Introduction
Gastric cancer (GC) is the 4th most commonly
diagnosed cancer with poor clinical outcomes, which is the second
leading cancer-related death worldwide, but the first leading cause
of cancer death in China (1).
Therefore, there is a need to develop novel agents for treatment of
this type of cancer. Advances in high-throughput technologies have
uncovered a flood of biomarkers and targets, and numerous of them
have been under investigation for GC, including the S-phase
kinase-associated protein 2 (SKP2) (2,3).
SKP2 is an F box protein that has been
well-characterized as oncoprotein, which plays a crucial role in
the development and progression of many cancers (4). It is involved in cell cycle
progression and senescence, in which it acts as an Skp1-Cullin-1-F
box (SCF) ubiquitin ligase substrate recognition factor, by which
SKP2 is able to positively regulate the cell-cycle by targeting
cell-cycle regulators and tumor suppressor proteins, including p27,
for ubiquitin-mediated degradation (2,3).
Mounting evidence has suggested that dysregulation of SKP2
expression is often associated with poor prognosis and metastasis
in many types of cancer, including the breast cancer (5) and GC (6). Repression of SKP2 expression by RNAi
or shRNA has shown a capability to inhibit the proliferation and
migration of gallbladder carcinoma cells by enhancing p27
expression (7), and the growth and
metastasis of gastric cancer MGC803 and SGC-7901 cells in
vitro and in vivo (8).
These studies implied that SKP2 was thus a potential therapeutic
target for developing novel agents for treatment of cancers,
including the GC (2–4,9,10).
MicroRNAs (miRNAs or miRs) are a class of endogenous
non-coding single-stranded small RNAs of ~22 nucleotides, which
have been demonstrated to play a crucial role in the regulation of
gene expression at the post-transcriptional level, and function as
either oncogenes or tumor suppressors in various cancers (11). Increasing evidence suggests that
miRNAs are involved in the initiation, progression and metastasis
of GC, in which they can function as oncomirs or tumor suppressor
gene, i.e., some miRNAs are downregulated, while some are
upregulated in GC (12,13). Li et al recently analyzed
the miRNA profile in GC by a quantitative PCR assay and identified
22 differential expressed miRNAs, 6 of them were downregulated and
might be tumor suppressors, the rest 16 miRNAs were upregulated and
could be putative oncomirs (14).
The miR-223 and miR-10a were further identified to correlate with
the metastasis of GC (14,15). Most recently, Zhao et al
identified miR-133b was a tumor suppressor in GC, in which miR-133b
was downregulated, and an overexpression of miR-133b led a
reduction of metastatic potential for GC cells by targeting Gli1
transcription factor (16). These
studies clearly suggested that miRNAs were novel biomarkers for the
diagnosis, prognosis and prediction of therapeutic efficacy of GC,
and new targets for developing novel agents for treatment of this
dreadful disease.
In the present study, we found that miR-508-5p was
downregulated in GC, and an overexpression of miR-508-5p could
inhibit the cell proliferation, and reduce the migration and
invasion of GC cells by targeting SKP2 and induce cell-cycle arrest
at G0/G1 phase.
Materials and methods
Ethics statement
Human gastric tissue was collected with a protocol
approved by the Ethic Committee for the Conduct of Human Research
at Ningxia Medical University. Written consent was obtained from
every individual according to the Ethic Committee for the Conduct
of Human Research protocol. All participants were over 18 years of
age and provided written informed consent for the publication of
the data. The Human Research Ethic Committee at Ningxia Medical
University approved this study.
Human gastric tissue samples
Twelve tumor samples with histologic evidence of
gastric adenocarcinoma and matched adjacent non-tumor tissues
(close to adjacent tissues about 2 cm) were collected, ten gastric
tissues diagnosed as antral or gastric inflammation and their
adjacent tissues were archival samples from the Department of
Medical Pathology, General Hospital of Ningxia Medical University
(Table I). The pathologic tumor
staging was determined according to the International Union Against
Cancer (2009) (16).
 | Table I.Profile of miR-508-5p transcript in
various stages of gastric cancer. |
Table I.
Profile of miR-508-5p transcript in
various stages of gastric cancer.
| Subjects | Ages | Gender | Histological
type | Clinical
stages | miR-508-5p
transcript | SKP2 IHC |
|---|
| T1 | 58 | F | A | I | 0.6 | + |
| T2 | 65 | F | A | I | 0.6 | + |
| T3 | 66 | M | A | I | 0.56 | + |
| T4 | 59 | F | A | I | 0.46 | + |
| T5 | 60 | M | A | II | 0.22 | ++ |
| T6 | 65 | M | A | II | 0.2 | ++ |
| T7 | 74 | F | A | III |
0.067 | +++ |
| T8 | 64 | M | A | III |
0.066 | +++ |
| T9 | 65 | F | A | III |
0.054 | +++ |
| T10 | 74 | M | A | III |
0.048 | +++ |
| T11 | 71 | F | A | III |
