Introduction
Lung cancer is the most common malignancy in humans
and is the primary cause of cancer-related mortality in both men
and women globally (1,2). Previous studies demonstrated that
accumulation of permanent genetic alterations in combination with
dynamic epigenetic alterations results in lung cancer formation and
progression (3,4). Lung cancer can be divided into two
major groups based on pathological features: Small cell lung cancer
(SCLC) and non-small cell lung cancer (NSCLC) (5). NSCLC is clinically characterised by
rapid progression, strong invasiveness and high mortality rate
(6). NSCLC accounts for ~85% of
all lung cancer cases and includes squamous cell carcinoma,
adenocarcinoma, and large cell carcinoma (7). Although considerable progress in
surgery, diagnostic method, radiotherapy and new chemotherapy
regimens has been made, the prognosis of NSCLC patients remains
poor (8). Recurrence and
metastasis, even after radiation therapy and/or chemotherapy, are
the major causes of death among NSCLC patients (5). Therefore, further understanding of
the underlying molecular mechanisms during NSCLC initiation and
progression is necessary. Moreover, the development of novel
efficient therapeutic strategies for patients with this malignancy
is urgently needed.
MicroRNAs (miRNAs) are a large group of endogenous,
single-strand, non-coding and short RNAs that are between
approximately 19 and 25 nucleotides in length (9). MiRNAs serve as key regulators of gene
expression through direct binding to the 3′-untranslated region
(UTR) of their target genes in a sequence-specific manner, leading
to either translation inhibition or messenger RNA (mRNA)
degradation (10). In recent
years, miRNAs have emerged as powerful regulators of various
physiological and pathological processes, such as growth,
apoptosis, differentiation, angiogenesis, inflammation and
tumourigenesis (11). Increasing
evidence has shown that more than half of miRNA genes are located
at fragile sites (12–14). Several studies reported that
numerous miRNAs are aberrantly expressed in various human cancers
and play significant roles in tumourigenesis and tumour
development, including NSCLC (15–17).
Obviously, miRNAs can function as either tumour suppressors or
oncogenes in different human cancers depending on the
characteristics of their target genes (18). Therefore, miRNAs are potential
biomarkers for diagnosis, treatment and prognosis of human
malignancies owing to their tissue- and disease-specific expression
and regulatory functions (19).
MicroRNA-661 (miR-661) has been reported to be
abnormally expressed in several tumours (20–22).
However, the miR-661 expression and functions in NSCLC are yet to
be investigated. The present study aims to elucidate the miR-661
expression, clinical significance and biological roles in NSCLC and
to investigate its underlying molecular mechanisms.
Materials and methods
Tissue samples
Forty-seven pairs of NSCLC tissues and adjacent
non-cancer tissues were obtained from patients who had undergone
surgical resection at The First Affiliated Hospital of Jinzhou
Medical University (Liaoning, China) between February 2013 and June
2015. All of these patients had not been treated with any
pre-operative cancer treatment, such as radiotherapy or
chemotherapy. All tissues were rapidly snap-frozen in liquid
nitrogen and stored at −80°C for subsequent experiments. The
protocol of the present study was reviewed and approved by the
Ethics Committee of The First Affiliated Hospital of Jinzhou
Medical University. Written informed consent was also provided by
all patients who enrolled in the present study.
Cell lines, culture and
transfection
Non-tumorigenic bronchial epithelium BEAS-2B cells
and four NSCLC cell lines (A549, H460, SK-MES-1 and SPC-A1) were
purchased from Shanghai Institute of Biochemistry and Cell Biology
(Shanghai, China). BEAS-2B cells were cultured in LHC-9 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.). NSCLC cell lines were maintained in Dulbecco's
Modified Eagle's Medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc.) containing 10% FBS and 1% penicillin-streptomycin (Sigma
Aldrich, Saint Louis, MO, USA). All cells were grown at 37°C in a
humidified atmosphere with 5% CO2.
MiR-661 inhibitor and corresponding scramble miRNA
inhibitor negative control (NC inhibitor) were synthesized by
GenePharma (Shanghai, China). Small interfering RNA (siRNA)
targeting RUNX3 (si-RUNX3) and an unrelated sequence used as a
negative control of siRNA (si-NC) were obtained from Ribobio
Technology Co., Ltd. (Guangzhou, China). Cells were collected,
counted and seeded into 6-well plates the day before transfection.
Cell transfection was performed using lipofectamine 2000
(Invitrogen, Carlsbad, CA, USA), in accordance with the
manufacturer's protocol. DMEM with 10% FBS was added to each well
at 6 h following transfection.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from tissues or cells with
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) following to the manufacturer's protocols. The
concentration of the total RNA was examined using the ND-2000
spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific,
Inc., Pittsburgh, PA, USA). For the analysis of miR-661 expression,
RNA was reverse-transcribed to cDNA using a TaqMan® MicroRNA
Reverse Transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and qPCR was carried out using
the TaqMan MicroRNA Assay kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) on an Applied Biosystems 7500 Real-time PCR
System (Thermo Fisher Scientific, Inc.). For quantification of
RUNX3 mRNA, cDNA was synthesized using PrimeScript™ RT Reagent kit
(Takara Biotechnology Co., Ltd., Dalian, China), followed by qPCR
with SYBR Premix Ex Taq mastermix (Takara Biotechnology Co, Ltd.).
RUN6B and GAPDH were used as internal control for miR-661 and RUNX3
mRNA, respectively. The primers used in the present study were as
follows: miR-661 forward, 5′-GTGCCTGGGTCTCTGGCCT-3′, and reverse,
5′-CGTCATGATGTTGCGTCACC-3′; RUN6B forward,
5′-CTCGCTTCGGCAGCACATATACT-3′, and reverse,
5′-ACGCTTCACGAATTTGCGTGTC-3′. RUNX3 forward,
5′-GACAGCCCCAACTTCCTCT-3′, and reverse, 5′-CACAGTCACCACCGTACCAT-3′;
GAPDH forward, 5′-GGGTGTGAACCATGAGAAGT-3′, and reverse,
5′-GACTGTGGTCATGAGTCCT-3′. The 2−∆∆Ct method was used to
calculate the relative expression of miRNA and mRNA (23).
