Introduction
Cardiovascular disease (CVD) is the leading cause of
mortality worldwide and according to the 2014 statistics, an
estimated 17.5 million non-communicable disease deaths have been
caused by CVD (1,2). Myocardial remodeling in
post-myocardial infarction is one of the pathophysiological bases
of CVD and eventually results in many serious complications.
Myocardial remodeling refers to changes in myocardial structure,
function and phenotype caused by a series of complex molecular and
cellular mechanisms. The renin-angiotensin system (RAS) is an
important mediator of atherosclerosis, hypertension (HT),
myocardial fibrosis, left ventricular (LV) hypertrophy, myocardial
infarction and congestive heart failure (3–5).
The classic RAS can be defined as the
angiotensin-converting enzyme (ACE)-Ang II-AT1R axis that promotes
vasoconstriction, water intake, sodium retention and other
mechanisms, as well as increase oxidative stress, fibrosis,
cellular growth and inflammation in pathological conditions
(6). Numerous studies have
indicated that the noncanonical RAS pathway
ACE2-angiotensin-(1–7) [Ang (1–7)]-Mas
receptor (MasR) axis counteracts the adverse effects of the
classical pathway (6–8). ACE2 is a pleiotropic mono-carboxy
peptidase, and belongs to the family of zinc metalloproteases, that
can be found in a soluble form in plasma and tissues ubiquitously
(9). ACE2 is involved in the
structural and functional regulation of human heart. ACE2
overexpression significantly prevents myocardial fibrosis, reduces
the development of LV hypertrophy (LVH) and improves cardiac
function (10–13). Ang (1–7), one
of the major enzymatic products of ACE2, can reduce Ang II-induced
cardiac hypertrophy and remodeling and pressure-overload-induced
heart failure (6,7,14).
The cardioprotective effects of Ang (1–7) are
mediated by MasR through different signaling pathways (15,16).
Therefore, the ACE2-Ang (1–7)-MasR
axis, known as the second metabolic axis of RAS, serves a pivotal
role in cardiovascular disease.
To elaborate the expression of the ACE2-Ang
(1–7)-Mas axis in animals of ventricular
remodeling in post-MI, the authors established a rat model of
myocardial infarction. The expression of Ang II remarkably
increased following development of myocardial infarction. In
addition, Tel and Olm inhibited local level of myocardial Ang II,
reduced myocardial collagen deposition and improved myocardial
remodeling by upregulating the expression of ACE2, Ang (1–7) and
MasR. The effect of Olm was greater that the effect of Tel. Thus,
the ACE2-Ang (1–7)-Mas axis serve an important role in the
development of ventricular remodeling in post-MI, which may provide
a new target for CVD drug design and therapeutic strategy.
Materials and methods
Experimental animals and
treatment
A total of 40 male Sprague-Dawley (SD) rats (weight
200±20 g, 2-months-old) were purchased from the Experimental Animal
Center of Xinjiang Medicine University (Urumqi, China).
Experimental protocols for animal studies were performed based on
the ‘Guide for the Care and Use of Laboratory Animals’ and have
been approved by the Institutional Animal Care and Use Committee at
the Xinjiang Medicine University (Ethical review number:
IACUC-20121127010). Male SD rats had free access to food and water,
and were maintained in a temperature (25°C) controlled room with a
12-h dark/light cycle. Male SD rats were randomly divided into a
sham group (n=10), a myocardial infarction (MI) group (n=10), Tel
group (5 mg/kg/d; n=10) and Olm group (5 mg/kg/d; n=10). In the Tel
and Olm groups, intragastric administration of Tel and Olm were
performed daily (15:00-16:00 pm) for 4 weeks following surgery. All
rats in which the induction of remodeling had been successful were
identified in each group.
The establishment myocardial
infarction model
Rats were fasted for 6 h before the operation, and
the rats' body weight was measured. Following full anesthesia
through the intraperitoneal injection of 10% chloral hydrate
(100–200 mg/kg), intubated and placed on a standard rodent
ventilator. The adequacy of anesthesia was verified using tail
pinch. An incision was made between the 3rd and 4th intercostal
space, and a rib spreader was used to allow visualization of the
heart. A 7–0 suture was used to ligate the left coronary artery at
a location approximately 1–2 mm distal to the left atrium.
Successful occlusion of the left anterior descending branch (LAD)
was confirmed by elevation of the ST segment on the
electrocardiogram and cyanosis of anterior LV wall. Immediately
before or after surgery, morphine (0.1 mg/kg) was administered
intraperitoneally to reduce pain and penicillin (4×104
U/rat) was administered intramuscularly for three days to prevent
infection. The mortality of the animals operated on in this fashion
was 33.7% within 24 h.
