Introduction
Esophageal cancer is the eighth most common cancer
and the sixth most common cause of cancer deaths worldwide
(1). A total of 70% of all
esophageal cancer worldwide occurs in China, of which 90% are
esophageal squamous cell carcinoma (ESCC). The main treatment of
the cancer has been the combination of surgery, radiation and
chemotherapy, yet the 5 year survival rate is <15% (2). Invasion and metastasis is the main
contributor to the outcome (3).
miRNAs are small non-coding RNAs that regulate mRNA
levels at the post-transcriptional level by either degrading mRNA
or blocking its translation, thus affecting protein production.
Growing evidence suggests that miRNAs serve a key role in the
expression regulation of multiple genes and related signal
transduction pathway synchronization, as well as in various
diseases, including cancer development and progression (4,5).
Using Ambion bioarrays, Feber et al (6) compared the expression patterns of 328
miRNAs among adenocarcinoma, ESCC, Barrett esophagus, high-grade
dysplasia and normal epithelium samples, and demonstrated miR-203
and miR-205 were downregulated and miR-21 was upregulated in both
ESCC and adenocarcinoma sample compared to the normal epithelium.
Furthermore, Guo et al (7)
identified potentially important miRNAs in ESCC. The expression of
three miRNAs were upregulated, including hsa-miR-25, hsa-miR-424
and hsa-miR-151, and four were downregulated, including
hsa-miR-100, hsa-miR-99a, hsa-miR-29c and mmu-miR-140. These miRNAs
may be used as diagnostic biomarkers to separate ESCC from normal
epithelium. In addition, elevated expression of miR-103/107 was
strongly correlated with poor survival in patients with ESCC from
mono-variant and multi-variant analysis. Increased expression of
miR-129 was reported as associated with poor survival of surgically
treated ESCC patients (8).
In spite of these advances, consistent findings
across studies are lacking. Most studies do not have functional or
mechanistic investigation for the potential markers identified;
association with overall survival can be challenging as it can be
confounded by many other factors. In the present study, miRNAs in
ESCC tissues were compared with lymph node metastasis and those
without, and associated with tumor metastasis by whole genome miRNA
microarray analysis. miR-612 was indicated as highly expressed in
tumors with metastasis. In addition, the authors investigated the
mechanisms of miR-612 in tumor invasion and metastasis in both cell
lines and tumor tissues. The result demonstrated that miR-612 is
positively correlated with tumor aggressiveness, which was aborted
by an miR-612 inhibitor. The expression of miR-612 is inversely
correlated with P53 mRNA and protein expression.
Materials and methods
Selection of miRNAs associated with
tumor metastasis
The research was approved by the Ethics Committee of
Qianfoshan Hospital (Jinan, China), and all clinical investigation
were conducted according to the principles expressed in the
Declaration of Helsinki. All participants in the study had signed
written informed consents.
To identify the candidate miRNAs that are associated
with tumor metastasis, the authors first conducted miRNA profiling
for tumors with lymph node metastasis and for those without. The
fresh frozen tissues of 10 primary ESCC, four with lymph node
metastasis and six without, were selected from the tumor tissue
collection bank in the Department of Pathology, Qianfoshan Hospital
(Jinan, China). The tissue samples were obtained from radical
esophagectomy performed in the same hospital, and patient
information was retrieved from medical records and pathological
reports. No other treatments were given prior to surgery. Tumor
tissue (~100 mg) was taken from the region without hemorrhage and
necrosis. The normal esophageal epithelium was taken from ~3–5 cm
away from the edge of the tumor. The samples were fast frozen in
nitrogen within 15 min following being resected and stored at −80°C
for later use.
RNA extraction
Tissue (30 µg) was taken from tumor and normal
tissues for each case and total RNA was isolated using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
miRNeasy mini kit (Qiagen GmbH, Hilden, Germany) according to
manufacturer's instructions, which efficiently recovered all RNA
species, including miRNAs. RNA quality and quantity was measured by
using a Nanodrop spectrophotometer (ND-1000, Nanodrop; Thermo
Fisher Scientific, Inc., Wilmington, DE, USA), and RNA integrity
was determined by agarose gel electrophoresis. The
OD260/OD280 ratios ranged from 1.8 to 2.01
and no DNA contamination and RNA degradation were observed.
RNA labeling
miRCURY™ isolation (Exiqon A/S, Vedbaek, Denmark)
was used. Of each sample, 1 µg was 3′-end-labeled with Hy3TM
fluorescent label (Exiqon A/S), using T4 RNA ligase by
the following procedure: RNA in 2.0 µl water was combined with 1.0
µl CIP buffer and CIP (Exiqon A/S). The mixture was incubated for
30 min at 37°C, using T4 RNA ligase, 1.5 µl fluorescent
label (Hy3TM), 2.0 µl DMSO (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China), 2.0 µl labeling enzyme were
added into the mixture. The labeling reaction was incubated for 1 h
at 16°C, and terminated by incubation for 15 min at 65°C.
miRNA microarray
The fifth generation of miRCURY™ LNA Array (version,
14.0; Exiqon A/S) was used, which contains >1,891 capture
probes, covering all human, mouse and rat microRNAs annotated in
miRBase 14.0 (http://www.mirbase.org/), as well as
all viral microRNAs related to these species. Following the
completion of the labeling procedure, the Hy3TM-labeled samples
were hybridized on the miRCURY™ LNA Array, according to
manufacturer's instructions. The total 25 µl mixture from
Hy3TM-labeled samples with 25 µl hybridization buffer were first
denatured for 2 min at 95°C, then placed on ice for 2 min and then
hybridized to the microarray for 16–20 h at 56°C in a 12-Bay
Hybridization Systems (NimbleGen; Roche Diagnostics, Basel,
Switzerland), which provides an active mixing action and constant
incubation temperature to improve hybridization uniformity and
enhance the signal. Following hybridization, the slides were washed
several times using Washing buffer kit (Exiqon A/S), and finally
dried by centrifugation for 5 min at 95 × g. Then the slides were
scanned using the Axon GenePix 4000B microarray scanner (Molecular
Devices, LLC, Sunnyvale, CA, USA).
miRNA array data analysis
Scanned images were then imported into GenePix Pro
6.0 software (Molecular Devices, LLC) for grid alignment and data
extraction. Replicated miRNAs were averaged and miRNAs with
intensities >50 arbitrary units in all samples were chosen for
calculating normalization factor. Expressed data were normalized
using the Median normalization. Hierarchical clustering was
performed using TIGR MeV software (version, 4.6; J. Craig Venter
Institute, Rockville, MD, USA). Differentially expressed miRNAs
between normal and tumor tissues were identified by combination of
volcano plot filtering and PAM (Prediction Analysis of Microarrays;
version 2.1; http://statweb.stanford.edu/~tibs/PAM/) (9). The selected genes were illustrated in
heat map by MEV software. Genes with differential a P<0.05 and
fold change >2 were selected for further investigation.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) validation
To validate miR-612 by RT-qPCR, the authors chose
additional 70 samples, including 26 cases of ESCC with lymph node
metastasis, 24 cases without, and 20 cases of normal esophageal
epithelial tissues. Together with the 20 samples, which had been
analyzed by miRNA microarray, a total of 90 samples were used for
RT-qPCR validation. U6 was used as reference for normalization
(10). Directional primers were
designed according to the known miRNA sequences: hsa-miR-612
forward, 5′GCTGGGCAGGGCTTCT3′ and reverse, 5′CAG TGC GTG TCG TGG
AGT3′; U6 forward, 5′GCT TCG GCA GCA CAT ATA CTA AAAT3′ and
reverse, 5′CGC TTC ACG AAT TTG CGT GTC AT3′. RNA was reverse
transcribed to cDNA and then measured by RT-qPCR at 90°C, denatured
for 10 sec, 60°C for 20 sec, 72°C for 20 sec and 78°C for 20 sec
for 45 cycles to establish miR-612 and U6 standard and melting
curves for each sample.