Cell Counting Kit-8 (CCK-8) assay
Cell proliferation was measured with CCK8 assay
(Dojindo Laboratories, Kumamoto, Japan). Cells were collected and
seeded into 96-well plates at a density of 3000 cells per well the
day before transfection. Cells were then transfected with miR-661
inhibitor, NC inhibitor, si-RUNX3 or si-NC. Subsequent to being
incubated at 37°C in a 5% CO2 incubator for 0–72 h, 10
µl CCK-8 reagent was added into each well and incubated at 37°C for
additional 4 h. The optical density (OD) at 450 nm for each well
was determined using an ELISA reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). All experiments were repeated in
triplicate.
Cell invasion assay
Cell invasion assays were performed using Transwell
chambers (pore size, 8 mm; Millipore Corp., Billerica, MA, USA)
pre-coated with Matrigel (BD Biosciences, Bedford, MA, USA).
Transfected cells were harvested at 48 h post-transfection,
re-suspended in FBS-fee DMEM and then seeded into the upper
Transwell chambers. In the lower chamber, DMEM containing 10% FBS
was added. After 48 h of incubation at 37°C with 5% CO2,
the non-invasive cells were removed carefully with cotton swabs.
The cells that had invaded through the chamber membrane were fixed
with 4% paraformaldehyde and stained with 0.1% crystal violet.
Subsequent to washing with PBS, cells in five randomly selected
visual fields were photographed and counted under an inverted
microscope (Olympus Corporation, Tokyo, Japan).
miRNA target prediction
Human miRNA target prediction algorithms: PicTar
(http://pictar.mdc-berlin.de/) and
TargetScan (http://www.targetscan.org/) were used to predicate the
potential targets of miR-661.
Luciferase reporter assays
For reporter assays, the human RUNX3 wild type or
mutated 3′-UTR sequence containing the miR-661 binding site was
inserted into psiCHECK-2 vector to develop psiCHECK2-RUNX3-3′UTR-Wt
and psiCHECK2-RUNX3-3′UTR-Mut. Cells were seeded into 24-well
plates at 5×104 cells per well and transfected with miR-661
inhibitor or NC inhibitor, together with psiCHECK2-RUNX3-3′UTR-Wt
or psiCHECK2-RUNX3-3′UTR-Mut. Following incubation at 37°C for 48
h, the Firefly and Renilla luciferase activities were determined
using Dual-luciferase reporter system according to the
manufacturer's instructions (Promega, Madison, WI, USA). Luciferase
activities were normalized to Renilla activities.
Western blot analysis
Tissues or cells were solubilized in cold
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China) and protein concentrations were
measured by the standard BCA method (BCA™ Protein Assay kit, USA).
Equal amounts of protein were separated by 10% SDS-PAGE
electronically and transferred onto a PVDF membrane (EMD Millipore,
Billerica, MA, USA). Subsequently, the membranes were blocked with
5% non-fat dry milk in Tris-buffered saline with 0.05% Tween 20
(TBST) buffer and incubated overnight at 4°C with the following
primary antibodies: mouse anti-human monoclonal RUNX3 antibody
(sc-376591; 1:1,000 dilution; Santa Cruz Biotechnology, CA, USA)
