Introduction
The cochlear blood-labyrinth-barrier (BLB), located
in the stria vascularis, is analogous to the blood-brain-barrier
(BBB) and serves a critical role in maintaining homeostasis of
cochlear solutes and ion transport (1,2).
Tracer studies of uptake of sodium, calcium and albumin from blood
into the perilymph have indicated how difficult it is for these
substances to penetrate the BLB into the inner ear (3). The low permeability of the BLB limits
the entry of inflammatory and infectious agents into the central
nervous system (2).
It is well known that tight junction (TJ) proteins
between adjacent microvascular endothelial cells in brain are the
primary contributor to low paracellular permeability and high
electrical resistance of the BBB (4). Downregulation of TJ proteins disrupts
the BBB (5). Similarly, strial
capillaries are also enriched in TJ and cell adhesion proteins,
which suggests they serve a role in the impermeability of the BLB
(6). Significant disruption of the
BLB occurs early in noise-induced hearing loss, and a loosening of
TJs and significantly increased BLB permeability are also observed
(7–9).
TJs are transmembrane proteins are linked to the
actin cytoskeleton through cytoplasmic accessory proteins,
including zonula occludens (ZO) (10). ZO proteins, particularly ZO-1, are
ubiquitous as scaffolds which provide the foundation for assembly
of multi-protein complexes on the cytoplasmic surface of the plasma
membrane. ZO proteins are actively involved in the remodeling of
junctional complexes in a number of cellular systems (11). As ZO-1 often serves as a crucial
central regulator of structural organization, it is used as an
observation index of blood-tissue-barrier function (5,10).
A previous study demonstrated that matrix
metalloproteinase (MMP)-2 and −9, secreted by leukemic cells,
affect ZO-1 and disrupt the integrity of the BBB (5). MMP-2 and −9 are known to degrade
collagen IV, the major component of extracellular matrix (ECM)
(12). Previous studies have also
revealed MMP-2 and −9 to be extensively expressed in the basal
membrane, spiral ganglion and stria vascularis. Expression of MMP-2
and −9 dynamically alters following noise exposure (13,14).
However, the involvement of MMP-2 and −9 in noise-induced
impairment of the BLB remains to be fully demonstrated. Based on
the results of previous studies, the present study hypothesized
that degradation of the TJ protein ZO-1 by MMP-2 and −9 is an
important mechanism in the noise-induced breakdown of the BLB. This
hypothesis was tested in noise-exposed guinea pigs and subsequently
assessed by RNA-sequencing (RNA-seq) and immunofluorescence.
Materials and methods
Animals
40 Adult guinea pigs (weight, 250–400 g; 20 male and
20 female) were purchased from Chinese PLA General Hospital
Laboratory Animal Center (Beijing, China) and normal tympanic
membrane, and Preyer's reflex were used in our experiments. All
animals were maintained in the same conditions, constant
temperature of 21–24°C and humidity of 40–70%, 12-h light/12-h dark
cycle, with free access to food and water. The experiment protocol
on animal use and care was reviewed and approved by the
Institutional Animal Care and Use Committee of the Chinese People's
Liberation Army General Hospital (Beijing, China).
Noise exposure
After evaluating baseline hearing, the guinea pigs
were randomly assigned to noise-exposure or control groups (n=20
per group). Animals in the noise-exposure group were placed in a
wire mesh cage and exposed to white noise at 120 dB SPL for 4 h on
2 consecutive days. This noise exposure regime is routinely used in
this laboratory and produces a permanent loss in cochlear
sensitivity.
Auditory brainstem response (ABR)
ABRs to pure tone bursts were used to evaluate
hearing function prior to and following noise exposure. Each animal
was anesthetized with an intraperitoneal injection of 10% chloral
hydrate (4 ml/kg, Sinopharm Chemical Reagent Co., Ltd., Shanghai,
China) and placed in a sound-isolated chamber. The body temperature
was maintained at 37.5°C with a warming blanket. Stainless-steel
needle electrodes were placed subdermally at the vertex
(noninverting input) and behind the stimulated and non-stimulated
ear (inverting input and ground, respectively). Each ear was
stimulated separately with a closed tube sound delivery system
sealed into the ear canal. ABRs were induced with clicks at 90 dB
SPL and the stimulus level was decreased in 10 dB steps until no
response was identifiable. The signal was band-pass filtered
(100~3,000 Hz), amplified (x50,000), and averaged using Tucker
Davis Technologies (TDT) System II hardware and SigGen/BioSig
version 4.4.1 (TDT, RX6, Alachua, FL, USA) software. Responses were
stored and displayed on a computer. The ABR threshold was defined
as the lowest stimulus intensity which reliably induces a
detectable response.
Collection of the stria vascularis
tissue
Animals were anesthetized with 10% chloral hydrate
and decapitated. The cochlea was quickly removed from the skull as
previously described (15). For
analysis of the transcriptional expression pattern of the MMPs and
associated genes, the cochlea was perfused with an RNA
stabilization reagent (RNAlater; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and dissected in the same reagent. Each turn of
the bony cochlear lateral wall, including the spiral ligament and
stria vascularis, was separated from the organ of Corti, and the
stria vascularis was gently peeled away from the spiral ligament.
