Introduction
Gastric cancer is one of the most malignant,
aggressive and common cancers worldwide (1). According to recent statistics, the
yearly estimated number of new gastric cancer cases was 26,370, and
the yearly estimated number of gastric cancer-related deaths was
10,730 in the USA alone (2). These
numbers were much higher in China: 679,100 and 498,000,
respectively (3). Although the
diagnosis and management of gastric cancer have greatly improved,
this disease still accounts for 10% of all deaths caused by
malignant tumors annually (4,5). The
primary curative treatment of gastric cancer is surgical resection.
However, Chinese gastric cancer patients are often diagnosed at
advanced stages, making surgery much more difficult and less
effective (6). Because advanced
stages of gastric cancer are generally associated with greater
invasion and metastasis, studies have suggested that inhibition of
cell signalling pathways could greatly affect the invasion and
metastasis of gastric cancer cells (7–12).
Therefore, the new prevention strategy of targeting cell signalling
pathways of invasion and metastasis in gastric cancer will likely
be of great clinical value.
PTEN, which is also known as TGF-β-regulated and
epithelial cell enriched phosphatase (TEP1) or muted in multiple
advanced cancers (MMAC1), was isolated and identified by three
laboratories in 1997 (13–15). As a tumor suppressor gene, its
protein is ubiquitously expressed among human cells. PTEN can
function as a phosphatase to dephosphorylate phosphatidylinositol
(3,4,5)-trisphosphate (PIP3) into
the biphosphate product PIP2 (16). PIP3 is a primary
activator of Akt, and dephosphorylation of PIP3 by PTEN results in
inhibition of Akt signalling, which is critical in various cancer
cellular functions, including cell transcription, proliferation,
metastasis and invasion (16).
PTEN also functions as a protein phosphatase by dephosphorylating
FAK, restricting cancer cell invasion and metastasis (17,18).
Studies have suggested that upstream signalling regulates invasion
and metastasis of cancer cells through regulation of PTEN (19,20).
Notch is vitally important in controlling cell fate.
The critical roles of Notch in tumorigenesis have been reported in
many malignant tumors (21,22).
Studies have indicated that Notch signalling has variable effects
on different cancer cells. Notch signalling can either act as a
tumor suppressor or tumor promoter (23–25).
The function of Notch signalling in gastric cancer, especially its
effect on invasion and metastasis, remains unclear. Evidence has
shown that Notch signalling regulates PTEN in cancer cells
(12,26,27).
However, the relationships between Notch and PTEN in gastric cancer
cells require further research.
The aim of this study was to investigate the role
and mechanism of the Notch1 signalling pathway on cell invasion and
metastasis and possible downstream regulation during this process
in gastric cancer cells in vitro.
In this study we determined that downregulation of
Notch1 signalling using siRNA inhibits invasion and metastasis of
gastric cancer cell lines SGC7901 and MKN74 in vitro. PTEN
activation and decreased expression of phosphorylated forms of FAK
and Akt was also observed after Notch1 depletion. Our data suggest
that the Notch1 signalling pathway may provide an effective
treatment in gastric cancer patients.
Materials and methods
Cell culture
The gastric cell line SGC7901 was kindly provided by
the Second Affiliated Hospital of Harbin Medical University. The
gastric cell line MKN74 was obtained from Harbin Medical University
Cancer Hospital. Both cell lines were cultured in high glucose
Dulbecco's modified Eagle's medium (DMEM; GE Healthcare Life
Sciences; Hyclone, Logan, UT, USA) containing 10% foetal bovine
serum (FBS; Biowest SAS, Nuaillé, France). All cells were incubated
in 5% CO2 and 37°C in a humidified chamber.
RNA extraction and quantitative
PCR
Total RNA was harvested from cell lines using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer's instructions. Reverse transcription were performed
using the Golden 1st cDNA Synthesis kit (HaiGene, Harbin, China).
Quantitative real-time PCR was performed with Mini-Opticon2 (MJ) by
using the Golden HS SYBR-Green qPCR Mix (HaiGene). β-actin was used
as an internal control and β-actin qPCR primer was obtained from
HaiGene. The specific primers for each gene were synthesized by
HaiGene. Specific primer sequences used were as follows: Notch1
forward, 5′-TCGGAGTGTGTATGCCAAGAG-3′ and reverse,
5′-TGATGCCTACATTTCAAGAACGG-3′; PTEN forward,
5′-GTGTGGAATGAAGTGAGGCTTG-3′ and reverse,
5′-TTGGACAACTGGATAGAGTAGGC-3′; Akt forward,
5′-CCATCACACCACCTGACCAAG-3′ and reverse,
5′-CGCCTCTCCATCCCTCCAAG-3′; FAK forward,
5′-GAGATTGAGATGGCACAGAAGC-3′ and reverse,
5′-TGAGCAGCAGTCAGCATTTG-3′. Specificity of amplification products
was determined by melting curve analysis. Independent experiments
were done in triplicate. The 2−ΔΔCt was presented as the
relative expression of the gene expression.
