Introduction
Cerebrovascular disease is the third most common
cause of mortality in the world (1). It has three major characteristics:
High morbidity, high disability rate and high mortality rate
(1). The morbidity of cerebral
ischemia-reperfusion injury (CIRI) accounts for ~70% of
cerebrovascular diseases, and this trend is increasing (1). Many patients lose the ability to work
and function normally, which causes heavy economic and
psychological burdens for the patients, families and society
(2). Therefore, it is important to
prevent and control the incidence and development of CIRI.
The therapeutic principle of ischemic stroke is to
restore blood perfusion in ischemia, improve the blood circulation
of the brain tissue, increase blood flow and oxygen supply at
ischemic penumbra, control encephaledema and to prevent and cure
further complications (3,4). However, reperfusion further
aggravates brain dysfunction, and structural failure results from
ischemia. Brain tissue injuries cause irreversible injury of brain
cells (5); this is known as
ischemic CIRI. Consequently, investigations are focused on
developing methods to reduce reperfusion injury, save brain cells
and restore brain functions (6),
potentially by detecting markers of the degree of CIRI (7). The development of novel
psychotherapeutic drugs for the prevention and treatment of
cerebrovascular diseases therefore has great significance.
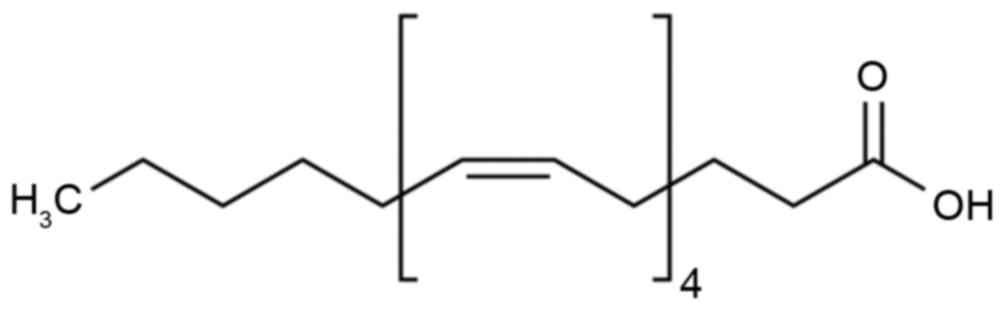
Epoxyeicosatrienoic acid (EET; Fig. 1) was initially identified from
investigations into the low incidence of cardiovascular disease in
eskimos (8). Docosahexaenoic acid,
eicosapentaenoic acid and other long-chain unsaturated fatty acids
have the efficacy to prevent cholesterol deposition on the arterial
vascular wall, and to prevent the incidence of cardiovascular
disease (9). EET has been
demonstrated to exert anti-inflammatory effects through nuclear
factor (NF)-κB, adipocyte protein 2 and other signaling pathways
(10). The present study used a
rat CIRI model to investigate the inhibition effects of EET, and
the potential underlying mechanisms.
Materials and methods
Animals and experimental groups
Specific pathogen-free male Sprague-Dawley rats
(age, 7 weeks; weight, 200–240 g; n=50) were purchased from the
Center for Animal Experiments, Renmin Hospital of Wuhan University
(Wuhan, China) and housed at 23±2°C in a 12 h light/dark cycle in
50±10% humidity with free access to food and water. All experiments
were conducted in accordance with the National Institute of Health
Guide for the Care and Use of Laboratory Animals (Bethesda, MD,
USA) and the experiments were approved by the Animal Care and Use
Committee of Wuhan University. The rats were randomly divided into
three groups: Sham, (n=10), CIRI (n=20) and EET (n=20). In the EET
group, rats were administered 6.24×10-7 mol/l 10 ml/kg 11,12-EET
via the jugular vein for 20 min. Rats in the other groups were
administered with saline. All rats were intraperitoneally
anesthetized with 30 mg/kg pentobarbital sodium (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) and were fixed in a supine position
at 37–38°C. A 4/0 surgical nylon filament with a silicone-beaded
tip was inserted into the right internal carotid artery via the
external carotid artery to occlude the origin of the middle
cerebral artery. The occlusion was released for 24 h following
ischemia at 2 h.
Neurological deficit score
Rats were suspended by the tail and neurological
testing was conducted independently by 3 researchers. The
neurological deficit scores were as follows: 0, forelimb flexion
and body twisting; 1, inability to extend the left forepaw; 2, rat
circled to the left; 3, inability to move the left forepaw; 4,
inability to walk.
Cerebral infarct volume and cerebral
edema
To assess cerebral infarct volume, the rats were
sacrificed and brain tissue samples were obtained and weighed as
wet weight (WW). Subsequently, tissue samples were sectioned into 4
mm-thick pieces and stained using 2% 2,3,5-triphenyltetrazolium
chloride (Amresco, LLC; Solon, OH, USA) at room temperature for 30
min. The infarct volume is expressed as the percentage of the
contralateral hemisphere. Tissue samples were dried at 110°C
overnight in an electric oven and weighed again to obtain the dry
weight (DW). Water content (%)=[(WW-DW)/WW]x100% was calculated as
brain water content.