The strial tissue was immediately frozen in dry ice for 10 min
before being stored at −80°C. Dissection of the strial vasculature
from both cochleae was completed within 30 min to ensure the
quality of the subsequent RNA analysis. For immunohistological and
histological examinations, the cochlea was fixed in 4%
paraformaldehyde overnight. The cochlea was then dissected in PBS
to harvest the stria vascularis.
RNA-seq
The stria vascularis from 8 cochleae (4 animals) was
pooled to generate one sample. A total of three biological repeats
were performed. Total RNA was extracted from the tissue using an
Agilent RNA 6000 Pico kit (Agilent Technologies, Inc., Santa Clara,
CA, USA) as per the manufacturer's protocol. Total RNA of the
collected sample was assessed with an Agilent Bioanalyzer 2100
(Agilent Technologies, Santa Clara, CA) and a RiboMinus Eukaryote
kit (Ambion; Thermo Fisher Scientific, Inc.).
Synthesis of cDNA from 8–10 ng of total RNA per
sample was performed with an Agilent High Sensitivity DNA kit
(Agilent Technologies, Inc.). A sequencing library was prepared for
each cDNA sample with an Ion PI™ Template OT2 200 Kit v2 (Ambion;
Thermo Fisher Scientific, Inc.) used according to the
manufacturer's protocol. The average insert size of the libraries
was 124 bp. Each cDNA library was sequenced in a 50-cycle single
read flow cell lane on an Illumina HiSeq 2000 system.
Immunofluorescence confocal
microscopy
Primary antibodies used in the experiment included
monoclonal mouse anti-MMP-2 (catalog no. MAB3308; EMD Millipore,
Billerica, MA, USA), polyclonal rabbit anti-MMP-9 (catalog no.
AB19016; EMD Millipore) and polyclonal rabbit anti-ZO-1 (catalog
no. 617300; Invitrogen; Thermo Fisher Scientific, Inc.). Secondary
antibodies included Alexa Fluor 488-conjugated goat anti-mouse IgG
(catalog no. A11001) and Alexa Fluor 568-conjugated donkey
anti-rabbit IgG antibodies (catalog no. A10042), both purchased
from Invitrogen; Thermo Fisher Scientific, Inc. Stria vascularis
samples were fixed in 4% paraformaldehyde at 4°C for 2 h, washed in
PBS for 30 min, permeabilized in 0.5% Triton X-100 for 1 h, and
immunoblocked in a solution of 5% goat serum (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China) in PBS for 1 h.
The specimens were incubated overnight at 4°C with the primary
antibody diluted (1:200) in PBS. After several washes in PBS,
tissues were incubated with secondary antibodies (1:200) at room
temperature for 1 h. The fluorescence was visualized under an
Olympus IX81 inverted microscope fitted with an Olympus Fluoview
FV1000 confocal laser system. The samples were examined as above,
and Z-series stacks were acquired at 1-um intervals. The Z-series
images were visualized using Image J 1.30 software (National
Institutes of Health, Bethesda, MD, USA).
Evaluation of BLB permeability
BLB integrity was assessed by evaluating the
extravasation and diffusion of a non-permeable dye (Evan's blue;
EBD) around strial capillaries. Under deep anesthesia with 10%
chloral hydrate as aforementioned, 2% EBD (20 mg/ml/kg; E2129;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was injected into
the femoral vein 2 h prior to sacrifice. The cochlea was perfused
with 4% paraformaldehyde in 0.1 M PBS in the vicinity of the round
and oval windows, and the stria vascularis gently dissected from
the bony cochlear lateral wall and fixed overnight in 4%
paraformaldehyde at 4°C. The degree of EBD extravasation in the