and mouse anti-human monoclonal GAPDH antibody (sc-47724; 1:1,000
dilution; Santa Cruz Biotechnology). Subsequent to washing three
times with TBST, the membranes were incubated with goat anti-mouse
horseradish peroxidase (HRP)-conjugated secondary antibody
(sc-2005; 1:5,000 dilution; Santa Cruz Biotechnology) at room
temperature for 2 h. The immunoblot was detected by an enhanced
chemiluminescence solution (Pierce Biotechnology, Inc., Rockford,
IL, USA). GAPDH was used as an internal control for RUNX3.
Densitometric analysis was conducted using Image-Pro Plus software
version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
All data were presented as mean ± standard errors.
Statistical significance betwen groups were evaluated by Student's
t-test or one-way analysis of variance (ANOVA).
SPSS 17.0 software (SPSS Inc., Chicago, IL, USA) was
used for statistical analysis. P<0.05 was considered
statistically significant.
Results
MiR-661 is upregulated in NSCLC
tissues and cell lines
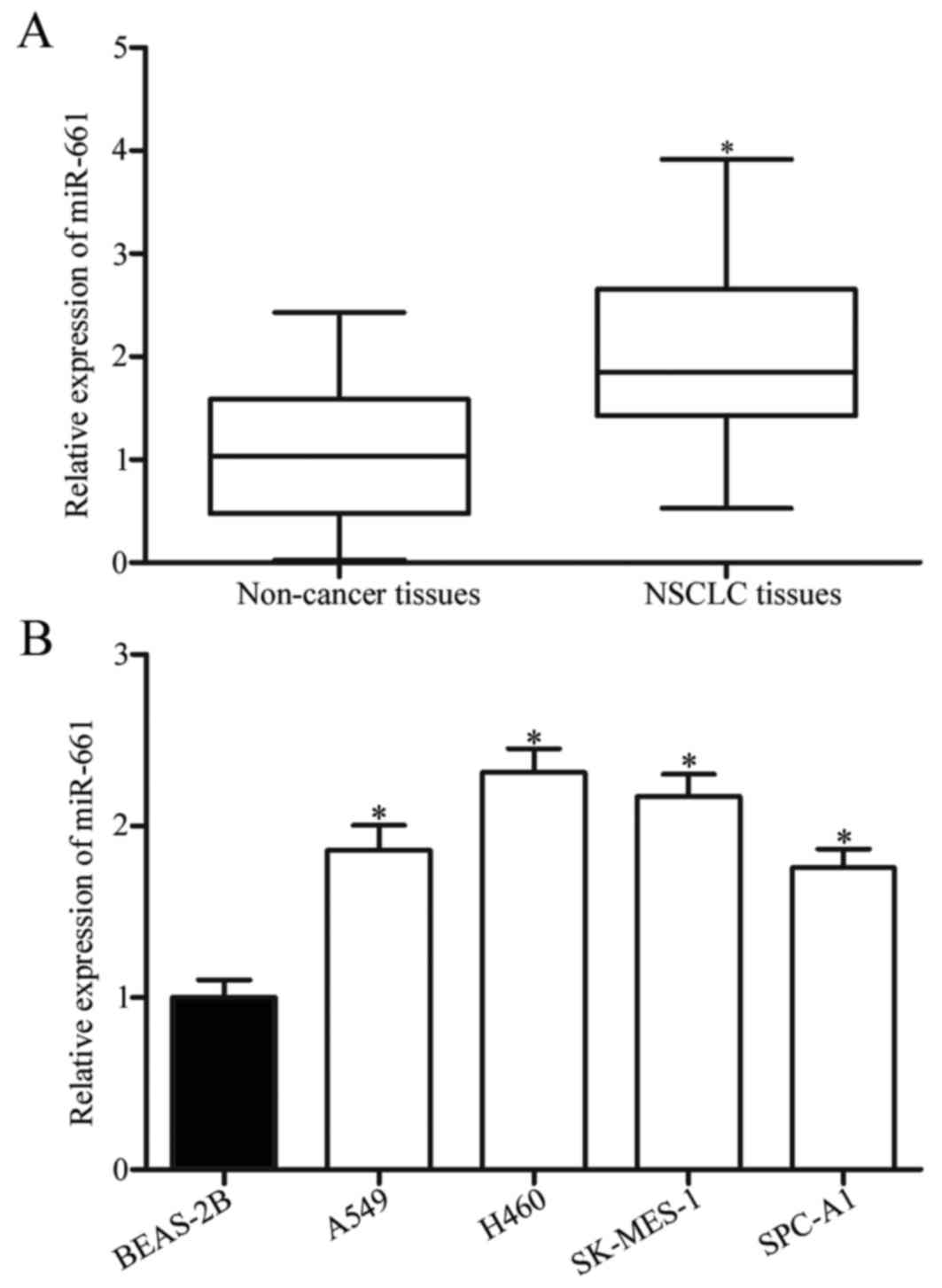
To investigate whether or not miR-661 levels were
altered in NSCLC, we measured miR-661 expression in 47 pairs of
NSCLC tissues and adjacent non-cancer tissues through RT-qPCR. The
results showed that miR-661 was significantly upregulated in NSCLC
tissues compared with adjacent non-cancer tissues (Fig. 1A, P<0.05). The association
between miR-661 expression and clinicopathological factors of NSCLC
patients was analysed. As shown in Table I, the miR-661 expression level was
strongly correlated with differentiation (P=0.011), tumor stage
(P=0.013) and lymph node metastasis (P=0.029), but not with gender,
age, smoker and tumour size (all P>0.05).
 | Table I.Association between microRNA-661
expression and clinicopathologic factors of non-small cell lung
cancer. |
Table I.
Association between microRNA-661
expression and clinicopathologic factors of non-small cell lung
cancer.
|
|
| microRNA- 661
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
factors | Cases | High | Low | P-value |
|---|
| Gender |
|
|
| 0.642 |
|
Male | 20 | 11 | 9 |
|
|
Female | 27 | 13 | 14 |
|
| Age (years) |
|
|
| 0.676 |
|
<60 | 19 | 9 | 10 |
|
|
≥60 | 28 | 15 | 13 |
|
| Smoker |
|
|
| 0.440 |
|
Yes | 28 | 13 | 15 |
|
| No | 19 | 11 | 8 |
|
| Tumor size
(cm) |
|
|
| 0.423 |
|
<3 | 25 | 12 | 13 |
|
| ≥3 | 22 | 12 | 10 |
|
|
Differentiation |
|
|
| 0.011a |
|
Moderate-Well | 28 | 10 | 18 |
|
|
Poorly | 19 | 14 | 5 |
|
| Tumor stage |
|
|
| 0.013a |
|
I–II | 24 | 8 | 16 |
|
|
III–IV | 23 | 16 | 7 |
|
| Lymph node
metastasis |
|
|
| 0.029a |
|
Negative | 21 | 7 | 14 |
|
|
Positive | 26 | 17 | 9 |
|
RT-qPCR was further carried out to quantify miR-661
expression levels in non-tumourigenic bronchial epithelium BEAS-2B
cells and four NSCLC cell lines (A549, H460, SK-MES-1 and SPC-A1).
Consistent with the results observed in clinical tissues, NSCLC
cell line exhibited significantly higher miR-661 expression levels
compared with BEAS-2B (Fig. 1B,
P<0.05). These results suggested that miR-661 might play key
roles in NSCLC formation and progression.