0.045 | +++ |
| T12 | 68 | M | A | III |
0.038 | +++ |
| N1 | 51 | F | Antral | Non-tumor | 1.66 | − |
| N2 | 65 | M | Antral | Non-tumor | 1.00 | − |
| N3 | 56 | M | Gastric
inflammation | Non-tumor | 2.91 | − |
| N4 | 59 | F | Antral | Non-tumor | 2.54 | − |
| N5 | 64 | M | Gastric
inflammation | Non-tumor | 3.14 | − |
| N6 | 65 | F | Antral | Non-tumor | 1.98 | − |
| N7 | 58 | F | Gastric
inflammation | Non-tumor | 2.38 | − |
| N8 | 66 | M | Gastric
inflammation | Non-tumor | 3.12 | − |
| N9 | 65 | M | Antral | Non-tumor | 2.46 | − |
| N10 | 54 | M | Antral | Non-tumor | 2.74 | − |
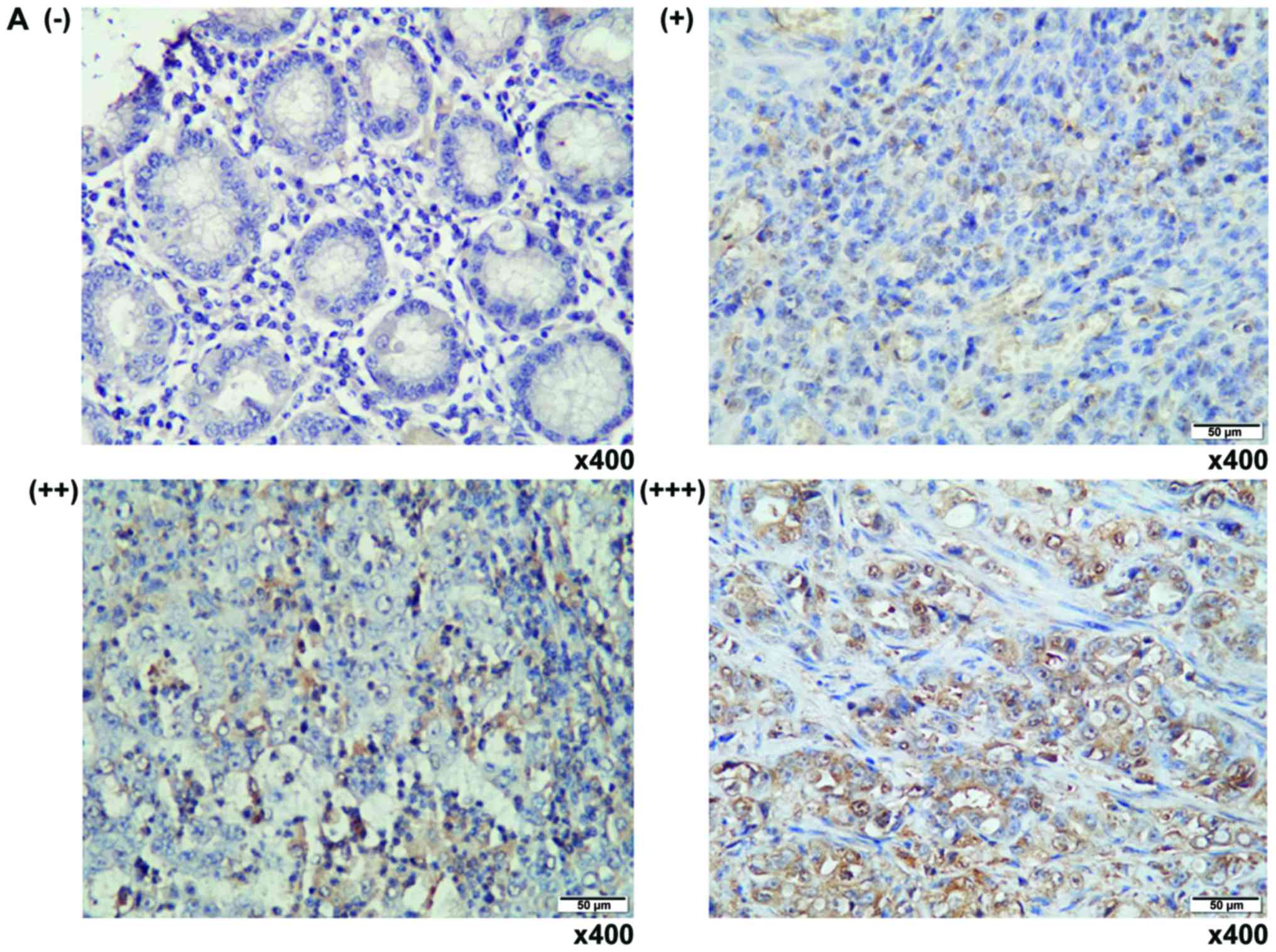
Immunohistochemistry staining
The expression of SKP2 in clinic human GC and
matched adjacent non-tumor tissues was evaluated by
Immunohistochemistry (IHC) staining using mouse anti-human SKP2
antibody (1:100; Abgent Biotech Co., Ltd., Suzhou, China) as
previously described (6,9,17,18).
The expression of SKP2 protein was arbitrarily scored from
-(<25% positive cells), +(25–50% positive cells), ++(50–75%
positive cells) and +++(>75% positive cells), based on the
intensity and number of positive cells by a single experienced
pathologist (Table I).
Cell culture and transfection
Cell lines of human embryonic kidney 293 and human
gastric cancer cell SGC-7901 were purchased from American Type
Culture Collection (Mannasas, VA, USA). The cells were cultured and
maintained at 37°C in a humidified atmosphere of 5% CO2
95% air in dulbecco's modified eagle medium (DMEM) supplemented
with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin.
Plasmid DNA transfection was performed using TransLipid
Transfection Reagent (Beijing TransGen Biotech Co., Ltd., Beijing,
China) per manufacturer's instruction.
Experimental validation of miR-508-5p
target
In order to validate the SKP2 mRNA was a target of
miR-508-5p, a reporter plasmid containing luciferase with the 3′UTR
sequence of SKP2 mRNA was generated. The following primers were
designed based on GenBank database (NM_001243120), and were used
for amplification of wild-type and mutated 3′UTR of SKP2 mRNA: The
sequence of common forward primer was
5′-CGACGCGTCGTTTATTGCAGGATGGTGT-3′, reverse primer for the
wild-type of SKP2 mRNA 3′UTR was 5′-GACTAGTCAGAATGAAGGCAAAGGGA-3′,
and the reverseec primer for mutated SKP2 mRNA 3′UTR was
5′-GACTAGTCACTCACGGATAACCCGAAGGACGGATAAAA-3′. The cDNA generated
from SGC7901 RNA was used as templates for amplification of SKP2
3′UTR fragment by a PCR argeting SKP2 mRNA was ascertained by
co-transfection plasmid DNA of pSicoR/massay as previously
described (19,20). The specificity of miR-508-5p
tiR-508-5p, miR-508-5p/inhibitor or pSicoR/NC and pMIR-Report/SKP2
or pMIR-Report/Mut-SKP2 into 293T cells and determined by the
relative activity of firefly luciferase unit (RLU) at 48 h
post-transfection using a dual-luciferase Reporter assay kit
(Promega Corp., Madison, WI, USA). A Renilla luciferase expressing
plasmid pRL-TK (Promega Corp.) was always included in the
transfection to normalize the efficiency of each transftion.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from infected cells was isolated with a
Miniprep kit, and small RNA was purified with an RNAiso kit per
manufacturer's recommendations (Takara Bio, Dalian, China). Total
RNA from archival paraffin sections was isolated using a Mag-Bind®
FFPE RNA Kit (Omega Bio-Tek, Norcross, GA, USA). The sequence of
primer set for amplification of miR-508-5p was
5′-ACACTCCAGCTGGGTACTCCAGAGGGCGTCACT-3′ (forward) and
5′-TGGTGTCGTGGAGTCG-3′ (reverse). The sequences of RT-PCR primer
set for SKP2 was as: Forward primer 5′-TGTGACTGGTCGGTTGC-3′;
reverse primer 5′-GGAGGGTGGACACTTCTAT-3′. The RT-qPCR was performed
using a Bio-Rad iQ5 lightcycler using a TaKaRa SYBR RT-PCR kit
(Takara Bio). Relative expression was calculated as previously
described using real-time PCR efficiencies and the crossing point
deviation of unknown sample vs. control using the ΔΔCq method
(21).
Generation and infection of lentiviral
vector expressing miR-508-5p
In order to construct a lentiviral vector expressing
miR-508-5p, oligonucleotides of sense strand
(5′-TTACTCCAGAGGGCGTCACTCATGTTCAAGAGACATGAGTGACGCCCTCTGGAGTATTTTTTC-3)
and antisense strand
(5′-TCGAGAAAAAATACTCCAGAGGGCGTCACTCATCTCTCTTGAACATGAGTGACGCCCTCTGGAGTAA-3′)
were synthesized, which were based on the sequence of human
miR-508-5p (MIMAT0004778: 5′-UACUCCAGAGGGCGUCACUCAUG-3′) from
miRBase database. Restriction endonuclease HpaI and
XhoI were introduced at 5′-ends of these oligonucleotides,
respectively. The miR-508-5p precursor was modified with
appropriate restriction enzymes, and cloned into a miRNA expressing
plasmid, pSicoR (Department of Biological Chemistry, School of
Medicine, Fudan University, Shanghai, China) to generate the vector
expressing miR-508-5p, which was designated as pSicoR/miR-508-5p in
this study. Using the same approch, a negative control vector was
also generated, the primer of miR-508 −5p/inhibitor was
5′-CAUGAGUGACGCCCUCUGGAGUA-3′.