The echocardiographic examination
Transthoracic echocardiography was performed
noninvasively to assess systolic and diastolic function, as
described previously using a Doppler ultrasound instrument with a
12-Hz transducer (Philips 21380A Ultra Band XDCR S12) M-mode
echocardiography was performed in the parasternal short-axis view
at the level of the papillary muscles. Mice were placed on a
heating pad and a nose cone with 1.5% isoflurane in 100% oxygen was
applied. Ultrasound gel was placed on the chest of the anesthetized
mouse. End-diastolic interventricular septal thickness,
end-diastolic left ventricular posterior wall thickness, left
ventricular end-diastolic diameter, left ventricular ejection
fraction were measured and recorded. All measurements were based on
the average of three consecutive cardiac cycles.
Histological analysis
Left ventricular myocardial specimens were taken
from each group and sectioned transversely parallel to the coronary
sulcus. Masson staining was used to observe the degree of
myocardial fibrosis; myocardial cells were red and collagen was
blue. Five sections were selected for each specimen, and four views
were taken for each section (the selected views were away from the
papillary muscle and perivascular collagen). The Image-Pro Plus
software (Media Cybernetics, Inc., Rockville, MD, USA) was used to
calculate the myocardial collagen volume fraction.
ELISA
An ELISA double-antibody sandwich assay kit for Ang
(1–7) was purchased from Cloud-Clone Corp
Company (cat. no. CES085Ra; Katy, TX, USA). According to the kit
instructions, the reaction system and standard curve were
established. The absorbance of each sample was measured by
enzyme-linked immunosorbent analyzer. The content of Ang (1–7) was
calculated in accordance with the standard curve.
Immunohistochemical determination of
myocardial ACE2, Ang (1–7), MasR and Ang II
4% paraformaldehyde-fixed, paraffin-embedded cardiac
tissue sections were used for immunohistochemical staining.
Following deparaffinization and hydration, the slides were washed
in PBS. Endogenous peroxidase activity was quenched by incubating
the slides in 3% H2O2 for 10 min. For antigen
retrieval, the sections were treated with sodium citrate buffer for
20 min at 37°C. Following overnight incubation with the primary
antibodies [rabbit polyclonal anti-ACE2 antibody (cat. no. ab15348;
Abcam, Cambridge, MA, USA; dilution, 1:200); rabbit polyclonal
anti-Ang (1–7) antibody (cat. no. PAS085Ra01;
Cloud-Clone Corp; dilution, 1:200); rabbit polyclonal anti-Ang II
antibody (cat. no. 15348; Abcam; dilution, 1:200); rabbit
polyclonal anti-Mas receptor antibody (cat. no. sc-135063; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA; dilution, 1:200)] at
4°C, the slides were washed in PBS, then a goat anti-rabbit
horseradish peroxidase-conjugated secondary antibody (cat. no.
sc-2007; Santa Cruz Biotechnology, Inc.; dilution, 1:1,000) was
added, and the slides were further incubated at room temperature
for 30 min. The color was developed with 0.05%
3,3′-diaminobenzidine tetrahydrochloride and 0.01%
H2O2, and counterstained with hematoxylin. A
negative control without primary antibody was included in the
experiment to ensure the antibody specificity. Myocardial
immunoreactivity for ACE2, Ang (1–7), Mas
receptor and Ang II was performed in 20 randomly selected fields of
heart sections at ×400 magnification by light microscopy.
RNA preparation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
Total RNA was prepared from rat cardiac tissue using TRIzol reagent
(Roche Diagnostics, Basel, Switzerland). A total of 1 µg RNA was
reverse transcribed using the cDNA Synthesis kit (THUNDERBIRD SYBR
qPCR Mix, Toyobo Co., Ltd., Osaka, Japan). The primers used in the
present study were as follows: ACE2, MasR, Ang II (Shanghai Sangon
Pharmaceutical Co., Ltd., Shanghai, China). The primer sequences
are listed in Table I. RT-qPCR
assays was performed using a miScript SYBR® Green PCR kit (Qiagen
GmbH, Hilden, Germany) and a ABI 7500 Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
according to the manufacturer's instructions. PCR reactions were
performed at 95°C for 10 min, followed by 40 cycles of 95°C for 15
sec and 60°C for 1 min. Densitometric analysis of PCR was performed
using the Digital Imaging System (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The use of equal amounts of mRNA in the RT-qPCR
assays was confirmed by analyzing the expression levels of
house-keeping gene β-actin. The results were calculated using the
2∆∆Cq method (17).