RT-qPCR examining TP53 mRNA expression
in EC109 cells transfected with miR-612
EC109 cells were transferred into a 25 ml flask the
day prior to transfection when cells were ~80% confluent. These
cells were transfected with 2 µg pSilencer/miR-612 (to express
miR-612) and 2 µg control plasmid pSi1encer/NC, respectively. At 24
h, RNAs were extracted from the EC109 cells by TRIzol reagent and
reverse transcribed to cDNA as before, to detect the TP53
expression levels in the EC109 cells.
mRNA target prediction
The authors applied TargetScan (http://www.targetscan.org/), Pictar (http://www.pictar.org/), miRanda (http://www.microrna.org/microrna/home.do) and
MirTarget 2 (http://mirdb.org/miRDB/download.html) analysis tools
to predict the miRNA targets and selected the more reliable targets
that were predicted by at least three out of the four tools.
Cell lines
Human esophageal carcinoma cell lines EC109 and
EC9706 were purchased from the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China), which were cultured
in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine
serum at 37°C and 5% CO2. All experiments were carried
out when the cells reached 60% confluency.
Main reagents
miR-612 inhibitor (anti-sense) and its negative
control, TRIzol reagent, Lipofectamine 2000, and plasmids either
with miR-612 (pSilencer/miR-612) or with negative control
(pSilencer/NC) were purchased from Shanghai GenePharma Co., Ltd
(Shanghai, China). The MicroRNA extracting kit (miRNeasy Mini Kit)
was from Qiagen. The TaqMan® MicroRNA Reverse Transcription kit and
Real-time PCR reagent (including miR-612 Assay, U6 Assay and TaqMan
Universal Master Mix II, no UNG) were from Thermo Fisher
Scientific, Inc. The miR-612 validation primer and the internal
reference U6 were bought from Guangzhou Funeng Gene Company
(Guangzhou, China). Transwell (Millicell Hanging Cell Culture
Inserts) and ECM matrix (ECM Cell Attachment Matrix) were from EMD
Millipore (Billerica, MA, USA). Fetal bovine serum (FBS) was
obtained from Tianjin Haoyang Biological Products Technology Co.,
Ltd. (Tianjin, China). DMEM was purchased from Gibco; Thermo Fisher
Scientific, Inc. The primary antibody against TP53 was a mouse
anti-human monoclonal antibody, the internal reference was a rabbit
anti-human GAPDH monoclonal antibody (Beijing Biosynthesis
Biotechnology Co., Ltd., Beijing, China), and the secondary
antibodies were rabbit anti-mouse IgG and sheep anti-rabbit IgG
(Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China)
The luciferase reporter assay kit was from Invitrogen; Thermo
Fisher Scientific, Inc.
Scratch test to measure migration
ability of cells
EC109 cells were divided into 3 groups, with three
replicates per group: miR-612 inhibitor group, negative control
group and blank group. At 24 h following transfection and
incubation at 37°C with 5% CO2 to form a single layer of
cells, a scratch line was made at the bottom of each well with a 10
µl pipette tip. The cells were washed gently to remove the
deciduous cells and culture medium was replaced with serum-free
medium. Migration vs. scratch distance was observed and measured at
0, 36 and 72 h, respectively.
Tumor cell migration test
EC109 cells were separated into 3 groups, three
replicates each: miR-612 inhibitor group, negative control group
and blank group. After being transfected for 24 h, the EC109 cells
were digested with trypsin and suspended with serum-free DMEM. The
cell suspension, (2×104 cells/ml/well in 200 µl), was added to the
upper chamber of the Transwell and 900 µl DMEM with 5% FBS was
added to lower chamber. After having been incubated for 6 h at
37°C, the remaining cells in the upper chamber were wiped off with
a wet cotton ball. The cells on the wet cotton ball were collected
on a glass slide, fixed with carbinol for 20 min, stained with 0.1%
crystal violet for 20 min., and then washed with PBS 3 times. The
number of cells that migrated to the lower chamber was counted at
×200 magnification using an inverted microscope. A total of 10
random fields of view for each sample were used for counting.
Tumor cell invasion test
The tips and Eppendorf tubes were pre-cooled at 4°C
overnight. Matrigel (1,000 µg/ml) was diluted to 0.2 µg/µl with
DMEM operated on ice. A total of 50 µl Matrigel was added to the
upper chamber of the Transwell and air dried at 4°C overnight. The
excess DMEM was removed and then hydrated with DMEM at 37°C for 1
h. EC109 cells were divided into 3 groups, three replicated each:
The miR-612 inhibitor group, negative control group and blank
group. After being incubated for 24 h at 37°C, the remaining cells
in the upper chamber were wiped off with a wet cotton ball. The
number of cells which migrated to the lower chamber was counted at
×200 magnification in an inverted microscope. A total of 10 views
for one sample were used for counting.
Enhanced green fluorescent protein
(EGFP) fluorescent reporter carrier assay
This experiment was conducted to demonstrate that
TP53 is the direct target of miR-612. EC109 cells were plated on a
48-well plate (1.5×104 cells/well) one day before transfection,
with the cell concentration at about 80% confluency, and then
co-transfected with Lipofectamine 2000 and pSilencer/miR-612 or
pSilencer/NC. Additionally, TP53 3′UTR mutant
(pcDNA3-Egfp-P53-3′UTR mut) and wild-type (pcDNA3-Egfp-P53-3′UTR;
0.3 µg) plasmids were also included to test whether the mutation at
the binding site affects miR-612 binding and subsequent TP53
activity. There were nine parallel wells in each group. Serum was
added to the wells 4 h after transfection to a final serum
concentration of 10%. At 48 h following transfection, cells were
treated with radioimmunoprecipitation assay buffer (150 mmol/l
NaCI, 50 mmol/l Tris-HCl, pH 7.2,1% Triton X-100 and 0.1% SDS),
centrifuged to separate the supernatant at 9,503 × g for 10 min.
The expression levels of green fluorescent proteins were measured
with fluorescence spectrophotometer F-4500 at 484 nm for excitation
and 510 nm for emission.
Western blotting
Western blotting was conducted to measure TP53
protein expression in tumor and normal tissues. The same 90 samples
that had been used for RT-qPCR validation were used for the
measurement of TP53 protein expression. Fresh frozen tissue (1–2 g)
of each sample was added to the precooled buffer solution.
Ultrasonic dispersion was performed in an ice-water bath and then
centrifuged at 13,684 × g at 4°C. Protein (~100 µg) was separated
by electrophoresis in 12% SDS-PAGE gel, then transferred to a
polyvinylidene difluoride (PVDF) membrane, blocked with 5% skim
milk at 4°C overnight. The primary antibodies [mouse anti-human
wild type TP53 monoclonal antibody (cat. no. D199442; 1:50,000;
Beijing Biosynthesis Biotechnology Co., Ltd.) and rabbit anti-human
GAPDH monoclonal antibody (cat. no. D110016; 1:2,000; Beijing
Biosynthesis Biotechnology Co., Ltd.)] were added, incubated for 2
h at 37°C. The PVDF membrane was washed with 0.1% TBS-Tween-20
three times, each for 10 min at room temperature. The secondary
antibodies [rabbit anti-mouse IgG (cat. no. ZDR-5109; horseradish
peroxidase-conjugated; 1:300,000; Beijing Zhongshan Jinqiao
Biotechnology Co. Ltd.) sheep anti-rabbit IgG (cat. no. ZB-5118;
1:10,000; Beijing Zhongshan Jinqiao Biotechnology Co. Ltd.)] were
added, and incubated for 2 h at 37°C. The membrane was washed with
TBS-Tween 20 and Immobilon Western Chemiluminescent HRP substrate
(EMD Millipore) was added. Clear protein bands on transfected
membrane indicated positive results. A ChemiDoc XRS+ image analyzer
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) and Image Lab 4
software (Bio-Rad Laboratories, Inc.) was used to measure the gray
level of the samples.
Western blotting identification of
TP53 protein expression in EC109 cells transfected with
miR-612
EC109 cells were lysed 48 h after the transfection
with miR-612, centrifuged for supernatant at 13,684 × g for 10 min,
electrophoresed on a 10% SDS-PAGE gel, transferred to
nitrocellulose membrane, blocked with 5% skim milk at 4°C
overnight. The primary antibodies and the secondary antibodies were
added, respectively, as described earlier.