For production of the lentiviral vector, the
proviral plasmid (1.5 µg) was co-transfected with packaging
plasmids pCMV-VSV-G (0.5 µg) and pCMV-dR8.91 (1 µg) (Department of
Biological Chemistry) with TransLipid Transfection Reagent as
suggested by the manufacturer. The medium was replaced with 2 ml of
DMEM/10% FBS at 6 h post transfection. The viral particles were
titrated in 293T cells by counting EGFP-positive cells. SGC7901
cells were infected with vector-containing supernatant directly
after 1 day in culture, which was designated as LV-miR-508-5p,
LV-NC and inhibitor.
Western blot analysis
Whole cell lysates (75 µg) were prepared in a lysis
buffer, and were resolved by a 10% sodium dodecyl
sulfate-polyacrylamide gel (SDS-PAGE), followed by being
transferred to a PVDF membrane (Millipore Corp., Billerica, MA,
USA). The membranes were probed with mouse anti-SKP2 antibody and
anti-GAPDH antibody (Boster, Wuhan, China) were for the interested
protein SKP2 and endogenous GAPDH for loading control,
respectively. The blots were developed using the enhanced
chemiluminescence (ECL) reagent (Advansta Inc., Menlo Park, CA,
USA) after they were incubated with the appropriate peroxidase
labeled secondary antibodies.
MTT assay
Cell proliferation was determined by using the MTT
cell proliferation kit (Solarbio, Beijing, China). 5×103 of SGC
7901 cells were seeded in each 96-well plate and allowed to adhere
overnight. The cells were then infected with lentiviral vector for
the indicated times prior to they were used for MTT assay per the
manufacturer's instruction (Bio-Rad Laboratories, Inc., Irvine, CA,
USA).
Assay of Hoechst staining
For the preparation of Hoechst staining, SGC 7901
cells were plated with 1×105 cells/ml in 6-well plates. The cells
were infected with lentiviral vectors for directly stained with
Hoechst kit from Beyotime Institute of Biotechnology (Shanghai,
China). The nuclear morphology was observed under a fluorescent
microscope (Leica, Mannheim, Germany).
Cell scratch assay
The SGC-7901 cells were seeded at 80% confluent and
infected with lentiviral vectors for 48 h (cells were grown to
confluence) in 6-well culture plates. The cells were then scratched
with 200 ml pipette tips. The resultant unattached cells were
removed by washing with pre-warmed PBS for three times, the wounded
monolayers were cultured for additional 48 h prior to be stained
with 0.1% crystal violet solution. The closure of the wounded areas
was observed under a microscope at ×40 magnification (Leica) and
photographed. The distance of closure was quantified with the NIH
Image J image processing program. These experiments were performed
in triplicate. Each condition was tested in duplicate and each
experiment was repeated at least three times.
Transwell assay
Transwell assay was used for accessing the migration
and invasion of cells. For cell migration, 2×104 lentiviral
infected cells suspended in serum-free medium were seed on onto
uncoated 8-µm transwell filter inserts (Corning Inc., Corning, NY,
USA) of 24-well plates in duplicate. The lower chambers contained
500 µl medium containing 10% FBS as a chemoattractant and cultured
for additional 24 h. For the invasion assay, the inserts were
previously coated with Matrigel (BD Biosciences, Bedford, MA, USA)
and cultured for 48 h. At the end of the experiments, the cells in
the upper chamber were removed with a cotton swab, and the cells on
the lower surface were fixed and stained with 0.1% crystal violet
solution. Cells of three randomly visual fields of each insert were
counted under a light microscope. Each condition was tested in
duplicate and each experiment was repeated for three times.
Flow cytometry assay
The apoptosis and cell cycle were determined by a
flow cytometry assay. SGC-7901 cells were infected with various
lentiviral vectors prior to be collected and stained with Annexin V
and PI using an Apoptosis Detection kit I (BD Pharmigen, San Jose,
CA, USA) for flow cytometry analysis. The flow cytometry analysis
was performed on a BD FACSCanto II, and data was analyzed with
FlowJo 8.8.6 software (Tree Star Inc, Ashland, OR, USA). All
experiments were performed with biological triplicates and data are
representative of at least three independent experiments.
Statistical analysis
All data collected in this study was obtained from
at least three independent experiments for each condition. SPSS
17.0 analysis software (SPSS, Inc., Chicago, IL, USA) and PRISM 5
(GraphPad Software, Inc., La Jolla, CA, USA) were used for the
statistic analysis. Statistical evaluation of the data was
performed by one-way ANOVA when more than two groups were compared
with a single control, and t-test for comparison of differences
between the two groups. Significant differences were assigned to
P<0.05, P<0.01 and P<0.0001 denoted by *, ** and ***,
respectively. Data was presented as the mean ± standard deviation
(SD).
Results
SKP2 is upregulated in human gastric
adenocarcinoma
Since SKP2 expression is positively correlated with
poor prognosis and metastasis in many cancers, including the GC
(6). To further interrogate
clinical relevance of SKP2 with the pathogenesis in human GC, its
expression was first evaluated in GC tumor tissues and the adjacent
non-tumor tissues by IHC staining against anti-SKP2 antibody. IHC
staining displayed a predominantly nuclear staining of SKP2
(Fig. 1A). Notably, the expression
of SKP2 was strikingly provoked with the malignant stages of tumors
from the twelve examined archival GC samples, relative to the
adjacent non-tumor tissues and antral or inflammatory gastric
tissues (Fig. 1B), supporting the
previous findings of that SKP2 was an important signaling molecule
that plays an oncogenic role in the carcinogenesis of GC, and an
attractive potential target for treatment of this disease (6,8,17).
miR-508-5p is downregulated in human
GC
Previous miRNA microarray analysis has demonstrated
a downregulation of miR-508-5p in many cancers, including the GC
(22–24). In order to further validate a
correlation of the expression of miR-508-5p and clinicopathologic
stages of GC, the relative expression of miR-508-5p in tissues of
various stages of GC, antral and grastric inflammation was
evaluated by a RT-qPCR assay (Fig.
1B and Table I). The results
revealed an inverse correlation of miR-508-5p transcripts with the
progression of GC (Table I);
miR-508-5p was dramatically downregulated in GC relative to other
non-cancer gastric disorders (Fig.
1B and Table I).