Antibodies and reagents
The following primary antibodies were purchased from
Cell Signaling Technology (CST; Danvers, MA, USA): Notch1, PTEN,
Akt, FAK, phospho-PTEN (Ser380/Thr382/383), phospho-Akt (Ser473)
and phospho-FAK (Tyr397). β-actin and all secondary antibodies were
provided by Santa Cruz Biotechnology (Dallas, Texas, USA).
Lipofectamine RNAiMAX, Notch1 small interfering RNA (siRNA),
control siRNA, and related chemicals were purchased from
Invitrogen.
siRNA transfection
The putative Notch1 candidate sequences and the
control sequence were designed and provided by Invitrogen. The
siRNA sequences are as follows: Sequence 1 forward,
5′-CCGCCUUUGUGCUUCUGUUCUUCGU-3′ and reverse,
5′-ACGAAGAACAGAAGCACAAAGGCGG-3′; sequence 2 forward,
5′-CCACCAGUUUGAAUGGUCAAUGCGA-3′ and reverse,
5′-UCGCAUUGACCAUUCAAACUGGUGG-3′; sequence 3 forward,
5′-CCGCCAAAUUCAACGGGCUCUUGUG-3′) and reverse,
5′-CACAAGAGCCCGUUGAAUUUGGCGG-3′). Control duplexes using Invitrogen
stealth RNAi negative control duplexes (High GC Duplex, cat no.
12935-400) were utilized. The transfection procedure was performed
following manufacturer's instructions. Cells were harvested at the
same time for investigation after 24 to 48 h of growth.
Wound healing assay
Wound-healing assays were performed to assess the
effect of migration. Gastric cancer cells were seeded into 6-well
plates and treated with mitomycin C (Santa Cruz Biotechnology,
Dallas, Texas, USA) to inhibit cell proliferation. The cell
monolayer was disrupted with a pipette tip. DMEM medium was used to
wash away floating cells. Photographs were captured using an
inverted microscope (same magnification) at the same time 48 h
after the scratch. Six fields for each point were recoded. Relative
wound size was calculated to assess migration activity.
Cell invasion assay
For invasion assay, a Transwell assay was performed
(8 µm; Corning Inc., New York, NY, USA). The membranes were coated
with 200 µl Matrigel at 200 µg/ml. The upper chamber was seeded
with cells in serum-free DMEM medium, and DMEM with 10% FBS was
added in the lower chamber. After incubation for 24 h, cells were
removed at the same time from the upper surface of the filter by
scraping gently with a swab. Cells that invaded the bottom of
membrane were fixed and stained. The numbers of invaded cells were
calculated.
Western blot analysis
Cells were lysed in buffer [1% nonidet P-40, 100
mg/l phenylmethylsulfonyl fluoride, 50 mmol/l Tris-Cl (pH 8.0),
0.02% sodium azide, and 1 mg/l aprotinin]. After centrifugation for
20 min, the supernatant was collected, and the BCA protein assay
kit (Beyotime Institute of Biotechnology, Shanghai, China) was used
to measure protein concentrations following manufacturer's
instructions. Equivalent amounts of protein were separated by 10%
SDS-polyacrylamide gel electrophoresis. Then, proteins were
transferred to a polyvinylidene fluoride (PVDF) membrane (Amersham
Biosciences, Piscataway, NJ, USA) and blocked for 2 h at 37°C. The
membranes were then incubated overnight at 4°C with primary
antibodies. Immunocomplexes were incubated at room temperature with
anti-mouse or anti-rabbit IgG for 1 h (diluted at 1:1,000). The
results were visualized using an ECL kit (Amersham Biosciences).
All antibodies and reagents were used based on manufacturer's
instruction.
Statistical analysis
Data were analysed and presented as the means ±
standard deviation (SD) of at least 3 independent experiments using
one-way analysis of variance (one-way ANOVA). All analyses were
performed using SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). A
p-value <0.05 was considered to indicate a significant
difference.