Enzyme-linked immunosorbent assay
(ELISA)
Cells were lysed using a radioimmunoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China) at 4°C for 15–20 min, the homogenate was centrifuged for 10
min at 30,000 × g at 4°C and the supernatant was collected. Protein
concentrations were detected with a bicinchoninic acid (BCA) assay
kit (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
levels of TNF-α (cat no. ab46070), interleukin (IL)-6 (cat no.
ab100772), NF-κB (cat no. ab176647) and inducible nitric oxide
synthase (iNOS; cat no. ab196266) were determined using commercial
ELISA kits (Abcam, Shanghai, China).
Western blot analysis
Cells were lysed using a radioimmunoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology) at 4°C for
15–20 min, the homogenate was centrifuged for 10 min at 30,000 × g
at 4°C and the supernatant was collected. Protein concentrations
were detected with a BCA kit. Proteins (50 µg) were separated by
10–12% SDS-PAGE and transferred to a polyvinylidene difluoride
membrane (EMD Millipore, Billerica, MA, USA). Membranes were
blocked using 5% skim milk powder in TBS containing 0.05% Tween-20
(TBST) for 1 h at 37°C and incubated overnight with the following
primary antibodies at 4°C: Anti-cleaved caspase-3 (1:3,000; cat no.
9664), anti-phospholipase A2 (PLA2; 1:3,000; cat no. 2832),
anti-cyclooxygenase-2 (COX-2; 1:3,000; cat no. 12282),
anti-pannexin-1 (1:3,000; cat no. 91137), purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA); anti-prostaglandin
E2 (PGE2; 1:300; cat no. D262741) and anti-β-actin (1:3,000; cat
no. D110007), obtained from Shanghai Sangong Pharmaceutical Co.,
Ltd. (Shanghai, China). Membranes were washed with TBST, and
subsequently probed with horseradish peroxidase-labeled secondary
antibodies (1:5,000; cat no. BM2006; Wuhan Boster Biological
Technology, Ltd., Wuhan, China) at 37°C for 1 h. Blots were
visualized by enhanced chemiluminescence using the Pierce™ Fast
Western Blot kit (cat no. 35050; Thermo Fisher Scientific, Inc.)
and semi-quantified using Quantity One software version 3.0
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data were analyzed using SPSS software version 18.0
(SPSS Inc., Chicago, IL, USA). Data are expressed as the mean ±
standard deviation of 3 independent experiments. The statistical
significance of the differences between groups was assessed using a
one-way analysis of variance followed by a post hoc Tukey-Kramer
test for multiple comparisons. P<0.05 was considered to indicate
a statistically significant difference.
Results
EET ameliorates CIRI-induced
neurological deficit scores
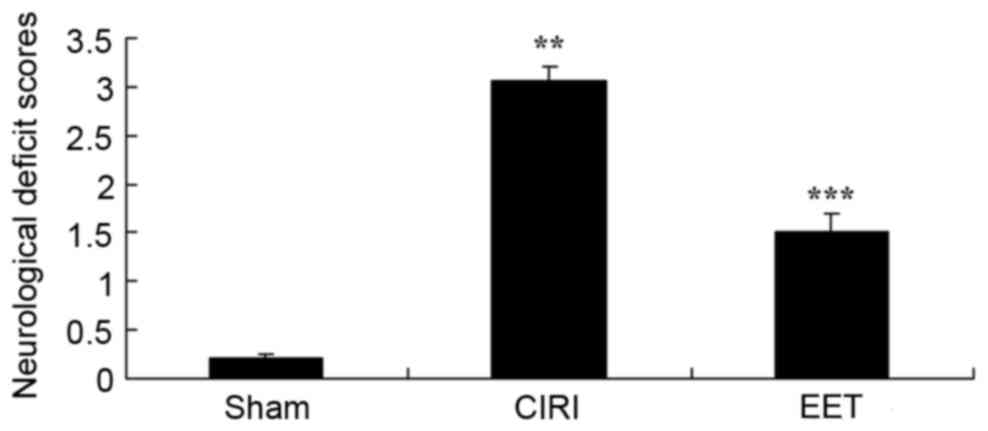
Neurological deficit scores from each group are
presented in Fig. 2. The
neurological deficit score of the CIRI model group significantly
increased compared with sham group. EET treatment caused a
significant decrease in the neurological deficit score, when
compared with the CIRI model group.