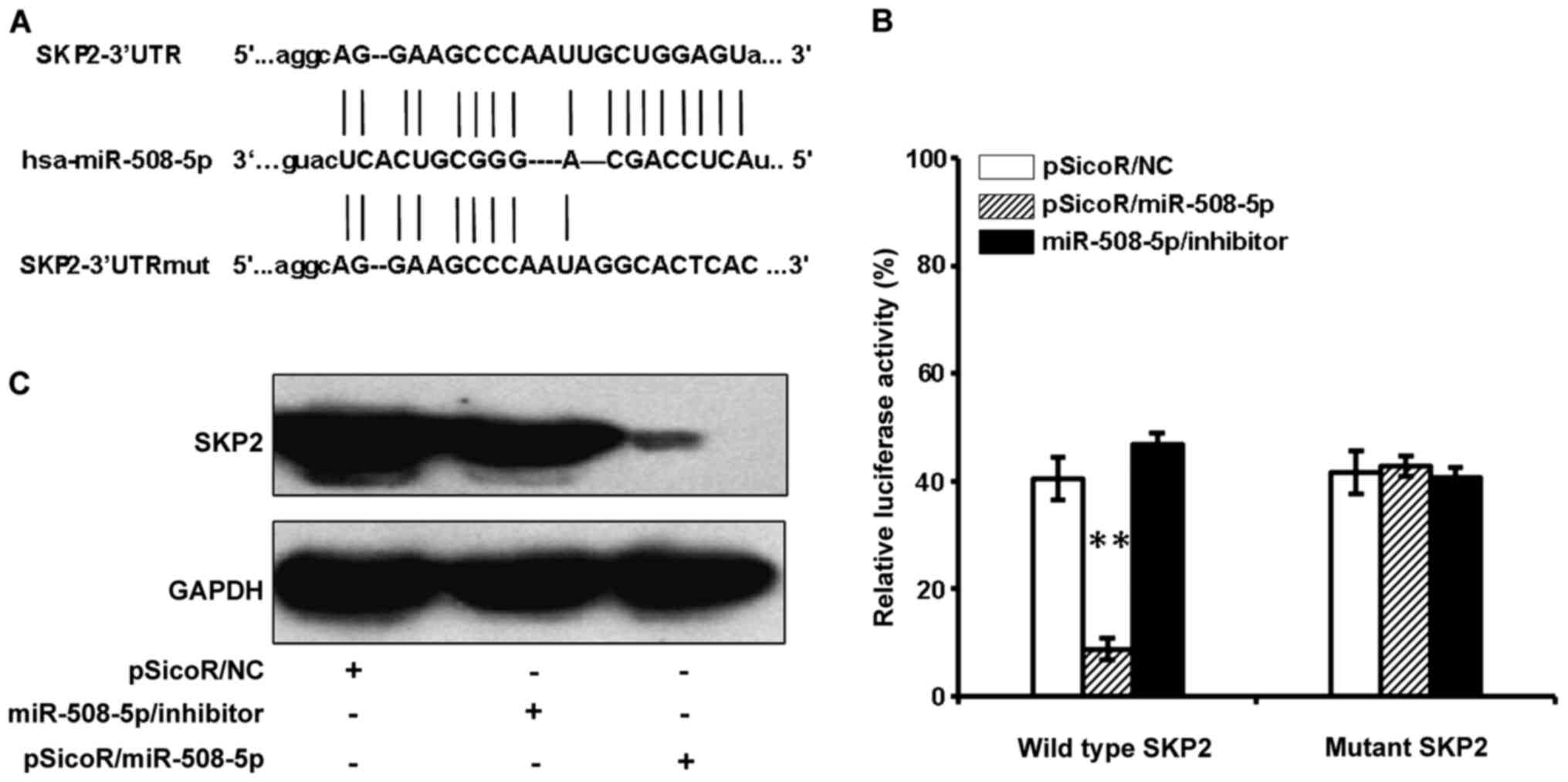
SKP2 mRNA is a target of
miR-508-5p
In order to identify the biological significances of
miR-508-5p in GC, the online computational miRNA target prediction
tool, TargetScan, was used to identify potential targets of
miR-508-5p. The SKP2 was thus identified as a candidate target of
miR-508-5p by virtue of possessing several seed sequences of
miR-508-5p within the 3′UTR of their mRNA. To experimentally
validate whether SKP2 is a potential target of miR-508-5p in GC.
Luciferase reporter vector containing a 3′UTR of SKP2 mRNA
(pMIR-Report/SKP2 3′UTR), or a mutated 3′UTR (pMIR-Report/Mut-SKP2
3′UTR) were first constructed (Fig.
2A). The 293T cells were co-transfected with pMIR-Report/SKP2
3′UTR or pMIR-Report/Mut-SKP2 3′UTR, and proviral plasmid
pSicoR/NC, pSicoR/miR-508-5p, miR-508-5p/inhibitor. The results of
dual luciferase assay showed a significant decrease and increase of
relative luciferase activity in the cells transfected with
pSicoR/miR-508-5p, miR-508-5p/inhibitor, respectively, in
comparison with the pSicoR/NC transfected cells (Fig. 2B). There was no significant change
of luciferase activity in the cells transfected with pSicoR/NC or
pMIR-Report/Mut-SKP2 3′UTR plasmid DNA (Fig. 2B). Immunoblotting assay further
confirmed that the endogenous SFP2 protein was also strikingly
suppressed in the 293T cells transfected with pSicoR/miR-508-5p
(Fig. 2C). This data indicated
that SKP2 might be a potential target for tumor suppressor
miR-508-5p in GC.
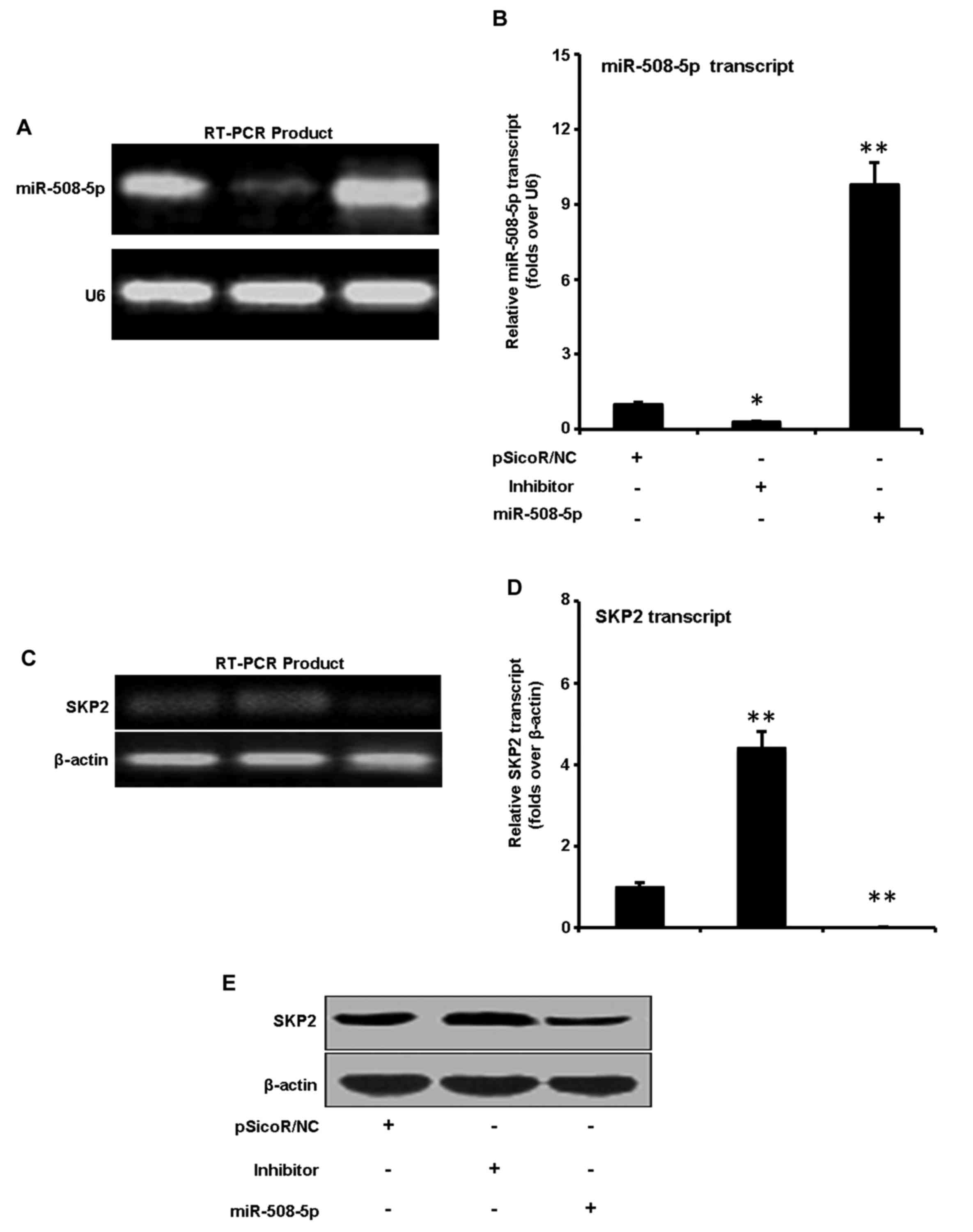
miR-508-5p represses SKP2 expression
in GC SGC-7901 cells
We next sought to explore the capacity of miR-508-5p
to regulate SKP2 in GC. SGC-7901 cells were infected with LV-NC,