stria vascularis was assessed by reading the fluorescence under an
Olympus IX81 inverted microscope fitted with an Olympus Fluoview
FV1000 confocal laser system. Image processing and fluorescence
analysis of the images were performed using Image J 1.30
software.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Comparisons between two groups were analyzed by Student's t-test
and one-way analysis of variance with Turkey honest significant
difference post hoc test was used for multiple comparisons on SPSS
version 17 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
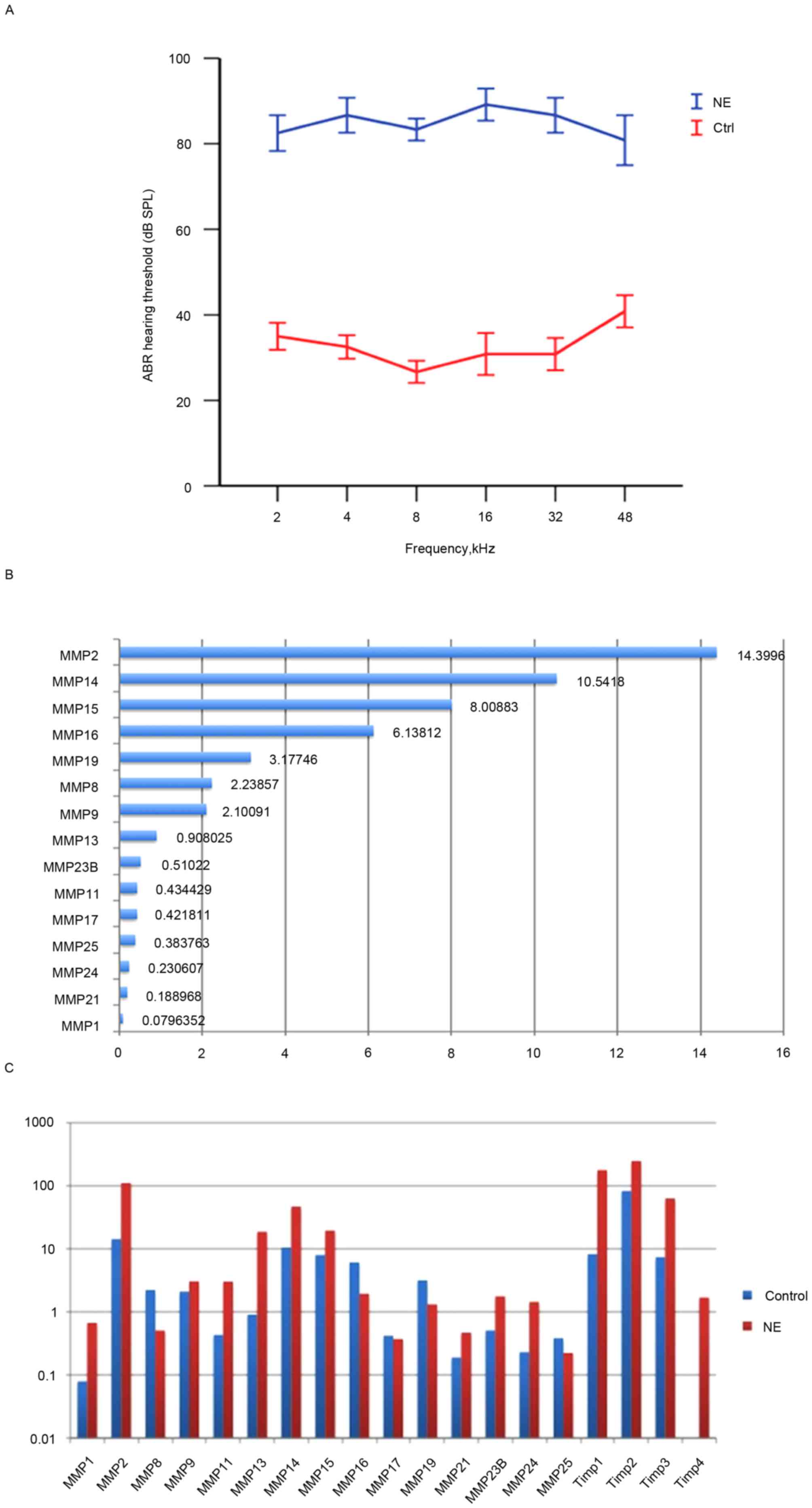
Noise exposure causes loss in cochlear
sensitivity
Exposure to white noise at 120 dB SPL for 4 h on 2
consecutive days caused significant loss in hearing sensitivity in
the experimental animals. A total of 2 days after noise exposure,
ABR thresholds at all test frequencies were significantly elevated
(P<0.05; Fig. 1A), a result
consistent with previous studies (9,16).
Multiple MMPs and associated genes are
constitutively expressed in healthy guinea pig strial tissue
The healthy cochlea has been demonstrated to be
enriched in MMP enzymatic activity (13). However, a comprehensive
understanding of the distribution and expression patterns of MMPs
and their associated genes in the stria vascularis has remained
elusive. Therefore, the transcriptional expression of these genes
was profiled in the healthy stria vascularis.
RNA-seq was used to screen mRNA transcripts in the
cochlear stria vascularis. A total of three biological repeats were
performed, and transcript levels were quantified in Reads Per
Kilobase of exon model per Million mapped reads (RPKM). An RPKM
value >0.1 was considered the threshold. The expression of 15
MMP genes and 3 tissue inhibitor of matrix metalloproteinase (TIMP)
genes in the stria vascularis were assessed. Among the 15 MMP genes
detected were 2 gelatinases (MMP-2 and −9), 3 collagenases (MMP-1,
−8 and −13), 2 stromelysins (MMP-11 and −19), 6 membrane-type MMPs
(MMP-14, −15, −16, −17, −24 and −25), and 2 other MMPs (MMP-21 and
−23B), data not shown.
To further assess the expression pattern, RNA-seq
was performed to assess the transcriptional expression levels of
the 15 MMP and 3 TIMP genes. A total of 7 high expression genes
were identified, including Timp2, Mmp2, Mmp14, Timp1, Mmp15, Timp3,
Mmp16, Mmp19, Mmp8 and Mmp9. Interestingly, Timp-2 was also highly
expressed in healthy stria vascularis, consistent with Hu's data
(13). In contrast, the expression
levels of Mmp13, Mmp23B, Mmp11, Mmp17, Mmp25, Mmp24, Mmp21 and Mmp1
were low (Fig. 1B).
Noise trauma causes dynamic
alterations in the expression level of MMP-associated genes in the
stria vascularis
To determine alterations in the expression of MMPs
and their associated genes following acoustic trauma, qRT-qPCR was
performed immediately after noise exposure to assess MMP
expression. The results revealed that 10 MMP genes were upregulated
(Mmp1, Mmp2, Mmp9, Mmp11, Mmp13, Mmp14, Mmp15, Mmp21, Mmp23B,
Mmp24), while 5 MMP genes were downregulated (Mmp8, Mmp16, Mmp17,
Mmp19, Mmp25). Notably, expression levels of the two gelatinases
(Mmp2 and −9) were both elevated, while the other MMP genes
demonstrated a mixed pattern. In addition, the 3 MMP inhibitors
(Timp1, Timp2 and Timp3) were upregulated. No genes appeared to
remain unaltered, which highlighted the marked effect that noise
trauma has on MMP-associated genes (Fig. 1C).