EET ameliorates CIRI-induced cerebral
infarct volume and cerebral edema
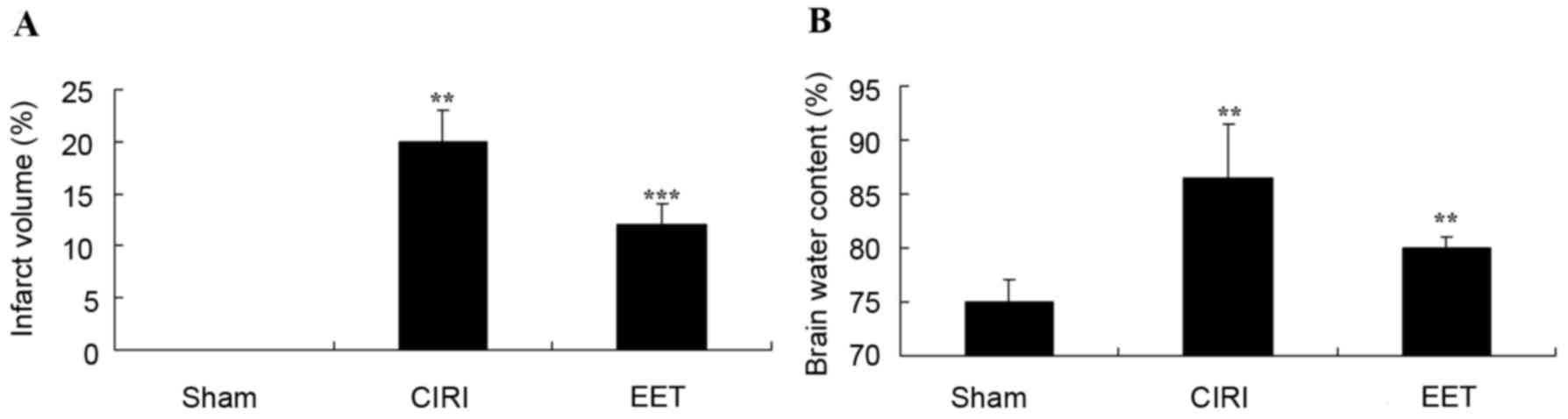
CIRI-induced cerebral infarct volume and cerebral
edema (brain water content) in all groups are presented in Fig. 3. The cerebral infarct volume in the
sham group was negligible, and it was significantly increased in
the CIRI group in comparison. There was a significant inhibition in
cerebral infarct volume in the EET group compared with the CIRI
model group (Fig. 3A). Cerebral
edema was increased in the CIRI group compared with the sham group;
however, treatment with EET significantly reduced this effect
(Fig. 3B).
EET ameliorates CIRI-induced cleaved
caspase-3
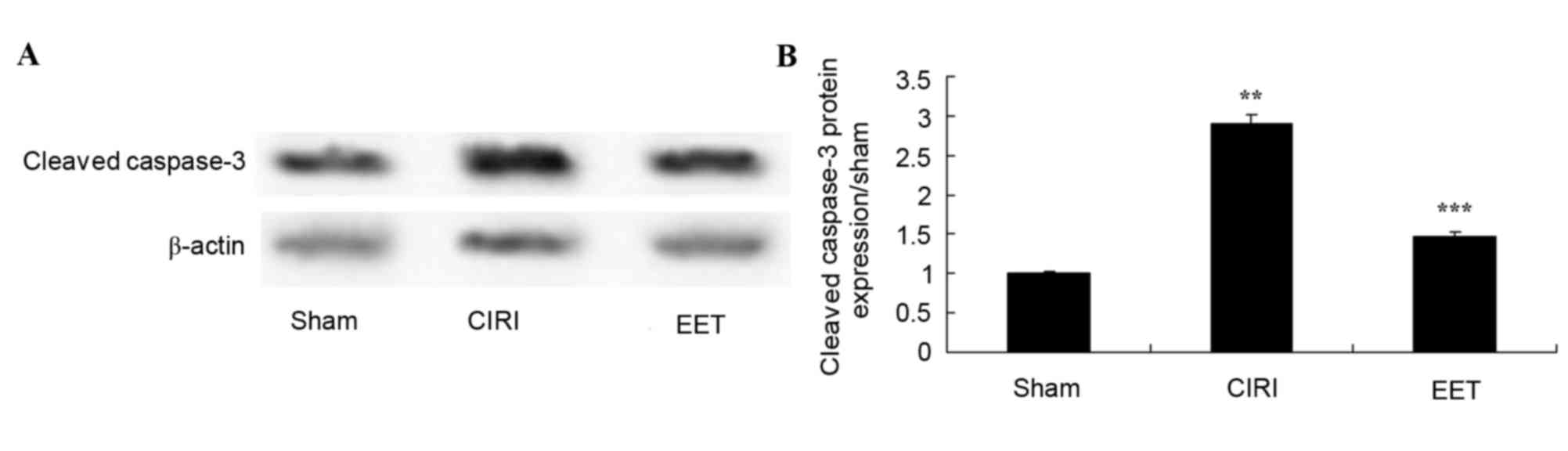
As assessed by western blot analysis (Fig. 4A), cleaved caspase-3 protein
expression levels in CIRI tissue homogenates was significantly
increased compared with the sham group. Pretreatment with EET
significantly reduced CIRI-induced cleavedcaspase-3 protein
expression levels (Fig. 4B).
