Introduction
Most of the testosterone in the male is synthesized
by the testicular Leydig cells (1,2),
which is mainly involved in the male reproductive system,
participating in the regulation of sexual differentiation,
spermatogenesis, maintaining sexual behavior and promoting the
development of the accessory sex gland (3–5). The
recent study shows that the testosterone secretion by Leydig cells
is regulated by multiple genes (6–8).
Therefore, identification of novel regulatory genes is important
for elucidating the regulation mechanism of testosterone secretion
in Leydig cells and spermatogenesis in testis.
Zinc finger protein is one kind of transcription
factor, which is widely existed in organisms, and plays an
important role in cell proliferation, cell differentiation and cell
apoptosis (9–12). At present, several studies
demonstrated that zinc finger protein was expressed specifically in
testis, such as ZNF230 (13),
ZNF105 (14) and ZNF300 (15), and closely associated with
spermatogenesis and male reproduction.
ZNF185, also known as ZFP185, is belonging to the
ZNF family and identified by Heiss for the first time in 1997
(16). ZNF185 is located on DXS52
region of the long arm of chromosome Xq28 (16), with a LIM zinc-binding domain at
the C terminus and an actin-targeting domain at the N terminus
(17). The previous study
indicates that ZNF185 is involved in cell proliferation and cell
differentiation (18,19). However, the relationship between
ZNF185 and male reproduction is unknown.
In this study, we investigated the expression and
localization of ZNF185 in mouse testis by qPCR, western blotting
and immunofluorescence. Finally, we studied the effects of ZNF185
on testosterone secretion, cell cycle and cell apoptosis by
lentiviral mediated RNA interference.
Materials and methods
Animals and tissue preparation
Thirty ICR male mice (2-week, 10-week and
60-week-old; n=10 in each group) were purchased from Weifang
Medical University Animal Center (Weifang, China). The mice were
sacrificed to obtain testis tissue and sperm used for following
study. Animal care and experimental procedures were carried out
according to the Animal Research Committee guidelines of Weifang
Medical University. The protocol was approved by the Ethics
Committee of Animal of Shandong (permit no. 20100326).
The isolation and culture of Leydig
cell and Sertoli cell
The isolation and culture of Leydig cell was
performed as previous study (20).
Briefly, the mouse testes were transferred to a centrifuge tube
containing 0.75 mg/ml collagenase IV (Sigma, St. Louis MO, USA) and
digested for 20 min at 37°C. After termination of digestion, the
supernatant was centrifuged at 200 g for 5 min. The supernatant was
discarded, and then the pellet was resuspended in Dulbecco's
modified Eagle's medium (DMEM; Invitrogen, Shanghai, China)
supplemented 10% fetal bovine serum (FBS; Gibco, Shanghai, China)
at 37°C and 5% CO2.
The isolation and culture of Sertoli cell was
performed as previous study (21).
In brief, the seminiferous tubules of testes were washed by
phosphate-buffered saline (PBS) and incubated using 3 mg/ml
collagenase I for 20 min at 37°C on the shaker, and then washed
three times using DMEM medium. After washing three times, the
samples were further digested using 3 mg/ml collagenase I, 1 mg/ml
trypsin (Sigma) and 2 mg/ml hyaluronidase (Sigma) for 20 min at
37°C. Finally, the pellet was resuspended and transferred into DMEM
supplemented 10% fetal bovine serum and incubated at 37°C and 5%
CO2.
Immunofluorescence
Immunofluorescence studies were performed on mouse
testis sections and cells (Leydig cells, Sertoli cells and sperm)
using anti-ZNF185 antibodies (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), as described previous study (22).
qPCR
The mRNA level of ZNF185 expression in mouse testis
was detected by qPCR using SYBR Premix Ex Taq kit (Takara Bio,
Dalian, China). The primer sequence of ZNF185 was as follows:
Forward, 5′-CTCCCAGCATCGCCCCTCTAAG-3′; reverse,
5′-GCCTGGGACCTCCGTTTCTGCT-3′. The primer sequence of β-actin was as
follows: Forward, 5′-CGTTGACATCCGTAAAGACC-3′; reverse,
5′-ACAGTCCGCCTAGAAGCAC-3′. The total volume of reaction mixture is
20 µl, containing 2 µl of cDNA, 0.4 µl Dye, 0.6 µl of each primer,
10 µl SYBR and 6.4 µl H2O. Each reaction was performed
in triplicate. The data was analyzed by the 2−∆∆Cq
method (23).