Steady MMP-2 and MMP-9 immunolabeling
in the healthy stria vascularis, and marked alterations in
immunoreactivity following noise trauma
MMP-2 and −9 genes were demonstrated in the healthy
stria vascularis by RNA-seq. An immunolabeling assay was employed
to assess the distribution of MMP-2 (Fig. 2A) and −9 (Fig. 2B) in the stria vascularis. As both
MMP-2 and −9 have been demonstrated to degrade the ECM, the present
experiment focused on these two MMPs. In the healthy stria
vascularis, weak MMP-2 and −9 immunoreactivity was present in
marginal cells and basal cells, with no reactivity in intermediate
cells, consistent with RNA-seq results. In noise-traumatized
cochleae, there was a significant increase in MMP-2 and −9
immunoreactivity in the stria vascularis (Fig. 2A and B, respectively). Labeling was
intense not only in marginal cells and basal cells, but significant
immunoreactivity was also seen in intermediate cells (Fig. 2C).
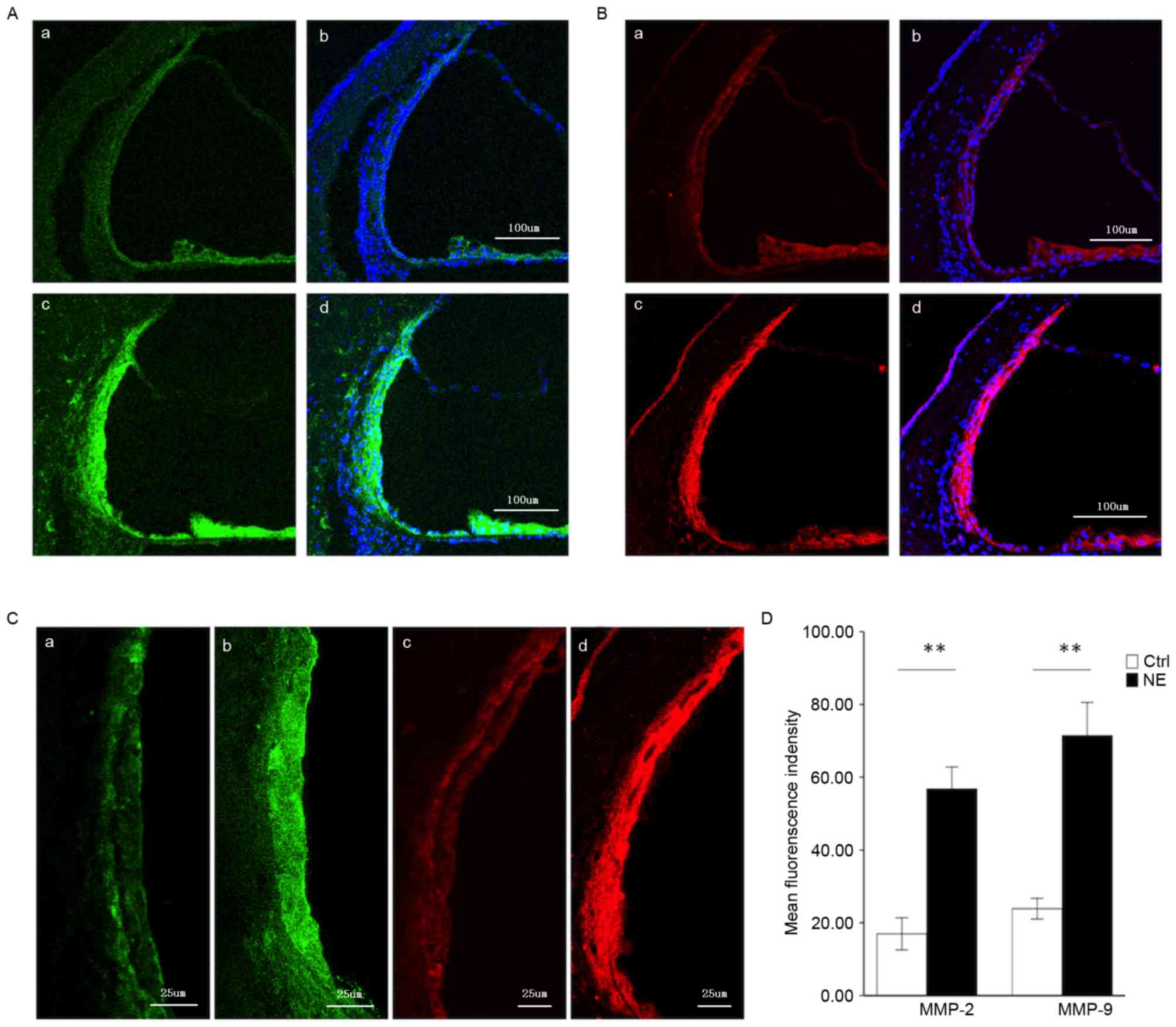 | Figure 2.Immunofluorescence assessment of MMP-2
and MMP-9 expression in the stria vascularis prior to and following
NE. (A) Tissue double-labeled for MMP-2 (green) and DAPI (blue)
prior to (a, b) and following (c, d) noise exposure. Scale bar, 100
µm. (B) Tissue double-labeled for MMP-9 (red) and DAPI (blue) prior
to (a, b) and following (c, d) noise exposure. Scale bar, 100 µm.
