Introduction
Age-related macular degeneration (AMD) is the
leading cause of visual loss among individuals >65 years of age
in developed countries (1). The
most common type of AMD, also termed the ‘dry-type’, is initiated
by the death of retinal pigment epithelium (RPE) cells and
eventually results in the degeneration of photoreceptors, which
leads to visual loss (2,3). AMD is a multifactorial disease;
aging, genetic background, cigarette smoking, oxidative damage and
chronic inflammation are all factors, which contribute to its onset
and progression (4–6).
It is well established that oxidative stress is
important in the pathogenesis of AMD (7,8). The
retina requires a higher oxygen concentration, compared with other
organs, in order to maintain the high metabolic rate of
photoreceptors. The higher the level of oxygen consumed, the more
reactive oxygen species (ROS) is produced. In addition, the daily
phagocytosis of shed photoreceptor outer segments leads to the
generation of free radicals and toxic oxidized materials in RPE
cells. Therefore, RPE cells are susceptible to long-term oxidative
stress, and oxidative stress induces the dysfunction of RPE cells,
contributing to the development of AMD (2,9).
There remains no effective treatment for the dominant type of AMD,
and current interventions are commonly focused on prevention rather
than treatment. Antioxidant supplements have been used to reduce
the risk of AMD, and dietary lutein is considered to act as a
protector against visual impairment from AMD (10).
Lutein is a type of carotenoid, which forms human
macular pigments with zeaxanthin in the retina, inhibiting noxious
blue light into retina and contributing to strengthening of the
antioxidant defense of RPE cells (11,12).
The human body cannot synthesize lutein. The sources of lutein are
primarily dietary in origin, for example, green leafy vegetables,
including spinach and cabbage; fruits, including grapes and kiwis;
egg yolks, and corn (13). It is
reported that the risks of the onset and progression of AMD are
negatively correlated with lutein concentration in the macula
(5,14).
Lutein has already been used in the healthcare
setting (15), however, the exact
molecular mechanism underlying the protective effect of lutein
against stress remains to be fully elucidated. To better understand
the function of lutein, the present study aimed to examine its
underlying mechanism and widen its areas of application.
Materials and methods
Cell culture
The acute retinal pigment epithelial 19 (ARPE-19)
human RPE cell line was obtained from the American Type Culture
Collection (Manassas, VA, USA). The cells were cultured in high
glucose Dulbecco's modified Eagle's medium (DMEM; HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) with 10% fetal bovine
serum (Thermo Fisher Scientific, Inc., Waltham, MA, USA),
penicillin (100 U/ml) and streptomycin (50 U/ml) in a 5%
CO2-humidified environment at 37°C.
Lutein and H2O2
treatment
The cells were seeded at a density of
4×103 per well in 96-well plates and 8×105
per dish in 60 mm dishes, and then cultured with lutein (Aladdin
Chemical Co., Ltd., Shanghai, China) at concentrations of 0, 1, 5,
10 and 15 µM for 12 h at 37°C. Lutein was dissolved in dimethyl
sulfoxide (DMSO; MP Biomedicals, Illkirch, France) with a stock
concentration of 1 mM and maintained in the dark. Following washing
once with PBS, the RPE cells were incubated in culture media
containing 0, 200, 400, 600, 800, 1,000, 1,200, 1,600 and 2,000 µM
H2O2 (Guangzhou Chemical Reagent Factory,
Guangzhou, China) for 12 or 24 h at 37°C prior to the specific
assays.
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium-bromide
(MTT) assay
Following treatment with lutein or
H2O2, the RPE cells were washed in PBS and
incubated at 37°C in DMEM containing 0.25 mg/ml MTT. After 4 h, the
MTT solution was removed and 150 µl DMSO was added to each well.
The optical densities at 490 nm were read on a microplate
spectrophotometer (Biotek Instruments, Inc., Winooski, VT,
USA).
Measurement of apoptosis, ROS levels
and cell cycle
The RPE cells pretreated with lutein for 24 h were
incubated with 800 µM H2O2 for another 24 h.
The cell apoptosis, ROS levels and cell cycle were detected using a
multicaspase kit, oxidative stress kit and cell cycle kit,
respectively (Muse™; Merck Millipore, Darmstadt, Germany). All
procedures were performed according to the manufacturer's
protocols. The Muse™ Cell Analyzer software (version 1.3) was used
for accurate statistical analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted with TRIzol reagent (Takara
Bio, Inc., Shiga, Japan). A 1 µg sample of RNA was reverse
transcribed using a PrimeScript™ RT reagent kit (Takara Bio, Inc.),
and the mixtures were incubated at 37°C for 15 min and 85°C for 5
sec. Subsequently, 1 µl (10 ng/µl) DNA was added to 5 µl SYBR Green
I, 0.5 µl (10 µM) forward primer, 0.5 µl (10 µM) reverse primer and
3 µl ddH2O, using the Universal qPCR kit (Kapa
Biosystems, Wilmington, MA, USA). The qPCR was performed on a
LightCycler® 96 sequence detection system (Roche Diagnostics,
Basel, Switzerland) according to the manufacturer's protocol. The
LightCycler® 96 application software version 1.1 was used for data
collection and analysis. Relative quantitative analysis of
interleukin (IL)-6, IL-8 and tumor necrosis factor-α (TNF-α) mRNAs
were performed using the 2−ΔΔCq method with
normalization to glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
mRNA (16). The system settings
were as follows: Preincubation at 95°C for 60 sec and amplification
at 72°C for 10 sec for 45 cycles, and melting at 97°C for 1 sec.