Western blotting
Mouse testis tissue and cells were homogenized and
treated by lysis buffer kit (Beyotime Institute of Biotechnology,
Shanghai, China). The concentration of protein was determined using
BCA assay kit (Beyotime Institute of Biotechnology). The proteins
were subjected to SDS-PAGE and then transferred to the PVDF
membrane. The membrane was blocked by 5% milk and incubated
overnight with anti-ZNF185 and anti-β-actin (Beyotime Institute of
Biotechnology) at 4°C. After washing by PBST, the membrane was
incubated with the secondary antibody (Beyotime Institute of
Biotechnology) for 1.5 h at room temperature. Finally, the reaction
bands were detected using the enhanced chemiluminescence reaction
kit (ECL; Beyotime Institute of Biotechnology).
Cell preparation
To investigate the relationship between ZNF185 and
testosterone secretion, different concentrations of LH were used to
stimulate the testosterone secretion of Leydig cells. After the
addition of LH, Leydig cells were cultured for 24 h, and then the
supernatants were collected for testosterone assay and the cells
were collected to detect the expression of ZNF185.
To further study the role of ZNF185 in regulation of
testosterone secretion, the lentiviral mediated RNA interference
targeting ZNF185 was constructed by Sangon Biotech (Shanghai,
China), which was transfected into Leydig cells to knockdown the
ZNF185 expression.
Testosterone measurement
To determine the effect of ZNF185 knockdown on
testosterone secretion in Leydig cells, the cells were cultured for
48 h. The cells were trypsinized and centrifuged at 500 g for 10
min. Subsequently, the culture supernatants were collected for
testosterone analysis using the testosterone test kit (Mlbio,
Shanghai, China) according to the manufacturer's instructions.
Cell cycle and cell apoptosis
analysis
Leydig cells were harvested and centrifuged at 200 g
for 15 min. After washing by PBS, the cells were fixed by 70%
ethanol. Subsequently, the cells were incubated in PBS with
propidium iodide (PI, 5 µg/ml) and RNase (200 µg/ml) for 30 min at
room temperature. Finally, the cell cycle was assessed by flow
cytometric analysis.
Leydig cells were collected and centrifuged at 200 g
for 15 min. After removing the supernatant, the cells were washed
by ice-cold PBS. The cells were then resuspended in binding buffer,
and incubated in binding buffer containing PI (5 µg/ml) and Annexin
V (5 µg/ml) for 30 min at room temperature. Ultimately, the cell
apoptosis were assessed by flow cytometric analysis, according to
the manufacturer's instructions.
Statistical analysis
Statistical comparisons were evaluated by an
independent sample t-test. Data were expressed as mean ± SE, and
P<0.05 was considered to indicate a statistically significant
difference. The SPSS software version 19.0 (SPSS, Inc., Chicago,
IL, USA) was used for analysis.