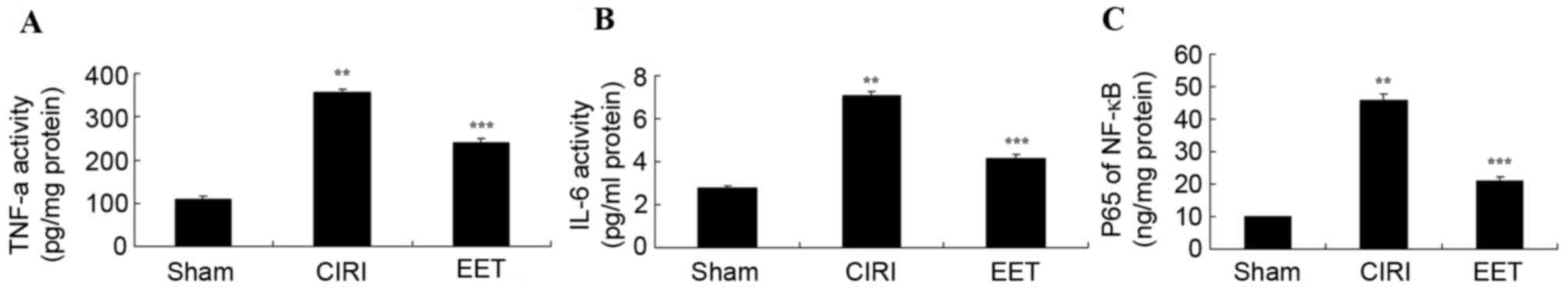
EET ameliorates CIRI-induced
inflammation
The levels of TNF-α (Fig. 5A), IL-6 (Fig. 5B) and NF-κB (Fig. 5C) measured in CIRI group samples
were significantly increased compared with the sham group; however,
treatment with EET significantly ameliorated this effect.
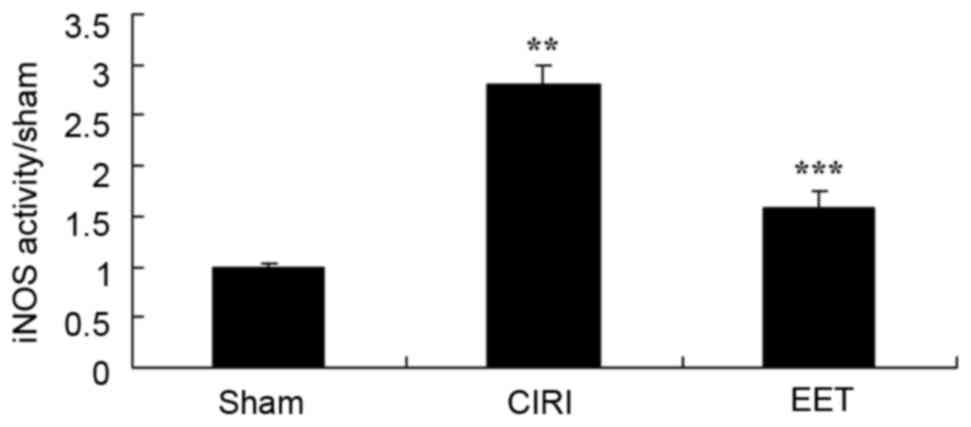
EET ameliorates CIRI-induced iNOS
activity
CIRI-induced iNOS activity was markedly increased
compared with the sham group, and EET treatment significantly
attenuated this effect (Fig.
6).
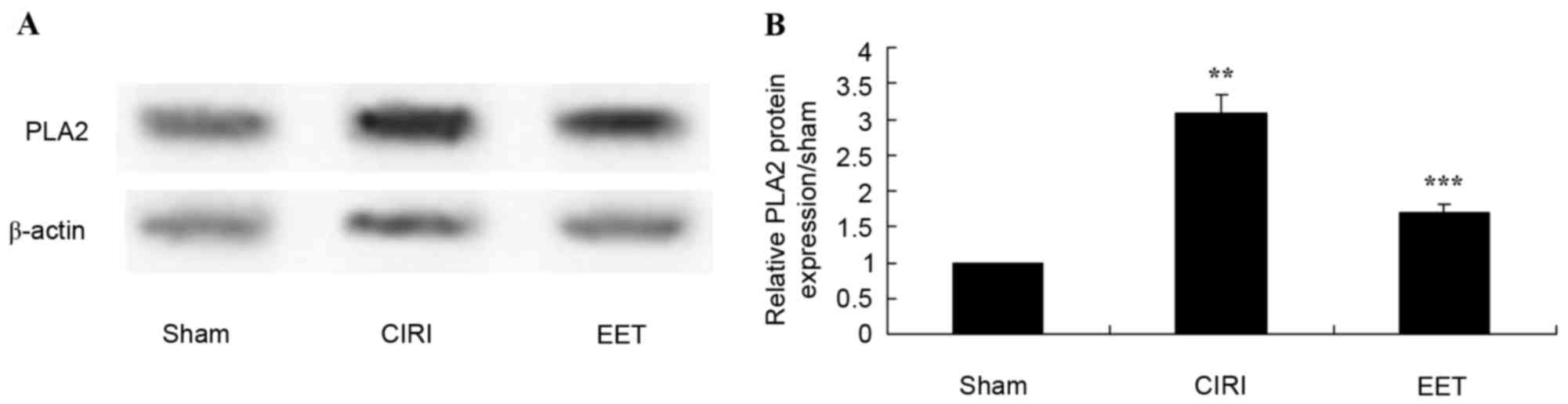
EET ameliorates CIRI-induced PLA2
protein expression
PLA2 protein expression levels were determined by
western blot analysis (Fig. 7A).