The primer sets were designed as shown in Table I.
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Primer | Sequence
(5′→3′) |
|---|
| GAPDH |
F:CCCGCTTCGCTCTCTGCTCC |
|
|
R:ACCAGGCGCCAATACGACC |
| IL-6 |
F:ACAGCCACTCACCTCTTCAG |
|
|
R:GAAGCATCCATCTTTTTCAGCCA |
| IL-8 |
F:GAGCTCTGTCTGGACCCCA |
|
|
R:TCTTCACTGATTCTTGGATACCA |
| TNF-α |
F:GGGACCTCTCTCTAATCAGCC |
|
|
R:GGTTTCGAAGTGGTGGTCTTG |
Western blot analysis
The cell proteins were extracted on ice using lysis
buffer solution (20 mM Tris HCl, 150 mM NaCl, 10% glycerol, 1%
NP-40, 2.1 g NaF, 1 mM PMSF, 1 mM Na3VO4, 10
µg/ml aptotin, 2 µg/ml leupeptin and 420 ml H2O)
containing phosphatase inhibitor (PhosSTOP; Merck Millipore).
Protein concentrations were measured using a bicinchoninic acid
assay kit (catalog no. C503021, Sangon Biotech Co., Ltd., Shanghai,
China). A total of 20 µg protein from each sample was loaded onto
10% gels and subjected to SDS-PAGE, prior to transfer onto a
nitrocellulose membrane for 75 min. The membrane was sealed with 5%
defatted milk for 1 h, incubated with primary antibodies at 4°C
overnight, and washed with 1X TBST for 10 min three times. The
membrane was then incubated with secondary antibodies for 1 h at
room temperature and washed with 1X TBST for 10 min three times.
The ECL reagent Immobilon™ Western (EMD Millipore, Billerica, MA,
USA) was added to the membranes for 1–3 min, and the
immunofluorescence reaction was observed using a western blot
luminescence imaging system (Tanon-5200; Tanon, Shanghai, China)
and image analysis software (Gel Image System Ver. 4.2.5; Tonon).
The antibodies used included tubulin α (cat. no. AF7010; Affinity