LV-miR-508-5p, inhibitor vectors. Although miR-508-5p transcript
was detected in GC SGC-7901 cells, a significant augmentation and
inhibition of miR-508-5p transcript were observed in cells infected
with LV-miR-508-5p, inhibitor vectors as determined by a qRT-PCR
assay, in comparison with the cells infected with LV-NC,
respectively (Fig. 3A and B). The
total cell lysates were harvested for immunoblotting analysis
against anti-SKP2 antibody at 48 h post infection. The result
demonstrated that the expression of SKP2 was downregulated and
upregulated at both mRNA (Fig. 3C and
D) and protein levels (Fig.
3E) in cells infected with LV-miR-508-5p, inhibitor vectors, as
compared with the LV-NC, respectively. These results suggested that
miR-508-5p was capable of downregulating SKP2 expression in GC
cells at both of transcriptional and post-transcriptional levels,
indicative of an underlying mechanism of miR-508-5p in
carcinogenesis of GC.
miR-508-5p arrests the SGC-7901 cell
proliferation by promoting cell apoptosis
To better characterize the functionality of miR-508
in GC cell proliferation, the SGC-7901 cells were infected with
LV-NC, LV-miR-508-5p, or inhibitor vectors, the cell proliferation
and apoptosis was determined by an MTT, Hoechst staining and flow
cytometric assays. The infection of LV-miR-508-5p showed a
significant reduction of cell proliferation, in comparison with
those infected with LV-NC and inhibitor at 72 h post-infection
(P<0.05), as determined by an MTT assay (Fig. 4A). Flow cytometry analysis further
demonstrated an increased frequency of apoptosis cells in the
SGC-7901 cells infected with LV-miR-508-5p, in comparison with