CIRI rats exhibited significantly increased PLA2 expression levels
compared with the sham group; however, EET pretreatment
significantly inhibited this effect (Fig. 7B).
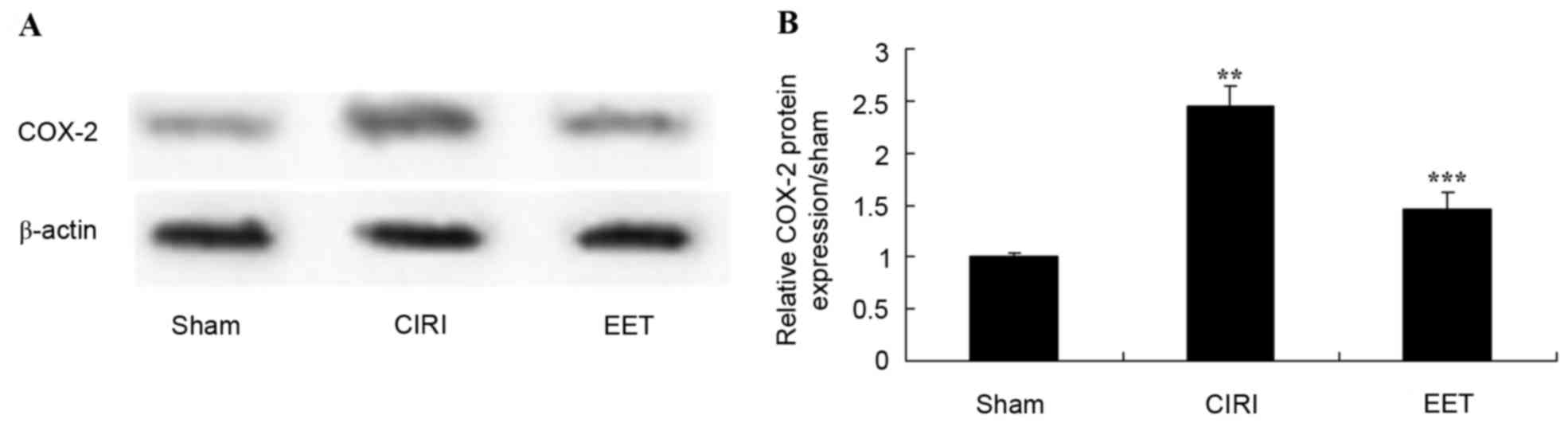
EET ameliorates CIRI-induced COX-2
protein expression
COX-2 protein expression levels were examined by
western blot analysis (Fig. 8A).
CIRI led to a significant increase in COX-2 protein expression
levels compared with sham-operated rats; however, EET treatment
significantly ameliorated this effect (Fig. 8B).
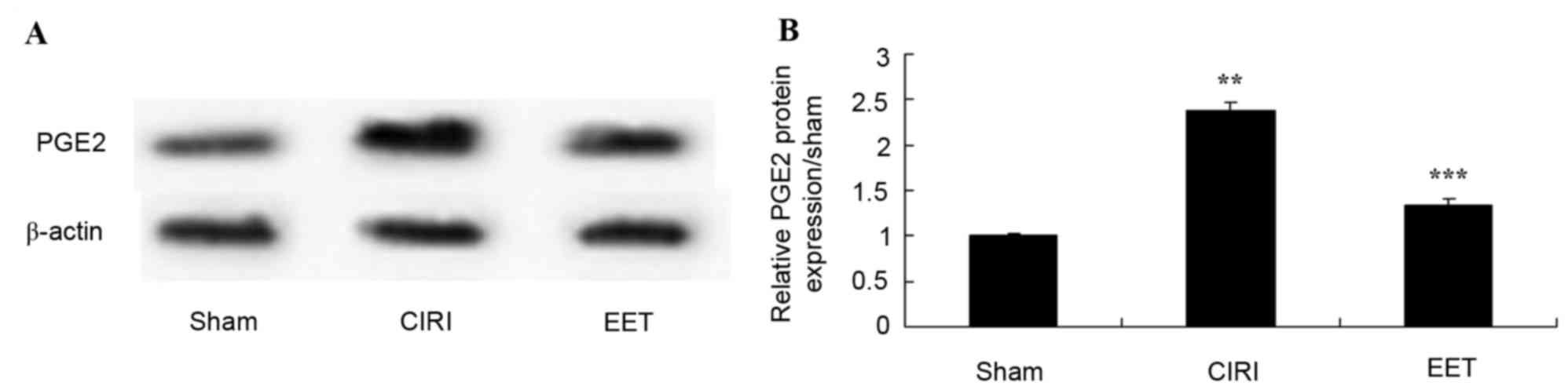
EET ameliorates CIRI-induced PGE2
protein expression
The protein expression levels of PGE2 was evaluated
in CIRI tissue (Fig. 9A). PGE2 was
highly expressed in CIRI tissue but not in sham-operated rats. In
addition, this activation of PGE2 protein expression was
significantly inhibited by treatment with EET (Fig. 9B).