 | Table I.Specific primers for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Specific primers for reverse
transcription-quantitative polymerase chain reaction.
| Gene name | Primer sequence
(forward) | Primer sequence
(reverse) |
|---|
| Ang II |
5′-TTGGGTGCTGAGGCAAATCT-3′ |
5′-TTGGGTGCTGAGGCAAATCT-3′ |
| ACE2 |
5′-GTGGAGCACTGACTGGAGC-3′ |
5′-GACAGGAGGCTCGTAAGGTG-3′ |
| MasR |
5′-TGACAGCCATCAGTGTGGAGA-3′ |
5′-GCATGAAAGTGCCCACAGGA-3′ |
| β-actin |
5′-CCCTGTGCTGCTCACCGA-3′ |
5′-ACAGTGTGGGTGACCCCGTC-3′ |
Western blot analysis
Myocardial protein expression was detected by
western blotting. The bicinchoninic acid (BCA) method was used to
detect protein concentration using an Enhanced BCA Protein Assay
kit (Beyotime Institute of Biotechnology, Haimen, China), according
to the manufacturer's instructions. Heart protein samples (50 µg)
were subjected to 10% SDS-PAGE, transferred to nitrocellulose
membrane, and immunoblotted using ACE2 antibodies (Abcam; dilution,
1:1,000), MasR antibodies (Santa Cruz Biotechnology, Inc.; 1:1,000)
and Ang II antibodies (Abcam; dilution, 1:1,000), Collagen I
antibodies (Abcam; dilution, 1:1,000), Collagen III antibodies
(Abcam; dilution, 1:2,000), β-actin (Abcam; dilution, 1:1,000) was
used as loading control to determine the relative expression levels
of the target protein in the sample. Western blotting bands of the
target protein were analyzed; the results are presented as the
ratio of integrated optical density, and statistical analysis was
conducted.
Statistical analysis
Experimental data are shown as mean ± standard
deviation. Main and interactive effects were analyzed by one-way
analysis of variance (ANOVA) using SPSS software (version, 22.0;
IBM SPSS, Armonk, NY, USA). When justified by one-way ANOVA,
differences between individual group means were analyzed by
Fisher's LSD test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Cardiac remodeling and left ventricle
dysfunction in post-myocardial infarction
Compared with the rats in the sham group, the left
ventricular remodeling progression increased [left ventricular mass
(LVM), left ventricular internal end-systolic diameter (LVIDs),
left ventricular internal end-diastolic diameter indicators
increased (LVIDd); P<0.05], and systolic function abnormalities
started to appear [left ventricular ejection fraction (EF)
decreased; P<0.05]. The left ventricular mass, the systolic
function abnormalities, the left ventricular internal end-systolic
diameter and left ventricular internal end-diastolic diameter are
improved in the Tel and Olm treated groups when compared with the
MI group (P<0.05), however, there is no significant difference
between the two groups. However, Olm could not completely improve
the cardiac function state and restore it to normal values
(compared with the sham group on LVIDs, LVIDd, LVM and EF,
P<0.05; Table II).
 | Table II.Cardiac function test of
post-myocardial infarction rats. |
Table II.
Cardiac function test of
post-myocardial infarction rats.