Downregulation of miR-661 suppresses
NSCLC proliferation and invasion
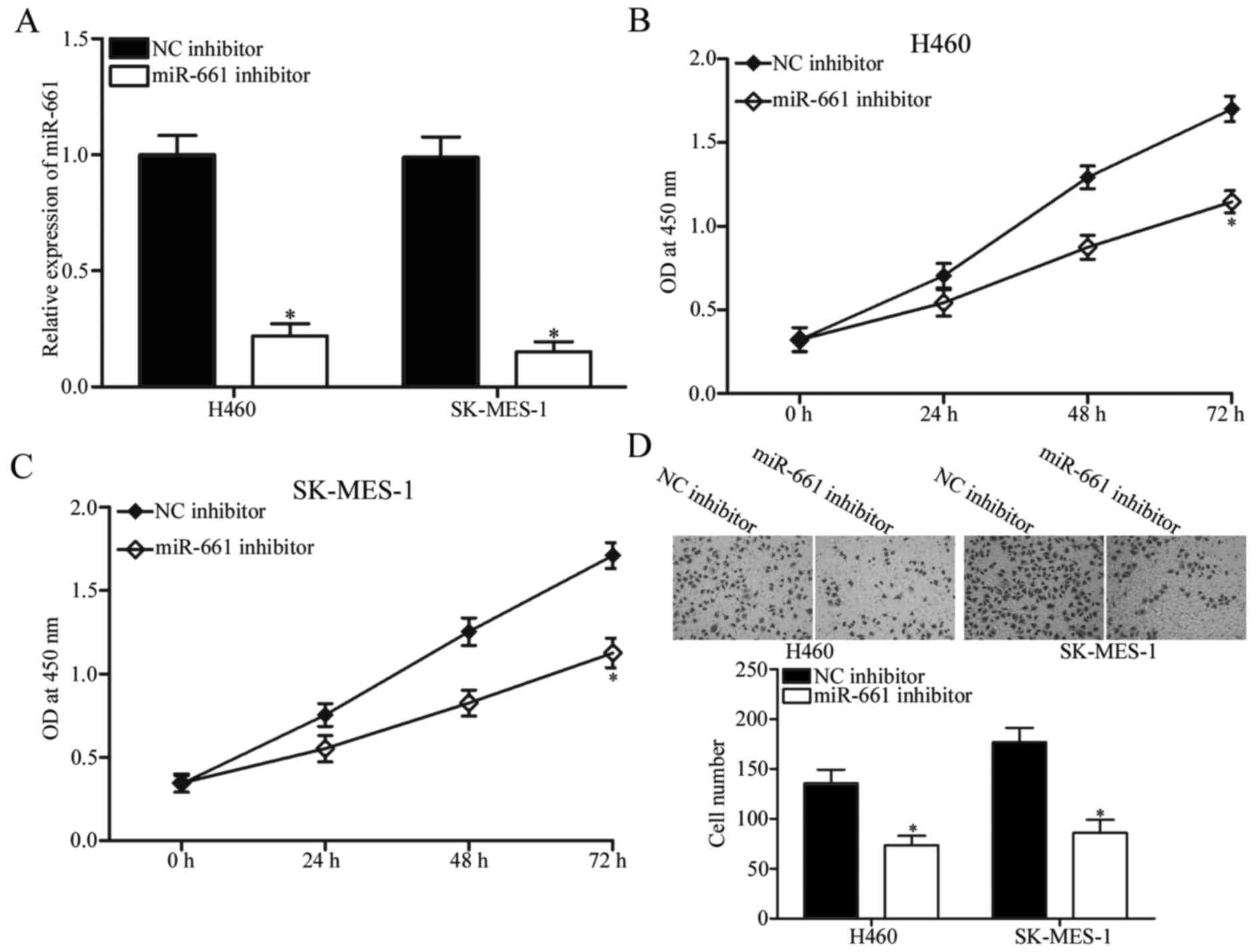
To investigate the potential role of miR-661 in
NSCLC, miR-661 inhibitor was transfected into H460 and SK-MES-1
cells, which expressed relatively higher miR-661 expression.
RT-qPCR analysis confirmed that miR-661 was markedly downregulated
in H460 and SK-MES-1 cells after transfection with miR-661
inhibitor (Fig. 2A, P<0.05).
The effect of miR-661 underexpression on NSCLC cell proliferation
was evaluated using CCK-8 assay. As shown in Fig. 2B and C, miR-661 downregulation
inhibited H460 and SK-MES-1 cell proliferation compared with NC
inhibitor. We also assessed the ability of miR-661 to regulate
NSCLC cell invasion using cell invasion assay. The results
indicated that miR-661-underexpressed H460 and SK-MES-1 cells
showed significantly less invasiveness than cells transfected with
NC inhibitor (Fig. 2D, P<0.05).
These findings suggested that miR-661 may play an oncogenic role in
NSCLC.
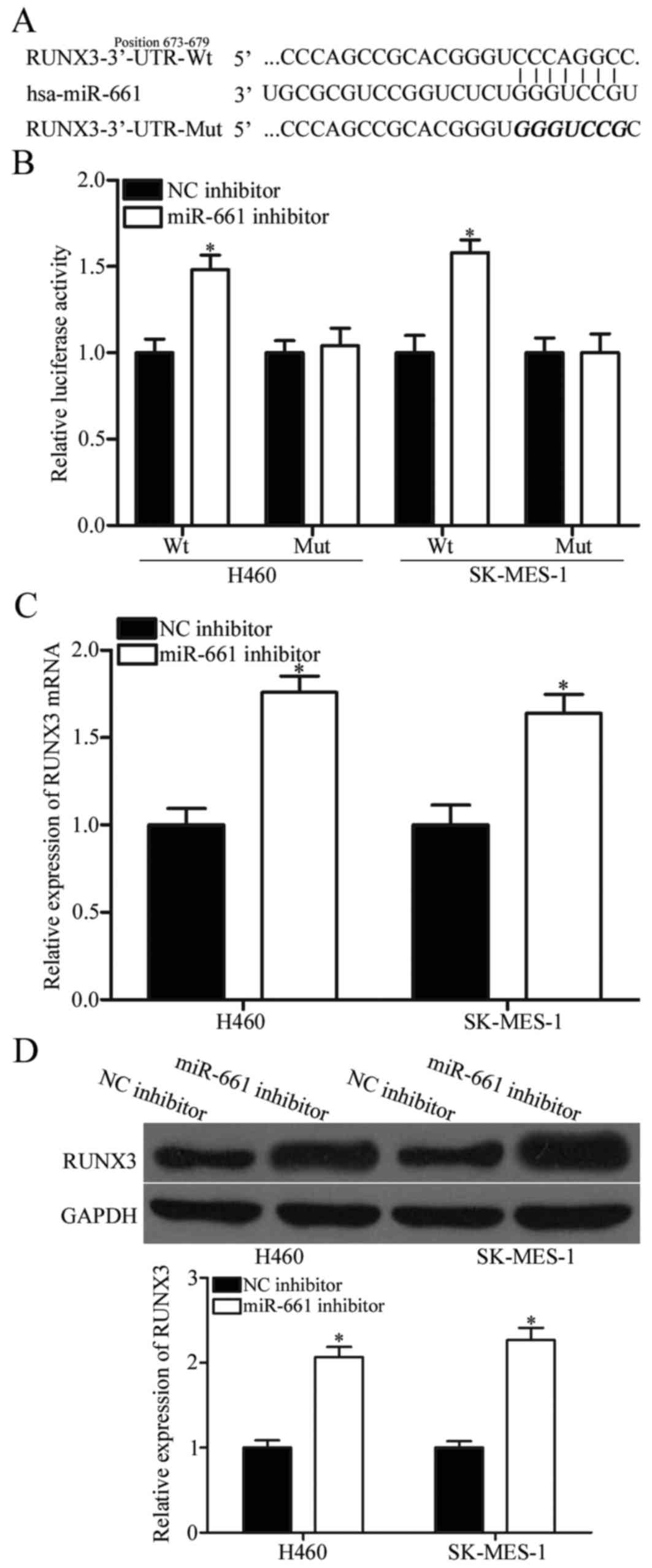
RUNX3 is a direct target of miR-661 in
NSCLC
We sought to identify the direct target genes of
miR-661 to explore the mechanism underlying its tumour-promoting
activity in NSCLC. A considerable number of potential targets were
predicted using bioinformatic analysis, and RUNX3 was selected for
further confirmation (Fig. 3A)
because it was downregulated in NSCLC tissues and contributed to
NSCLC initiation and progression (24,25).