Results
The localization and expression of
ZNF185 in the testis of mouse
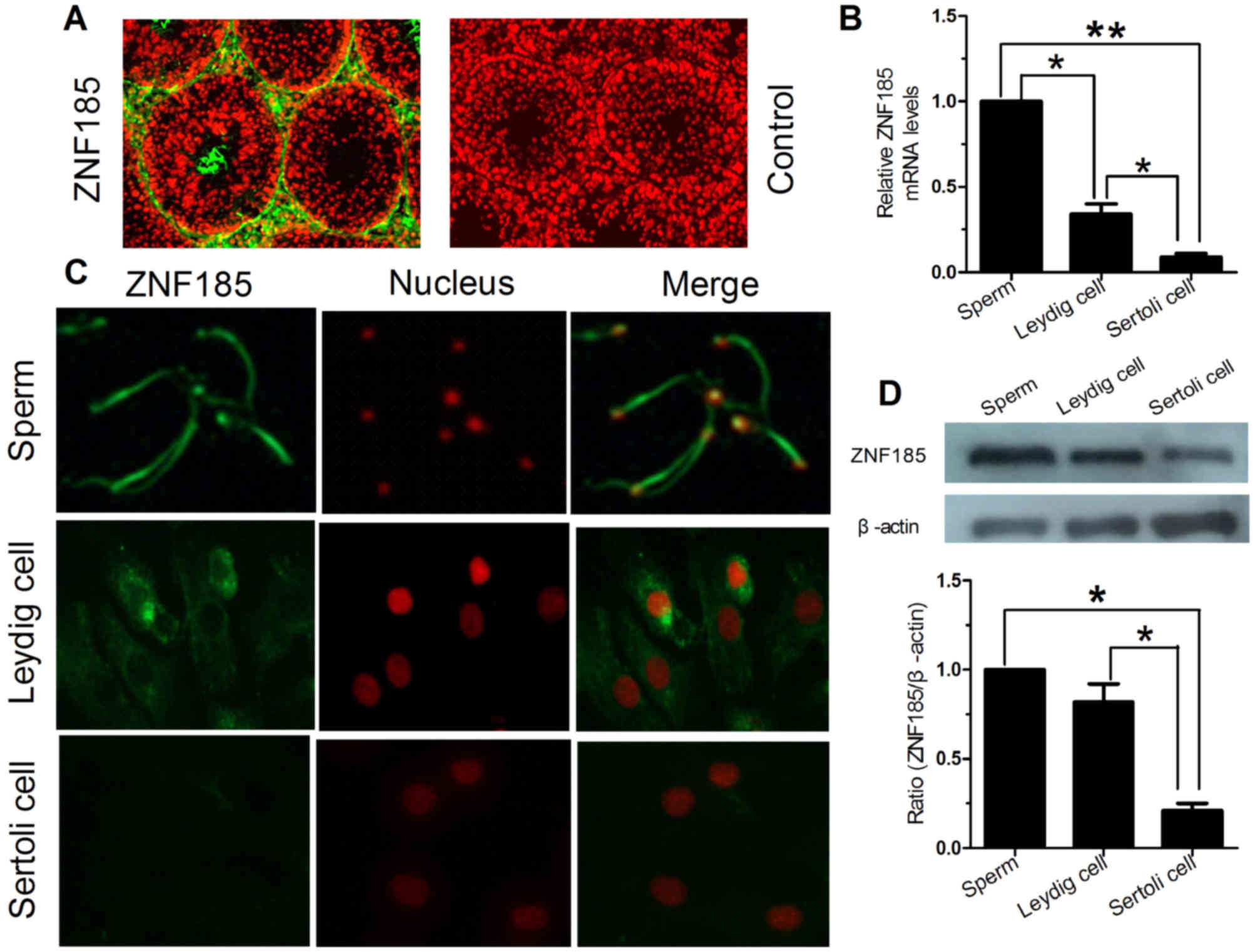
The localization of ZNF185 in the testis of mouse
was detected by immunofluorescence (Fig. 1). The in vivo experimental
results showed that ZNF185 was present in Leydig cell, Sertoli cell
and sperm. Nevertheless, the expression of ZNF185 in Leydig cell
and sperm was significantly higher than Sertoli cell (P<0.05,
Fig. 1A). The in vitro
experimental results showed that ZNF185 was mainly localized in the
cytoplasm of Leydig cell and Sertoli cell, as well as the head and
tail of the sperm (Fig. 1C).
Subsequently, the expression of ZNF185 in Leydig
cell, Sertoli cell and sperm was studied by real-time PCR and
western blot. The PCR results showed that the expression of ZNF185
in sperm was significantly higher than Leydig cell and Sertoli cell
(P<0.05), while the expression of ZNF185 in Leydig cell was
significantly lower than sperm, but higher than Sertoli cell
(P<0.05, Fig. 1B). Western blot
was used to further validate the expression of ZNF185. As shown in
Fig. 1D, the protein expression
level of ZNF185 was significantly higher in Leydig cell and sperm
compared with Sertoli cell (P<0.05), and there was no
significant difference between them (P>0.05).
