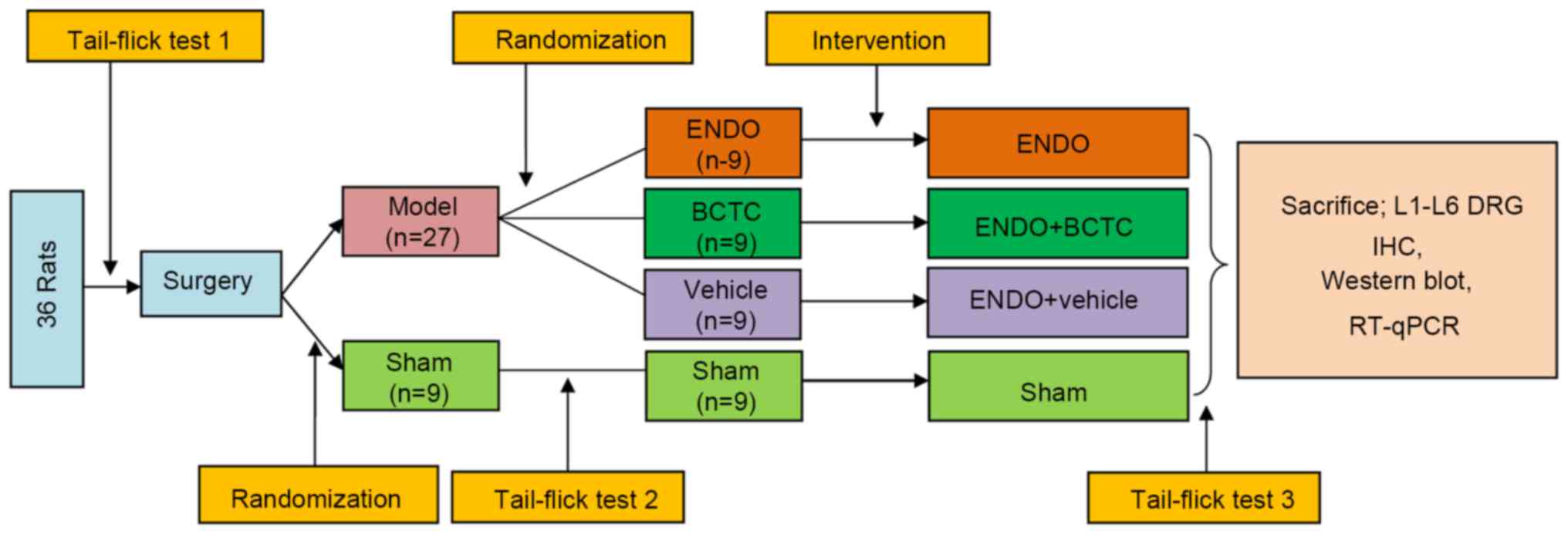
Experiment protocol
Following 3 days of acclimatization, an
endometriosis-inducing surgery was performed. Then all rats were
randomly divided into two groups: the model group (n=27) and the
sham group (n=9). At 4 weeks following surgery, model rats were
further randomly divided into three groups (n=9): ENDO group, BCTC
group and vehicle group. BCTC (3875, Tocris Bioscience, Bristol,
UK) dissolved in 25% cyclodextrin (332593; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) was administered to rats at 10 mg/kg body
weight, as previously reported (15–17).
The vehicle group were administered with 0.5 ml/100 g cyclodextrin.
A tail-flick test was performed prior to surgery, 1 h before and
after treatment of BCTC or vehicle groups. Finally, all rats were
sacrificed by cervical dislocation and decapitation, and the L1-L6
DRG were rapidly excised. Half of the DRG were fixed in 4%
paraformaldehyde for immunohistochemistry. The other half was
stored at −80°C for subsequent western blotting and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
L1-L6 DRG were used in the present study for the reason that the
tail and pelvic visceral pain afferents project to this area
(18). The flow chart of the
experiment is illustrated in Fig.
1. All the experimental procedures were approved by the
institutional experimental animals review board of Shanghai
Gynaecology and Obstetrics Hospital, Fudan University (Shanghai,
China).
Surgical procedures
The rats were anesthetized with 300 mg/kg chloral
hydrate. For the model group, following laparotomy, left uterine
horns were ligated using 7/0 silk suture and excised. Tubular
segment was incised to expose the endometrium, then cut into two
small pieces (~3 mm in diameter). For the sham group, same size of
fat tissues were excised from each abdomen. Two pieces of uterine
or fat tissues were sutured onto the peritoneum with a 7/0 silk
suture. At the end, the midline incision was closed with a 4/0 silk
suture. In order to stimulate the growth of implanted endometrium,
all rats received 0.2 mg/kg 17β-estradiol (E2758; Sigma-Aldrich;
Merck KGaA) in subcutaneous injection every 2 days for the next 2
weeks. Meanwhile, all rats were given penicillin (40,000 U/d)
intramuscularly for 3 days to avoid intraperitoneal infection
(19).
Tail-flick test
A beam of high-intensity light of tail-flick Meter
(Anhui Zhenghua Biological Instrument E quipment Co., Ltd., Anhui,
China) was given focused on the tail 2 cm distal to the tip while
gently restraining the animal by hand. The time (in sec) from the
start of the lighting to the flick of tail from the heat source was
evaluated. The average of three readings was used as the tail-flick
response latency for each animal. The basal response latency was
set as 2 sec, and 10 sec was set as the cut-off time to avoid
tissue damage. Tail flick latency was presented as the percentage
of maximal possible effect (MPE). MPE%=[(test response time-basal
response time)/(cut-off time-basal response time)]x100%.