those infected with LV-NC (P<0.01) (Fig. 4B); and an increased abundance of
cells with characteristics of chromosome condensation was also
found in the cells infected with LV-miR-508-5p (Fig. 4C and D).
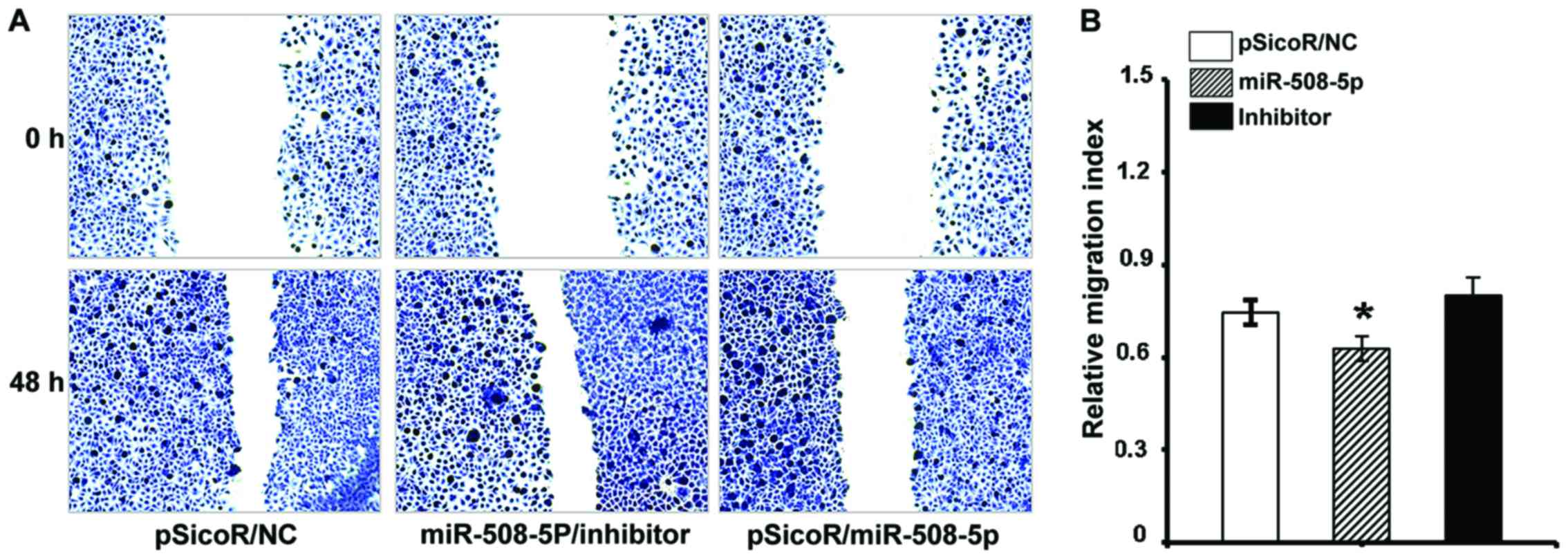
miR-508-5p inhibits SGC-7901 cell
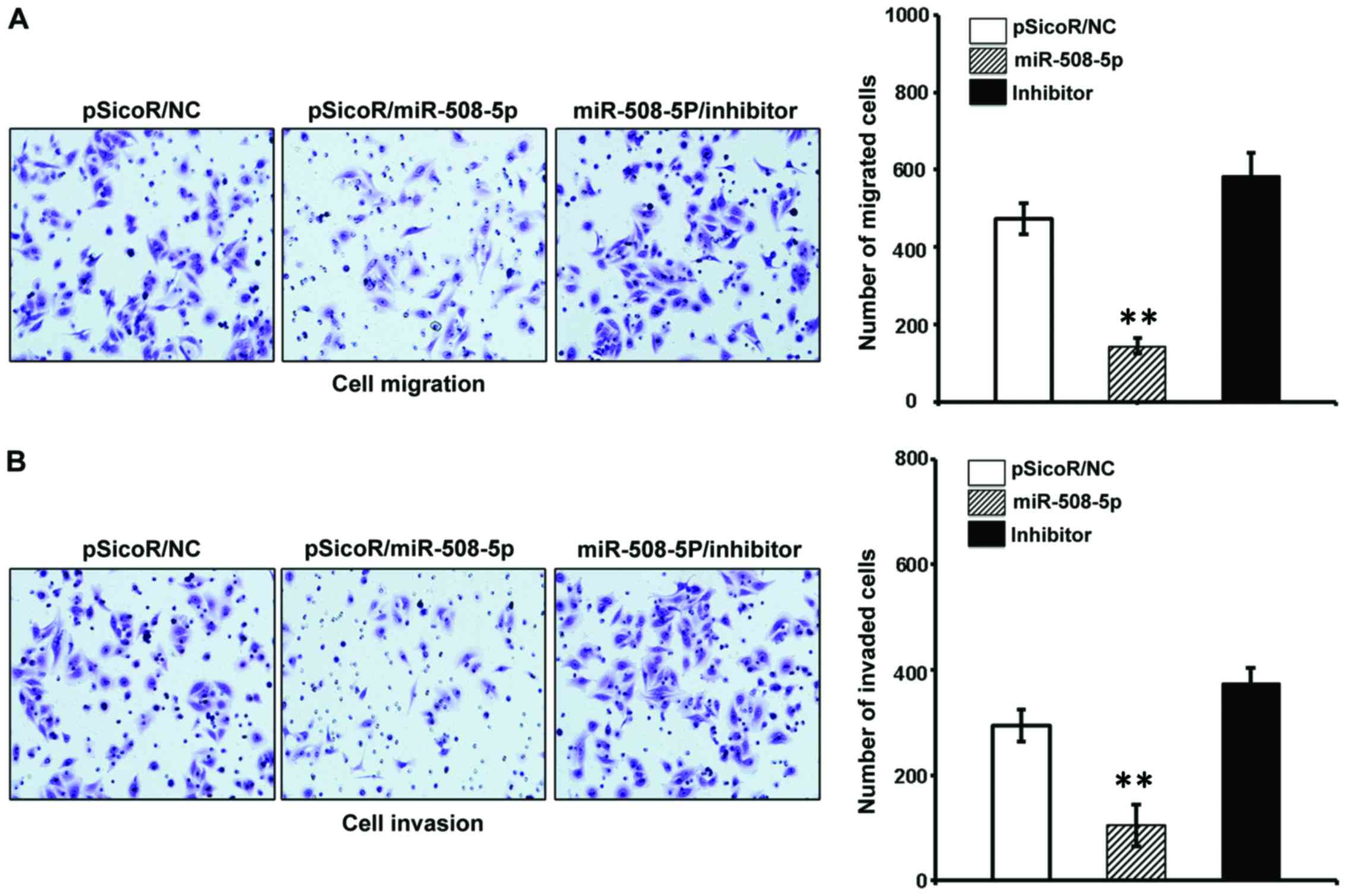
migration and invasion in vitro
The aforementioned result of inverse relationship
between miR-508-5p and clinical pathogenesis of GC led us to
investigate the impact of miR-508-5p in the metastasis of GC by a
cell scratch assay and a transwell assay in vitro. The
migration capacity in the SGC-7901 cells infected with
LV-miR-508-5p was significantly reduced, as compared with those
infected with LV-NC or inhibitor, as determined by both of scratch
assay (Fig. 5) and transwell assay
(Fig. 6A) (P<0.05 and
P<0.01, respectively). In addition, the ability of invasion
through the Matrigel was also dramatically decreased in the
SGC-7901 cells overexpressed miR-508-5p, in comparison with the
controls (P<0.01) (Fig.
6B).
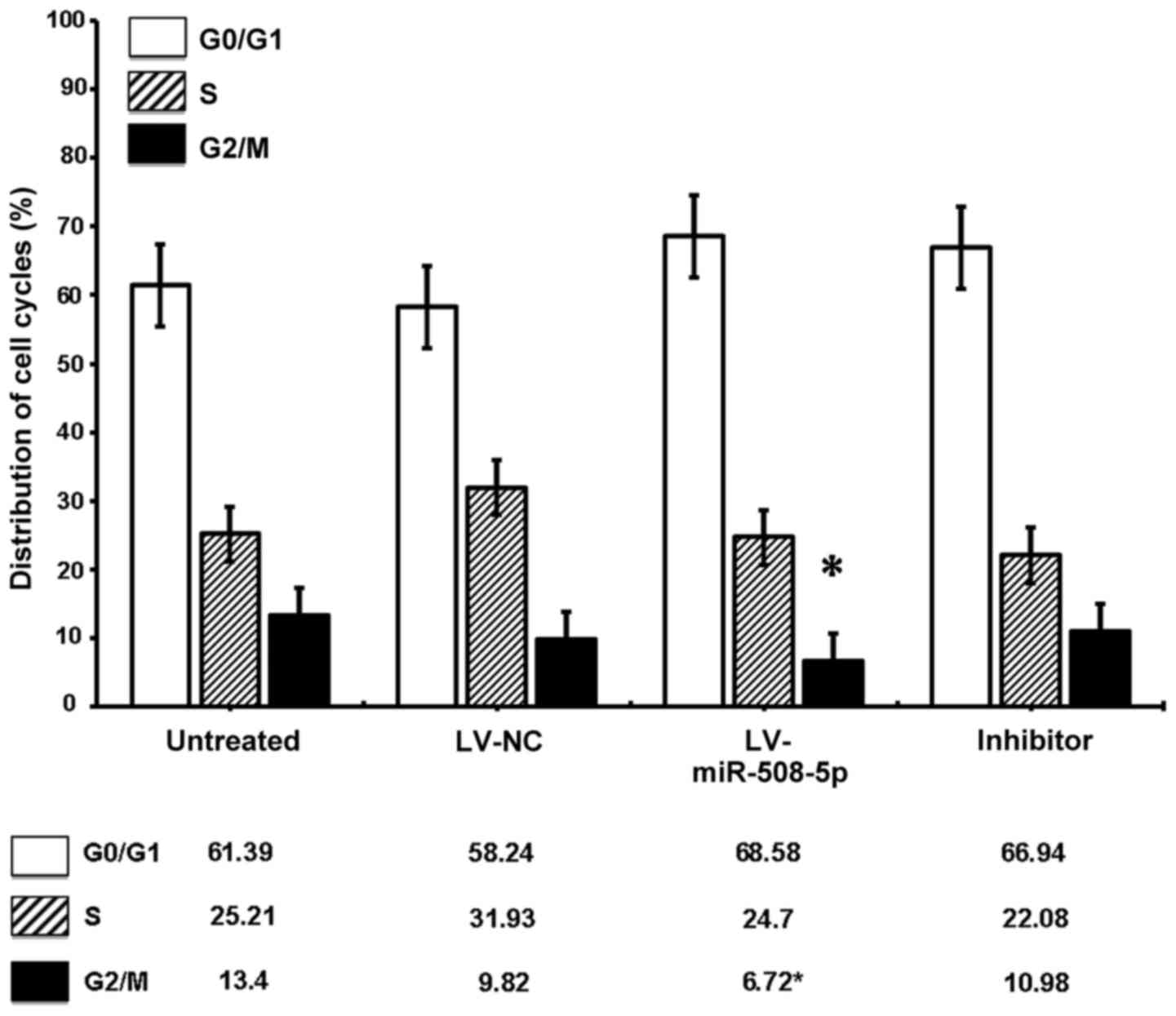
miR-508-5p regulates SGC-7901 cell
cycle progression
Since the SKP2 was a regulator of cell cycle, we
next sought to determine whether the miR-508-5p regulated the cell
cycle of GC cells in vitro. Flow cytometric assay
demonstrated a significantly decreased G2/M phase cell fraction in
LV-miR-508-5p cells, as compared with the controls (P<0.05)
(Fig. 7). Of interest, an
overexpression of miR-508-5p also showed a trend toward cell arrest
at G0/G1 phase (Fig. 7). These
data suggested that miR-508-5p was capable of regulating SGC-7901
cell cycle progression by inducing cell cycle arrest at the G0/G1
phase in part through a mechanism of targeting SKP2.