(C) Weak MMP-2 (green) and −9 (red) immunoreactivity was observed
in marginal cells and basal cells in the stria vascularis of
controls (a, c), which significantly increased following
noise-trauma (b, d). Scale bar, 25 µm. (D) Quantification of
localized fluorescence density of MMP-2 (green) and −9 (red) in the
stria vascularis. Data are expressed as the mean ± standard
deviation. **P<0.01. MMP, matrix metalloproteinase; NE, noise
exposure; Ctrl, control. |
Fig. 2D compares
MMP-2 and −9 immunoreactivity in the stria vascularis prior to and
following noise exposure (images were visualized using Image J
software).
Noise trauma causes alterations in the
expression of the TJ protein, ZO-1
It was hypothesized that upregulation of MMP-2 and
−9 following noise trauma would induce increased permeability of
the BLB by disrupting the TJ protein ZO-1. To test this hypothesis,
ZO-1 in the stria vascularis was immunolabeled. Confocal imaging
demonstrated that ZO-1 formed a compact linear structure on the
plasma membrane in areas of cell-cell contact between marginal
cells in healthy cochlear stria vascularis (Fig. 3A). Following noise trauma, the ZO-1
structure became loose and intermittent regional breaks were seen
(Fig. 3B).
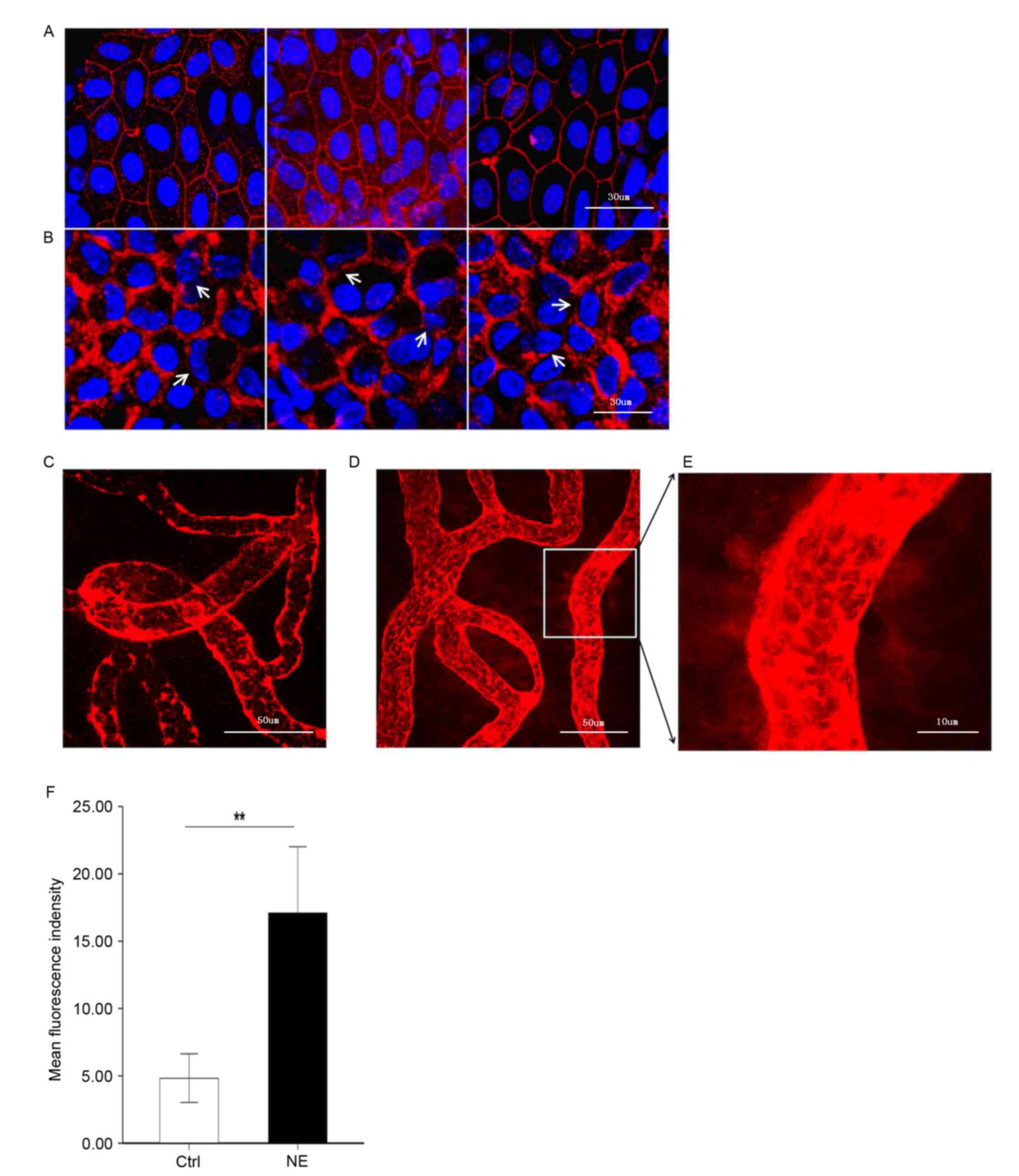 | Figure 3.Noise trauma upregulates expression of
ZO-1 (red) and causes alterations in BLB permeability, nuclei are
labeled by DAPI (blue). Magnification, ×60. (A) Representative
confocal microscope images demonstrating the compact linear
structure of ZO-1 (red) on the plasma membrane in the stria
vascularis. Scale bar, 30 µm. (B) Noise exposure caused the
structure of ZO-1 (red) to loosen, and regional breaks were
observed (white arrows). Scale bar, 30 µm. BLB integrity was
assessed by noting the degree of EBD extravasated around strial
capillaries (C) prior to and (D) following noise exposure. Scale
bar, 50 µm. (E) EBD outside capillaries was observed further away
from the vessel with noise exposure, demonstrative of the
significantly increased permeability following loud noise exposure.