To determine whether RUNX3 is a direct target of miR-661,
luciferase reporter assay was performed in H460 and SK-MES-1 cells
co-transfected with psiCHECK2-RUNX3 3′UTR Wt or psiCHECK2-RUNX3
3′UTR Mut and miR-661 or NC inhibitor. As shown in Fig. 3B, co-transfection with miR-661
inhibitor increased the luciferase activities of wild-type RUNX3
3′-UTR plasmid compared with NC inhibitor (P<0.05). However, the
luciferase activities of mutant RUNX3 3′-UTR constructs was not
affected by miR-661 inhibitor. Further RT-qPCR and Western blot
demonstrated that RUNX3 expression at both mRNA (Fig. 3C, P<0.05) and protein (Fig. 3D, P<0.05) levels in H460 and
SK-MES-1 cells was significantly increased after transfection with
miR-661 inhibitor. These results revealed that RUNX3 is the direct
target of miR-661 in NSCLC.
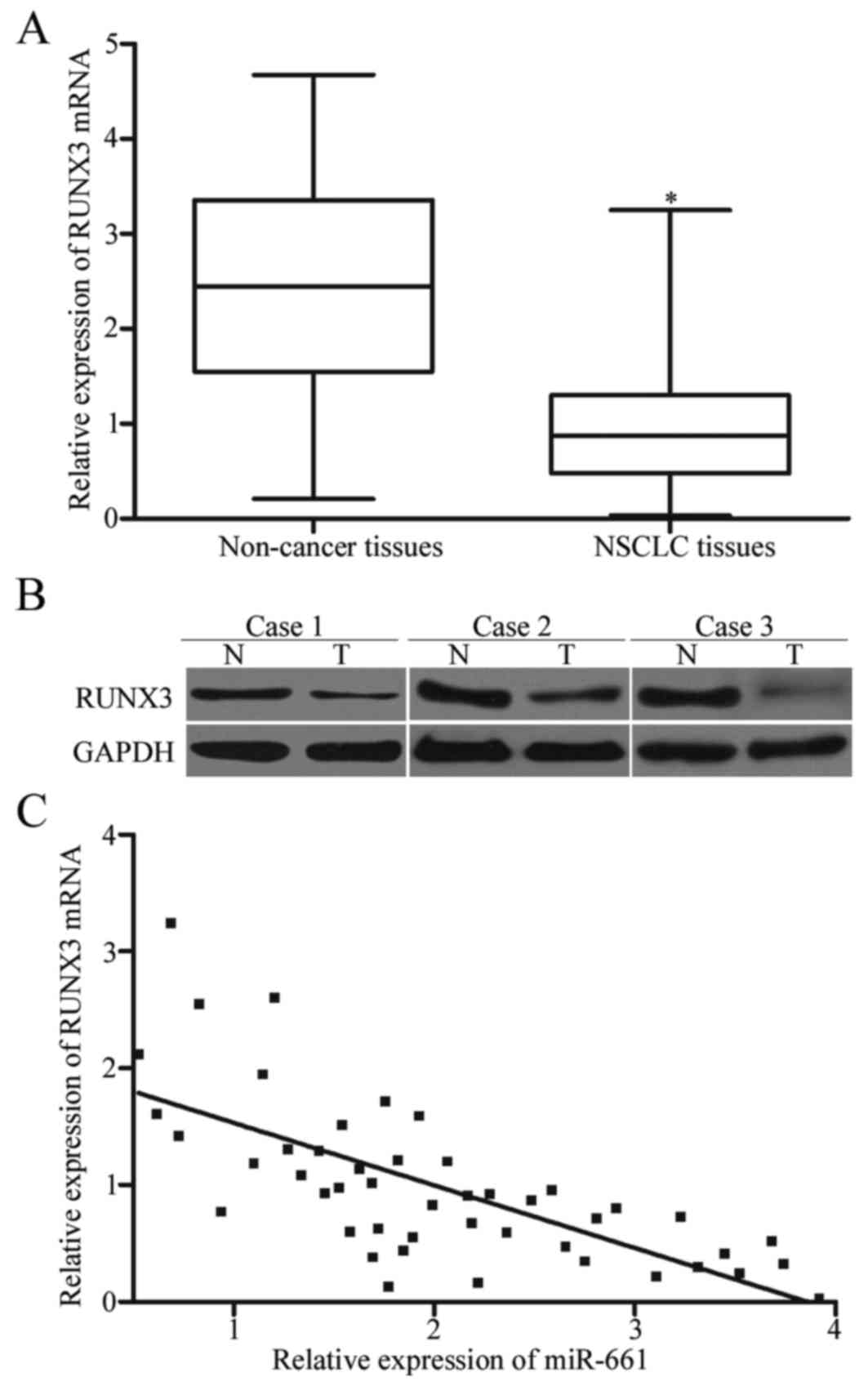
RUNX3 is downregulated and negatively
correlated with miR-661 in NSCLC tissues
To further determine the relationship between
miR-661 and RUNX3, RT-qPCR and Western blot were performed to
measure RUNX3 expression at mRNA and protein levels in NSCLC
tissues and adjacent non-cancer tissues. We found that RUNX3
expression level was decreased at both mRNA (Fig. 4A, P<0.05) and protein (Fig. 4B, P<0.05) levels in NSCLC
tissues compared with that in adjacent non-cancer tissues.
Additionally, we assessed the association between RUNX3 mRNA and
miR-661 expression levels in NSCLC tissues. The results of
Spearman's correlation analysis indicated a statistically inverse
association between RUNX3 mRNA and miR-661 expression levels in
NSCLC tissues (Fig. 4C; r=−0.6960,
P<0.0001).