Biotech, Kansas, MO, USA, GAPDH (cat. no. 60004–1-Ig; Proteintech
Group, Inc., Wuhan, China), cyclin-dependent kinase 1 (CDK1; cat.
no. BM1028; Boster Biotech, Wuhan, China), cyclin B1 (cat. no.
BM0766; Boster Biotech) and cell division cycle 25C (CDC25C; cat.
no. BM2728; Boster Biotech). The primary antibodies were diluted
1:500 and an anti-mouse IgG horseradish peroxidase-conjugated
secondary antibody (catalog. no. 7076S) or an anti-rabbit IgG Alexa
Fluor® 555-conjugated secondary antibody (catalog no. 4413) from
Cell Signaling Technology, Inc., Danvers, MA, USA, were diluted
1:2,000.
Statistical analysis
Each experiment was performed in triplicate. All
analyses were performed using SPSS version 22.0 (IBM SPSS, Armonk,
NY, USA). The data are expressed as the mean ± standard deviation
and were statistically compared using Student's t-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Cell viability of RPE cells following
treatment with lutein and H2O2
The cytotoxicity of lutein was assessed using an MTT
assay for 3 days. Different concentrations of lutein were added to
the cultural media of RPE cells. Compared with the control group of
RPE cells cultured without treatment, the cell viability and
proliferation of the RPE cells remained unchanged with
concentrations of lutein up to 15 µM (Fig. 1A).
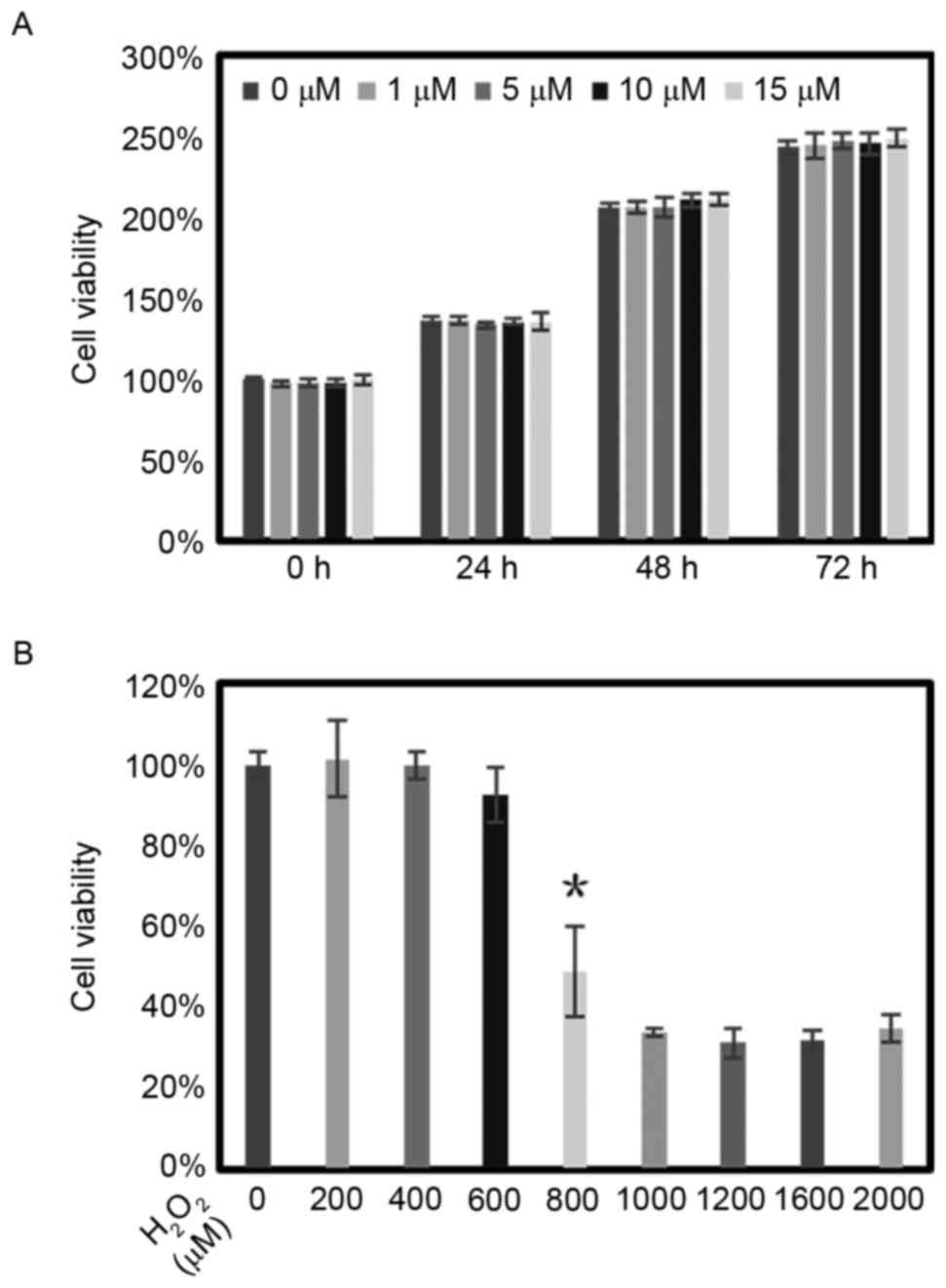 | Figure 1.Viabilities of RPE cells following
lutein and H2O2 treatment. (A) RPE cells were
treated with lutein (0, 1, 5, 10 and 15 µM) for 3 days and cell
viability was assessed using an MTT assay. (B) Dose-responses of
RPE cells to treatment with H2O2 (0–2,000 µM)
were detected using an MTT assay, with viabilities expressed as a