Scale bar, 10 µm. (F) Mean fluorescence density outside capillaries
in the stria vascularis, as assessed with Image J software. Data
are expressed as the mean ± standard deviation. **P<0.01. EBD,
Evans blue dye; ZO-1, zona-occludens 1; Ctrl, control; NE, noise
exposure; Ctrl, control. |
BLB permeability alters following
noise trauma
EBD (961 Da) is widely used as a tracer to study
blood-tissue-barrier permeability. Once injected into the
circulation, EBD binds strongly to serum albumin (69,000 Da) and
becomes a high molecular weight protein tracer. Unlike sodium
fluorescein, EBD becomes fully albumin-bound within 5 min of
post-intravenous bolus injection (17,18).
Consistent with previous reports on the BBB, EBD quickly colored
the eyes, nose and paws of the guinea pigs a deep blue, and the
coloration persisted for the 2-h duration of the experiment. The
BLB is well known to restrict extravasation of inert tracers
(3). Accordingly, in the control
group, the red-fluorescence of albumin-bound EBD was mostly limited
to the capillary lumen in the stria vascularis, which was expected
as the BLB is non-permeable (Fig.
3C). Animals in the noise exposure group displayed markedly
increased EBD extravasation in the stria vascularis (Fig. 3D-F). EBD concentration outside the
capillaries exhibited marked alterations in distance from the
vessel, demonstrative of significantly increased permeability
following loud noise.
Discussion
The blood-labyrinth barrier (BLB) in the stria
vascularis comprises a dense capillary network of endothelial
cells, pericytes, basement membranes and perivascular resident
macrophages (16,19,20).
The capillary network in the stria vascularis is a sandwich of
epithelial marginal cells and mesodermal basal cells interconnected
by TJs. The network functions as a barrier, selectively excluding
most blood-borne substances from entering the ear and protecting it
from systemic influences (6,21).
Consistent with the BLB's impermeability, a previous study has
demonstrated that the stria vascularis is abundant with TJ proteins
(6). Noise trauma significantly
disrupts blood flow and diminishes the integrity of the BLB within
a quick time frame (19,22). Under a light microscope, acute
swelling of the stria vascularis is seen within 24 h of noise
exposure, and electron microscopy has demonstrated the acute strial
swelling is largely due to an increase in extracellular space
between marginal and intermediate cells (8).
In previous studies of the central nervous system,
disruption of TJ proteins, including occludin and ZO-1, and
dissociation of ZO-1 from the junctional complex, have been
associated with increased BBB permeability (5). Increased matrix metalloproteinase
(MMP) activity, especially MMP-2 and −9, is a consequence of
degradation of the TJ protein ZO-1, and occludin may also serve an
important role in the BBB breakdown (5,23).
As MMPs can degrade the ECM, MMPs are involved in regulation of
tissue remodeling, embryonic development, modulation of
inflammation, tumor invasion and metastasis, and wound healing
(24–27). Healthy cochlea has been
demonstrated to be rich in MMP enzymatic activity, and MMPs and
their associated gene products were identified in the modulation of
cochlear sensory epithelium response to acoustic trauma in rats
(13,14). The expression profile of MMPs and
their associated genes in healthy cochlear stria vascularis, as
well as the role the MMPs play in BLB pathogenesis following
acoustic trauma, remains largely unknown. The present study
provided novel evidence that MMP-2 and −9 are involved in
noise-induced disruption of the BLB by downregulating the TJ
protein ZO-1.
Numerous members of the MMP family have been
identified in the cochlear sensory epithelium (13). The present study used RNA-seq and
RT-qPCR assays to profile the expression pattern of MMP-associated
genes. A set of MMP genes were identified to be constitutively
active and expressed in the stria vascularis. The gene types
identified were slightly different from the previously known gene
types in the cochlear sensory epithelium (13).
Based on their substrate specificity, MMPs can be
subdivided into six groups: Interstitial collagenases (MMP-1, −8,
−13 and −18); type IV collagenases or gelatinases (MMP-2 and −9);
stromelysins (MMP-3, −10, −11 and −19); matrilysins (MMP-7 and
−26); membrane-type MMPs (MMP-14, −15, −16, −17, −24 and −25); and
6) other MMPs (MMP-12, −20, −21, −22, −23, −27 and −28) (28,29).