The expression of ZNF185 in different
developmental stages of mouse testis
The ZNF185 expression in mouse testis aged 2 weeks,
10 weeks and 60 weeks was evaluated by real-time PCR and western
blot. The results showed that ZNF185 was expressed in all detected
time points, and the protein expression pattern of ZNF185 was
similar to the mRNA expression, with age-dependent manner (Fig. 2). The expression of ZNF185 in the
testis of 10 week old mouse was significantly higher than 2 week
old group and 60 week old group (P<0.05). The expression of
ZNF185 in the testis of 60 week old mouse was lower than 10 week
old group (P>0.05), while significantly higher than 2 week old
group (P<0.05).
LH treatment up-regulated the ZNF185
expression and testosterone secretion
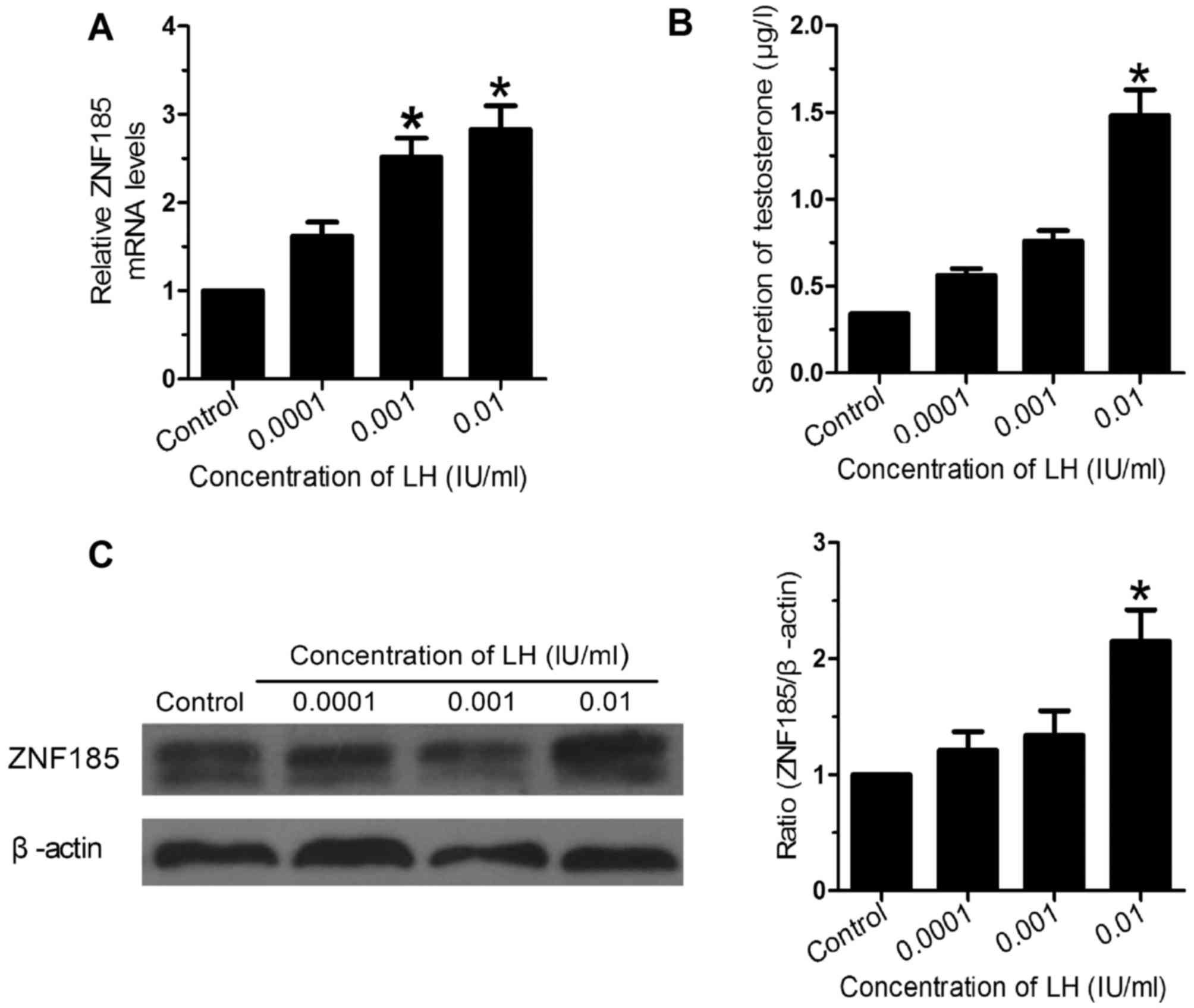
Using different concentrations of LH to stimulate
the Leydig cell, subsequently, the ZNF185 expression was studied by
real-time PCR and western blot, and the testosterone concentration
was detected by ELISA. The results showed that LH could up-regulate
the expression of ZNF185 and testosterone secretion, with
concentration-dependent manner (Fig.