Statistical analysis
Measurement data are expressed as the mean ±
standard deviation. Comparisons among three groups were performed
by one-way analysis of variance (ANOVA) followed by Fisher's least
significant difference and Student-Newman-Keuls post hoc tests.
Comparisons between two groups were made with unpaired Student's
t-test. In all cases, P<0.05 was considered to indicate a
statistically significant difference.
Results
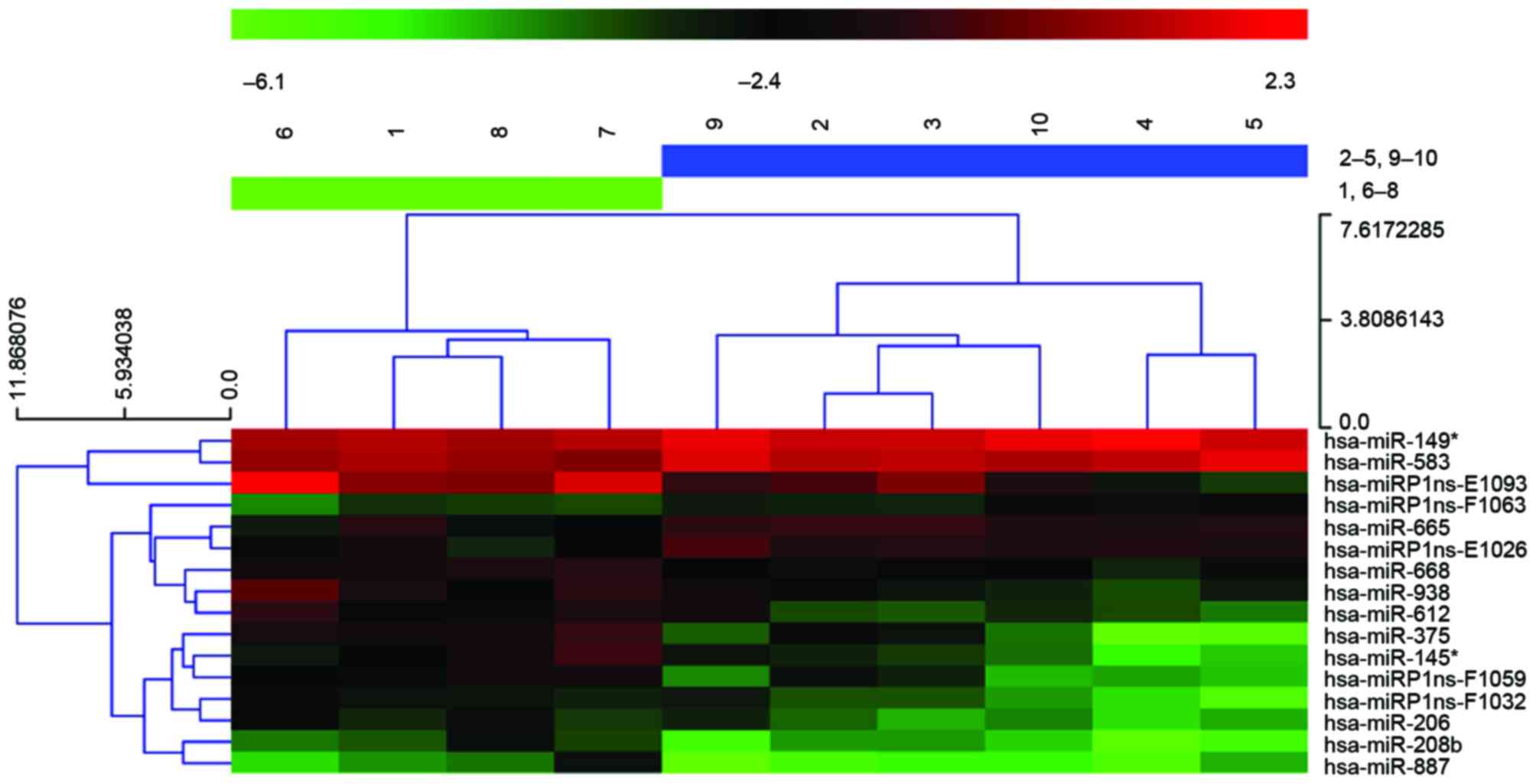
miR-612 is highly expressed in
metastatic tumor tissues
miRNA expression profiling was investigated for 10
ESCC samples, five with metastasis and five without, using an
miRCURY LNA Array. Differential expression analysis indicates that
16 miRNAs are significantly different between the two groups,
P<0.05 and fold change (FC)>2, among which 13 were
upregulated and three were downregulated in the tumors with
metastasis. The authors further conducted non-parametric PAM
analysis, and miR-612 remained a strong predictor for metastasis
phenotype (Fig. 1).
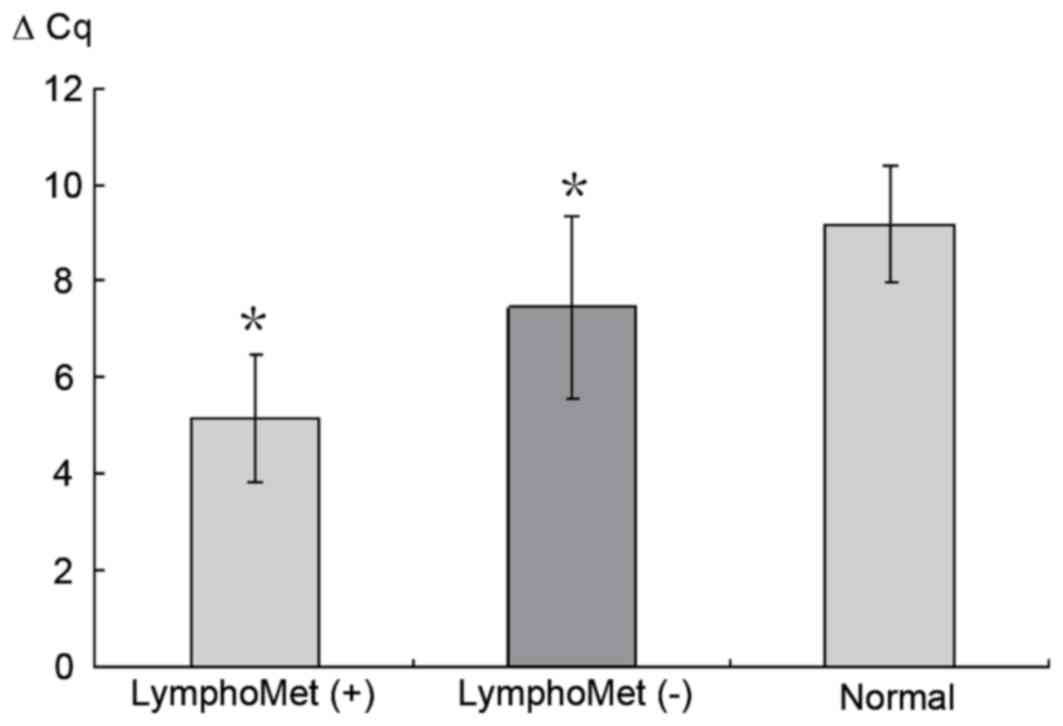
Validation of miR-612 by RT-qPCR
RT-qPCR was performed to validate the expression of
miR-612 in 70 samples. The Cq value was first obtained by comparing
the amplification curves with threshold value. The FC between
miR-612 and U6 was obtained by the 2−ΔΔCq method where a
value >2 indicates upregulation and <0.5 for downregulation.
Consistent with microarray results, miR-612 increased
>five-folds in tumors with lymph node metastasis (LymphoMet),
when compared with the tumors without (P<0.05; Fig. 2).