percentage of the control. *P<0.05 for cell viability reduction
by 50%, compared with the control at 24 h. The data are presented
as the mean ± standard deviation of results from six samples in
each group. RPE, retinal pigment epithelium; MTT,
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium-bromide. |
The RPE cells were treated with different
concentrations of H2O2 (0–2,000 µM) for 24 h.
Cell viability was also evaluated using an MTT assay. The results
revealed that the cell viability reduced to ~50% of that in the
control when the concentration of H2O2
reached 800 µM (Fig. 1B). Based on
this result, 800 µM was selected as the concentration for inducing
apoptosis and the production of ROS.
Lutein increases cell viability, and
decreases apoptosis and ROS in RPE cells exposed to
H2O2 stress
In the experiments, H2O2
reduced the cell viability of the RPE cells to 43.66% of the
control. Lutein reversed the reduction in cell viability in a
dose-dependent manner. When pretreated with lutein at
concentrations of 5, 10 and 15 µM, the cell viability of the RPE
cells was increased to 49.95, 65.39 and 74.32 of the control,
respectively (Fig. 2A).
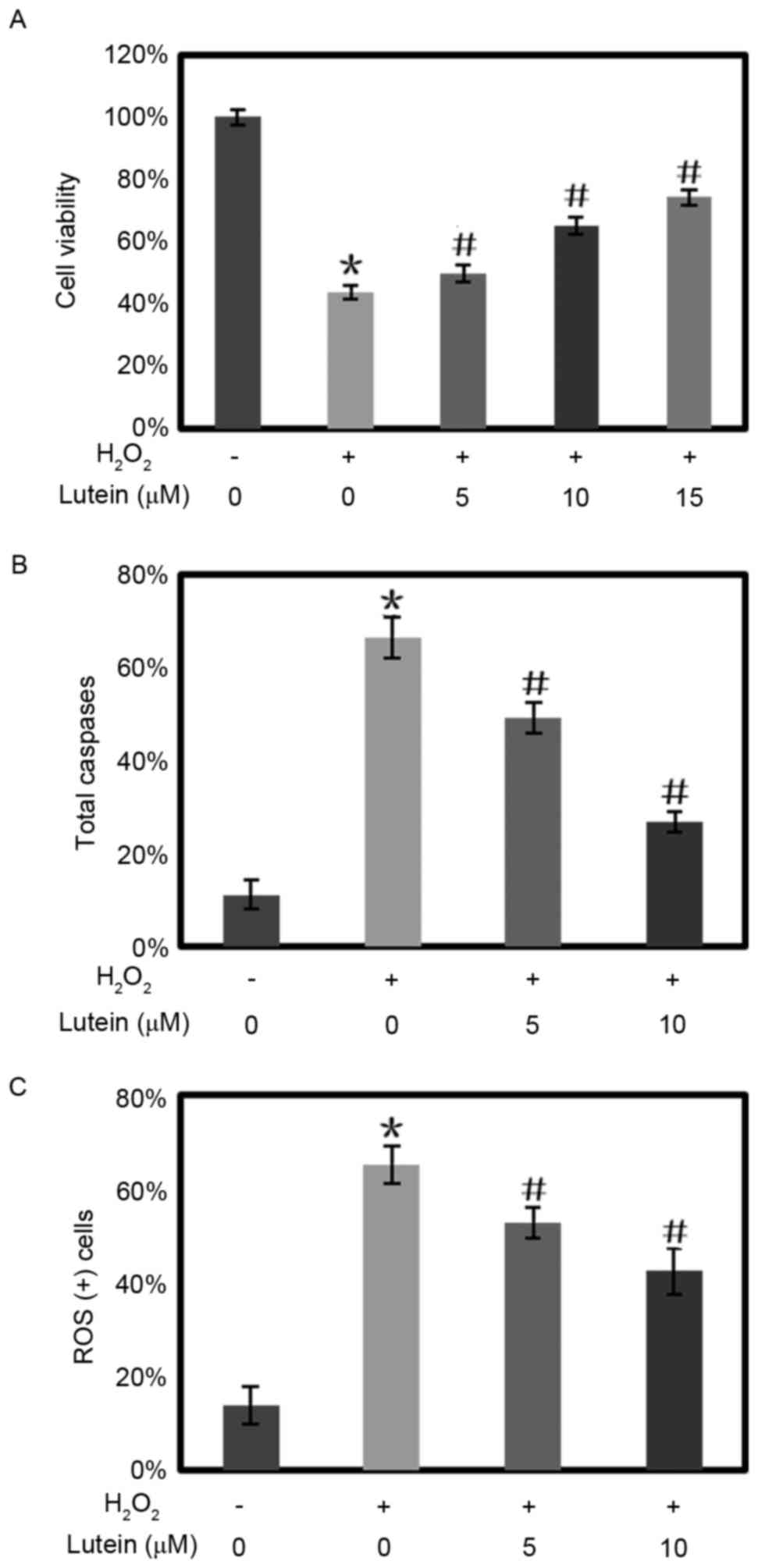 | Figure 2.Lutein protects RPE cells from cell
toxicity, cell apoptosis and intracellular ROS elevation induced by
H2O2. (A) Different doses of lutein were
added 12 h prior to treating the RPE cells with
H2O2. After 24 h, the cell viability was
quantified using a
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium-bromide
assay. RPE cells were treated with lutein (0, 5, 10 and 15 µM) for
12 h and then challenged with H2O2 (800 µM).
The levels of (B) caspases and (C) ROS were quantified using flow