Results
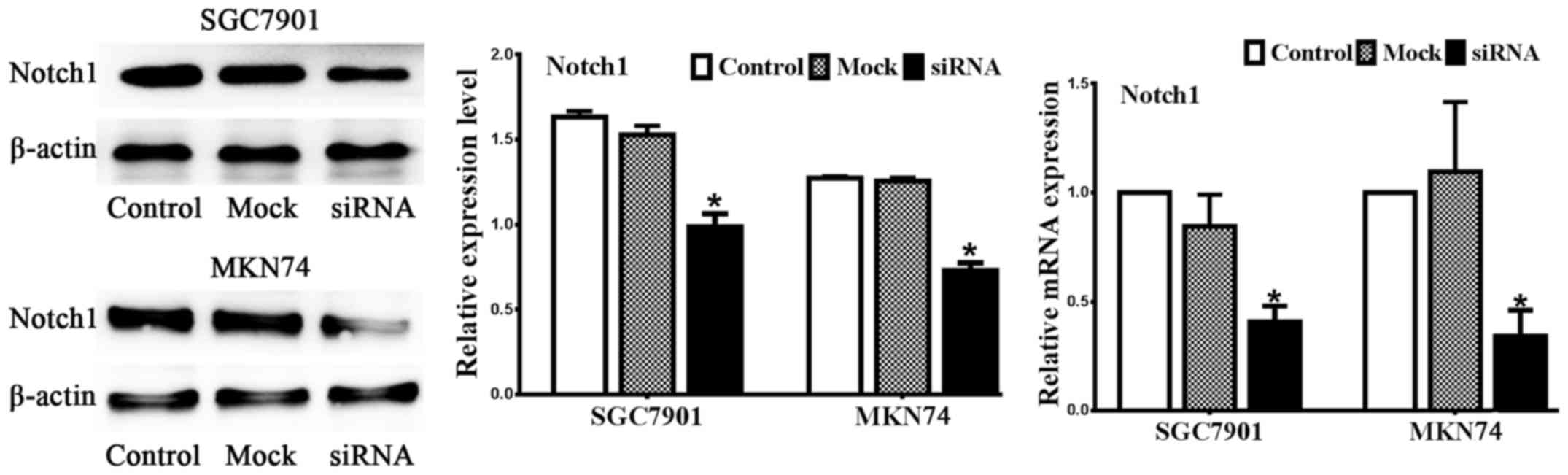
Notch1 is silenced by siRNA
The gastric cancer cell lines SGC7901 and MKN74 were
transiently transfected with Notch1 siRNA and mock siRNA. We
designed candidate Notch1 siRNA and negative control sequences
(mock). Real-time PCR and western blot analysis were performed to
assess the efficiency of Notch1 siRNA. As illustrated in Fig. 1, Notch1 was expressed in both
SGC7901 and MKN74 cell lines, and the candidate sequence inhibited
both Notch1 mRNA and protein expression compared to the control and
mock treatments. Collectively, the expression of Notch1 protein was
markedly decreased in the cells transfected with Notch1 siRNA
compared with control (no siRNA) and mock (negative control siRNA)
treatments in both cell lines (n=3, P<0.05).
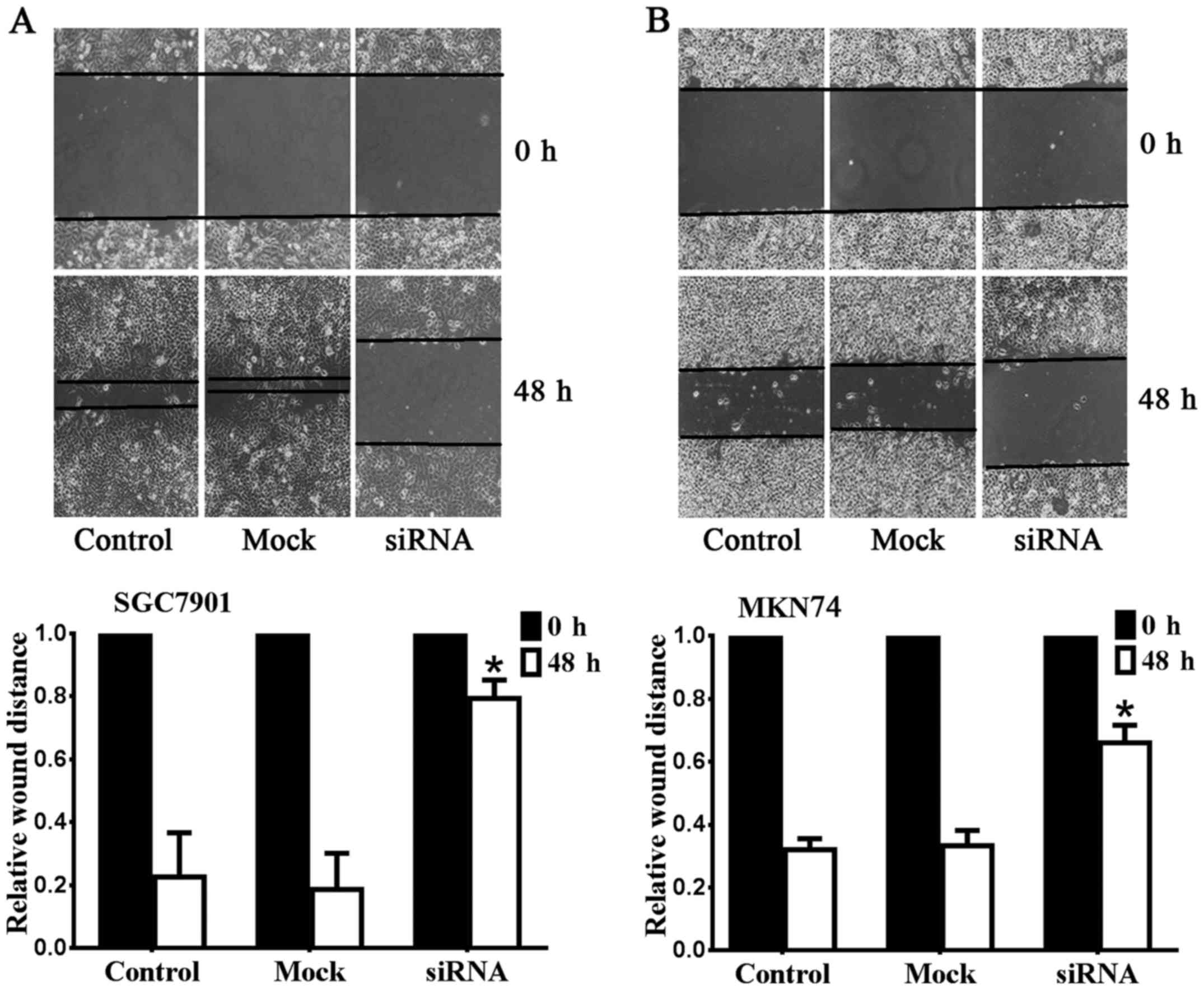
The metastasis and invasion of gastric
cancer cell lines were inhibited after downregulation of Notch1
expression
To determine whether the migratory abilities of
SGC7901 and MKN74 cell lines were affected by Notch1 depletion, we
performed wound-healing assays as presented in Fig. 2. The metastasis of SGC7901 was
significantly suppressed after downregulation of Notch1 (Fig. 2A). The relative wound size of the
Notch1 siRNA group (0.79±0.06 mm) was larger than the control
(0.23±0.14 mm) and mock groups (0.19±0.12 mm) (n=6, P<0.05). A
similar result was observed for MKN74 (Fig. 2B). The relative wound size of the
Notch1 siRNA group (0.66±0.06 mm) was larger than the control
(0.32±0.04 mm) and mock groups (0.33±0.05 mm) (n=6, P<0.05).