3). With the increase of LH concentration, the expression of
ZNF185 and testosterone secretion was also elevated. Compared with
the control group, the expression of ZNF185 and testosterone
secretion in 0.01 IU/ml group was significantly higher (P<0.05).
Further statistical analysis showed that ZNF185 expression was
significantly positively correlated with testosterone secretion
(r2=0.92, P<0.05).
Lentiviral mediated RNA interference
inhibited the ZNF185 expression and testosterone secretion
In order to further study the role of ZNF185 in
testosterone secretion, the lentiviral mediated RNA interference
targeting ZNF185 was constructed (Fig.
4). The Leydig cells of testis were transfected by the
lentivirus, subsequently, the ZNF185 expression was investigated by
qPCR and western blotting. The results showed that the lentivirus
could significantly inhibit the ZNF185 expression in mRNA and
protein level compared to the control (P<0.05, Fig. 4A and C).
After transfection by the lentivirus, the
testosterone secretion level in Leydig cells was detected by ELISA.
The results showed that the testosterone secretion level in the
lentiviral mediated RNA interference group was significantly lower
than the control group (P<0.05, Fig. 4B). This result indicated that
ZNF185 was closely associated with the testosterone secretion.
Knockdown of ZNF185 expression did not
affect cell cycle and cell apoptosis of Leydig cells
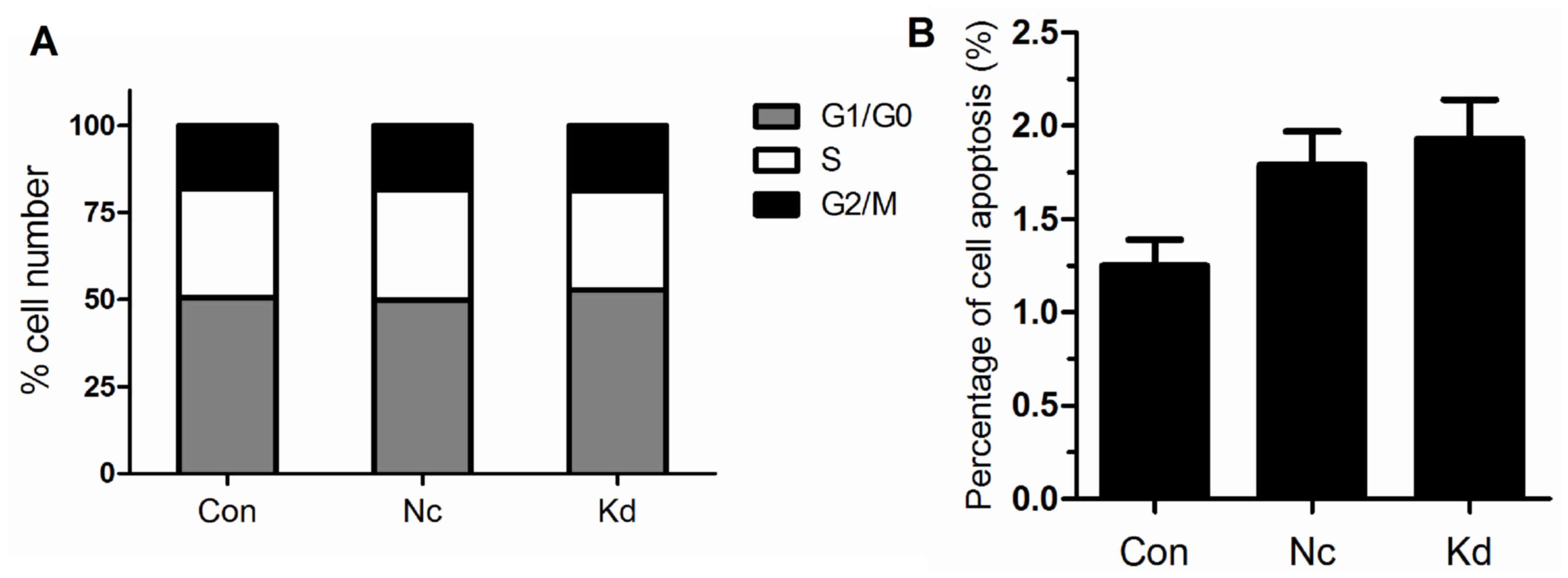
After transfection by the lentivirus, the cell cycle
and cell apoptosis were tested by flow cytometry. The results
showed that knockdown of ZNF185 expression did not significantly
affect cell cycle and cell apoptosis compared to the control
(P>0.05, Fig. 5).