Immunohistochemistry
Following tissue collection, DRG tissues were fixed
in 4% paraformaldehyde overnight at room temperature, prior to
sectioning into 4-µm thick sections, which was performed by
Servicebio (Wuhan, China). The paraffin sections were dried at 65°C
for 2 h, following routine deparaffinization and rehydration
procedures. Tissue slides were immersed in citric acid (0.1 mol/l)
and boiled at 98°C for 20 min before left cooling naturally at room
temperature. The slides were incubated with 10% goat serum in order
to block nonspecific binding agents. The rabbit polyclonal
antibodies against CGRP (ab47027; Abcam, Cambridge, MA, USA), TRPV1
(ab63083; Abcam), SP (sc9758; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) using as primary antibodies, were diluted to
1:200, 1:100, 1:50, respectively. Sections were incubated with
antibody overnight at 4°C in a humidified chamber. The biotinylated
secondary antibody (1:300; MR-M100, MingRui BioTech, Shanghai,
China) was incubated for 30 min at room temperature. The
streptavidin-peroxidase system was used. DAB was stained until
appropriate for microscopic examination. For negative control
slides, the primary antibody was replaced with PBS.
Western blot analysis
Following the manufacturer's instructions, total
proteins were extracted using radioimmunoprecipitation assay
reagent (Beyotime Institute of Biotechnology, Haimen, China), the
bicinchoninic acid assay (Beyotime Institute of Biotechnology) was
used to determine concentration of the protein lysate. Following
denaturation in loading buffer, the protein lysate was separated by
15% SDS-PAGE gel and transferred onto nitrocellulose membranes (EMD
Millipore, Billerica, MA, USA). The membranes were blocked with 5%
non-fat milk for 1 h and then incubated with the primary antibody
against TRPV1 (1:2,000), SP (1:500), CGRP (1:500) and β-actin
(1:4,000; ab8229; Abcam) overnight at 4°C. Following washing in TBS
containing 1% Tween-20 (TBST), the membranes were incubated with an
anti-rabbit horseradish peroxidase-conjugated secondary antibody
(1:5,000; sc2357; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. Following three washes with TBST, the blots were
visualized using an electrochemiluminescence system (Image Quant
LAS 4000 mini analyzer; General Electric, Fairfield, CT, USA) with
Image Quant LAS 4000 mini control software (version 1.2; General
Electric). The relative band intensities were analyzed using Image
Lab software (version 4.0; Bio-Rad Laboratories, Inc., Hercules,
CA, USA). The experiment was repeated three times.
RT-qPCR
Total RNA was extracted from L1-L6 DRG using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to the manufacturer's protocols. First Strand cDNA
was synthesized using Maxima First Strand cDNA Synthesis kit
(Thermo Fisher Scientific, Inc.). The RT-qPCR was performed in an
ABI PRISM 7000HT system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) in triplicates using the SYBR Green Master Mix
(Takara Biotechnology Co., Ltd., Dalian, China). The primers used
in RT-qPCR reactions are presented in Table I. The PCR conditions were as
follows according to the protocol: 15 sec at 95°C, 1 cycle; 5 sec
at 95°C and 34 sec at 60°C, for 40 cycles. β-actin served as the
internal control. The 2−ΔΔCq method was used to
calculate the relative mRNA levels (20).
 | Table I.Primers used in reverse
transcription-quantitative polymerase chain reactions. |
Table I.
Primers used in reverse
transcription-quantitative polymerase chain reactions.
| Gene name | Direction | Primer sequence | Length (bp) |
|---|
| TRPV1 | Forward |
AGCCAACGCAAGGAGTATGTG | 22 |
|
| Reverse |
CAGTAACAGGATGATGAAGACAGC | 25 |
| CGRP | Forward |
AAGTTCTCCCCTTTCCTGGTTG | 22 |
|
| Reverse |
TCCTGTTCCTCCTCCTGCTC | 20 |
| SP | Forward |
AACCCTGTAACGCACTATCTATTC | 24 |
|
| Revere |
CAGCAGCCTTTCTGTCTTTGG | 21 |
| β-actin | Forward |
TTGTCCCTGTATGCCTCTGGTC | 21 |
|
| Reverse |
CTTTAATGTCACGCACGATTTCCC | 23 |
Statistical analysis
Data are expressed as the mean ± standard deviation,
Student's t test was used to determine the significance of
differences between two groups. The comparison among three groups
was analyzed by one-way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference. All
computations were made with SPSS software (version, 17.0; Chicago,
IL, USA).