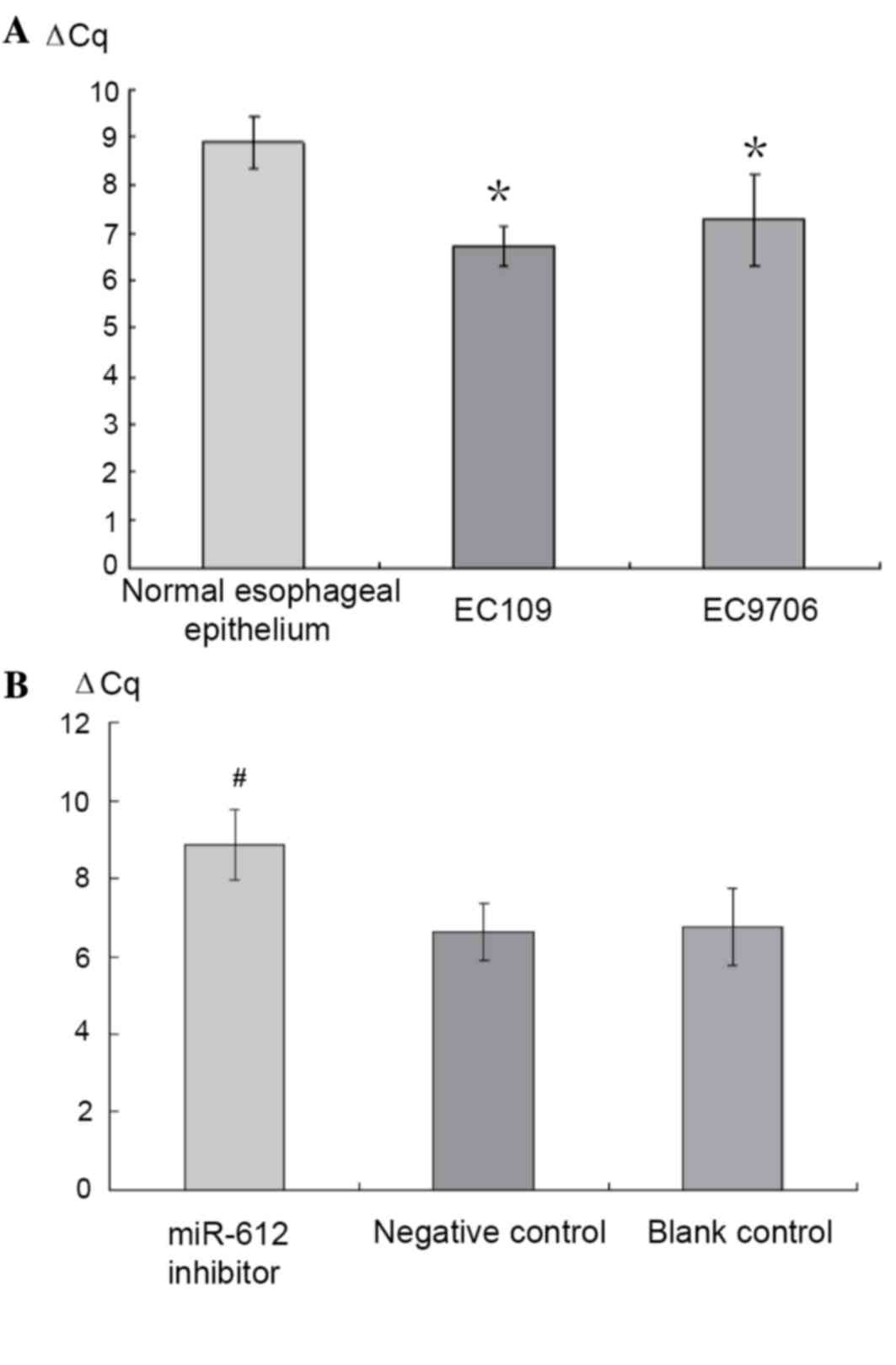
miR-612 inhibitor transfection into
EC109 and EC9706 cell lines
The expression of miR-612 in the normal esophageal
epithelium and cancer cell lines EC109 and EC9706 was measured
using RT-qPCR. Compared to the normal esophageal epithelium cell
line (established in the authors' laboratory), both EC109 and
EC9706 cell lines presented increased expression of miR-612, 4.49-
and 3.05-fold higher, respectively (P<0.05; Fig. 3A). EC109 was then selected for the
transfection experiment with three conditions groups created:
miR-612 inhibitor (150 nmol/l), negative control with scrambled
miRNA (NC), and a blank control (culture media only). After 72 h of
the treatments, RT-qPCR was used to measure the miR-612 levels.
Compared to those prior to the treatment, the group with the
miR-612 inhibitor had significantly reduced expression of miR-612
(P<0.05), while the two control groups saw no change (P>0.05;
Fig. 3B).
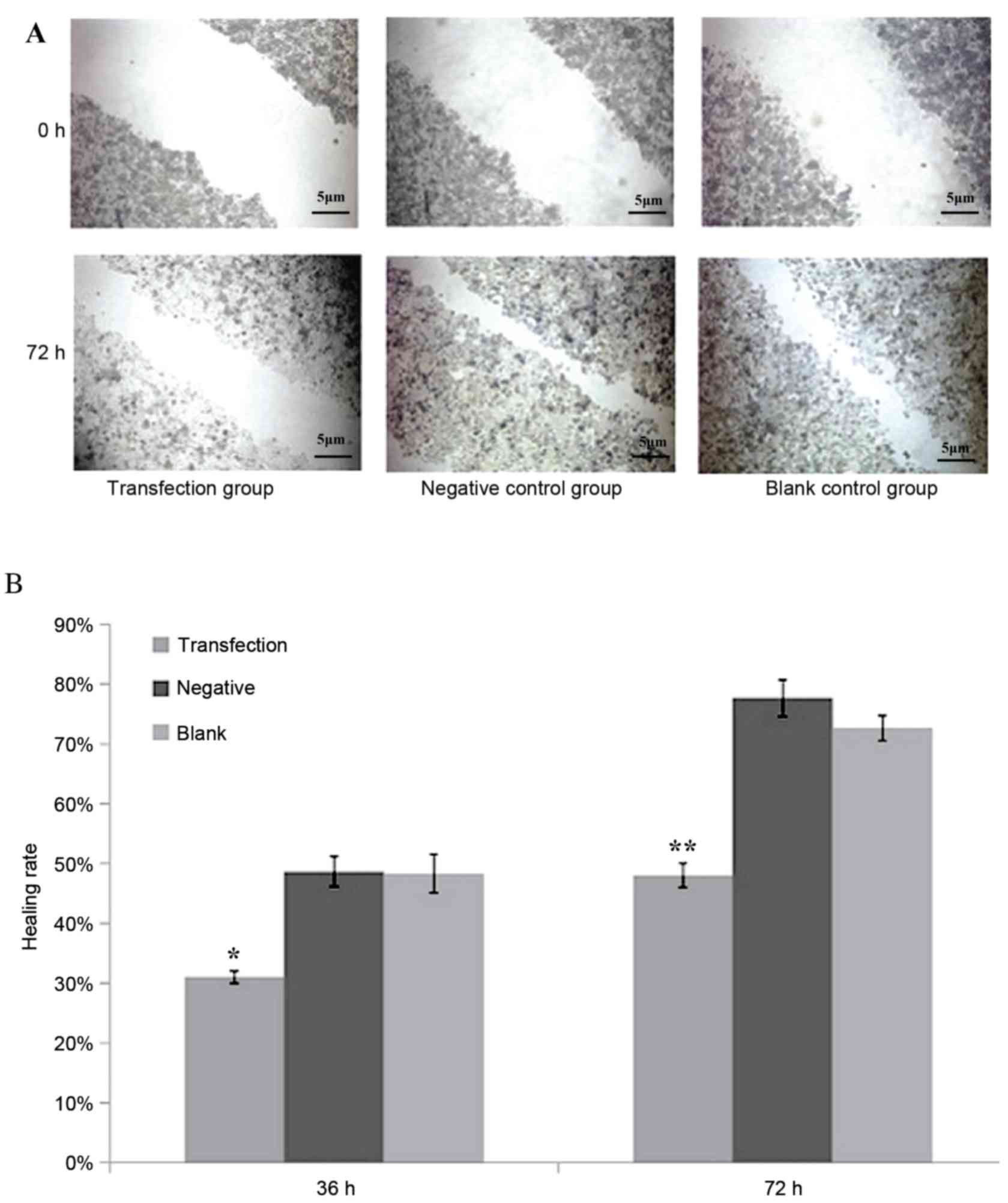
Reduced healing in the miR-612
suppressed group as a result of scratch healing
After scratching, the experimental group whose
miR-612 was inhibited by siRNA had a significantly reduced healing
rate than in the negative control or blank control group (P<0.05
and P<0.01, respectively; Fig.