These data demonstrated that Notch1 depletion inhibits the
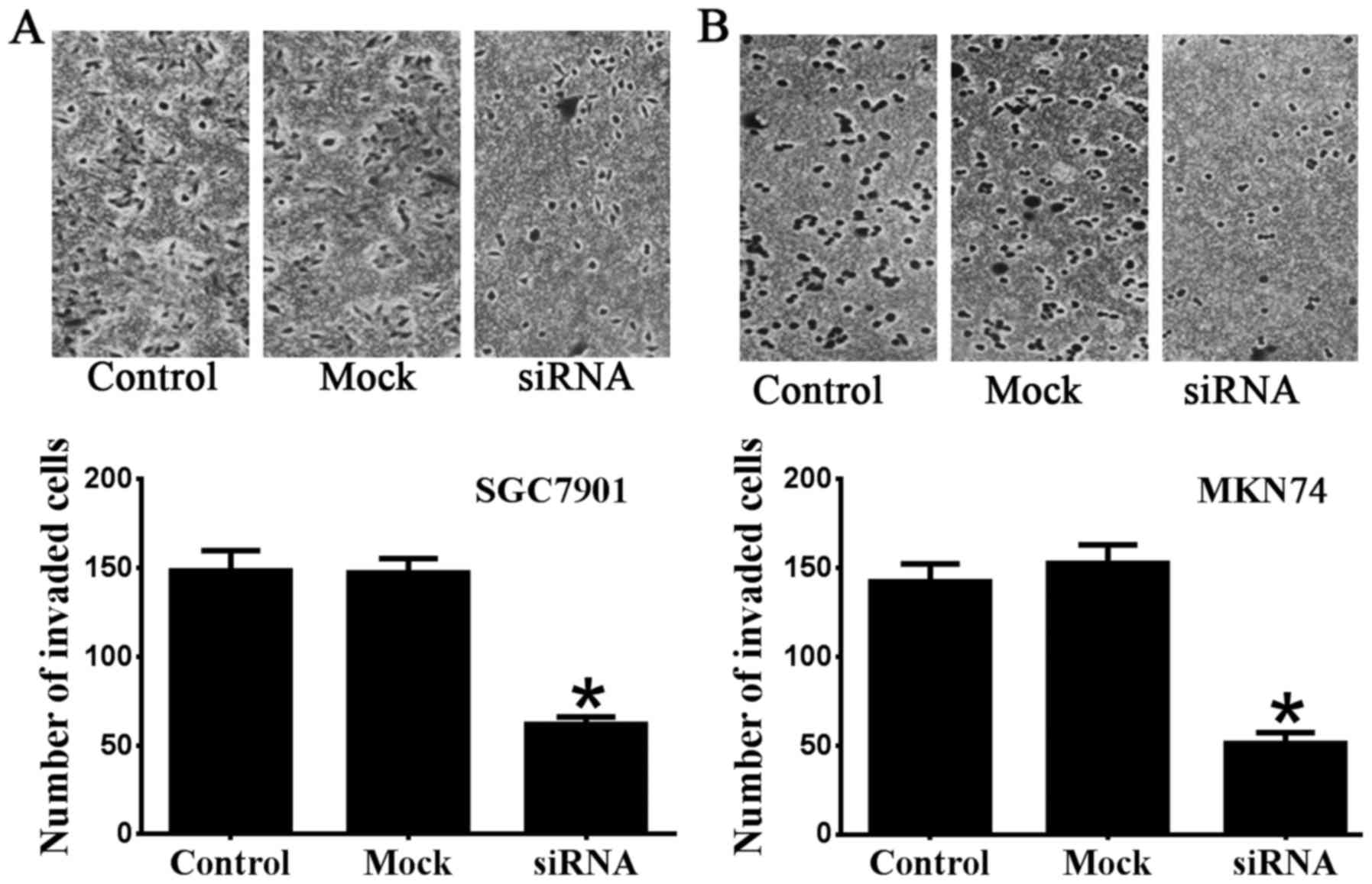
migration of SGC7901 and MKN74 cells. The results of Transwell
invasion assays were consistent with the wound-healing assay
results (Fig. 3). The number of
invaded SGC7901 cells transfected with Notch1 siRNA (62±4.1) was
significantly reduced compared with the control (148.5±11.4) and
mock groups (147.3±8.0) (n=6 P<0.05) (Fig. 3A). The number of invaded MKN74
cells transfected with Notch1 siRNA (51.3±6.0) was also
significantly reduced compared with the control (142.3±10.0) and
mock groups (152.7±10.4) (n=6 P<0.05) (Fig. 3B). Taken together, our data
indicate the role of Notch1 regarding invasion and metastasis in
SGC7901 and MKN74 gastric cancer cells.
Inhibition of Notch1 alters expression
of PTEN, pPTEN, pAkt and pFAK
As shown in Fig. 4,
both mRNA and protein expression of PTEN was upregulated in the
Notch1 siRNA group compared with the control and mock groups,
whereas phospho-PTEN expression was downregulated after inhibition
of Notch1 (n=3, P<0.05) in SGC7901 and MKN74 gastric cancer cell
lines. The mRNA and protein expression of total Akt and FAK showed
no significant changes. These results demonstrate that PTEN
function is activated by the depletion of Notch1. Decreased
expression of phospho-Akt and phospho-FAK but not total expression
of Akt and FAK was also observed following reactivation of PTEN in
both cell lines as shown in Fig. 4
(n=3, P<0.05).
Discussion
Invasion and metastasis are both vital causes of
mortality in gastric cancer patients. Therefore, developing new