cytometry. *P<0.05, vs. control; #P<0.05, vs.
cells treated with H2O2 only. The experiments
were repeated at least three times. RPE, retinal pigment
epithelium; ROS, reactive oxygen species. |
The expression levels of total caspases in the RPE
cells increased to 66.3% when the cells were exposed to
H2O2, compared with 11.1% in the control
group. Lutein inhibited the increased expression of total caspases
in a concentration-dependent manner. Following retreatment with
lutein at concentrations of 5 and 10 µM, the expression of total
caspases in RPE cells reduced to 49.3 and 26.9%, respectively.
(Fig. 2B).
In the RPE cells treated with
H2O2, the ROS levels increased to 65.21%,
compared with 10.76% in the control group. Lutein reversed the
elevation in ROS levels. The ROS levels reduced to 52.8 and 42.4%
when the RPE cells were pretreated with 5 and 10 µM lutein,
respectively (Fig. 2C).
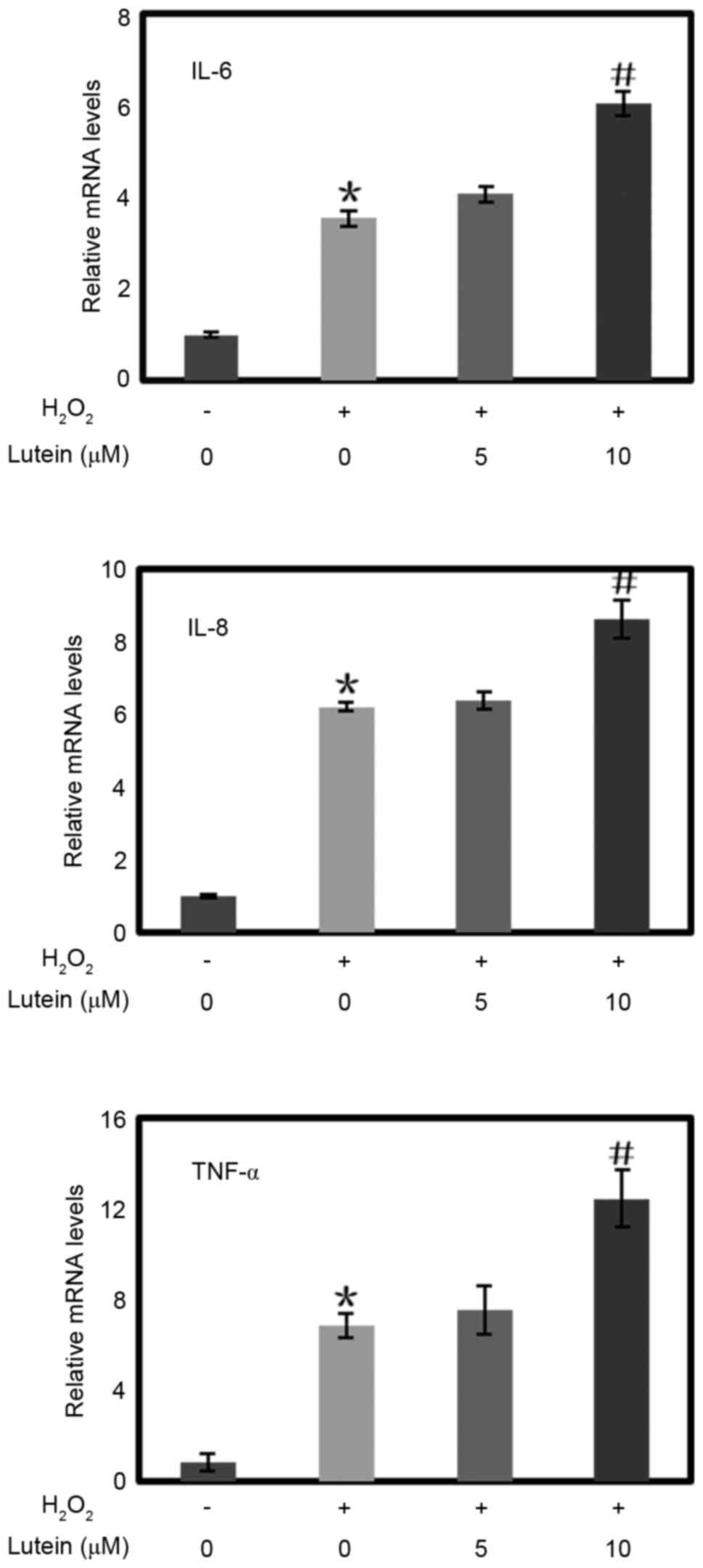
Lutein increases the expression of
IL-6, IL-8 and TNF-α inflammatory cytokines in RPE cells treated
with H2O2
In the present study, H2O2
markedly increased the expression levels of the IL-6, IL-8 and
TNF-α inflammatory cytokines in the RPE cells (Fig. 3). When the RPE cells were
pretreated with lutein at a concentration of 10 µM, the
transcription levels of these inflammatory cytokines were also
elevated, although pretreatment with lutein at a concentration of 5
µM did not alter the expression of these inflammatory
cytokines.
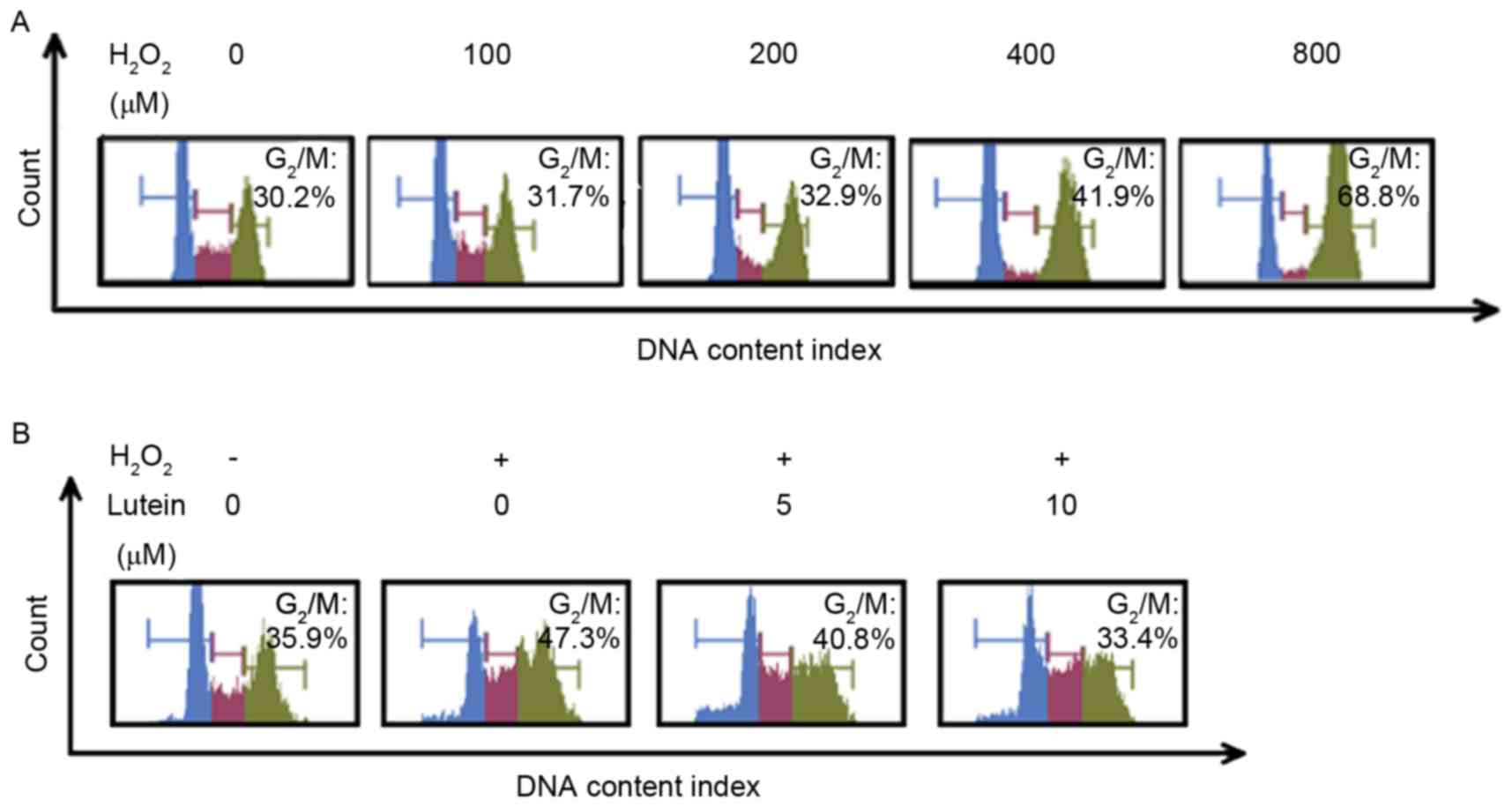
Lutein reduces RPE G2/M
phase arrest induced by H2O2
When the concentration of H2O2
reached 400 µM, cell cycle arrest of the RPE cells was observed in
the G2/M phase. (Fig.