4). Healing rate was measured by the width of the scratch,
which was defined as 0% at 0 h, and 100% when the cells were kept
confluent.
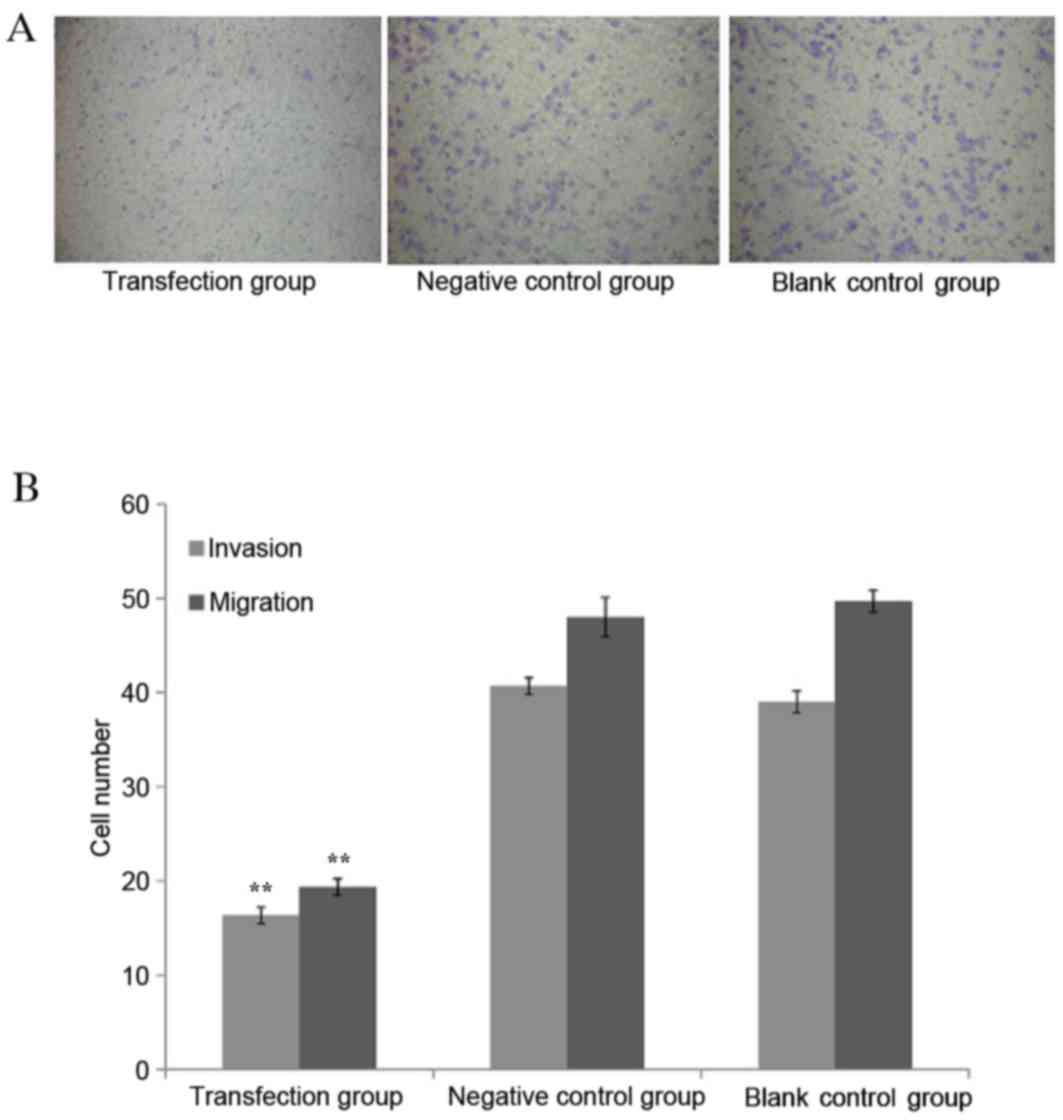
Cancer cells infected with miR-612
inhibitor had reduced invasive and migratory abilities
The invasive and migratory abilities for EC109 cells
were measured through the Transwell experiment following
transfecting the cells with the miR-612 inhibitor along with
scrambled siRNA as negative control and blank control. The cells
transfected with the miR-612 inhibitor had significantly reduced
invasive ability, as demonstrated by the fewer number of cells
migrated out of wells, when compared with the cells without
specific inhibition, either by scrambled miRNA or blank media (both
P<0.01; Fig. 5).
Prediction and evaluation of target
genes of miR-612
TargetScans, Pictar and miRanda and MirTarget 2 were
used to search for the mRNA targets of miR-612 and the targets that
were predicted by all the prediction tools were evaluated (45
predicted miRNA target genes). With consideration of the literature
reports for esophageal squamous cell carcinoma-associated genes
(11–17), it was identified that TP53 is
likely the most important target of miR-612 due to its significant
role in cancer development. The current research was focused on the
relationship of TP53 and miR-612.
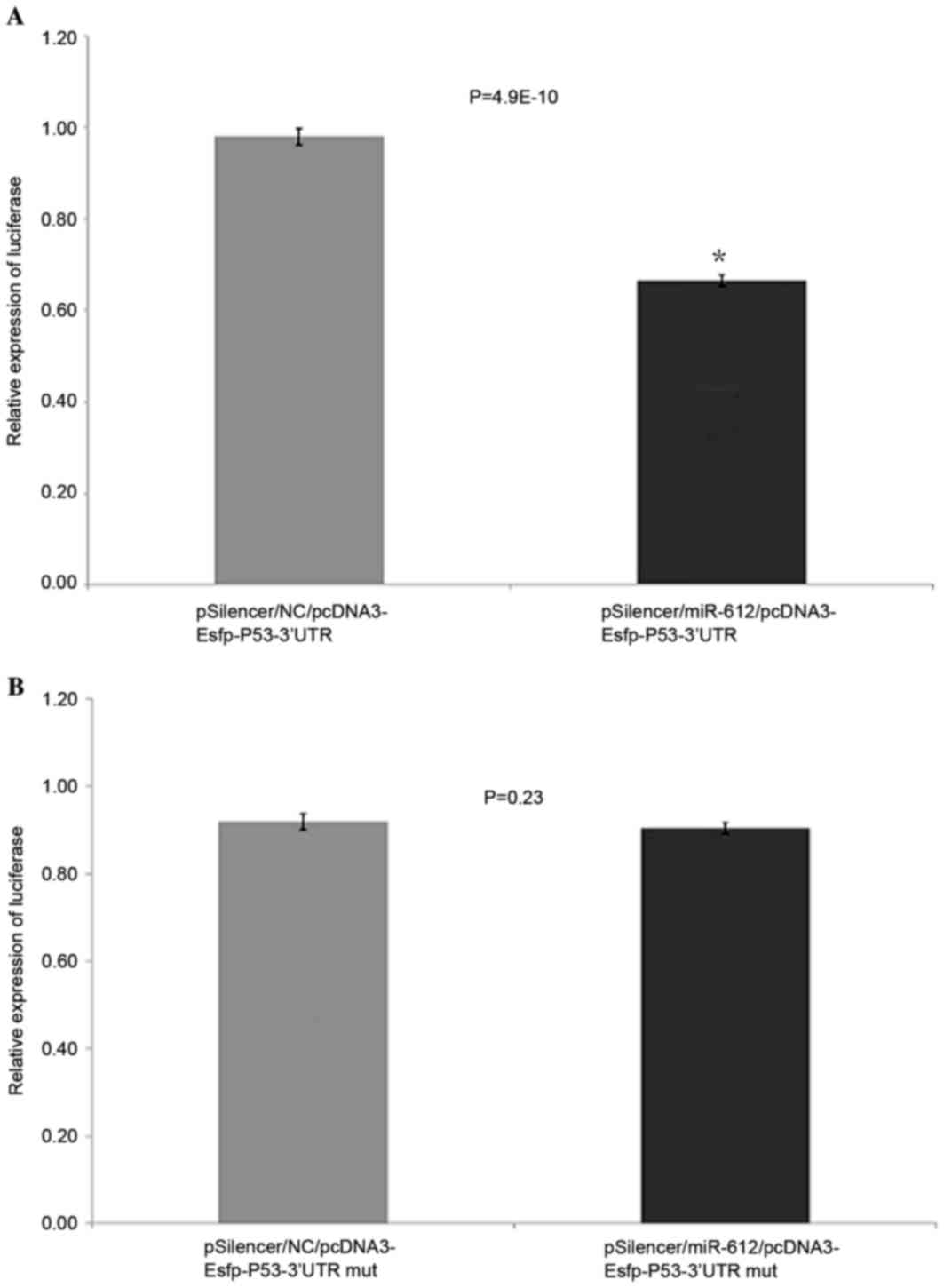
TP53 is targeted by miR-612 as
demonstrated by the EGFP fluorescent reporter carrier
experiment
The miR-612 expression plasmid pSiIencer/miR-612 was
mixed with the fluorescent reporter carrier plasmid
pcDNA3-Egfp-P53-3′UTR or pcDNA3-Egfp-P53-3′UTRmut and transfected
into EC109 cells. The pSilencer/NC plasmid with fluorescent
reporter carrier plasmid pcDNA3-Egfp-P53-3′UTR or
pcDNA3-Egfp-P53-3′UTRmut was used as the control. After 48 h
transfection, green fluorescent protein expression levels were
measured by a fluorescence spectrophotometer. The cells infected
with miR-612 expression plasmid had reduced TP53 3′UTR reporter
plasmid GFP by 29.3% (P<0.05), compared to the control group,
suggesting that the expressed miR-612 acted on the 3′ untranslated
region of TP53 transcripts and caused reduced expression of GFP.