Among the 15 MMP genes detected in healthy stria vascularis, there
are 2 gelatinases (MMP-2 and −9), 3 collagenases (MMP-1, −8 and
−13), 2 stromelysins (MMP-11 and −19), 6 membrane-type MMPs
(MMP-14, −15, −16, −17, −24 and −25), and 2 other type MMPs (MMP-21
and −23B). The abundance of MMP genes indicates the important role
MMPs serve in maintaining the integrity of the BLB.
The TIMP family is composed of four members. A total
of three TIMP genes (Timp1, 2 and 3) are detected in healthy stria
vascularis. Although TIMPs do not exhibit high specificity for any
particular MMP, TIMP-2 does preferentially bind with MMP-2, and
TIMP-1 with MMP-9. In addition, TIMP-2, −3 and −4, but not TIMP-1,
are effective inhibitors of membrane-type MMPs (30). In the present study, Timp2 was most
highly expressed in normal stria vascularis, consistent with Hu's
data on the sensory epithelium (13). Therefore, TIMP-2 may be the primary
endogenous inhibitor of MMPs in the cochlea.
Consistent with the RNA-seq data, weak
immunoreactivity for MMP-2 and −9 in marginal cells and basal cells
was observed in healthy stria vascularis, and no immunoreactivity
in intermediate cells. The distribution of MMP-2 and −9 in healthy
stria vascularis conforms with the ultrastructural morphology of
the three cell types in the BLB. Marginal cells comprise the
epithelial lining of the endolymphatic duct and form a TJ barrier
between endolymph and the intra-strial compartment, while basal
cell plasma membranes line the lateral surface of the stria and
form a TJ barrier between the intrastrial space and spiral ligament
(8). The present study revealed
that noise trauma causes a marked increase in immunoreactivity for
MMP-2 and −9 not only in marginal and basal cells, but also in
intermediate cells.
Previous studies have observed that noise exposure
leads to disruption of the BLB and increased permeability of the
stria vascularis (7,8,19,22).
Transmission electron microscope images of the stria vascularis in
noise-exposed animals reveals a reduced number of TJ contact points
(6). The results of the present
study are consistent with previous studies; albumin-bound EBD
served as a tracer to indicate a marked increase in the
permeability of the BLB, with destruction of TJ protein ZO-1
structures. As ECM components, including ZO-1, are major targets of
MMP-2 and −9, it was hypothesized that MMP-2 and −9-mediated
structural damage to ZO-1 is a potential underlying mechanism for
noise-induced disruption of the BLB, leading to aberrations in
cochlear ion transport and sensorineural hearing loss. Notably,
analysis of immunolabeled ZO-1 did not reveal obvious
downregulation in the stria vascularis following noise exposure, in
contrast to a previous observation that other TJ proteins,
including claudin-5 and occludin, were downregulated in the BLB
(5,9). Therefore, there may be a variety of
regulatory mechanisms involved in noise-induced disruption of the
BLB.
In conclusion, the present study used RNA-seq and
immunofluorescence analysis to demonstrated stable expression of
MMP-2 and −9 in healthy stria vascularis, and marked upregulation
of MMP-2 and −9 with noise trauma. The acoustic trauma caused the
compact structure of ZO-1 in the BLB to loosen and substantially
leak EBD out of the capillaries of the stria vascularis. These data
implicated MMP-2 and −9 in the structural damage to TJ proteins,
including ZO-1, with structural damage to TJ proteins contributing
to the breakdown of the BLB with acoustic trauma. More generally,
the present study demonstrated the involvement of MMPs in the
regulation of BLB integrity and permeability, which may provide a
theoretical basis for the prevention of noise-induced hearing
loss.
Acknowledgments
This work was supported by the National Natural
Science Foundation of China (grant nos. 81170908 and 81470683). The
authors would like to thank Dr Yongbing Shi for editing the
language of this paper.
References
|
1
|
Juhn SK, Hunter BA and Odland RM:
Blood-labyrinth barrier and fluid dynamics of the inner ear. Int
Tinnitus J. 7:72–83. 2001.PubMed/NCBI
|
|
2
|
Hirose K, Hartsock JJ, Johnson S, Santi P
and Salt AN: Systemic lipopolysaccharide compromises the
blood-labyrinth barrier and increases entry of serum fluorescein
into the perilymph. J Assoc Res Otolaryngol. 15:707–719. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Juhn SK, Rybak LP and Prado S: Nature of
blood-labyrinth barrier in experimental conditions. Ann Otol Rhinol
Laryngol. 90:135–141. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Romero IA, Radewicz K, Jubin E, Michel CC,
Greenwood J, Couraud PO and Adamson P: Changes in cytoskeletal and
tight junctional proteins correlate with decreased permeability
induced by dexamethasone in cultured rat brain endothelial cells.