Results
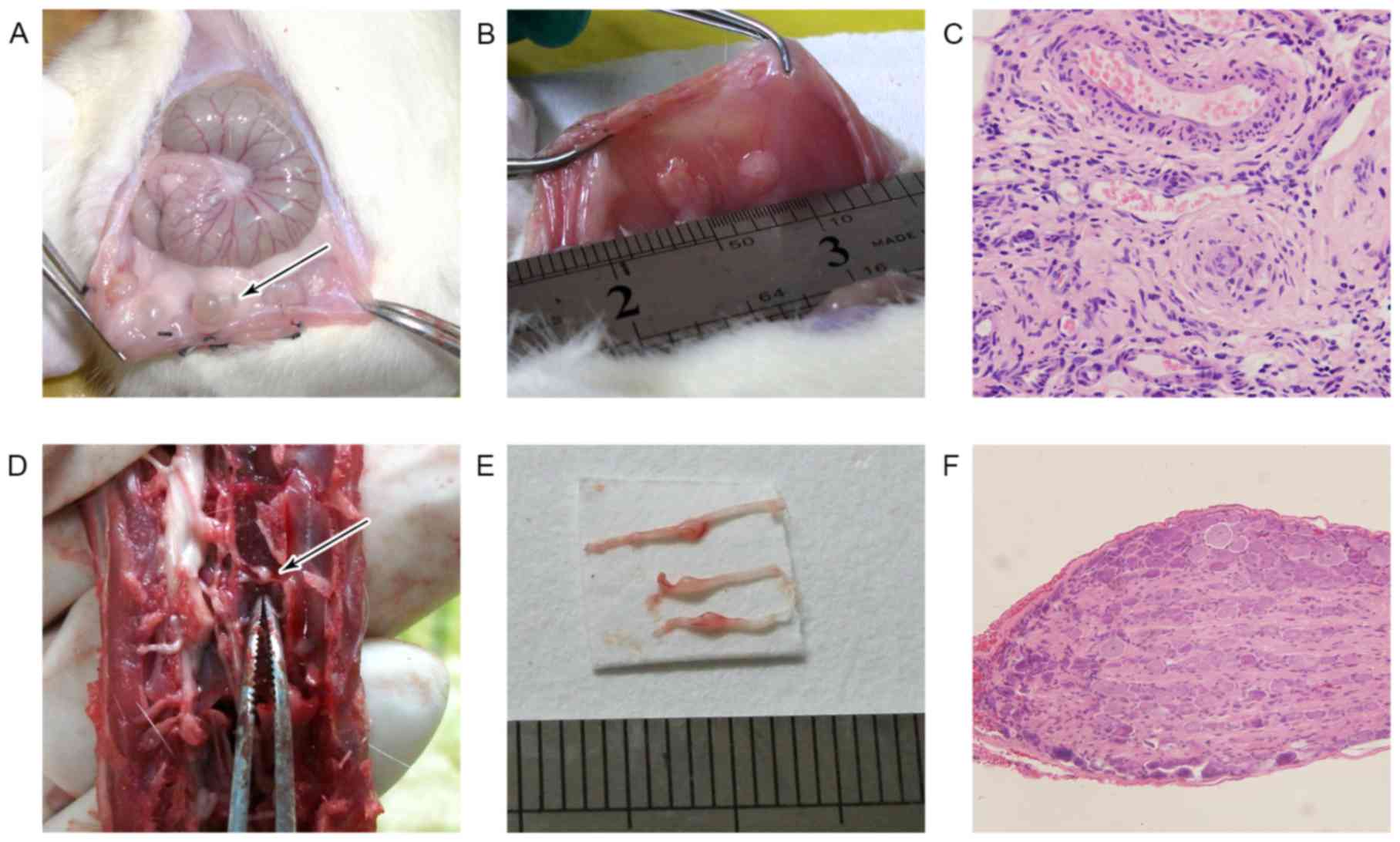
All rats under endometriosis surgery exhibited 1 or
2 cysts in transplanted sites (Fig.
2A) except one rat eliminated because of failing to form the
cyst in the vehicle group. There was no formed cyst in the rat of
sham group (Fig. 2B).
Tail-flick latency
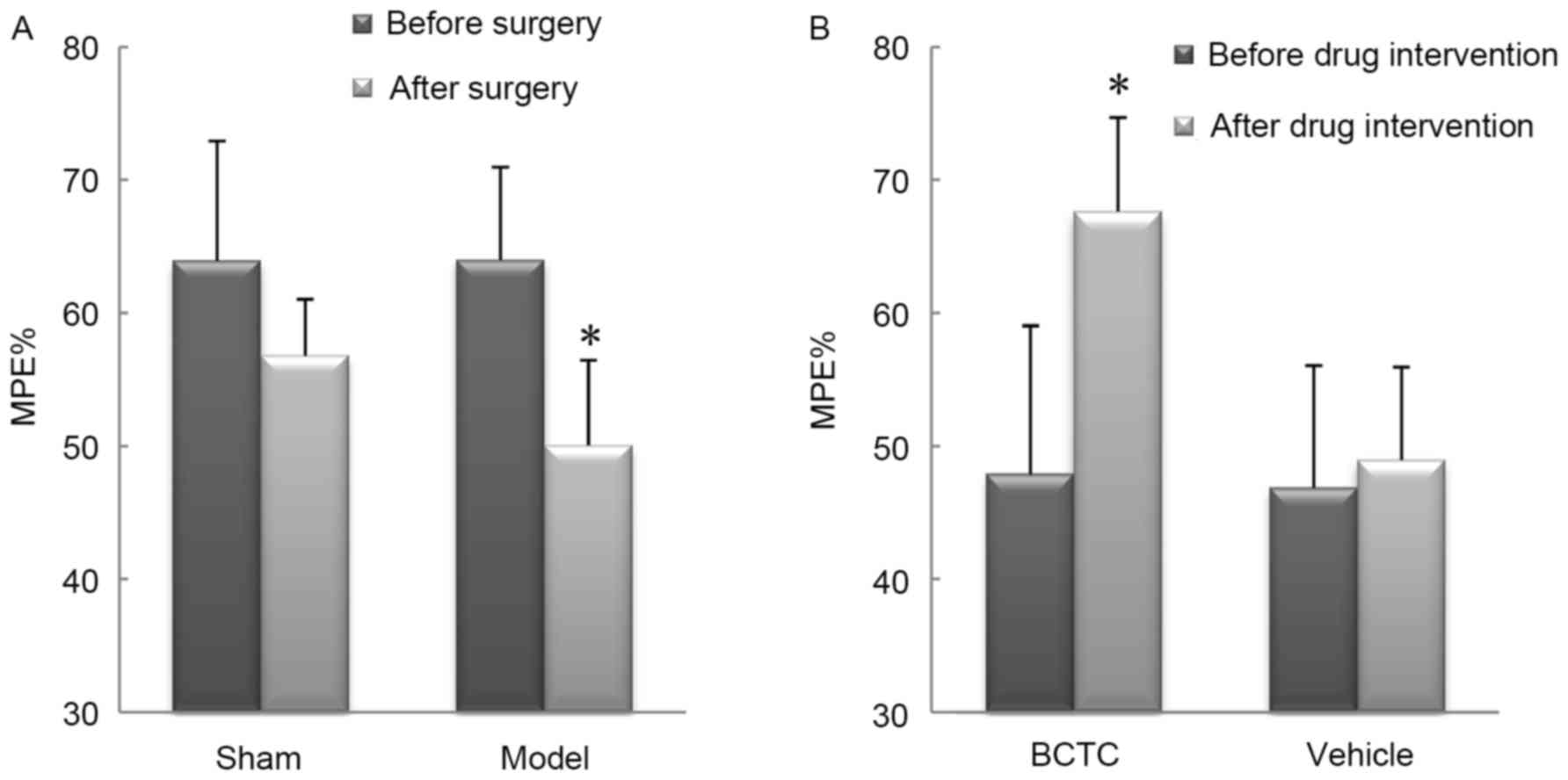
In tail-flick test 1, there was no significant
difference in MPE% between the sham group and the model group
(P=0.996) before sham or endometriosis surgery. In tail-flick test
2, four weeks following the surgery, the model group exhibited a
significant decrease in MPE% compared to pre-surgical baseline
(P<0.01). No significant changes were observed in MPE% of sham