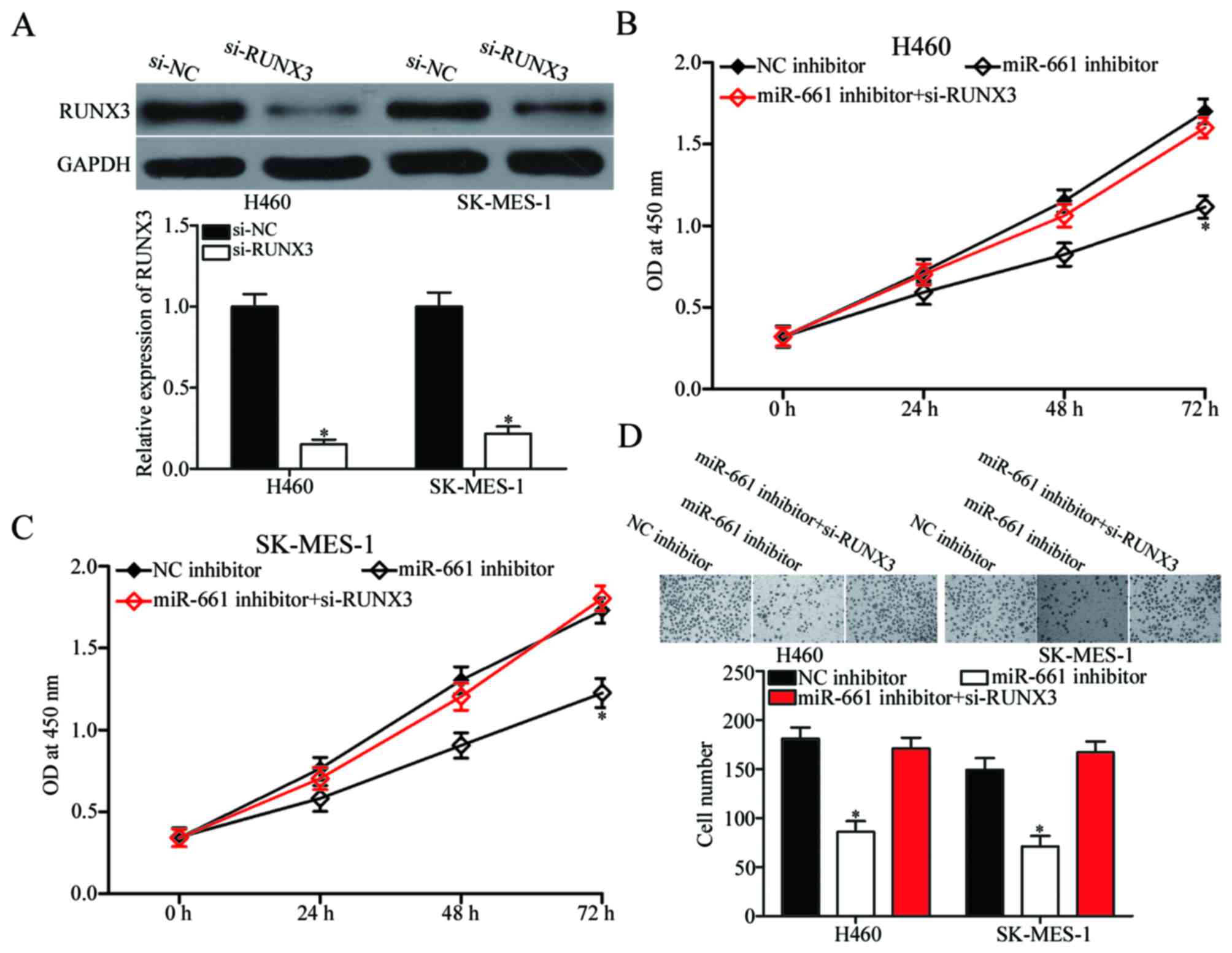
RUNX3 knockdown rescued the effects
induced by miR-661 inhibitor on NSCLC cells
Rescue experiments were carried out to explore
whether or not RUNX3 is responsible for the functional effect of
miR-661 in NSCLC cells. Si-RUNX3 or si-NC was introduced into H460
and SK-MES-1 cells, and Western blot confirmed that RUNX3 was
downregulated in si-RUNX3-transfected H460 and SK-MES-1 cells
(Fig. 5A, P<0.05).
Subsequently, CCK-8 and cell invasion assays were performed, which
identified that RUNX3 knockdown significantly rescued the effects
of miR-661 inhibitor on H460 and SK-MES-1 cell proliferation
(Fig. 5B and C, P<0.05) and
invasion (Fig. 5D, P<0.05).
These findings suggested that miR-661 exerts its biological roles
in NSCLC through negative regulation of RUNX3.
Discussion
Increasing evidence indicated that abnormal miRNA
expression contributes to the carcinogenesis and progression of
multiple human cancers (26–28).
Numerous studies have shed light on tumour-targeting therapies
using miRNAs as novel diagnostic and therapeutic tools (29–31).
Therefore, elucidating the expression level, clinical significance,
biological roles and underlying mechanisms of specific miRNA in
NSCLC will provide novel therapeutic targets for the diagnosis and
therapy of patients with this disease. In the present study, we
found that the miR-661 expression was upregulated in NSCLC tissues
and cell lines. The miR-661 expression level was significantly
correlated with differentiation, tumor stage and lymph node
metastasis of NSCLC patients. Functional assays indicated that
miR-661 underexpression attenuated NSCLC cell proliferation and
invasion in vitro. With regard to the mechanism, our results
demonstrated that RUNX3 functions as a direct downstream target of
miR-661 in NSCLC. These results demonstrated that miR-661 may play
significant roles in NSCLC.
Previously, miR-661 was revealed to be aberrantly
expressed in a number of human malignancies and play a crucial role
in tumourigenesis and cancer progression. For example, Li et
al (20) reported that miR-661
was lowly expressed in glioma tissues. Restoration of miR-661
expression inhibited glioma cell proliferation, migration and
invasion; induced cell cycle arrest and apoptosis in vitro;
and decreased cell growth in vivo. Zhu et al
(21) found that miR-661 was
upregulated in both ovarian cancer tissues and cell lines.