Discussion
Increasing evidence has suggested that miRNAs play
crucial roles in the development and progression of human GC. In
the present study, we identified miR-508-5p was a potential tumor
suppressor in GC. miR-508-5p was significantly downregulated in GC,
which was correlated with the progression and malignance of GC.
Overexpression of miR-508-5p showed an ability to promote cell
apoptosis, inhibit the migration and invasion of GC cells in
vitro, and induce cell cycle arrest at G0/G1 phase by directly
targeting SKP2.
SKP2 has been demonstrated as a key factor for
progression of cell from G to S phase by regulating cell cycle
progression in Gl/S and G2/M phases, through a ubiquitin-dependent
p27 degradation pathway (25). In
this regards, SKP2 could interrupt cell cycle progression, and thus
were involved in the cell proliferation, apoptosis, tumorigenesis
and malignant transformation, by ubiquitin-dependent degradation of
a variety of target proteins (7).
SKP2 can function as an oncoprotein that plays a crucial role in
the development and progression of many cancers (4). Several studies have revealed that the
expression of SKP2 was evoked in human gastrointestinal cancers,
which was inversely correlated with p27 protein level, and
positively associated with the progression of the cancer (6,26,27).
SKP2 is thus a potential target of developing novel agents for
treatment of cancers (3,4,28).
In agreement with these findings, the IHC result in the present
study also showed an augmented SKP2 protein expression in human GC
mucosal tissues, which was correlated with a reduced expression of
miR-508-5p transcript and the progression of the cancers.
miRNAs have been demonstrated to play roles in
development of GC (14,16,19,29–31).
For instance, Yao et al recently identified miR-146a acted
as a tumor suppressor by directly targeting WASF2 in GC (32). Mounting evidence suggested that
miRNAs could directly or indirectly regulate SKP2 by targeting
various genes. Consistent with this finding, we interestingly
identified which the miR-508-5p was able to exert a role of tumor
suppressor in GC by targeting SKP2. An overexpression of miR-508-5p
was able to downregulate SKP2 expression at both transcriptional
and post-transcriptional levels, repress GC cell migration and
invasion, and inhibit GC cell proliferation by inducing cell cycle
arrest at the G0/G1 phase. Such miRNA-regulated cell cycle arrest
was also reported by other group (25,33).
Thus, a downregulation of SKP2 may inhibit cell proliferation and
induce cell cycle arrest at G0/G1 phase.
miR-508-5p has been demonstrated to be involved in
chronic heart failure (CHF) and several types of cancer, including
the GC (22–24,34).
These datas suggested miR-508-5p could be a useful target for the
diagnosis, prevention and treatment of CHF (34). In human GC, miR-508-5p was found to
be downregulated in lymph node metastatic tissues, relative to the
primary tumor tissue of GC (23);
overexpression of miR-508-5p was capable of reversing GC cell
resistance to multiple chemotherapeutics in vitro and
sensitizing tumors to chemotherapy in vivo (22). Together with the findings in this
study, the results concluded that a miR-508-5p played a critical
role in the tumorigenesis of GC, and could be served as a
prognostic factor for overall survival, drug resistance prediction
and treatment in gastric cancer.
Recently, Zhao et al found miR-133b was
downregulated in human GC, and overexpression of miR-133b reduced
the metastatic capacity of GC cells by directly targeting Gli1
transcription factor (16). In
line with this finding, we also observed reduced the abilities of
invasion and migration in SGC-7901 cells after over-expressed
miR-508-5p by both scratch and transwell assays. Previous study has
demonstrated that SKP2 binding sites resided in Sp1 promoter region
(20), and the Sp1 gene could
regulate the expressions of MMP2 and MMP9, consequently influence
the potentials of cell invasion and migration. Therefore, a
downregulation of SKP2 may also inhibit the Sp1 activity, and
sequentially reduce the metastatic ability of GC cells.
Collectively, abundant SKP2 protein was detected in
GC tissues, accompanied with striking downregulation of miR-508-5p
in GC. An overexpression of miR-508-5p exhibited an ability to
promote cell apoptosis, reduce metastatic potentials in
vitro, and induce cell cycle arrest at G0/G1 phase through a
mechanism by which the miR-508-5p directly target SKP2. We thus
identified miR-508-5p as a tumor suppressor in GC, which warranted
for further investigation as a novel target for prognosis,
prevention and treatment of this disease.
Acknowledgements
This study was supported by grants from the Natural
Science Foundation of China (no. 81460368), the Ningxia High
Education Science and Technology important project (2014, no.
2014–70), and the Science and Technology program of Ningxia
(2013).
References
|
1
|
Yang L: Incidence and mortality of gastric
cancer in China. World J Gastroenterol. 12:17–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chan CH, Li CF, Yang WL, Gao Y, Lee SW,
Feng Z, Huang HY, Tsai KK, Flores LG, Shao Y, et al: The Skp2-SCF
E3 ligase regulates Akt ubiquitination, glycolysis, herceptin
sensitivity, and tumorigenesis. Cell. 149:1098–1111. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pascal LE and Wang Z: Virtual drug design:
Skp1-Skp2 inhibition targets cancer stem cells. Asian J Androl.
15:717–718. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Z, Gao D, Fukushima H, Inuzuka H, Liu
P, Wan L, Sarkar FH and Wei W: Skp2: A novel potential therapeutic
target for prostate cancer. Biochim Biophys Acta. 1825:11–17.
2012.PubMed/NCBI
|
|
5
|
Sonoda H, Inoue H, Ogawa K, Utsunomiya T,
Masuda TA and Mori M: Significance of skp2 expression in primary
breast cancer. Clin Cancer Res. 12:1215–1220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma XM, Liu Y, Guo JW, Liu JH and Zuo LF:
Relation of overexpression of S phase kinase-associated protein 2
with reduced expression of p27 and PTEN in human gastric carcinoma.