group (P=0.081; Fig. 3A). In
tail-flick test 3, rats in the BCTC group had a significant
improvement in tail-flick latency 1 h following BCTC intervention
(P<0.01), while there were no significant changes for rats in
the vehicle group compared with MPE% before intervention (P=0.069;
Fig. 3B).
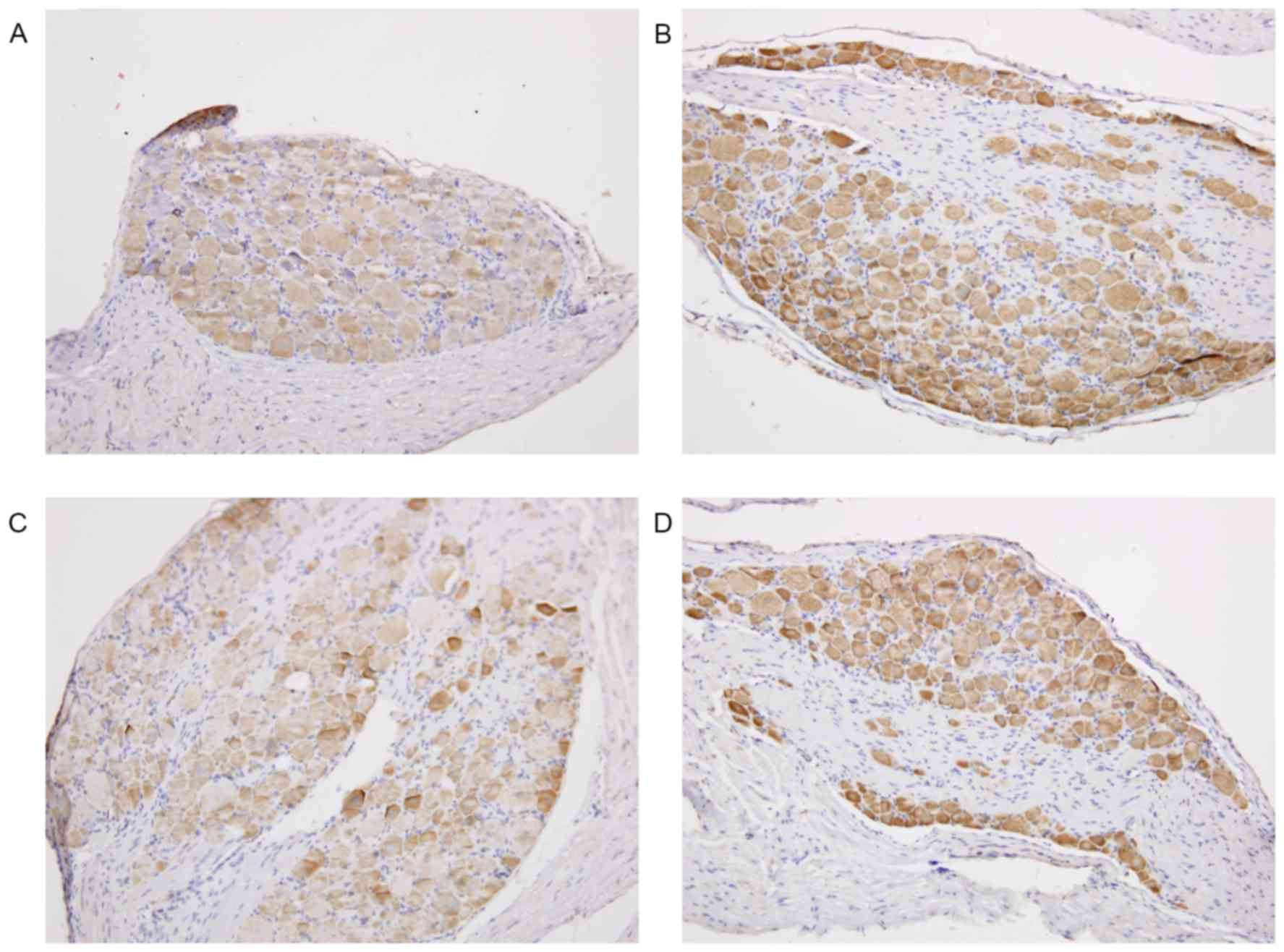
Immunostaining of TRPV1 protein
TRPV1 staining was primarily localized in the
cytoplasm of DRG cells. Increased immunostaining of TRPV1 was
observed in the DRG cells of rats with endometriosis compared with
rats in sham group. Treatment with BCTC resulted in a reduction of
immunoreactivity of TRPV1 in the DRG of rats with endometriosis
(Fig. 4).