Upregulation of miR-661 promoted cell proliferation, colony
formation and anchorage-independent growth in vitro. Reddy
et al (22) demonstrated
that ectopic miR-661 expression repressed cell migration, invasion
and anchorage-independent growth and tumourigenicity of breast
cancer. These findings indicated that miR-661 may be investigated
as an effective therapeutic target for these types of cancer.
The identification of the direct target gene of
miR-661 is important in understanding its roles in NSCLC and is
also essential in the investigation of novel therapeutic target for
NSCLC patients. Several targets of miR-661 have been reported,
including hTERT (20) in glioma,
INPP5J (21) in ovarian cancer and
MTA1 (22) in breast cancer. In
the present study, RUNX3 was identified as a direct target gene of
miR-661 in NSCLC. Initially, the bioinformatic analysis revealed
that RUNX3 may be a potential target for miR-661. Subsequently,
this predication was further confirmed by the luciferase report
assay. The results demonstrated that the 3′-UTR of RUNX3 could be
directly targeted by miR-661 in NSCLC cells. Notably, the
regulatory effect of miR-661 on endogenous RUNX3 expression in
NSCLC cells was determined using RT-qPCR and western blot, and
demonstrated that downregulation of miR-661 significantly increased
RUNX3 expression at the mRNA and protein levels in NSCLC cells.
Besides, RUNX3 was downregulated and negative correlated with
miR-661 expression level in NSCLC tissues. Finally, RUNX3 knockdown
rescued the effects observed as a result of miR-661 underexpression
in NSCLC cells.
Runt-related gene family includes three members,
namely, RUNX1, RUNX2, and RUNX3, and all play key roles in the
normal developmental process and tumourigenesis (32). RUNX3, which is located on
chromosome 1p36, has been reported to be downregulated in many
human cancer types, such as bladder cancer (33), oesophageal squamous cell carcinoma
(34), breast cancer (35), gastric cancer (36) and glioma (32). In addition, RUNX3 is regarded as a
tumour suppressor gene that inhibits cell proliferation, migration
and invasion and induces cell cycle arrest and apoptosis (37–39).
Previous studies also found that RUNX3 was reduced in NSCLC
tissues, which was associated with promoter hypermethylation
(24). Additionally, RUNX3
methylation was obviously correlated with NSCLC clinical stage,
lymph node metastasis and differentiation (40). Functional experiments revealed that
RUNX3 is involved in crucial regulation of cell proliferation
(41), epithelial-mesenchymal
transition (42) and
tumourigenesis (25) in NSCLC.
These findings indicated that RUNX3 may contribute to NSCLC
progression. The present study identified RUNX3 as a direct gene
target of miR-661, suggesting that the miR-661/RUNX3 axis may be a
promising therapeutic target for the treatment of NSCLC
patients.
In conclusion, we found that miR-661 expression
level was increased in NSCLC tissues and cell lines. In addition,
miR-661 expression level was significantly correlated with
differentiation, tumor stage and lymph node metastasis of NSCLC
patients. Furthermore, miR-661 acted as an oncogene in NSCLC, at
least in part through RUNX3 targeting. These observations suggest
that miR-661 may serve as a novel therapeutic target for the
treatment of NSCLC.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cushing L, Jiang Z, Kuang P and Lü J: The
roles of microRNAs and protein components of the microRNA pathway
in lung development and diseases. Am J Respir Cell Mol Biol.
52:397–408. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ebrahimi A and Sadroddiny E: MicroRNAs in
lung diseases: Recent findings and their pathophysiological
implications. Pulm Pharmacol Ther. 34:55–63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Islam KM Monirul, Shostrom V, Kessinger A
and Ganti AK: Outcomes following surgical treatment compared to
radiation for stage I NSCLC: A SEER database analysis. Lung Cancer.
82:90–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xue C, Hu Z, Jiang W, Zhao Y, Xu F, Huang
Y, Zhao H, Wu J, Zhang Y, Zhao L, et al: National survey of the
medical treatment status for non-small cell lung cancer (NSCLC) in
China. Lung Cancer. 77:371–375. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Laskin JJ and Sandler AB: State of the art
in therapy for non-small cell lung cancer. Cancer Invest.
23:427–442. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang Q, Xiao B, Ma X, Qu M, Li Y,
Nagarkatti P, Nagarkatti M and Zhou J: MicroRNAs associated with
the pathogenesis of multiple sclerosis. J Neuroimmunol 295–296.
148–161. 2016. View Article : Google Scholar
|
|
12
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M
and Croce CM: Human microRNA genes are frequently located at
fragile sites and genomic regions involved in cancers. Proc Natl
Acad Sci USA. 101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meltzer PS: Cancer genomics: Small RNAs
with big impacts. Nature. 435:745–746. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bao L, Wang L, Wei G, Wang Y, Wuyun G and
Bo A: Role of microRNA-4458 in patients with non-small-cell lung
cancer. Oncol Lett. 12:3958–3966. 2016.PubMed/NCBI
|
|
15
|
Hu W, Jin P, Ding C and Liu W: miR-19a/b
modulates lung cancer cells metastasis through suppression of MXD1
expression. Oncol Lett. 12:1901–1905. 2016.PubMed/NCBI
|
|
16
|
Sahay D, Leblanc R, Grunewald TG,
Ambatipudi S, Ribeiro J, Clézardin P and Peyruchaud O: The
LPA1/ZEB1/miR-21-activation pathway regulates metastasis in basal
breast cancer. Oncotarget. 6:20604–20620. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maugeri-Saccà M, Coppola V, Bonci D and De
Maria R: MicroRNAs and prostate cancer: From preclinical research
to translational oncology. Cancer J. 18:253–261. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jones KB, Salah Z, Del Mare S, Galasso M,
Gaudio E, Nuovo GJ, Lovat F, LeBlanc K, Palatini J, Randall RL, et
al: miRNA signatures associate with pathogenesis and progression of
osteosarcoma. Cancer Res. 72:1865–1877. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bartels CL and Tsongalis GJ: MicroRNAs:
Novel biomarkers for human cancer. Clin Chem. 55:623–631. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Z, Liu YH, Diao HY, Ma J and Yao YL:
MiR-661 inhibits glioma cell proliferation, migration and invasion
by targeting hTERT. Biochem Biophys Res Commun. 468:870–876. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu T, Yuan J, Wang Y, Gong C, Xie Y and
Li H: MiR-661 contributed to cell proliferation of human ovarian
cancer cells by repressing INPP5J expression. Biomed Pharmacother.