Discussion
Recently, studies demonstrated that several zinc
finger proteins including ZNF230 (13), ZNF105 (14) and ZNF300 (15) were expressed specifically in testis
and closely related to spermatogenesis and male reproduction.
ZNF185 also belongs to the zinc finger protein family. However, the
role of ZNF185 in male reproduction is unknown.
In our present study, we demonstrated that ZNF185
was highly expressed in Leydig cells of the mouse testis and
involved in the secretion of testosterone for the first time. This
conclusion was based on the results of molecular and immunological
experiments. The results of immunofluorescence indicated that
ZNF185 was highly expressed in Leydig cells of the mouse testis
in vivo. The results of real-time PCR and western blot
further validated that ZNF185 expressed significantly higher in
Leydig cells and sperm compared with Sertoli cells in vitro
(P<0.05). Furthermore, the results of immunofluorescence showed
that ZNF185 was mainly localized in the cytoplasm of Leydig cells
and Sertoli cells.
Previous studies have indicated that, in order to
satisfy the physiological functions of different developmental
stages, the testis show stage-specific gene expression pattern
(24–26). Consequently, we investigated the
expression pattern of ZNF185 in mouse testis at different
developmental stages in this study. The results showed that ZNF185
was expressed with age-dependent manner. The expression of ZNF185
was highest in the testis of 10 week old mouse. The expression of
ZNF185 in the testis of 60 week old mouse was lower than
10-week-old group (P>0.05), while significantly higher than
2-week-old group (P<0.05). Taken together, the current results
suggested that ZNF185 plays an important role in male
reproduction.
To verify whether there was a relationship between
ZNF185 and male reproduction, we used different concentrations of
LH to deal with Leydig cells, and then detect the expression of
ZNF185 and testosterone concentration. The results revealed that LH
could up-regulate the expression of ZNF185 and testosterone
secretion. Accompanying with the increase of LH concentration, the
expression of ZNF185 and testosterone secretion was raised. Further
statistical analysis showed that ZNF185 expression was
significantly positively correlated with testosterone secretion
(r2=0.92, P<0.05).
It is well known that most of the testosterone in
the male is synthesized by the testicular Leydig cells and
contribute to the development of Sertoli cells and germ cells
(26). Accordingly, Leydig cells
are essential for maintaining normal reproductive activity and the
testosterone is involved in the regulation of sexual
differentiation, spermatogenesis, maintaining sexual behavior and
promoting the development of the accessory sex gland (3–5,27).
The testosterone secretion of Leydig cells is regulated by various
factors which involved in endocrine and paracrine signaling, and
these factors are crucial for reproductive activity (26). Therefore, a variety of specific
genes of Leydig cells have been identified as pivotal regulatory
factors in the process of reproduction (6–8). In
this study, the results demonstrated that ZNF185 was highly
expressed in Leydig cells of the testis and expressed highest in
adult mouse. In addition, we also found that ZNF185 expression
pattern was significantly positively correlated with testosterone
secretion. As a result, we speculated that ZNF185 was critical for
testosterone secretion of Leydig cells.