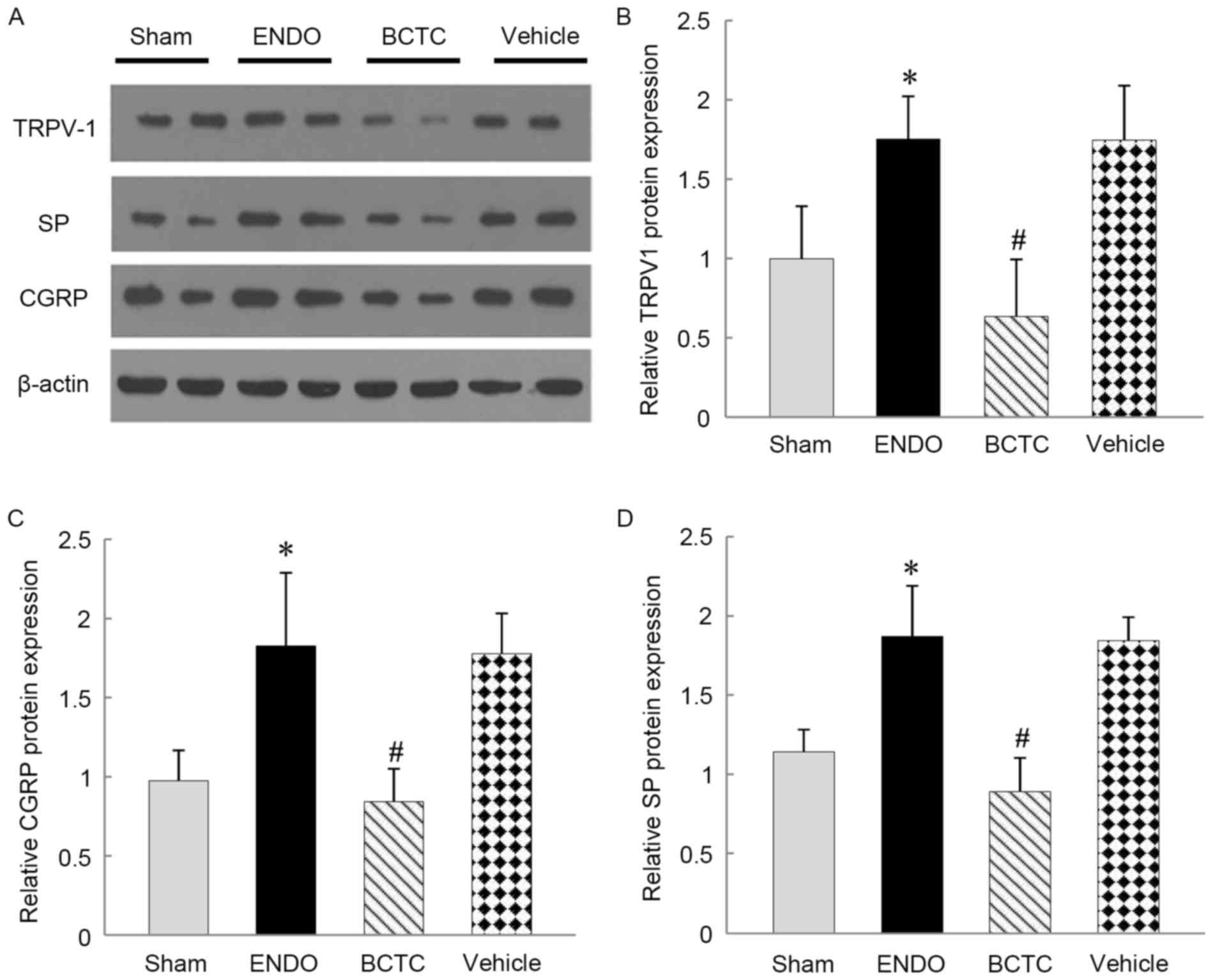
Western blot analysis
As is presented in the bar graph of Fig. 5, the relative expression levels of
TRPV1, SP and CGRP protein in DRG were significantly higher in the
ENDO group compared with sham group (all P<0.05). TRPV1, SP and
CGRP expressions were decreased in the BCTC group compared with the
ENDO group (P<0.01). There was no significant difference in the
relative expression levels of TRPV1 (P=0.998), SP (P=0.841) and
CGRP (P=0.801) in the vehicle group compared with the ENDO
group.