treatments targeting invasion and metastasis are of great
importance. Inhibition of cell signalling pathways shows great
promise. As an upstream signalling pathway, the importance of Notch
activation has been reported in numerous cancers (21,22).
Increasing evidence has indicated that Notch1 is aberrantly
activated and highly expressed in gastric cancer tissue (22,28).
Notch1 plays an important tumor progression role in gastric cancer
(22,29). Several studies have found that
Notch1 promotes invasion and metastasis in cancer cells (30–34).
To examine whether Notch1 affects invasion and metastasis in
gastric cancer cells, we used siRNA to inhibit Notch1 signalling in
SGC7901 and MKN74. Notch signalling can be inhibited by prevention
of ligand binding using gamma-secretase inhibitors (GSIs) or
transcriptional activity inhibition. The methods we used to inhibit
Notch1 in this study are widely effective, and we investigated the
effect of inhibition using real-time PCR and western blot analysis.
We also employed wound-healing and Transwell assays to assess the
effect of cell invasion and metastasis after Notch1 downregulation.
The data indicate that siRNA downregulates the expression of Notch1
mRNA and protein following the suppression of cell invasion and
metastasis of SCG7901 and MKN74 gastric cancer cells. This result
indicates the role of Notch1 in gastric cancer cell lines SCG7901
and MKN74 regarding invasion and metastasis in vitro and
also suggests that Notch1 could be a potential therapeutic target
in gastric cancer treatment.