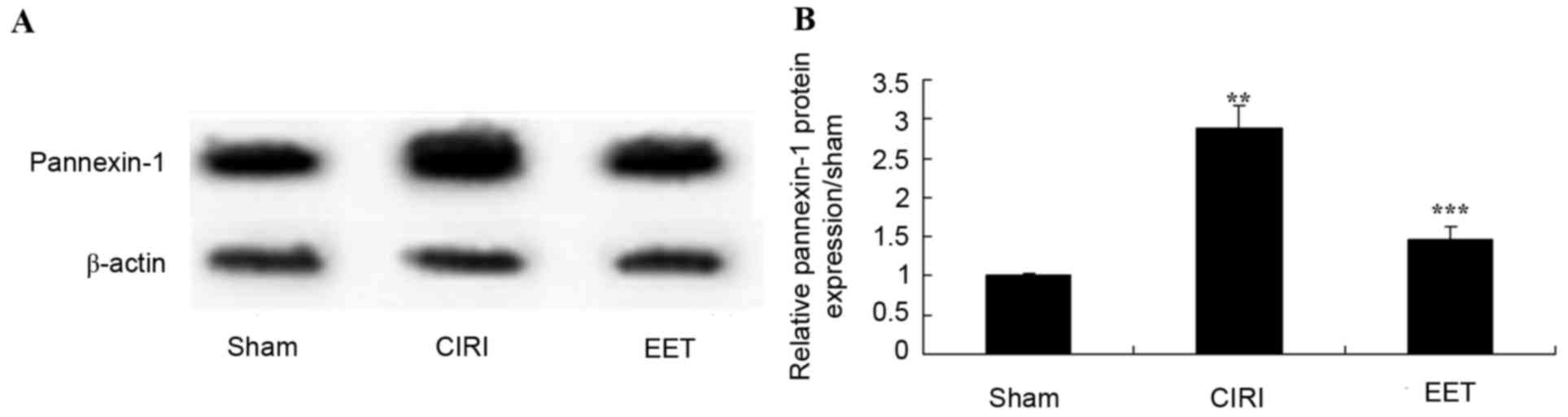
EET ameliorates CIRI-induced
pannexin-1 protein expression
Pannexin-1 protein expression levels were examined
by western blot analysis (Fig.
10A). These results demonstrated that, following CIRI,
pannexin-1 protein expression levels were increased compared with
the sham group. Treatment with EET significantly inhibited
CIRI-induced pannexin-1 protein expression in rats (Fig. 10B).
Discussion
CIRI refers to cellular function metabolic disorders
and organizational structure damage aggravation following blood
reperfusion of ischemic damage (11). Although timely blood reperfusion
may rescue certain cells close to the site of infarction,
reperfusion causes a greater extent of tissue damage than pure
ischemia injury (11). Previous
studies have indicated that CIRI is primarily associated with
inflammatory reactions, excitability amino acids, intracellular
friction overload, oxygen free radicals and cell apoptosis
(4,12). These various factors interact
during cerebral ischemia reperfusion, and interlink to form a
vicious circle. It eventually leads to ischemia reperfusion injury
(13). Among these factors, the
inflammatory reaction is considered to be one of the most important
causes of CIBI (13). TNF is a
cytokine with key effects in the network of inflammation. It is
considered as an initiating medium for systemic inflammatory
reaction (14). It may directly
lead to reduced circulation resistance, increased vasopermeability
and decreased numbers of vascular endothelial cells. In addition,
it may induce a cascade release of IL-1, IL-6 IL-8 and additional
cytokines, which forms amplification effects on inflammatory injury
(14). The present study
demonstrated that EET treatment significantly ameliorated
CIRI-induced neurological deficit scores, cerebral infarct volume
and cerebral edema, and inhibited the expression levels of cleaved
caspase-3, TNF-α, IL-6 and NF-κB in CIRI rats. Previous studies
have demonstrated that EET attenuates IL-8 production in bronchial
epithelial cells (15) and
inflammation in the cardiovascular system (16).
PLA2 has been suggested as an inflammatory marker
closely associated with atherosclerosis (17). A combination of PLA2 and
low-density lipoproteins in the blood generates a great amount of
blood transportation, lecithin and free fatty acid oxidation,
promoting atherosclerosis (18).
The primary pathological basis of ischemic stroke, particularly
cerebral thrombosis, is atherosclerosis (19). Consequently, it is a predictive
index for atherosclerosis cerebral infarction. The present study
revealed that EET treatment significantly ameliorates CIRI-induced
PLA2 protein expression in rats. Jiang et al (20) reported that the release of EETs
from red blood cells may be via cytosolic PLA2.
Increased expression levels of PLA2 may induce
genetic expression of COX-2 for numerous inflammation-stimulating
factors, including lipopolysaccharide, IL-1, TNF, epidermal growth
factor α and platelet activating factor (21), and increase levels of PGE2,
prostacyclin and PGE1 at inflammatory sites. A positive association
between COX-2 mRNA and PGE2 levels at inflammatory sites and
severity of inflammation has been verified in numerous experiments
(22). At present, PGE2 is
considered as an important mediator of inflammation. It has been
demonstrated that the expression of COX-2 in the brain is the
highest among all visceral organs, and is primarily distributed in
the hippocampus and temporal lobe cortex (23,24).