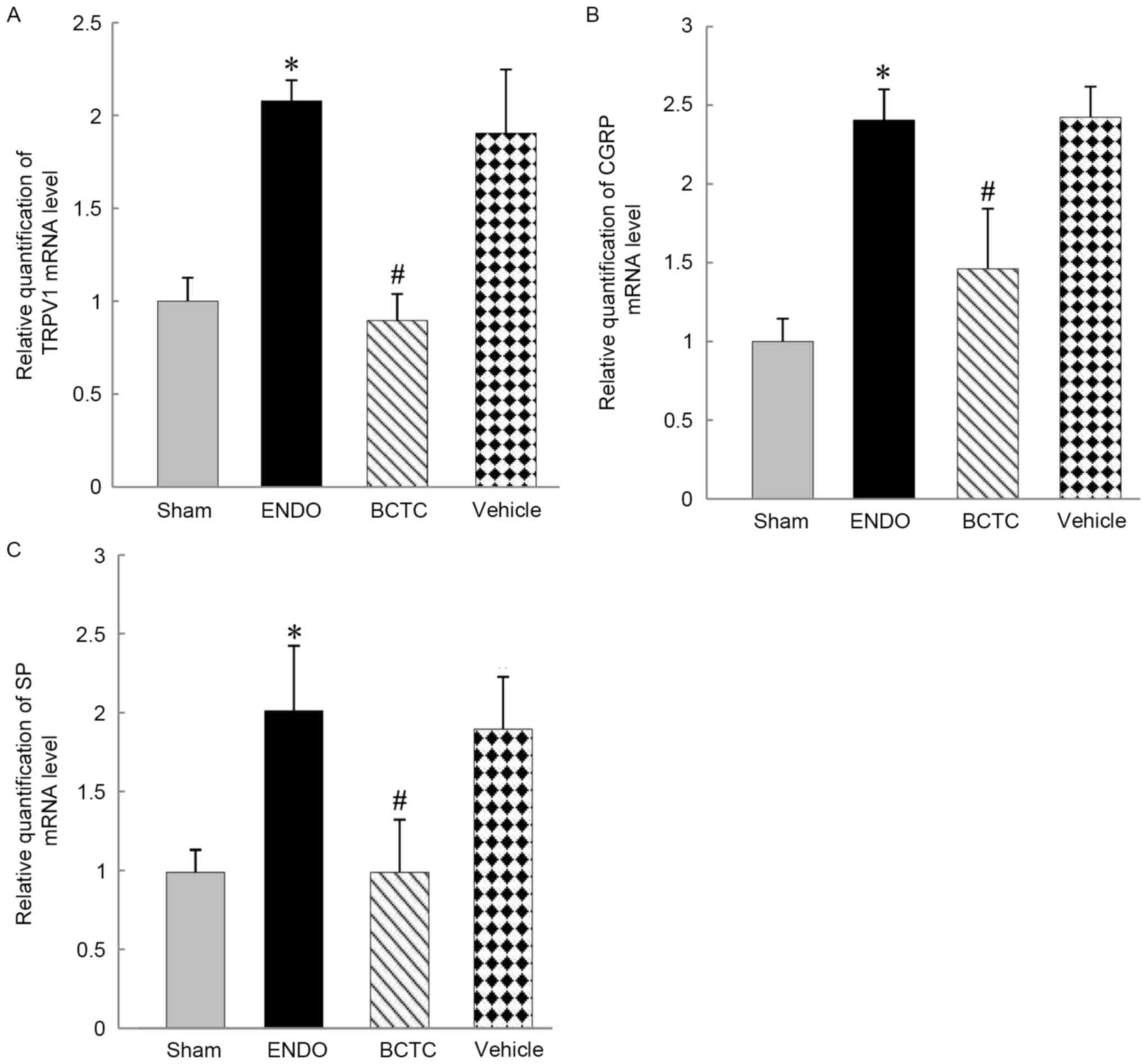
RT-qPCR analysis
The mRNA levels of TRPV1, SP and CGRP in DRG were
significantly higher in the ENDO group compared with sham group
(all P<0.01). Expressions of TRPV1, SP and CGRP mRNA were
significantly lower in the BCTC group compared with the ENDO group
(all P<0.01). There were no significant differences in TRPV1, SP
and CGRP mRNA levels between vehicle group and ENDO groups
(P=0.273, 0.629, 0.928, respectively) (Fig. 6A).
Discussion
Women with endometriosis suffer from different kinds
of pain. Pain is usually caused by activity of unmyelinated and
thinly myelinated primary afferent neurons (21). Primary afferent neurons are
normally silent without stimulation; DRG neurons that receive
afferent input from irritated structures will develop pathological
activity in case of nerve injury, inflammation or neuropathy in
pelvic organs. The pathological changes include great molecular and
cellular changes at the level of the primary afferent (22,23).
Increased TRPV1 mRNA and protein in DRGs have been described in
several inflammation and nerve injury models (24–26).
In the present study, the authors indicated that rats with
endometriosis exhibited a significant decrease in thermal response
latency, and the expression of TRPV1, SP and CGRP in L1-L6 DRG
significantly increased compared with rats in sham group, which
provides an important insight into the action of central
sensitization in spinal cord of endometriosis rat, an important
addition to peripheral sensitization.
The authors further investigated the function of
TRPV1 in endometriosis by using BCTC, a selective antagonist of the
rat TRPV1 receptor. The plasma half-life of BCTC was 1 h, BCTC was
orally bioavailable and could significant penetrate into the
central nervous system of the rat. Besides, BCTC could block the
activation of rat TRPV1 not only by capsaicin, but also by low pH,
which made it a good candidate for exploring the role of TRPV1 in
rat models of human disease (27).
In the present study, BCTC significantly enhanced the thermal
response latency, while there was no significant improvement in the
vehicle group. Moreover, the expression of TRPV1, SP and CGRP
proteins and mRNA levels were significantly downregulated in DRG
tissues of endometriotic rat 1 h following BCTC administration,
while that did not significantly differ from that of the vehicle
group. The results provide clues to hypothesize that TRPV1 may
serve an important role in central sensitization of
endometriosis-associated pain.
A large number of studies indicate that TRPV1
involves the triggering of signal transduction cascades of pain
sensitization (9,28–30).