4A). It was found that, in the RPE cells treated with 600 µM
H2O2, the proportion of cells in the
G2/M phase was 47.3%, compared with 35.9% in the control
group. Lutein reversed the increased proportion of cells in the
G2/M phase in a concentration-dependent manner. When the
cells were pretreated with 5 and 10 µM lutein, the proportions of
RPE cells in the G2/M phase were reduced to 40.8 and
33.4%, respectively (Figs. 4B and
5A).
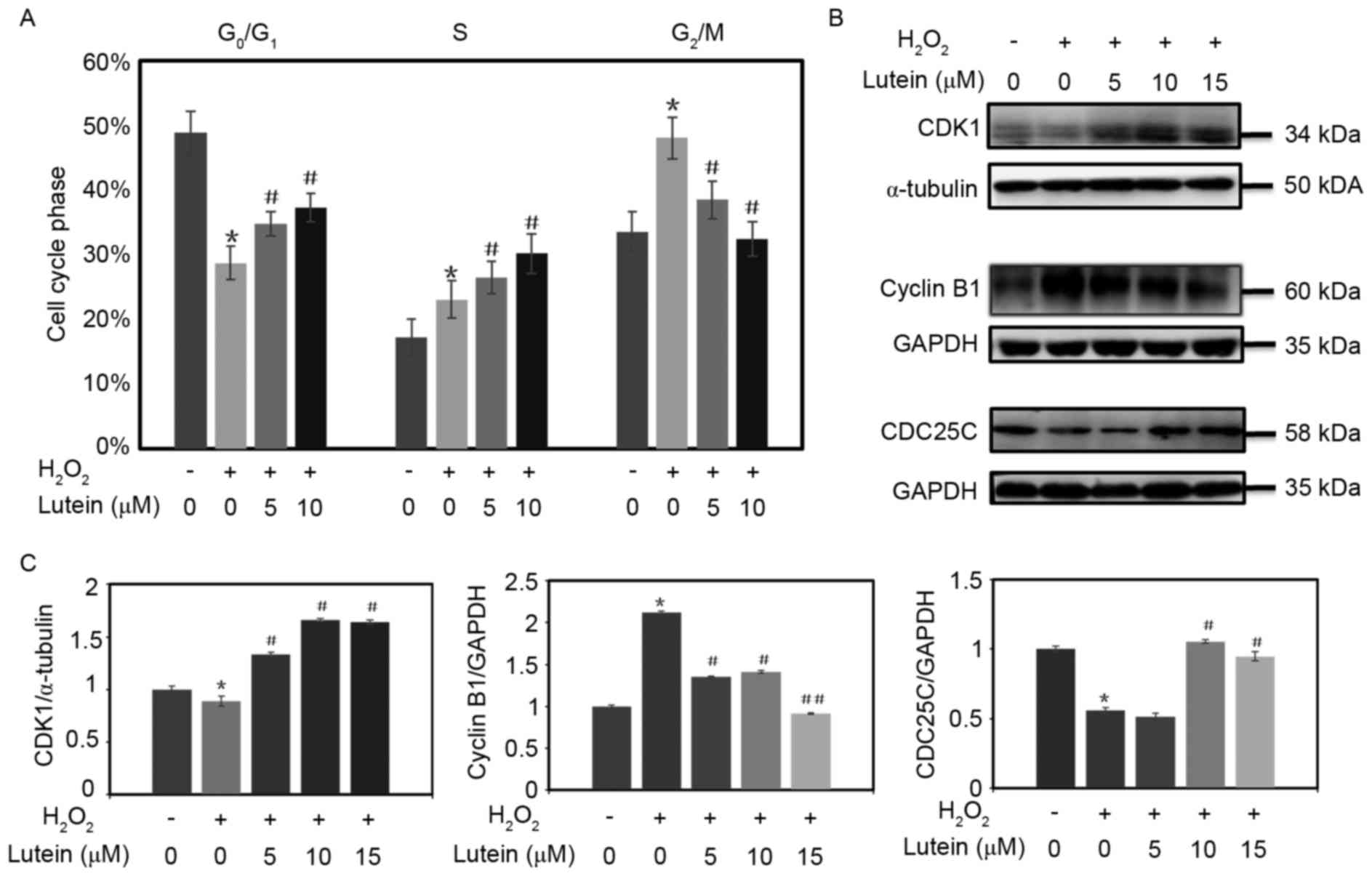 | Figure 5.Lutein attenuates the G2/M
phase arrest induced by oxidative stress. (A) RPE cells were
pretreated with lutein (0, 5, 10 and 15 µM) for 24 h and then
challenged with or without H2O2 for 24 h. A
histogram of the cell cycle phases of the RPE cells is shown. (B)
Expression levels of CDK1, CDC25C and cyclin B1 were determined
using western blot analysis; α-tubulin and GAPDH were used as
internal controls. (C) Densitometric analyses of the protein
expression levels of CDK1, cyclin B1 and CDC25C from the western
blots are shown. *P<0.05, vs. control; #P<0.05 and
##P<0.01, vs. cells treated with
H2O2 only. Analysis was repeated at least
three times. RPE, retinal pigment epithelium; CDK2,
cyclin-dependent kinase 1; CDC25C, cell division cycle 25C; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase. |
Lutein attenuates RPE cell cycle
arrest in the G2/M phase by activating CDK1 and CDC25C,
and decreasing cyclin B1
When the RPE cells were treated with
H2O2, the expression levels of CDK1 and
CDC25C were inhibited, and the protein expression of of cyclin B1
was increased in the cells. However, the inactivation of CDK1 and
CDC25C, and increase of cyclin B1 were attenuated when lutein was
added to the cells (Fig. 5B and
C).