This may be associated with the high density of excitatory amino
acid receptors in these regions. Overexpression of COX-2 in nervous
tissue may directly damage nerve cells (25). In the present study, COX-2 protein
expression levels were significantly reduced by treatment with EET.
Michaelis et al (26)
indicated that EET induces COX-2 protein expression in endothelial
cells.
The gap-junction connected gel networks of
astrocytes have been suggested to be involved in ischemia;
therefore, have attracted increasing attention (27). Pannexin, the primary protein
composition for close connection of astrocytes, is involved in
transmission of information within astrocyte networks via
communication between gap junctions (27). Gap junction intercellular
communication may disperse antioxidative, anti-apoptotic and growth
factors around the ischemia area via tight junctions to alleviate
ischemic injury. However, dying nerve cells in the ischemic core
area may disperse excitatory amino acids, free radical products,
apoptosis signals and other injurious factors to ischemia area to
induce cell injury and apoptosis in the peripheral zone, further
expanding the cerebral infarction volume (28,29).
The present study demonstrated that EET ameliorates CIRI-induced
PGE2 and pannexin-1 protein expression levels in rats. Nüsing et
al (30) suggested that EET
may affect renal tubular epithelial cells via PGE2 and COX-2.
In conclusion, the present study demonstrated that
EET inhibits CIRI-induced neurological deficit scores, cerebral
infarct volume and cerebral edema, and inhibited the levels of
cleaved caspase-3, TNF-α, IL-6 and NF-κB in CIRI rats via
inhibiting the activation of PLA2/COX-2/PGE2. Therefore, EET may
represent a potential therapeutic agent for the prevention and
treatment of CIRI.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Hubei Province (grant no. 2013BKB014).
References
|
1
|
Nasri H: Renal cell protection of
erythropoietin beyond correcting the anemia in chronic kidney
disease patients. Cell J. 15:378–380. 2014.PubMed/NCBI
|
|
2
|
Zager RA, Johnson AC and Becker K: Acute
unilateral ischemic renal injury induces progressive renal
inflammation, lipid accumulation, histone modification and
‘end-stage’ kidney disease. Am J Physiol Renal Physiol.
301:F1334–F1345. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu YF, Zhang Y, Dai D and Xu Z:
Expression of NF-κB, MCP-1 and MMP-9 in a cerebral aneurysm rabbit
model. Can J Neurol Sci. 41:200–205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang S, Liu K, Seneviratne CJ, Li X,
Cheung GS, Jin L, Chu CH and Zhang C: Lipoteichoic acid from an
clinical strain promotes TNF-a expression through the NF-kB and p38
MAPK signaling pathways in differentiated THP-1 macrophages. Biomed
Rep. 3:697–702. 2015.PubMed/NCBI
|
|
5
|
Ma XF, Zhang J, Shuai HL, Guan BZ, Luo X
and Yan RL: IKKa/NF-kB mediated the low doses of bisphenol A
induced migration of cervical cancer cells. Arch Biochem Biophys.
573:52–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li J, Deng Z, Wang Z, Wang D, Zhang L, Su
Q, Lai Y, Li B, Luo Z, Chen X, et al: Zipper-interacting protein
kinase promotes epithelial-mesenchymal transition, invasion and
metastasis through AKT and NF-kB signaling and is associated with
metastasis and poor prognosis in gastric cancer patients.
Oncotarget. 6:8323–8338. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuper C, Beck FX and Neuhofer W: NFAT5
contributes to osmolality-induced MCP-1 expression in mesothelial
cells. Mediators Inflamm. 2012:5130152012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lakkappa N, Krishnamurthy PT, Hammock BD,
Velmurugan D and Bharath MM: Possible role of Epoxyeicosatrienoic
acid in prevention of oxidative stress mediated neuroinflammation
in Parkinson disorders. Med Hypotheses. 93:161–165. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang X, Liang D, Guo L, Liang W, Jiang Y,
Li H, Zhao Y, Lu S and Chi ZH: Curcumin protects renal tubular
epithelial cells from high glucose-induced
epithelial-to-mesenchymal transition through Nrf2-mediated
upregulation of heme oxygenase-1. Mol Med Rep. 12:1347–1355.
2015.PubMed/NCBI
|
|
10
|
Mawdsley JE, Jenkins DG, Macey MG,
Langmead L and Rampton DS: The effect of hypnosis on systemic and
rectal mucosal measures of inflammation in ulcerative colitis. Am J
Gastroenterol. 103:1460–1469. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Inui Y, Mochida H, Yamairi F, Okada M,
Ishida J, Fukamizu A and Arakawa K: Effects of aging and
uninephrectomy on renal changes in Tsukuba hypertensive mice.