To further validate whether ZNF185 involved in
testosterone secretion, the lentiviral mediated RNA interference
was used to knock down the ZNF185 expression in Leydig cells, and
then the testosterone secretion was detected. The results showed
that the lentivirus mediated RNA interference could significantly
inhibit the ZNF185 expression in mRNA and protein level, meanwhile,
the testosterone secretion of Leydig cells was decreased obviously.
These results demonstrated that ZNF185 was directly involved in
testosterone secretion of Leydig cells. However, the mechanism of
testosterone secretion in Leydig cells regulated by ZNF185 need to
be further studied.
In addition, we investigated the cell biological
function, and the results showed that knockdown of ZNF185
expression did not significantly affect cell cycle and cell
apoptosis. These results suggested that ZNF185 was not related to
the cell cycle and cell apoptosis. Nevertheless, whether ZNF185
involved in other cell biological function need to be explored.
In conclusion, we demonstrated that ZNF185 was
highly expressed in Leydig cells of the testis and involved in the
secretion of testosterone for the first time. Our results
contributed to elucidation of the mechanism of male reproduction,
and might provide a target for the treatment of infertility and the
development of contraceptive vaccine.
Acknowledgements
The study was supported by the Natural Science
Foundation of Shandong Province (nos. ZR2013CM032 and ZR2014CL034),
the Science and Technology Development Plan of Shandong Province
(no. 2015GSF118178) and Project of Health and Family Planning
Commission of Shandong Province (no. 201411).
References
|
1
|
Alexandrova ML and Bochev PG: Reduced
extracellular phagocyte oxidative activity, antioxidant level
changes and increased oxidative damage in healthy human blood as a
function of age. Age. 31:99–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen H, Cangello D, Benson S, Folmer J,
Zhu H, Trush MA and Zirkin BR: Age-related increase in
mitochondrial superoxide generation in the testosterone-producing
cells of Brown Norway rat testes: Relationship to reduced
steroidogenic function? Exp Gerontol. 36:1361–1373. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharpe RM, Maddocks S, Millar M, Kerr JB,
Saunders PTK and Mckinnell C: Testosterone and spermatogenesis
identification of stage-specific, androgen-regulated proteins
secreted by adult rat seminiferous tubules. J Androl. 13:172–184.
1992.PubMed/NCBI
|
|
4
|
Zirkin BR and Tenover JL: Aging and
declining testosterone: Past, present, and hopes for the future. J
Androl. 33:1111–1118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Walker WH: Molecular mechanisms of
testosterone action in spermatogenesis. Steroids. 74:602–607. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ahn SW, Gang GT, Kim YD, Ahn RS, Harris
RA, Lee CH and Choi HS: Insulin directly regulates steroidogenesis
via induction of the orphan nuclear receptor DAX-1 in testicular
Leydig cells. J Biol Chem. 288:15937–15946. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matzkin ME, Yamashita S and Ascoli M: The
ERK1/2 pathway regulates testosterone synthesis by coordinately
regulating the expression of steroidogenic genes in Leydig cells.
Mol Cell Endocrinol. 370:130–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mishra J, Gautam M, Dadhich R, Kowtharapu
BS and Majumdar SS: Peritubular cells may modulate Leydig
cell-mediated testosterone production through a nonclassic pathway.
Fertil Steril. 98:1308–1317.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma J, Mi C, Wang KS, Lee JJ and Jin X:
Zinc finger protein 91 (ZFP91) activates HIF-1α via NF-κB/p65 to
promote proliferation and tumorigenesis of colon cancer.
Oncotarget. 7:36551–36562. 2016.PubMed/NCBI
|
|
10
|
Tseng KY and Lin S: Zinc finger factor 521
enhances adipogenic differentiation of mouse multipotent cells and
human bone marrow mesenchymal stem cells. Oncotarget.
6:14874–14884. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang F, Ma H, Feng L, Lian M, Wang R, Fan
E and Fang J: Zinc finger protein x-linked (ZFX) contributes to
patient prognosis, cell proliferation and apoptosis in human
laryngeal squamous cell carcinoma. Int J Clin Exp Pathol.