However, the pSilencer/miR-612 or control plasmid pSilencer/NC
transfected with mutant TP53 (3′UTR pcDNA3-Esfp-P53-3′UTRmut) had
no inhibitory effect on miR-612 (P>0.05; Fig. 6A and B).
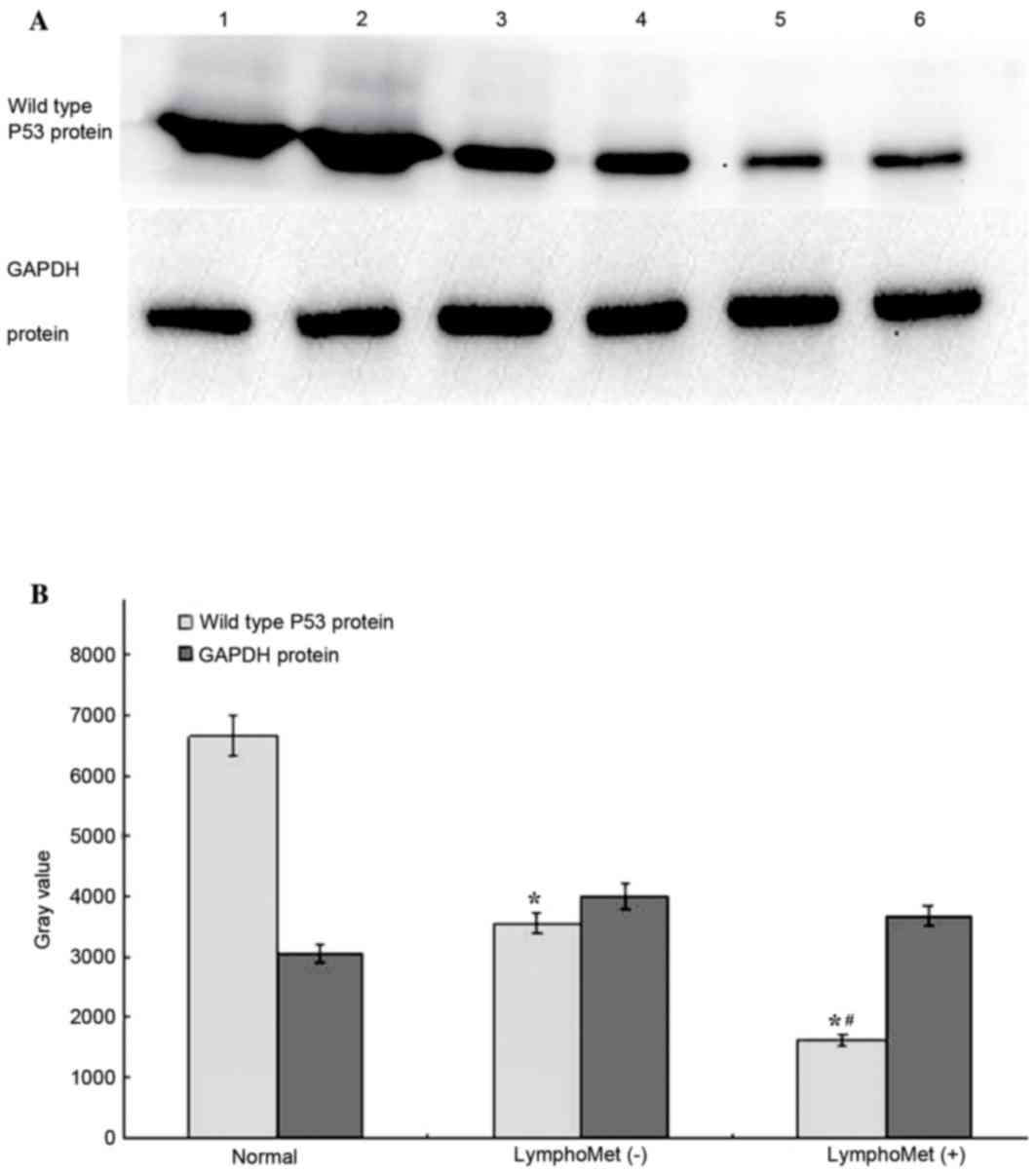
Reduced wild type P53 protein
expression in tumors and metastatic tumors of esophageal squamous
cell carcinoma by western blotting
A western blot analysis was conducted to measure the
wild type P53 protein expression in: 30 cases of ESCC with lymph
node metastasis, 30 cases without, and 10 cases of normal
esophageal epithelial tissues. The average grey value was obtained
for each case and one-way ANOVA, with post hoc Fisher's least
significant difference and Student-Newman-Keuls tests, was
performed for differential protein levels among the three groups.
Both the tumors with and without metastasis presented significantly
reduced TP53 protein expression compared to the normal tissues
(75.5%, 46.7% reduction, respectively, P<0.05, Fig. 7A and B). The tumors with metastasis
had further reduced wide type TP53 expression compared to the
tumors without metastasis.