75:123–128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reddy SD, Pakala SB, Ohshiro K, Rayala SK
and Kumar R: MicroRNA-661, a c/EBPalpha target, inhibits metastatic
tumor antigen 1 and regulates its functions. Cancer Res.
69:5639–5642. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee YS, Lee JW, Jang JW, Chi XZ, Kim JH,
Li YH, Kim MK, Kim DM, Choi BS, Kim EG, et al: Runx3 inactivation
is a crucial early event in the development of lung adenocarcinoma.
Cancer Cell. 24:603–616. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee KS, Lee YS, Lee JM, Ito K, Cinghu S,
Kim JH, Jang JW, Li YH, Goh YM, Chi XZ, et al: Runx3 is required
for the differentiation of lung epithelial cells and suppression of
lung cancer. Oncogene. 29:3349–3361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiao CZ, Wei W, Guo ZX, Zhang MY, Zhang
YF, Wang JH, Shi M, Wang HY and Guo RP: MicroRNA-34c-3p promotes
cell proliferation and invasion in hepatocellular carcinoma by
regulation of NCKAP1 expression. J Cancer Res Clin Oncol.
143:263–273. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang H, Ju H, Zhang L, Lu H and Jie K:
microRNA-577 suppresses tumor growth and enhances chemosensitivity
in colorectal cancer. J Biochem Mol Toxicol. Feb 2–2017.(Epub ahead
of print). View Article : Google Scholar
|
|
28
|
Wang H, Li Q, Niu X, Wang G, Zheng S, Fu G
and Wang Z: miR-143 inhibits bladder cancer cell proliferation and
enhances their sensitivity to gemcitabine by repressing IGF-1R
signaling. Oncol Lett. 13:435–440. 2017.PubMed/NCBI
|
|
29
|
Gandellini P, Giovannetti E and Nicassio
F: MicroRNAs in cancer management: Big challenges for small
molecules. Biomed Res Int. 2015:9821562015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mlcochova J, Faltejskova-Vychytilova P,
Ferracin M, Zagatti B, Radova L, Svoboda M, Nemecek R, John S, Kiss
I, Vyzula R, et al: MicroRNA expression profiling identifies
miR-31-5p/3p as associated with time to progression in wild-type
RAS metastatic colorectal cancer treated with cetuximab.
Oncotarget. 6:38695–38704. 2015.PubMed/NCBI
|
|
31
|
Parpart S, Roessler S, Dong F, Rao V,
Takai A, Ji J, Qin LX, Ye QH, Jia HL, Tang ZY and Wang XW:
Modulation of miR-29 expression by α-fetoprotein is linked to the
hepatocellular carcinoma epigenome. Hepatology. 60:872–883. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mei PJ, Bai J, Liu H, Li C, Wu YP, Yu ZQ
and Zheng JN: RUNX3 expression is lost in glioma and its
restoration causes drastic suppression of tumor invasion and
migration. J Cancer Res Clin Oncol. 137:1823–1830. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dodurga Y, Avci CB, Satiroglu-Tufan NL,
Tataroglu C, Kesen Z, Doğan ZO, Yilmaz S and Gündüz C: Detection of
deleted in malignant brain tumors 1 and runt-related transcription
factor 3 gene expressions in bladder carcinoma. Mol Biol Rep.
39:4691–4695. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sugiura H, Ishiguro H, Kuwabara Y, Kimura
M, Mitsui A, Mori Y, Ogawa R, Katada T, Harata K and Fujii Y:
Decreased expression of RUNX3 is correlated with tumor progression
and poor prognosis in patients with esophageal squamous cell
carcinoma. Oncol Rep. 19:713–719. 2008.PubMed/NCBI
|
|
35
|
Jiang Y, Tong D, Lou G, Zhang Y and Geng
J: Expression of RUNX3 gene, methylation status and
clinicopathological significance in breast cancer and breast cancer
cell lines. Pathobiology. 75:244–251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hsu PI, Hsieh HL, Lee J, Lin LF, Chen HC,
Lu PJ and Hsiao M: Loss of RUNX3 expression correlates with
differentiation, nodal metastasis, and poor prognosis of gastric
cancer. Ann Surg Oncol. 16:1686–1694. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jili S, Eryong L, Lijuan L and Chao Z:
RUNX3 inhibits laryngeal squamous cell carcinoma malignancy under
the regulation of miR-148a-3p/DNMT1 axis. Cell Biochem Funct.
34:597–605. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen F, Liu X, Cheng Q, Zhu S, Bai J and
Zheng J: RUNX3 regulates renal cell carcinoma metastasis via
targeting miR-6780a-5p/E-cadherin/EMT signaling axis. Oncotarget.
Nov 8–2016.(Epub ahead of print).
|
|
39
|
Kim BR, Kang MH, Kim JL, Na YJ, Park SH,
Lee SI, Kang S, Joung SY, Lee SY, Lee DH, et al: RUNX3 inhibits the
metastasis and angiogenesis of colorectal cancer. Oncol Rep.
36:2601–2608. 2016.PubMed/NCBI
|
|
40
|
Yu GP, Ji Y, Chen GQ, Huang B, Shen K, Wu
S and Shen ZY: Application of RUNX3 gene promoter methylation in
the diagnosis of non-small cell lung cancer. Oncol Lett. 3:159–162.
2012.PubMed/NCBI
|
|
41
|
Torshabi M, Faramarzi MA, Yazdi M
Tabatabaei, Ostad SN and Gharemani MH: Runx3 expression inhibits
proliferation and distinctly alters mRNA expression of Bax in AGS
and A549 cancer cells. Iran J Pharm Res. 10:355–361.
2011.PubMed/NCBI
|
|
42
|
Lee JM, Shin JO, Cho KW, Hosoya A, Cho SW,
Lee YS, Ryoo HM, Bae SC and Jung HS: Runx3 is a crucial regulator
of alveolar differentiation and lung tumorigenesis in mice.
Differentiation. 81:261–268. 2011. View Article : Google Scholar : PubMed/NCBI
|