8:13886–13899. 2015.PubMed/NCBI
|
|
12
|
Buchner DA, Charrier A, Srinivasan E, Wang
L, Paulsen MT, Ljungman M, Bridges D and Saltiel AR: Zinc finger
protein 407 (ZFP407) regulates insulin-stimulated glucose uptake
and glucose transporter 4 (Glut4) mRNA. J Biol Chem. 290:6376–6386.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang S, Qiu W, Wu H, Zhang G, Huang M,
Xiao C, Yang J, Kamp C, Huang X, Huellen K, et al: The shorter zinc
finger protein ZNF230 gene message is transcribed in fertile male
testes and may be related to human spermatogenesis. Biochem J.
359:721–727. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou H, Liu LH, Zhang H, Lei Z and Lan ZJ:
Expression of zinc finger protein 105 in the testis and its role in
male fertility. Mol Reprod Dev. 77:511–520. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao Y, Li JX, Ji CN, Xu XW and Wu M:
Molecular cloning and characterization of a novel splice variant of
human ZNF300 gene, which expressed highly in testis. DNA Seq.
18:312–315. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heiss NS, Gloeckner G, Bächner D, Kioschis
P, Klauck SM, Hinzmann B, Rosenthal A, Herman GE and Poustka A:
Genomic structure of a novel LIM domain gene (ZNF185) in Xq28 and
comparisons with the orthologous murine transcript. Genomics.
43:329–338. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Furukawa D, Chijiwa T, Matsuyama M, Mukai
M, Matsuo EI, Nishimura O, Kawai K, Suemizu H, Hiraoka N, Nakagohri
T, et al: Zinc finger protein 185 is a liver metastasis-associated
factor in colon cancer patients. Mol Clin Oncol. 2:709–713.
2014.PubMed/NCBI
|
|
18
|
Vanaja DK, Cheville JC, Iturria SJ and
Young CY: Transcriptional silencing of zinc finger protein 185
identified by expression profiling is associated with prostate
cancer progression. Cancer Res. 63:3877–3882. 2003.PubMed/NCBI
|
|
19
|
Zhang J, Gong A and Young C: ZNF185, an
actin-cytoskeleton-associated growth inhibitory LIM protein in
prostate cancer. Oncogene. 26:111–122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhong L, Sun J, Liu GH, Zhu YJ and Zhu J:
Research on the steroidogenesis of proliferated Leydig cells in
vitro. J Artif Organs. 16:229–233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang X, Zhang H, Shi Y, Yin S, Zhang Y,
Yang W, Zheng W, Wang L, Wang Z, Bukhari I, et al: Specific
deficiency of Plzf paralog, Zbtb20, in Sertoli cells does not
affect spermatogenesis and fertility in mice. Sci Rep. 4:70622014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Sosnik J, Brassard L, Reese M,
Spiridonov NA, Bates TC, Johnson GR, Anguita J, Visconti PE and
Salicioni AM: Expression and localization of five members of the
testis-specific serine kinase (Tssk) family in mouse and human
sperm and testis. Mol Hum Reprod. 17:42–56. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Johnston DS, Olivas E, DiCandeloro P and
Wright WW: Stage-specific changes in GDNF expression by rat Sertoli
cells: A possible regulator of the replication and differentiation
of stem spermatogonia. Biol Reprod. 85:763–769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Johnston DS, Wright WW, DiCandeloro P,
Wilson E, Kopf GS and Jelinsky SA: Stage-specific gene expression
is a fundamental characteristic of rat spermatogenic cells and
Sertoli cells. Proc Natl Acad Sci USA. 105:8315–8320. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang Q, Hao J, He M, Chen M and Li G:
Localization and expression patterns of prolactin-like protein J in
mouse testis. Mol Med Rep. 10:255–261. 2014.PubMed/NCBI
|
|
27
|
Midzak AS, Chen H, Papadopoulos V and
Zirkin BR: Leydig cell aging and the mechanisms of reduced
testosterone synthesis. Mol Cell Endocrinol. 299:23–31. 2009.
View Article : Google Scholar : PubMed/NCBI
|