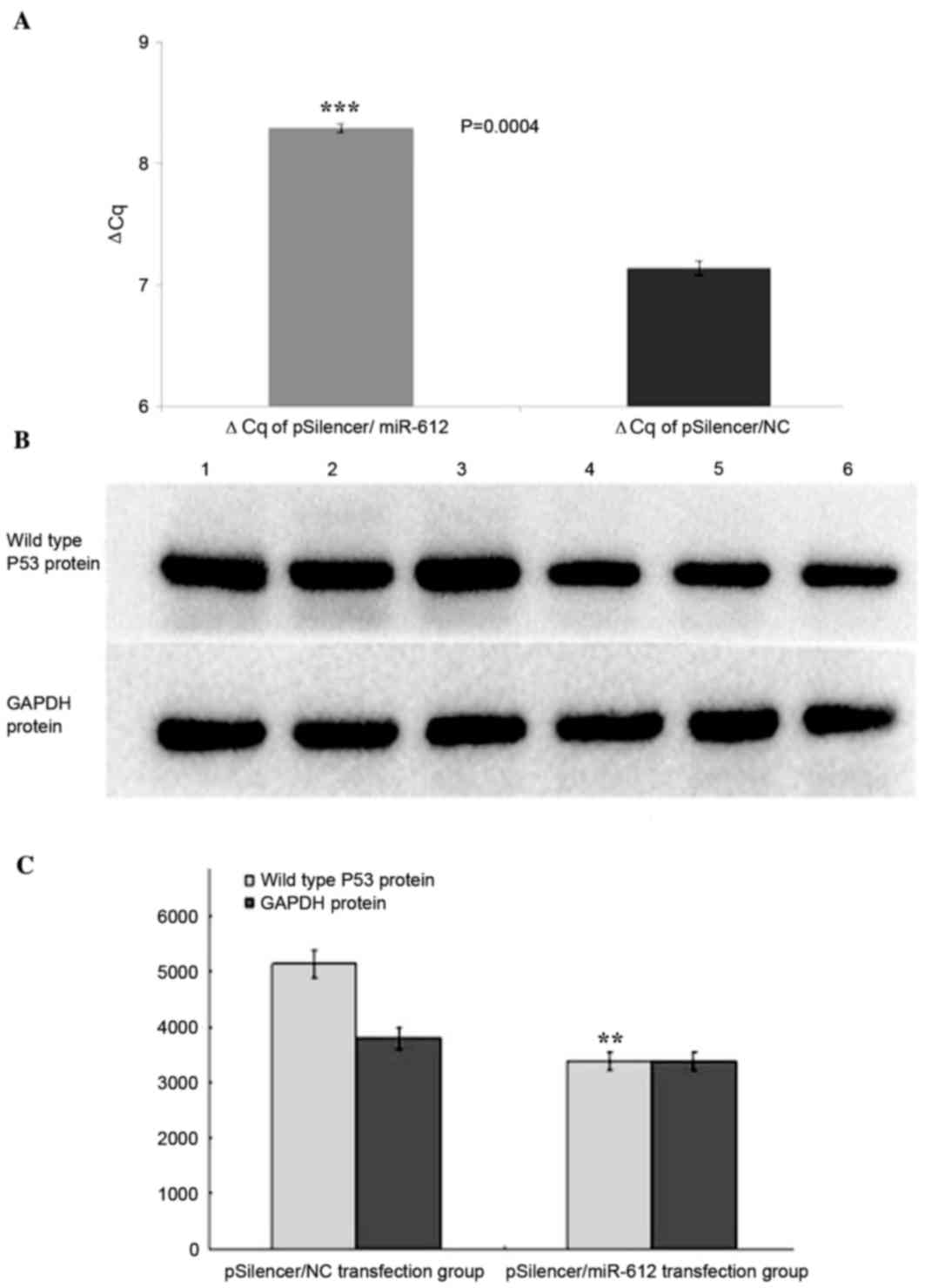
miR-612 downregulates wild type P53
mRNA expression in EC109 cells
When the pSilencer/miR-612 plasmid and the negative
control pSilencer/NC plasmid were used to transfect EC109 cells,
TP53 mRNA levels, measured by RT-qPCR, were significantly reduced
in the pSilencer/miR-612 infected cells compared to the negative
controls (2.2-fold less; P<0.001; Fig. 8A).
miR-612 downregulates wild type P53
protein expression in EC109 cells
The authors further measured the protein expression
of TP53 by western blotting. At 48 h following transfection with
the pSilencer/miR-612 plasmid and negative control pSilencer/NC
plasmid of EC109 cells, respectively, TP53 expression was measured
through western blotting and showed that comparing to the control
group, the TP53 expression in pSilencer/miR-612 group was
significantly reduced (P<0.01, Fig.
8B and C).
Discussion
A previous study demonstrated that >50% of miRNAs
are located in tumor-associated gene loci or fragile sites in the
genome (18). miRNAs can act as a
tumor suppressor that inhibits the activities of oncogenes or they
can involve in cell cycle, regulate apoptosis, angiogenesis and
tumor metastasis (4,5). However, our understanding for these
functions is limited to a very few miRNAs and more research on more
miRNAs is needed.
There has been growing interest in the role of
miRNAs in ESCC development for the past few years. The first genome
wide survey using miRNA microarray demonstrated that 46 miRNAs are
differentially expressed compared to its adjacent normal epithelium
(7). The study by Feber et
al (6) demonstrates that
miR-194, miR-192 and miR-200c express much higher in adenocarcinoma
than in squamous cell carcinoma while miR-21, miR-205, miR-203 and
miR-93 are dramatically different between tumor and normal tissue.
The expression pattern changes could be potentially used to
differentiate tumor from normal tissue for esophageal cancer
diagnosis (19). For example,
ANXA1 is a tumor suppressor gene that has a role in inhibiting cell
proliferation and promoting apoptosis. miR-196, a miRNA that can
target ANXA1, may inhibit the expression of ANXA1 and cause the
development of esophageal carcinoma (19). LATS2 is another tumor suppressor
and it targeting miR-373 may have a role in SCC development through
downregulating this gene (20) The
study by Hiyoshi (21) indicates
that miR-21 is not only overexpressed in primary tumors, but also
in metastatic tumors. miR-21 mediates ESCC development and
progression through suppressing the expression of PDCD4. In the
study of SSC sub-clone, Tian et al (22) find that miR-10b serves a role in
tumor cell invasion and metastasis through the inhibition of tumor
suppression gene KLF4. In spite of these progresses, prominent and
specific miRNAs for SSC are still lacking (23,24).
In the present study, miRNA expression was profiled
between ESCC with and without lymph node metastasis and identified
63 differentially expressed miRNAs. Further analyses through PAM
and target prediction revealed miR-612 as a potential regulator in
ESCC development and metastasis. The authors' functional validation
further indicated that miR-612 as a tumor promoter was likely
mediated through downregulating TP53. To the best of the authors'
knowledge, the present study is the first to establish the link of
miR-612 with ESCC metastasis.
The function of miR-612 and downstream regulation
pathway is largely unknown. This microRNA coding sequence is
located at chromosome 11q13.1, with mature sequence
gcugggcagggcuucugagcuccu. Tian et al (25) reported that the nucleotide sequence
of miR-612 is the same as miRNA-1285 but it does not bind to the
P53 3′UTR. Tao et al (26)
report that miR-612 suppresses the invasive-metastatic cascade in
hepatocellular carcinoma. They revealed that miR-612 has inhibitory
effects on cell proliferation, migration, invasion and metastasis
of hepatocellular carcinoma. This is opposite to what the present
study discovered. From multiple target prediction and subsequent
experiments, it was identified that miR-612 has an inhibitory
action on mRNA and protein expression of TP53. In the authors'
‘blast’ analysis, the miR-612 sequence at 5′ end matches with the
3′UTR of TP53. The current fluorescent reporter plasmid experiment
indicated that miR-612 reduced the protein expression of the TP53
3′UTR reporter plasmid but has no effect on P53 3′UTR with the
mutated reporter plasmid, which clearly presents the specific
binding of miR-612 with TP53. The present data demonstrated that
the inhibition of miR-612 significantly reduced EC109 cell invasion
and migration abilities and further demonstrated the promoting role
of miR-612 in ESCC progression and metastasis.
The role of miR-612 is likely more broad. In the
authors' target prediction by TargetScans, Pictar, miRanda and
MirTarget 2, there were 2,800 gene targets predicted by at least
three prediction tools. Gene Ontology analysis on these genes
suggests these genes are involved in several important cancer
related functions/pathways such as cell growth regulation,
differentiation, apoptosis, cell-cell adhesion and cell-matrix
adhesion, and cell invasion and migration (25–30).
The genes that participate in cell apoptosis and proliferation
include P53, MAPK, WNT and BCL2, and the genes that promote
angiogenesis include TGFα and TGFβ.