World J Gastroenterol. 11:6716–6721. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang B, Ji LH, Liu W, Zhao G and Wu ZY:
Skp2-RNAi suppresses proliferation and migration of gallbladder
carcinoma cells by enhancing p27 expression. World J Gastroenterol.
19:4917–4924. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei Z, Jiang X, Liu F, Qiao H, Zhou B,
Zhai B, Zhang L, Zhang X, Han L, Jiang H, et al: Downregulation of
Skp2 inhibits the growth and metastasis of gastric cancer cells in
vitro and in vivo. Tumor Biol. 34:181–192. 2013. View Article : Google Scholar
|
|
9
|
Tian YF, Chen TJ, Lin CY, Chen LT, Lin LC,
Hsing CH, Lee SW, Sheu MJ, Lee HH, Shiue YL, et al: SKP2
overexpression is associated with a poor prognosis of rectal cancer
treated with chemoradiotherapy and represents a therapeutic target
with high potential. Tumour Biol. 34:1107–1117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lv A, Li Z, Tian X, Guan X, Zhao M, Dong B
and Hao C: SKP2 high expression, KIT exon 11 deletions, and
gastrointestinal bleeding as predictors of poor prognosis in
primary gastrointestinal stromal tumors. PLoS One. 8:e62912013.
View Article : Google Scholar
|
|
11
|
Manikandan J, Aarthi JJ, Kumar SD and
Pushparaj PN: Oncomirs: The potential role of non-coding microRNAs
in understanding cancer. Bioinformation. 2:330–334. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao M, Yin H and Fei ZW: Clinical
application of microRNA in gastric cancer in Eastern Asian area.
World J Gastroenterol. 19:2019–2027. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao X, Li X and Yuan H: microRNAs in
gastric cancer invasion and metastasis. Front Biosci (Landmark Ed).
18:803–810. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li X, Zhang Y, Zhang H, Liu X, Gong T, Li
M, Sun L, Ji G, Shi Y, Han Z, et al: miRNA-223 promotes gastric
cancer invasion and metastasis by targeting tumor suppressor
EPB41L3. Mol Cancer Res. 9:824–833. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen W, Tang Z, Sun Y, Zhang Y, Wang X,
Shen Z, Liu F and Qin X: miRNA expression profile in primary
gastric cancers and paired lymph node metastases indicates that
miR-10a plays a role in metastasis from primary gastric cancer to
lymph nodes. Exp Ther Med. 3:351–356. 2012.PubMed/NCBI
|
|
16
|
Zhao Y, Huang J, Zhang L, Qu Y, Li J, Yu
B, Yan M, Yu Y, Liu B and Zhu Z: MiR-133b is frequently decreased
in gastric cancer and its overexpression reduces the metastatic
potential of gastric cancer cells. BMC Cancer. 14:342014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei Z, Jiang X, Qiao H, Zhai B, Zhang L,
Zhang Q, Wu Y, Jiang H and Sun X: STAT3 interacts with Skp2/p27/p21
pathway to regulate the motility and invasion of gastric cancer
cells. Cell Signal. 25:931–938. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hung WC, Tseng WL, Shiea J and Chang HC:
Skp2 overexpression increases the expression of MMP-2 and MMP-9 and
invasion of lung cancer cells. Cancer Lett. 288:156–161. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tong F, Cao P, Yin Y, Xia S, Lai R and Liu
S: MicroRNAs in gastric cancer: From benchtop to bedside. Dig Dis
Sci. 59:24–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tapias A, Ciudad CJ, Roninson IB and Noe
V: Regulation of Sp1 by cell cycle related proteins. Cell Cycle.
7:2856–2867. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shang Y, Zhang Z, Liu Z, Feng B, Ren G, Li
K, Zhou L, Sun Y, Li M, Zhou J, et al: miR-508-5p regulates
multidrug resistance of gastric cancer by targeting ABCB1 and
ZNRD1. Oncogene. 33:3267–3276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Z, Wang J, Yang Y, Hao B, Wang R, Li
Y and Wu Q: Loss of has-miR-337-3p expression is associated with
lymph node metastasis of human gastric cancer. J Exp Clin Cancer
Res. 32:762013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yan H, Wang S, Yu H, Zhu J and Chen C:
Molecular pathways and functional analysis of miRNA expression
associated with paclitaxel-induced apoptosis in hepatocellular
carcinoma cells. Pharmacology. 92:167–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lonjedo M, Poch E, Mocholí E,
Hernández-Sánchez M, Ivorra C, Franke TF, Guasch RM and Pérez-Roger
I: The Rho family member RhoE interacts with Skp2 and is degraded
at the proteasome during cell cycle progression. J Biol Chem.
288:30872–30882. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fukuchi M, Masuda N, Nakajima M, Fukai Y,
Miyazaki T, Kato H and Kuwano H: Inverse correlation between
expression levels of p27 and the ubiquitin ligase subunit Skp2 in
early esophageal squamous cell carcinoma. Anticancer Res.
24:777–783. 2004.PubMed/NCBI
|
|
27
|
Harada K, Kawaguchi S Supriatno, Kawashima
Y, Itashiki Y, Yoshida H and Sato M: High expression of S-phase
kinase-associated protein 2 (Skp2) is a strong prognostic marker in
oral squamous cell carcinoma patients treated by UFT in combination
with radiation. Anticancer Res. 25:2471–2475. 2005.PubMed/NCBI
|
|
28
|
Wei S, Chu PC, Chuang HC, Hung WC, Kulp SK
and Chen CS: Targeting the oncogenic E3 ligase Skp2 in prostate and
breast cancer cells with a novel energy restriction-mimetic agent.
PLoS One. 7:e472982012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He W, Li Y, Chen X, Lu L, Tang B, Wang Z,
Pan Y, Cai S, He Y and Ke Z: miR-494 acts as an anti-oncogene in
gastric carcinoma by targeting c-myc. J Gastroenterol Hepatol.
29:1427–1434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu T, Tang H, Lang Y, Liu M and Li X:
MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by
targeting prohibitin. Cancer Lett. 273:233–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takagi T, Iio A, Nakagawa Y, Naoe T,
Tanigawa N and Akao Y: Decreased expression of microRNA-143 and
−145 in human gastric cancers. Oncology. 77:12–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yao Q, Cao Z, Tu C, Zhao Y, Liu H and
Zhang S: MicroRNA-146a acts as a metastasis suppressor in gastric
cancer by targeting WASF2. Cancer Lett. 335:219–224. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sanchez N, Gallagher M, Lao N, Gallagher
C, Clarke C, Doolan P, Aherne S, Blanco A, Meleady P, Clynes M and
Barron N: MiR-7 triggers cell cycle arrest at the G1/S transition
by targeting multiple genes including Skp2 and Psme3. PLoS One.
8:e656712013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qiang L, Hong L, Ningfu W, Huaihong C and
Jing W: Expression of miR-126 and miR-508-5p in endothelial
progenitor cells is associated with the prognosis of chronic heart
failure patients. Int J Cardiol. 168:2082–2088. 2013. View Article : Google Scholar : PubMed/NCBI
|