Neurosci Lett. 344:112–116. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feng S, Cen J, Huang Y, Shen H, Yao L,
Wang Y and Chen Z: Matrix metalloproteinase-2 and −9 secreted by
leukemic cells increase the permeability of blood-brain barrier by
disrupting tight junction proteins. PLoS One. 6:e205992011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang Y, Dai M, Wilson TM, Omelchenko I,
Klimek JE, Wilmarth PA, David LL, Nuttall AL, Gillespie PG and Shi
X: Na+/K+-ATPase α1 identified as an abundant protein in the
blood-labyrinth barrier that plays an essential role in the barrier
integrity. PLoS One. 6:e165472011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dai M, Yang Y, Omelchenko I, Nuttall AL,
Kachelmeier A, Xiu R and Shi X: Bone marrow cell recruitment
mediated by inducible nitric oxide synthase/stromal cell-derived
factor-1alpha signaling repairs the acoustically damaged cochlear
blood-labyrinth barrier. Am J Pathol. 177:3089–3099. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hirose K and Liberman MC: Lateral wall
histopathology and endocochlear potential in the noise-damaged
mouse cochlea. J Assoc Res Otolaryngol. 4:339–352. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu YX, Zhu GX, Liu XQ, Sun F, Zhou K, Wang
S, Wang CM, Jia JW, Song JT and Lu LJ: Noise alters guinea pig's
blood-labyrinth barrier ultrastructure and permeability along with
a decrease of cochlear Claudin-5 and Occludin. BMC Neurosci.
15:1362014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fanning AS, Jameson BJ, Jesaitis LA and
Anderson JM: The tight junction protein ZO-1 establishes a link
between the transmembrane protein occludin and the actin
cytoskeleton. J Biol Chem. 273:29745–29753. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hervé JC, Derangeon M, Sarrouilhe D and
Bourmeyster N: Influence of the scaffolding protein Zonula
Occludens (ZOs) on membrane channels. Biochim Biophys Acta.
1838:595–604. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Klein G, Vellenga E, Fraaije MW, Kamps WA
and de Bont ES: The possible role of matrix metalloproteinase
(MMP)-2 and MMP-9 in cancer, e.g. acute leukemia. Crit Rev Oncol
Hematol. 50:87–100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu BH, Cai Q, Hu Z, Patel M, Bard J,
Jamison J and Coling D: Metalloproteinases and their associated
genes contribute to the functional integrity and noise-induced
damage in the cochlear sensory epithelium. J Neurosci.
32:14927–14941. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Setz C, Brand Y, Radojevic V, Hanusek C,
Mullen PJ, Levano S, Listyo A and Bodmer D: Matrix
metalloproteinases 2 and 9 in the cochlea: Expression and activity
after aminoglycoside exposition. Neuroscience. 181:28–39. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Neng L, Zhang W, Hassan A, Zemla M,
Kachelmeier A, Fridberger A, Auer M and Shi X: Isolation and
culture of endothelial cells, pericytes and perivascular resident
macrophage-like melanocytes from the young mouse ear. Nat Protoc.
8:709–720. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takeuchi S, Ando M, Sato T and Kakigi A:
Three-dimensional and ultrastructural relationships between
intermediate cells and capillaries in the gerbil stria vascularis.
Hear Res. 155:103–112. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wolman M, Klatzo I, Chui E, Wilmes F,
Nishimoto K, Fujiwara K and Spatz M: Evaluation of the dye-protein
tracers in pathophysiology of the blood-brain barrier. Acta
Neuropathol. 54:55–61. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yen LF, Wei VC, Kuo EY and Lai TW:
Distinct patterns of cerebral extravasation by evans blue and
sodium fluorescein in rats. PLoS One. 8:e685952013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi X: Cochlear pericyte responses to
acoustic trauma and the involvement of hypoxia-inducible
factor-1alpha and vascular endothelial growth factor. Am J Pathol.
174:1692–1704. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi X: Resident macrophages in the
cochlear blood-labyrinth barrier and their renewal via migration of
bone-marrow-derived cells. Cell Tissue Res. 342:21–30. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hibino H, Nin F, Tsuzuki C and Kurachi Y:
How is the highly positive endocochlear potential formed? The
specific architecture of the stria vascularis and the roles of the
ion-transport apparatus. Pflugers Arch. 459:521–533. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suzuki M, Yamasoba T, Ishibashi T, Miller
JM and Kaga K: Effect of noise exposure on blood-labyrinth barrier
in guinea pigs. Hear Res. 164:12–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hawkins BT, Lundeen TF, Norwood KM, Brooks
HL and Egleton RD: Increased blood-brain barrier permeability and
altered tight junctions in experimental diabetes in the rat:
Contribution of hyperglycaemia and matrix metalloproteinases.
Diabetologia. 50:202–211. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Szabova L, Son MY, Shi J, Sramko M, Yamada
SS, Swaim WD, Zerfas P, Kahan S and Holmbeck K: Membrane-type MMPs
are indispensable for placental labyrinth formation and
development. Blood. 116:5752–5761. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Page-McCaw A, Ewald AJ and Werb Z: Matrix
metalloproteinases and the regulation of tissue remodelling. Nat
Rev Mol Cell Biol. 8:221–233. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Parks WC, Wilson CL and López-Boado YS:
Matrix metalloproteinases as modulators of inflammation and innate
immunity. Nat Rev Immunol. 4:617–629. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rosenberg GA: Matrix metalloproteinases
and their multiple roles in neurodegenerative diseases. Lancet
Neurol. 8:205–216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
Structure, function, and biochemistry. Circ Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hernandez-Barrantes S, Bernardo M, Toth M
and Fridman R: Regulation of membrane type-matrix
metalloproteinases. Semin Cancer Biol. 12:131–138. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chelladurai P, Seeger W and Pullamsetti
SS: Matrix metalloproteinases and their inhibitors in pulmonary
hypertension. Eur Respir J. 40:766–782. 2012. View Article : Google Scholar : PubMed/NCBI
|