P53 is a tumor suppressor and serves an important
role in promoting cell apoptosis. P53 may be wild type or mutant
type. The former acts as a tumor suppressor and participates in
many cell activities, such as gene transcription, DNA repair, cell
cycle and apoptosis regulation, and cell proliferation and
differentiation. The wild type is the key for tumor sensitivity to
radiation and chemotherapy, induction to formation of TNF and IL-6
inflammatory agents, and inhibition of angiogenesis in tumors. The
mutant P53 may lead to unchecked cell proliferation and tumor
development. ~50% tumors harbor P53 point mutations, gene loss or
loss of function (23). The
current data indicated that wild type TP53 is reduced in primary
esophageal SCC and even more in the tumors with lymph node
metastasis. As demonstrated, this change is likely mediated through
the action of miR-612, thus promoting esophageal cancer invasion
and metastasis. The presented results provide a solid foundation
for further investigation of the therapeutic potential of
miR-612.
Acknowledgements
The present work was supported by the Natural
Scientific Foundation of Shandong (grant no. ZR2012HM085) and the
National Natural Science Foundation of China (grant no. 81272420)
and Shandong Project of Medicine and Health Technology Development
(grant no. 2015WSB04049). The authors would like to thank the
patients and investigators for their participation in the current
study. They would also like to thank Zhifu Sun and Lei Fang for
their technical and language-editing assistance.
References
|
1
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li D: The molecular epidemiologic research
of esophageal carcinoma in China. Chin J Epidemiology. 24:939–943.
2003.
|
|
3
|
Furihata T, Sakai T, Kawamata H, Omotehara
F, Shinagawa Y, Imura J, Ueda Y, Kubota K and Fujimori T: A new in
vivo model for studying invasion and metastasis of esophageal
squamous cell carcinoma. Int J Oncol. 19:903–907. 2001.PubMed/NCBI
|
|
4
|
Nelson KM and Weiss GJ: MicroRNAs and
cancer: Past, present, and potential future. Mol Cancer Ther.
7:3655–3660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Latronico MV, Catalucci D and Condorelli
G: MicroRNA and cardiac pathologies. Physiol Genomics. 34:239–242.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feber A, Xi L, Luketich JD, Pennathur A,
Landreneau RJ, Wu M, Swanson SJ, Godfrey TE and Litle VR: MicroRNA
expression profiles of esophageal cancer. J Thorac Cardiovasc Surg.
135:255–260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo Y, Chen Z, Zhang L, Zhou F, Shi S,
Feng X, Li B, Meng X, Ma X, Luo M, et al: Distinctive microRNA
profiles relating to patient survival in esophageal squamous cell
carcinoma. Cancer Res. 68:26–33. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ogawa R, Ishiguro H, Kuwabara Y, Kimura M,
Mitsui A, Katada T, Harata K, Tanaka T and Fujii Y: Expression
profiling of micro-RNAs in human esophageal squamous cell carcinoma
using RT-PCR. Med Mol Morphol. 42:102–109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tibshirani R, Hastie T, Narasimhan B and
Chu G: Diagnosis of multiple cancer types by shrunken centroids of
gene expression. Proc Natl Acad Sci USA. 99:6567–6572. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bass AJ and Meyerson M: Genome-wide
association study in esophageal squamous cell carcinoma.
Gastroenterology. 137:1573–1576. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang LD, Zhou FY, Li XM, Sun LD, Song X,
Jin Y, Li JM, Kong GQ, Qi H, Cui J, et al: Genome-wide association
study of esophageal squamous cell carcinoma in Chinese subjects
identifies susceptibility loci at PLCE1 and C20orf54. Nat Genet.
42:759–763. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu C, Hu Z, He Z, Jia W, Wang F, Zhou Y,
Liu Z, Zhan Q, Liu Y, Yu D, et al: Genome-wide association study
identifies three new susceptibility loci for esophageal
squamous-cell carcinoma in Chinese populations. Nat Genet.
43:679–684. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu C, Li D, Jia W, Hu Z, Zhou Y, Yu D,
Tong T, Wang M, Lin D, Qiao Y, et al: Genome-wide association study
identifies common variants in SLC39A6 associated with length of
survival in esophageal squamous-cell carcinoma. Nat Genet.
45:632–638. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shimada H, Ochiai T and Nomura F: Japan
p53 Antibody Research Group: Titration of serum P53 antibodies in
1,085 patients with various types of malignant tumors: A
multiinstitutional analysis by the Japan P53 antibody research
group. Cancer. 97:682–689. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gomes NP and Espinosa JM: Gene-specific
repression of the p53 targer gene PUMA via intragenic CTCF-Cohesin
binding. Genes Dev. 24:1022–1034. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Le MT, Teh C, Shyh-Chang N, Xie H, Zhou B,
Korzh V, Lodish HF and Lim B: Micro RNA-125b is anovel negative
regulator of p53. Genes Dev. 23:862–876. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M
and Croce CM: Human microRNA genes are frequently located at
fragile sites and genomic regions involved in cancers. Proc Natl
Acad Sci USA. 101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luthra R, Singh RR, Luthra MG, Li YX,
Hannah C, Romans AM, Barkoh BA, Chen SS, Ensor J, Maru DM, et al:
MicroRNA-196a targets annexin A1: A microRNA-mediated mechanism of
annexin A1 downregulation in cancers. Oncogene. 27:6667–6678. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee KH, Goan YG, Hsiao M, Lee CH, Jian SH,
Lin JT, Chen YL and Lu PJ: MicroRNA-373 (miR-373)
post-transcriptionally regulates large tumor suppressor, homolog 2
(LATS2) and stimulates proliferation in human esophageal cancer.
Exp Cell Res. 315:2529–2538. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kurashige J, Kamohara H, Watanabe M,
Tanaka Y, Kinoshita K, Saito S, Hiyoshi Y, Iwatsuki M, Baba Y, Baba
H, et al: Serum microRNA-21 is a novel biomarker in patients with
esophageal squamous cell carcinoma. J Surg Oncol. 106:188–192.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tian Y, Luo A, Cai Y, Su Q, Ding F, Chen H
and Liu Z: MicroRNA-10b promotes migration and invasion through
KLF4 in human esophageal cancer cell lines. J Biol Chem.
285:7986–7994. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsushima K, Isomoto H, Kohno S and Nakao
K: MicroRNAs and esophageal squamous cell carcinoma. Digestion.
82:138–144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu BL, Xu LY, Du ZP, Liao LD, Zhang HF,
Huang Q, Fang GQ and Li EM: MiRNA profile in esophageal squamous
cell carcinoma: Downregulation of miR-143 and miR-145. World J
Gastroenterol. 17:79–88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tian S, Huang S, Wu S, Guo W, Li J and He
X: MicroRNA-1285 inhibits the expression of p53 by directly
targeting its 3′ untranslated region. Biochem Biophys Res Commun.
396:435–439. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tao ZH, Wan JL, Zeng LY, Xie L, Sun HC,
Qin LX, Wang L, Zhou J, Ren ZG, Li YX, et al: miR-612 suppresses
the invasive-metastatic cascade in hepatocellular carcinoma. J Exp
Med. 210:789–803. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Landi D, Gemignani F, Naccarati A, Pardini
B, Vodicka P, Vodickova L, Novotny J, Försti A, Hemminki K, Canzian
F and Landi S: Polymorphisms within micro-RNA-binding sites and
risk of sporadic colorectal cancer. Carcinogenesis. 29:579–584.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Balaguer F, Moreira L, Lozano JJ, Link A,
Ramirez G, Shen Y, Cuatrecasas M, Arnold M, Meltzer SJ, Syngal S,
et al: Colorectal cancers with microsatellite instability display
unique miRNA profiles. Clin Cancer Res. 17:6239–6249. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim HK, Prokunina-Olsson L and Chanock SJ:
Common genetic variants in miR-1206 (8q24.2) and miR-612 (11q13.3)
affect biogenesis of mature miRNA forms. PLoS One. 10:e474542012.
View Article : Google Scholar
|
|
30
|
Tang J, Tao ZH, Wen D, Wan JL, Liu DL,
Zhang S, Cui JF, Sun HC, Wang L, Zhou J, et al: MiR-612 suppresses
the stemness of liver cancer via Wnt/β-catenin signaling. Biochem
Biophys Res Commun. 447:210–215. 2014. View Article : Google Scholar : PubMed/NCBI
|