To explore the mechanism by which Notch1 affects
invasion and metastasis in SGC7901 and MKN74 cells, we focused on
expression of PTEN and phospho-PTEN (Ser380/Thr382/383). PTEN acts
as a tumor suppressor by functioning as a dual-specificity protein
and phospholipid phosphatase (35). Its function depends on its protein
structure, which has five distinct domains: N-term, Phosphatase
domain, C2 domain, C-tail and PDZ. N-term contains the PIP binding
domain. The phosphatase domain is responsible for its enzymatic and
phosphatase activity. The C2 domain is responsible for its cellular
location and protein-protein interactions. The C-tail domain is
less defined but may be critical for the stability of PTEN and the
C-terminal PDZ domain (36). PTEN
is involved in numerous biological processes, and its regulation is
very complex. One of its important regulations is
posttranslational. The C-terminal tail of PTEN can be
phosphorylated at Ser380, Thy382 and Thy383. The result of this
regulation is inhibition of PTEN's critical phosphatase activity,
thus leading to cell growth promotion (37). Phospho-PTEN (Ser380/Thr382/383)
protein expression may inhibit PTEN activation.
In this study, increased total PTEN mRNA and protein
expression and decreased phospho-PTEN expression was observed
following Notch1 depletion. Notch can either be a tumor promoter or
tumor suppressor via differential regulation of PTEN protein
expression in different situations (38,39).
In this situation, Notch1 acts as a tumor promoter that negatively
correlates with PTEN expression and positively correlates with
phospho-PTEN (Ser380/Thr382/383) protein expression.
We hypothesize that Notch1 negatively regulates PTEN
activation not only by suppressing total PTEN expression but also
by phosphorylating the PTEN C-terminal tail at Ser380, Thy382 and
Thy383, thus causing inhibition of PTEN's phosphatase activity in
gastric cancer cell lines. This hypothesis is supported by Kim
et al research; they determined that Notch signalling
disables PTEN by phosphorylation and contributes to tumorigenesis
(40). We will investigate
relative mechanism of this regulation and focus on testing if PTEN
activation is required by inhibition of migration in gastric cancer
cells upon depletion of Notch1 in our further study.
Cancer cell invasion and metastasis involves many
mechanisms. Activation of Akt and FAK signalling pathways through
phosphorylation promotes invasion, metastasis and proliferation
(41–43). One of the classic PTEN functions
involves dephosphorylating PIP3, thus antagonizing the
(PI3K)/Akt signalling pathway (16). PTEN also downregulates the activity
of FAK by dephosphorylation (18).
In this study, decreased expression of phosphorylated Akt and FAK
was observed after the inhibition of Notch1. We hypothesized that
decreased expression of phosphorylated Akt and FAK directly leads
to suppression of cell invasion and metastasis and correlates with
re-activation of PTEN. However, further investigations are
required.
Collectively, our results demonstrate that invasion
and metastasis in SGC7901 and MKN74 gastric cancer cells are
inhibited in vitro after downregulation of the Notch1
signalling pathway by siRNA. Depletion of Notch1 leads to increased
PTEN and decreased phospho-PTEN (Ser380/Thr382/383) protein
expression in gastric cancer cells. Re-activation of PTEN by
inhibition of Notch1 leads to decreased expression of
phosphorylated Akt and FAK. The Notch1-PTEN-Akt&FAK signalling
axis may serve as a further treatment of gastric cancer targeting
invasion and metastasis.
Acknowledgements
We thank the Key Laboratory of Myocardial Ischemia,
Chinese Ministry of Education (Harbin Medical University). Our
entire experiment was conducted in this laboratory, and we were
given useful advice by lab working staff. We also thank The 2nd
Affiliated Hospital of Harbin Medical University and Harbin Medical
University Cancer Hospital for kindly providing SGC7901 and MKN74
gastric cell lines.
References
|
1
|
Karimi P, Islami F, Anandasabapathy S,
Freedman ND and Kamangar F: Gastric cancer: Descriptive
epidemiology, risk factors, screening and prevention. Cancer
Epidemiol Biomarkers Prev. 23:700–713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brenner H, Rothenbacher D and Arndt V:
Epidemiology of stomach cancer. Methods Mol Biol. 472:467–477.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang L: Incidence and mortality of gastric
cancer in China. World J Gastroenterol. 12:17–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang XB, Song L, Wen HJ, Bai XX, Li ZJ
and Ma LJ: Upregulation of microRNA-31 targeting integrin α5
suppresses tumor cell invasion and metastasis by indirectly
regulating PI3K/AKT pathway in human gastric cancer SGC7901 cells.