The sensitization of primary afferent nociceptive neurons due to
activation of TRPV1 could evoke the local release of
proinflammatory neuropeptides, such as CGRP and SP, which initiate
neurogenic inflammation (31,32).
CGRP and SP, in turn, can modulate TRPV1 through activating their
effector cell receptors in the peripheral nervous system, providing
a positive feedback mechanism that amplifies the effect of
neurogenic inflammation (33–36).
The transduction mechanism of TRPV1 involves that noxious
stimulation induce voltage-dependent Na+ and
Ca+ ions influx into the cytoplasm of the afferent
nociceptive neurons after the opening of nonselective cation
channels, leading to the depolarization of the afferent neurons. It
then causes exocytosis and the release of inflammatory mediators
into the periphery, resulting in a sensation of pain (37–39).
To the best of the authors' knowledge, the current
study is the first description of TRPV1 expression in DRG of an
endometriosis rat, and it has provided evidence that TRPV1 may
serve an important role in central sensitization of
endometriosis-associated pain. The results of this present study
also corroborate the deduction of previous literature wherein TRPV1
channel may be at least one of the important factors in the
occurrence of endometriosis-related pain (14). Due to the complexity of the
etiology of endometriosis, current targeted therapy remains
unsatisfactory, including analgesic, anti-inflammatory, hormonal
and surgical treatments (40).
Considering the mechanisms and therapeutic implications of
neuropathic pain, the authors propose potential treatment of
anti-TRPV1 therapy for the elimination of endometriosis-associated
pain.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 30901942).
Glossary
Abbreviations
Abbreviations:
|
TRPV1
|
transient receptor potential vanilloid
type 1
|
|
SP
|
substance P
|
|
CGRP
|
calcitonin gene-related peptide
|
|
DRG
|
dorsal root ganglia
|
References
|
1
|
Eskenazi B and Warner ML: Epidemiology of
endometriosis. Obstet Gyn Clin North Am. 24:235–258. 1997.
View Article : Google Scholar
|
|
2
|
Asante A and Taylor RN: Endometriosis: the
role of neuroangiogenesis. In: Annual Review of PhysiologyJulius D
and Clapham DE: Annual Reviews. 73. Palo Alto: pp. 163–182.
2011
|
|
3
|
Baron R: Mechanisms of disease:
Neuropathic pain-a clinical perspective. Nat Clinl Pract Neuro.
2:95–106. 2006. View Article : Google Scholar
|
|
4
|
Anaf V, El Nakadi I, de Moor V, Chapron C,
Pistofidis G and Noel JC: Increased nerve density in deep
infiltrating endometriotic nodules. Gynecol Obstet Inves.
71:112–117. 2011. View Article : Google Scholar
|
|
5
|
Tokushige N, Markham R, Russell P and
Fraser IS: Nerve fibres in peritoneal endometriosis. Hum Reprod.
21:3001–3007. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anaf V, Simon P, El Nakadi I, Fayt I,
Simonart T, Buxant F and Noel JC: Hyperalgesia, nerve infiltration
and nerve growth factor expression in deep adenomyotic nodules,
peritoneal and ovarian endometriosis. Hum Reprod. 17:1895–1900.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Berkley KJ, Rapkin AJ and Papka RE: The
pains of endometriosis. Science. 308:1587–1589. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rose KE, Lunardi N, Boscolo A, Dong X,
Erisir A, Jevtovic-Todorovic V and Todorovic SM: Immunohistological
demonstration of CaV3.2 T-type voltage-gated calcium channel
expression in soma of dorsal root ganglion neurons and peripheral
axons of rat and mouse. Neuroscience. 250:263–274. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y: The functional regulation of TRPV1
and its role in pain sensitization. Neurochem Res. 33:2008–2012.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen HS, He X, Wang Y, Wen WW, You HJ and
Arendt-Nielsen L: Roles of capsaicin-sensitive primary afferents in
differential rat models of inflammatory pain: A systematic
comparative study in conscious rats. Exp Neurol. 204:244–251. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hagenacker T and Büsselberg D: Modulation
of intracellular calcium influences capsaicin-induced currents of
TRPV-1 and voltage-activated channel currents in nociceptive
neurones. J Peripher Nerv Syst. 12:277–284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Caterina MJ, Schumacher MA, Tominaga M,
Rosen TA, Levine JD and Julius D: The capsaicin receptor: A
heat-activated ion channel in the pain pathway. Nature.
389:816–824. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Caterina MJ and Julius D: The vanilloid
receptor: A molecular gateway to the pain pathway. Annu Rev
Neurosci. 24:487–517. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nie JC, Liu XS and Guo SW:
Immunoreactivity of oxytocin receptor and transient receptor
potential vanilloid type 1 and its correlation with dysmenorrhea in
adenomyosis. Am J Obstet Gynecol. 202:346.e1–e8. 2010. View Article : Google Scholar
|
|
15
|
Tékus V, Bölcskei K, Kis-Varga A, Dézsi L,
Szentirmay E, Visegrády A, Horváth C, Szolcsányi J and Petho G:
Effect of transient receptor potential vanilloid 1 (TRPV1) receptor
antagonist compounds SB705498, BCTC and AMG9810 in rat models of
thermal hyperalgesia measured with an increasing-temperature water
bath. Eur J Pharmacol. 641:135–141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tanaka H, Shimaya A, Kiso T, Kuramochi T,
Shimokawa T and Shibasaki M: Enhanced insulin secretion and
sensitization in diabetic mice on chronic treatment with a
transient receptor potential vanilloid 1 antagonist. Life Sci.