Discussion
In the present study, it was demonstrated that the
oxidative stress triggered by H2O2 decreased
cell viability, increased intracellular ROS and increased apoptosis
in RPE cells. It was noted that marked G2/M phase arrest
occurred in the RPE cells when subjected to
H2O2 and for the first time, to the best of
our knowledge, it was found that lutein attenuated this
G2/M arrest in a concentration-dependent manner.
Lutein is present at a high concentration in the
macula of the eye (17). It
contains several double bonds, which react with ROS to scavenge
free radicals (1). Lutein
functions as a cytoprotective antioxidant in a direct
anti-apoptotic or indirect anti-oxidation manner (12,18).
In addition, the reversal of G2/M phase arrest observed
in oxidative stressed cells induced by lutein contribute to its
role in cell protection.
When DNA is damaged, the G2 checkpoint
inhibits cells entering mitosis. The cell cycle arrest provides an
opportunity for repair and inhibits proliferation of the damaged
cells (19). In Hl299 cells, DNA
damage and G2/M phase arrest were found to be induced by
oxidative damage, whereas an antioxidant in red seaweed
Gracilaria tenuistipitata protected the cells from DNA
damage and G2/M arrest (20). The results of the present study
demonstrated that lutein protected cell viability and reversed
G2/M arrest of RPE cells under oxidative stress.
Cell cycle progression is regulated by various
factors, including CDKs and cyclins. The cyclin B1/CDK1 complex
regulates cell cycle progression from the G2 to M phase,
and cyclins accumulate steadily during the G2 phase and
are rapidly eliminated as cells exit mitosis. The activation of
CDK1 kinase is an ordered process, which triggers the initiation of
mitosis. CDC25 is also a key regulator, which activates CDK1 and
drives cell cycle progression (21). In the present study, when the RPE
cells were subjected to oxidative stress, a significant increase in
cyclin B1 and deceases in CDK1 and CDC25C were observed, which
suggested that cell cycle progression was inhibited prior to
entering the mitosis phase. This suggestion was confirmed by the
analysis of cell cycle using flow cytometry, as RPE cells in the
G2/M phase increased when exposed to
H2O2. However, lutein protected the RPE cells
from G2/M phase arrest by degrading the cyclin B1
protein, and increasing the activities of CDK1 and CDC25C in a
concentration-dependent manner. As the results of the flow
cytometry indicated, fewer RPE cells were arrested in the
G2/M phase when treated with lutein.
Increasing evidence has indicated the role of
inflammation in the pathogenesis of AMD. Inflammatory proteins make
up the composition of drusen in AMD, and RPE cells are a rich
resource of inflammatory cytokines (11,22,23).
Lutein prevents the proteasome from inactivation by photo-oxidative
damage and alters the expression of the inflammatory-associated
genes, monocyte chemoattractant protein-1, IL-8 and complement
factor H in RPE cells (11).
Lutein also exerts an anti-inflammatory effect in the
ischemic/hypoxic retina by reducing the expression of IL-1β and
cyclooxygenase 2 in rMC-1 cells (24). The present study demonstrated that
H2O2 treatment upregulated the expression of
the inflammation-associated genes, IL-6, IL-8 and TNF-α. At
concentrations >10 µM, lutein increased the expression levels of
IL-6, IL-8 and TNF-α. These results improve current understanding
of the effect of lutein on inflammation and indicated the potential
cytotoxic effect of lutein; therefore, the use of large
concentrations of lutein requires caution (25).
In conclusion, the present study demonstrated that
lutein protected RPE cells from oxidative damage, and reversed
G2/M phase arrest through activating CDK1 and CDC25C,
and degrading the protein expression of cyclin B1. As AMD is a
disease prevailing worldwide and a socioeconomic burden requiring
resolution, dietary lutein supplementation may offer a suitable
measure for preventing AMD.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81270914 and
81670874) to H.X.S.
References
|
1
|
Koushan K, Rusovici R, Li W, Ferguson LR
and Chalam KV: The role of lutein in eye-related disease.
Nutrients. 5:1823–1839. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Holz FG, Schmitz-Valckenberg S and
Fleckenstein M: Recent developments in the treatment of age-related
macular degeneration. J Clin Invest. 124:1430–1438. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wenzel A, Grimm C, Samardzija M and Remé
CE: Molecular mechanisms of light-induced photoreceptor apoptosis
and neuroprotection for retinal degeneration. Prog Retin Eye Res.