Tumor Biol. 37:8317–8325. 2016. View Article : Google Scholar
|
|
8
|
Liu JJ, Liu JY, Chen J, Wu YX, Yan P, Ji
CD, Wang YX, Xiang DF, Zhang X, Zhang P, et al: Scinderin promotes
the invasion and metastasis of gastric cancer cells and predicts
the outcome of patients. Cancer Lett. 376:110–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deng QJ, Xie LQ and Li H: Overexpressed
MALAT1 promotes invasion and metastasis of gastric cancer cells via
increasing EGFL7 expression. Life Sci. 157:38–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tan C, Qiao F, Wei P, Chi Y, Wang W, Ni S,
Wang Q, Chen T, Sheng W, Du X and Wang L: DIXDC1 activates the Wnt
signaling pathway and promotes gastric cancer cell invasion and
metastasis. Mol Carcinog. 55:397–408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J, Deng Z, Wang Z, Wang D, Zhang L, Su
Q, Lai Y, Li B, Luo Z, Chen X, et al: Zipper-interacting protein
kinase promotes epithelial-mesenchymal transition, invasion and
metastasis through AKT and NF-κΒ signaling and is associated with
metastasis and poor prognosis in gastric cancer patients.
Oncotarget. 6:8323–8338. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bertrand FE, McCubrey JA, Angus CW, Nutter
JM and Sigounas G: NOTCH and PTEN in prostate cancer. Adv Biol
Regul. 56:51–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Steck PA, Pershouse MA, Jasser SA, Yung
WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T,
et al: Identification of a candidate tumour suppressor gene, MMAC1,
at chromosome 10q23.3 that is mutated in multiple advanced cancers.
Nat Genet. 15:356–362. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Yen C, Liaw D, Podsypanina K, Bose
S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al:
PTEN, a putative protein tyrosine phosphatase gene mutated in human
brain, breast, and prostate cancer. Science. 275:1943–1947. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li DM and Sun H: TEP1, encoded by a
candidate tumor suppressor locus, is a novel protein tyrosine
phosphatase regulated by transforming growth factor beta. Cancer
Res. 57:2124–2129. 1997.PubMed/NCBI
|
|
16
|
Milella M, Falcone I, Conciatori F, Incani
U Cesta, Del Curatolo A, Inzerilli N, Nuzzo CM, Vaccaro V, Vari S,
Cognetti F and Ciuffreda L: PTEN: Multiple functions in human
malignant tumors. Front Oncol. 5:242015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tamura M, Gu J, Takino T and Yamada KM:
Tumor suppressor PTEN inhibition of cell invasion, migration, and
growth: Differential involvement of focal adhesion kinase and
p130Cas. Cancer Res. 59:442–449. 1999.PubMed/NCBI
|
|
18
|
Tamura M, Gu J, Matsumoto K, Aota S,
Parsons R and Yamada KM: Inhibition of cell migration, spreading,
and focal adhesions by tumor suppressor PTEN. Science.
280:1614–1617. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang S, Tie J, Wang R, Hu F, Gao L, Wang
W, Wang L, Li Z, Hu S, Tang S, et al: SOX2, a predictor of survival
in gastric cancer, inhibits cell proliferation and metastasis by
regulating PTEN. Cancer Lett. 358:210–219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang H, Wu Q, Liu Z, Luo X, Fan Y, Liu Y,
Zhang Y, Hua S, Fu Q, Zhao M, et al: Downregulation of FAP
suppresses cell proliferation and metastasis through PTEN/PI3K/AKT
and Ras-ERK signaling in oral squamous cell carcinoma. Cell Death
Dis. 5:e11552014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lobry C, Oh P and Aifantis I: Oncogenic
and tumor suppressor functions of Notch in cancer: It's NOTCH what
you think. J Exp Med. 208:1931–1935. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Du X, Cheng Z, Wang YH, Guo ZH, Zhang SQ,
Hu JK and Zhou ZG: Role of Notch signaling pathway in gastric
cancer: A meta-analysis of the literature. World J Gastroenterol.
20:9191–9199. 2014.PubMed/NCBI
|
|
23
|
Baron M, Aslam H, Flasza M, Fostier M,
Higgs JE, Mazaleyrat SL and Wilkin MB: Multiple levels of Notch
signal regulation (Review). Mol Membr Biol. 19:27–38. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grishina IB: Mini-review: Does Notch
promote or suppress cancer? New findings and old controversies. Am
J Clin Exp Urol. 3:24–27. 2015.PubMed/NCBI
|
|
25
|
Yap LF, Lee D, Khairuddin A, Pairan MF,
Puspita B, Siar CH and Paterson IC: The opposing roles of NOTCH
signalling in head and neck cancer: A mini review. Oral Dis.