88:559–563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Winter BY, Bredenoord AJ, van Nassauw
L, de Man JG, de Schepper HU, Timmermans JP and Pelckmans PA:
Involvement of afferent neurons in the pathogenesis of
endotoxin-induced ileus in mice: Role of CGRP and TRPV1 receptors.
Eur J Pharmacol. 615:177–184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chaban VV: Visceral sensory neurons that
innervate both uterus and colon express nociceptive TRPv1 and P2×3
receptors in rats. Ethn Dis. 18 2 Suppl 2:S2-20-42008.PubMed/NCBI
|
|
19
|
Liu M, Liu X, Zhang Y and Guo SW: Valproic
acid and progestin inhibit lesion growth and reduce hyperalgesia in
experimentally induced endometriosis in rats. Reprod Sci.
19:360–373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hara T, Chiba T, Abe K, Makabe A, Ikeno S,
Kawakami K, Utsunomiya I, Hama T and Taguchi K: Effect of
paclitaxel on transient receptor potential vanilloid 1 in rat
dorsal root ganglion. Pain. 154:882–889. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jänig W and Koltzenburg M: On the function
of spinal primary afferent-fibers supplying colon and
urinary-bladder. J Auton Nerv Syst. 30 Suppl:S89–S96. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McMahon SB: Sensitisation of
gastrointestinal tract afferents. Gut. 53 Suppl 2:ii13–ii15. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu X, Wang P, Zou X, Li D, Fang L and Lin
Q: Increases in transient receptor potential vanilloid-1 mRNA and
protein in primary afferent neurons stimulated by protein kinase C
and their possible role in neurogenic inflammation. J Neurosci Res.
87:482–494. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Amaya F, Oh-Hashi K, Naruse Y, Iijima N,
Ueda M, Shimosato G, Tominaga M, Tanaka Y and Tanaka M: Local
inflammation increases vanilloid receptor 1 expression within
distinct subgroups of DRG neurons. Brain Res. 963:190–196. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tominaga M, Caterina MJ, Malmberg AB,
Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI and Julius
D: The cloned capsaicin receptor integrates multiple pain-producing
stimuli. Neuron. 21:531–543. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Valenzano KJ, Grant ER, Wu G, Hachicha M,
Schmid L, Tafesse L, Sun Q, Rotshteyn Y, Francis J, Limberis J, et
al:
N-(4-tertiarybutylphenyl)-4-(3-chloropyridin-2-yl)tetrahydropyrazine-1(2H)-carbox-amide
(BCTC), a novel, orally effective vanilloid receptor 1 antagonist
with analgesic properties: I. In vitro characterization and
pharmacokinetic properties. J Pharmacol Exp Ther. 306:377–386.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Khasar SG, Lin YH, Martin A, Dadgar J,
McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, et
al: A novel nociceptor signaling pathway revealed in protein kinase
C epsilon mutant mice. Neuron. 24:253–260. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jung J, Shin JS, Lee SY, Hwang SW, Koo J,
Cho H and Oh U: Phosphorylation of vanilloid receptor 1 by
Ca2+/calmodulin-dependent kinase II regulates its
vanilloid binding. J Biol Chem. 279:7048–7054. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aley KO, Martin A, McMahon T, Mok J,
Levine JD and Messing RO: Nociceptor sensitization by extracellular
signal-regulated kinases. J Neurosci. 21:6933–6939. 2001.PubMed/NCBI
|
|
31
|
Kilo S, Harding-Rose C, Hargreaves KM and
Flores CM: Peripheral CGRP release as a marker for neurogenic
inflammation: A model system for the study of neuropeptide
secretion in rat paw skin. Pain. 73:201–207. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kessler F, Habelt C, Averbeck B, Reeh PW
and Kress M: Heat-induced release of CGRP from isolated rat skin
and effects of bradykinin and the protein kinase C activator PMA.
Pain. 83:289–295. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qiao LY and Grider JR: Up-regulation of
calcitonin gene-related peptide and receptor tyrosine kinase TrkB
in rat bladder afferent neurons following TNBS colitis. Exp Neurol.
204:667–679. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Linhart O, Obreja O and Kress M: The
inflammatory mediators serotonin, prostaglandin E2 and bradykinin
evoke calcium influx in rat sensory neurons. Neuroscience.
118:69–74. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang N, Inan S, Cowan A, Sun R, Wang JM,
Rogers TJ, Caterina M and Oppenheim JJ: A proinflammatory
chemokine, CCL3, sensitizes the heat- and capsaicin-gated ion
channel TRPV1. Proc Natl Acad Sci USA. 102:4536–4541. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Olah Z, Karai L and Iadarola MJ: Protein
kinase C(alpha) is required for vanilloid receptor 1 activation.
Evidence for multiple signaling pathways. J Biol Chem.
277:35752–35759. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Y, Malmberg AB, Yaksh TL, Sjölund B,
Sundler F and Håkanson R: Capsaicin-evoked release of pituitary
adenylate cyclase activating peptide (PACAP) and calcitonin
gene-related peptide (CGRP) from rat spinal cord in vivo. Regul
Pept. 69:83–87. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Szallasi A and Blumberg PM: Vanilloid
(capsaicin) receptors and mechanisms. Pharmacol Rev. 51:159–212.
1999.PubMed/NCBI
|
|
39
|
Lin Q, Li D, Xu X, Zou X and Fang L: Roles
of TRPV1 and neuropeptidergic receptors in dorsal root
reflex-mediated neurogenic inflammation induced by intradermal
injection of capsaicin. Mol Pain. 3:302007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Stratton P and Berkley KJ: Chronic pelvic
pain and endometriosis: Translational evidence of the relationship
and implications. Hum Reprod Update. 17:327–346. 2011. View Article : Google Scholar : PubMed/NCBI
|