24:275–306. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Richer S, Stiles W, Statkute L, Pulido J,
Frankowski J, Rudy D, Pei K, Tsipursky M and Nyland J:
Double-masked, placebo-controlled, randomized trial of lutein and
antioxidant supplementation in the intervention of atrophic
age-related macular degeneration: The Veterans LAST study (Lutein
Antioxidant Supplementation Trial). Optometry. 75:216–230. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seddon JM, Ajani UA, Sperduto RD, Hiller
R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller
DT, et al: Dietary carotenoids, vitamins A, C, and E, and advanced
age-related macular degeneration. Eye Disease Case-Control Study
Group. JAMA. 272:1413–1420. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zarbin MA: Current concepts in the
pathogenesis of age-related macular degeneration. Arch Ophthalmol.
122:598–614. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cai J, Nelson KC, Wu M, Sternberg P Jr and
Jones DP: Oxidative damage and protection of the RPE. Prog Retin
Eye Res. 19:205–221. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang FQ and Godley BF: Oxidative
stress-induced mitochondrial DNA damage in human retinal pigment
epithelial cells: A possible mechanism for RPE aging and
age-related macular degeneration. Exp Eye Res. 76:397–403. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang KH, Lemke G and Kim JW: The PI3K-PTEN
tug-of-war, oxidative stress and retinal degeneration. Trends Mol
Med. 15:191–198. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tan JS, Wang JJ, Flood V, Rochtchina E,
Smith W and Mitchell P: Dietary antioxidants and the long-term
incidence of age-related macular degeneration: The Blue Mountains
Eye Study. Ophthalmology. 115:334–341. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bian Q, Gao S, Zhou J, Qin J, Taylor A,
Johnson EJ, Tang G, Sparrow JR, Gierhart D and Shang F: Lutein and
zeaxanthin supplementation reduces photooxidative damage and
modulates the expression of inflammation-related genes in retinal
pigment epithelial cells. Free Radic Biol Med. 53:1298–1307. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Silvan JM, Reguero M and de Pascual-Teresa
S: A protective effect of anthocyanins and xanthophylls on
UVB-induced damage in retinal pigment epithelial cells. Food Funct.
7:1067–1076. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sommerburg O, Keunen JE, Bird AC and van
Kuijk FJ: Fruits and vegetables that are sources for lutein and
zeaxanthin: The macular pigment in human eyes. Br J Ophthalmol.
82:907–910. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Obana A, Hiramitsu T, Gohto Y, Ohira A,
Mizuno S, Hirano T, Bernstein PS, Fujii H, Iseki K, Tanito M and
Hotta Y: Macular carotenoid levels of normal subjects and
age-related maculopathy patients in a Japanese population.
Ophthalmology. 115:147–157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Age-Related Eye Disease Study 2 Research
Group, . Lutein + zeaxanthin and omega-3 fatty acids for
age-related macular degeneration: The age-related eye disease study
2 (AREDS2) randomized clinical trial. JAMA. 309:2005–2015. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abdel-AAl el SM, Akhtar H, Zaheer K and
Ali R: Dietary sources of lutein and zeaxanthin carotenoids and
their role in eye health. Nutrients. 5:1169–1185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aimjongjun S, Sutheerawattananonda M and
Limpeanchob N: Silk lutein extract and its combination with vitamin
E reduce UVB-mediated oxidative damage to retinal pigment
epithelial cells. J Photochem Photobiol B. 124:34–41. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stark GR and Taylor WR: Control of the
G2/M transition. Mol Biotechnol. 32:227–248. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang JI, Yeh CC, Lee JC, Yi SC, Huang HW,
Tseng CN and Chang HW: Aqueous extracts of the edible Gracilaria
tenuistipitata are protective against H2O2-induced DNA damage,
growth inhibition and cell cycle arrest. Molecules. 17:7241–7254.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li L, Gu B, Zhou F, Chi J, Wang F, Peng G,
Xie F, Qing J, Feng D, Lu S and Yao K: Human herpesvirus 6
suppresses T cell proliferation through induction of cell cycle
arrest in infected cells in the G2/M phase. J Virol. 85:6774–6783.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Anderson DH, Radeke MJ, Gallo NB, Chapin
EA, Johnson PT, Curletti CR, Hancox LS, Hu J, Ebright JN, Malek G,
et al: The pivotal role of the complement system in aging and
age-related macular degeneration: Hypothesis re-visited. Prog Retin
Eye Res. 29:95–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cherepanoff S, McMenamin P, Gillies MC,
Kettle E and Sarks SH: Bruch's membrane and choroidal macrophages
in early and advanced age-related macular degeneration. Br J
Ophthalmol. 94:918–925. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li SY, Fung FK, Fu ZJ, Wong D, Chan HH and
Lo AC: Anti-inflammatory effects of lutein in retinal
ischemic/hypoxic injury: In vivo and in vitro studies. Invest
Ophthalmol Vis Sci. 53:5976–5984. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Murthy RK, Ravi K, Balaiya S, Brar VS and
Chalam KV: Lutein protects retinal pigment epithelium from
cytotoxic oxidative stress. Cutan Ocul Toxicol. 33:132–137. 2014.
View Article : Google Scholar : PubMed/NCBI
|