21:850–857. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kwon OJ, Zhang L, Wang J, Su Q, Feng Q,
Zhang XH, Mani SA, Paulter R, Creighton CJ, Ittmann MM and Xin L:
Notch promotes tumor metastasis in a prostate-specific Pten-null
mouse model. J Clin Invest. 126:2626–2641. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu YJ, Li HY, Qiu KJ, Li DC, Zhou JH, Hu
YH and Zhang FM: Downregulation of Notch1 inhibits the invasion of
human hepatocellular carcinoma HepG2 and MHCC97H cells through the
regulation of PTEN and FAK. Int J Mol Med. 34:1081–1086.
2014.PubMed/NCBI
|
|
28
|
Wu X, Liu W, Tang D, Xiao H, Wu Z, Chen C,
Yao X, Liu F and Li G: Prognostic values of four Notch receptor
mRNA expression in gastric cancer. Sci Rep. 6:280442016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yeh TS, Wu CW, Hsu KW, Liao WJ, Yang MC,
Li AF, Wang AM, Kuo ML and Chi CW: The activated Notch1 signal
pathway is associated with gastric cancer progression through
cyclooxygenase-2. Cancer Res. 69:5039–5048. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jia M, Jiang L, Wang YD, Huang JZ, Yu M
and Xue HZ: lincRNA-p21 inhibits invasion and metastasis of
hepatocellular carcinoma through Notch signaling-induced
epithelial-mesenchymal transition. Hepatol Res. 46:1137–1144. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao ZL, Ma SR, Wang WM, Huang CF, Yu GT,
Wu TF, Bu LL, Wang YF, Zhao YF, Zhang WF and Sun ZJ: Notch
signaling induces epithelial-mesenchymal transition to promote
invasion and metastasis in adenoid cystic carcinoma. Am J Transl
Res. 7:162–174. 2015.PubMed/NCBI
|
|
32
|
Sonoshita M, Itatani Y, Kakizaki F,
Sakimura K, Terashima T, Katsuyama Y, Sakai Y and Taketo MM:
Promotion of colorectal cancer invasion and metastasis through
activation of NOTCH-DAB1-ABL-RHOGEF protein TRIO. Cancer Discov.
5:198–211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang P, Yang Y, Zweidler-McKay P and
Hughes DP: Retraction: Critical role of notch signaling in
osteosarcoma invasion and metastasis. Clin Cancer Res.
14:2962–2969. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang P, Yang Y, Zweidler-McKay PA and
Hughes DP: Critical role of notch signaling in osteosarcoma
invasion and metastasis. Clin Cancer Res. 14:2962–2969. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu WT, Yang Z and Lu NH: Roles of PTEN
(phosphatase and tensin homolog) in gastric cancer development and
progression. Asian Pac J Cancer Prev. 15:17–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jerde TJ: Phosphatase and tensin
homologue: Novel regulation by developmental signaling. J Signal
Transduct. 2015:2825672015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Odriozola L, Singh G, Hoang T and Chan AM:
Regulation of PTEN activity by its carboxyl-terminal autoinhibitory
domain. J Biol Chem. 282:23306–23315. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Whelan JT, Kellogg A, Shewchuk BM,
Hewan-Lowe K and Bertrand FE: Notch-1 signaling is lost in prostate
adenocarcinoma and promotes PTEN gene expression. J Cell Biochem.
107:992–1001. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chappell WH, Green TD, Spengeman JD,
McCubrey JA, Akula SM and Bertrand FE: Increased protein expression
of the PTEN tumor suppressor in the presence of constitutively
active Notch-1. Cell Cycle. 4:1389–1395. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim SJ, Lee HW, Baek JH, Cho YH, Kang HG,
Jeong JS, Song J, Park HS and Chun KH: Activation of nuclear PTEN
by inhibition of Notch signaling induces G2/M cell cycle arrest in
gastric cancer. Oncogene. 35:251–260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao HF, Wang J, Jiang HR, Chen ZP and To
SS: PI3K p110β isoform synergizes with JNK in the regulation of
glioblastoma cell proliferation and migration through Akt and FAK
inhibition. J Exp Clin Cancer Res. 35:782016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gao H, Zhong F, Xie J, Peng J and Han Z:
PTTG promotes invasion in human breast cancer cell line by
upregulating EMMPRIN via FAK/Akt/mTOR signaling. Am J Cancer Res.
6:425–439. 2016.PubMed/NCBI
|
|
43
|
Egawa H, Jingushi K, Hirono T, Ueda Y,
Kitae K, Nakata W, Fujita K, Uemura M, Nonomura N and Tsujikawa K:
The miR-130 family promotes cell migration and invasion in bladder
cancer through FAK and Akt phosphorylation by regulating PTEN. Sci
Rep. 6:205742016. View Article : Google Scholar : PubMed/NCBI
|