Biomed Rep. 1:359–364. 2013.PubMed/NCBI
|
|
12
|
Yao L, Lu P, Li Y, Yang L, Feng H, Huang
Y, Zhang D, Chen J and Zhu D: Osthole relaxes pulmonary arteries
through endothelial phosphatidylinositol 3-kinase/Akt-eNOS-NO
signaling pathway in rats. Eur J Pharmacol. 699:23–32. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hua KF, Yang SM, Kao TY, Chang JM, Chen
HL, Tsai YJ, Chen A, Yang SS, Chao LK and Ka SM: Osthole mitigates
progressive IgA nephropathy by inhibiting reactive oxygen species
generation and NF-kB/NLRP3 pathway. PLoS One. 8:e777942013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsai YF, Yu HP, Chung PJ, Leu YL, Kuo LM,
Chen CY and Hwang TL: Osthol attenuates neutrophilic oxidative
stress and hemorrhagic shock-induced lung injury via inhibition of
phosphodiesterase 4. Free Radic Biol Med. 89:387–400. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mannon PJ, Hornung RL, Yang Z, Yi C,
Groden C, Friend J, Yao M, Strober W and Fuss IJ: Suppression of
inflammation in ulcerative colitis by interferon-b-1a is
accompanied by inhibition of IL-13 production. Gut. 60:449–455.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang SK, Jung HY, Kang GH, Kim YM, Myung
SJ, Shim KN, Hong WS and Min YI: Appendiceal orifice inflammation
as a skip lesion in ulcerative colitis: An analysis in relation to
medical therapy and disease extent. Gastrointest Endosc.
49:743–747. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xia Y, Kong L, Yao Y, Jiao Y, Song J, Tao
Z, You Z and Yang J: Osthole confers neuroprotection against
cortical stab wound injury and attenuates secondary brain injury. J
Neuroinflammation. 12:1552015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin VC, Chou CH, Lin YC, Lin JN, Yu CC,
Tang CH, Lin HY and Way TD: Osthole suppresses fatty acid synthase
expression in HER2-overexpressing breast cancer cells through
modulating Akt/mTOR pathway. J Agric Food Chem. 58:4786–4793. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang YZ, Tang YZ and Liu YH: Wogonoside
displays anti-inflammatory effects through modulating inflammatory
mediator expression using RAW264.7 cells. J Ethnopharmacol.
148:271–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang H, McGiff JC, Quilley J, Sacerdoti
D, Reddy LM, Falck JR, Zhang F, Lerea KM and Wong PY:
Identification of 5,6-trans-epoxyeicosatrienoic acid in the
phospholipids of red blood cells. J Biol Chem. 279:36412–36418.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang SM, Chan YL, Hua KF, Chang JM, Chen
HL, Tsai YJ, Hsu YJ, Chao LK, Feng-Ling Y, Tsai YL, et al: Osthole
improves an accelerated focal segmental glomerulosclerosis model in
the early stage by activating the Nrf2 antioxidant pathway and
subsequently inhibiting NF-kB-mediated COX-2 expression and
apoptosis. Free Radic Biol Med. 73:260–269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lucas AL, Ouellette SP, Kabeiseman EJ,
Cichos KH and Rucks EA: The trans-Golgi SNARE syntaxin 10 is
required for optimal development of Chlamydia trachomatis. Front
Cell Infect Microbiol. 5:682015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Finocchietti S, Cappagli G and Gori M:
Encoding audio motion: Spatial impairment in early blind
individuals. Front Psychol. 6:13572015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nordahl H, Osler M, Frederiksen BL,
Andersen I, Prescott E, Overvad K, Diderichsen F and Rod NH:
Combined effects of socioeconomic position, smoking, and
hypertension on risk of ischemic and hemorrhagic stroke. Stroke.
45:2582–2587. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Venturino A, Oda A and Perin P: Hair
cell-type dependent expression of basolateral ion channels shapes
response dynamics in the frog utricle. Front Cell Neurosci.
9:3382015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Michaelis UR and Fleming I: From
endothelium-derived hyperpolarizing factor (EDHF) to angiogenesis:
Epoxyeicosatrienoic acids (EETs) and cell signaling. Pharmacol
Ther. 111:584–595. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Groote RP, Budinčević I, Billowes J,
Bissell ML, Cocolios TE, Farooq-Smith GJ, Fedosseev VN, Flanagan
KT, Franchoo S, Ruiz RF Garcia, et al: Use of a continuous wave
laser and pockels cell for sensitive high-resolution collinear
resonance ionization spectroscopy. Phys Rev Lett. 115:1325012015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen Y, Qiao H, Fan Q, Zhang S and Tang T:
Potentiated osteoinductivity via cotransfection with BMP-2 and VEGF
genes in microencapsulated C2C12 cells. Biomed Res Int.
2015:4352532015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rodriguez DA, de Lima RF, Campos MS, Costa
JR, Biancardi MF, Marques MR, Taboga SR and Santos FC: Intrauterine
exposure to bisphenol A promotes different effects in both neonatal
and adult prostate of male and female gerbils (Meriones
unguiculatus). Environ Toxicol. Oct 7–2015.(Epub ahead of print)
doi: 10.1002/tox.22176. PubMed/NCBI
|
|
30
|
Nusing RM, Schweer H, Fleming I, Zeldin DC
and Wegmann M: Epoxyeicosatrienoic acids affect electrolyte
transport in renal tubular epithelial cells: Dependence on
cyclooxygenase and cell polarity. Am J Physiol Renal Physiol.
293:F288–F298. 2007. View Article : Google Scholar : PubMed/NCBI
|