|
| Sham (n=10) | MI (n=10) | Tel (n=10) | Olm (n=10) |
|---|
| BW (g) | 339.98±21.61 | 328.54±12.63 | 342.45±25.10 | 336.05±18.91 |
| HW (mg) | 1.00±0.23 |
1.25±0.12a |
1.12±0.09a,b |
1.12±0.08a,b |
| LVM (mg) | 0.81±0.34 |
1.08±0.10a |
0.96±0.08a,b |
0.99±0.05a,b |
| LV/BW (mg/g) | 2.38±0.17 |
3.28±0.33a |
2.82±0.35a,b |
2.95±0.18a,b |
| LVIDs (mm) | 3.47±0.82 |
6.38±0.66a |
5.01±0.78a,b |
4.49±0.79a,b |
| LVIDd (mm) | 6.11±0.38 |
8.34±0.78a |
7.49±0.61a,b |
7.09±0.45a,b |
| EF (%) | 84.27±4.69 |
52.14±5.47a |
57.98±6.04a,b |
65.78±7.79a,b,c |
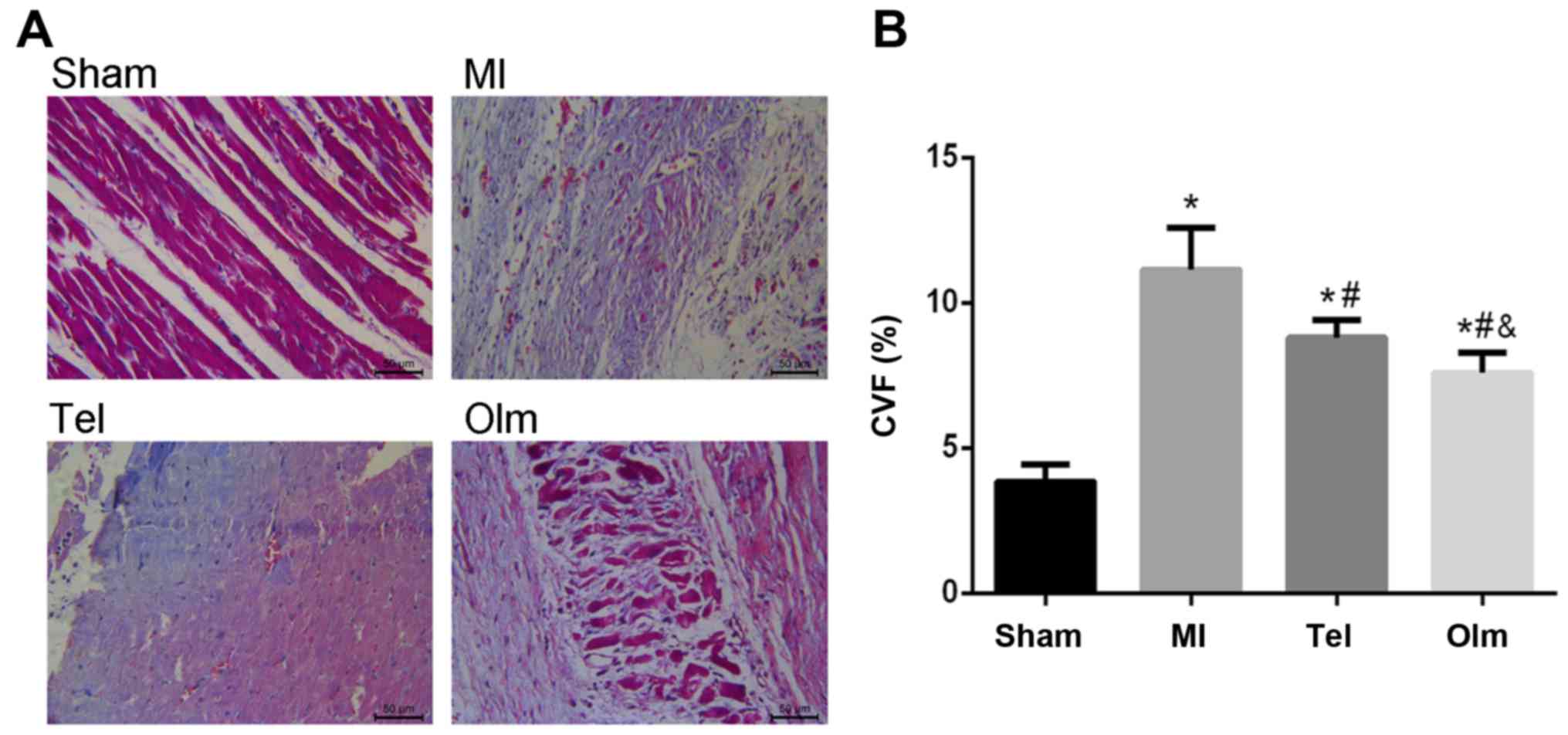
Collagen deposition in post-myocardial
infarction
Collagen deposition leads to myocardial fibrosis,
which can result in myocardial remodeling. The degree of collagen
deposition in the post-myocardial infarction rats could be
identified using Masson staining (Fig.
1A). Normal myocardial tissue was red, and the blue fibrin
component appeared in the myocardial interstitium in myocardial
infarction group. Collagen deposition induced myocardial
interstitial fibrosis could limit myocardial diastolic function.
The volume fraction of myocardial collagen was calculated using
Image-Pro Plus software. The collagen volume fraction of the MI
group increased significantly compared with that of the sham group
[Sham (3.85±0.60); MI (11.14±1.48), P<0.05; Fig. 1B]. However, Tel and Olm
significantly reduced the degree of myocardial fibrosis in
post-myocardial infarction rats; the effect of Olm is more marked
[Tel (8.81±0.6); Olm (7.6±0.69), P<0.05; Fig. 1B].
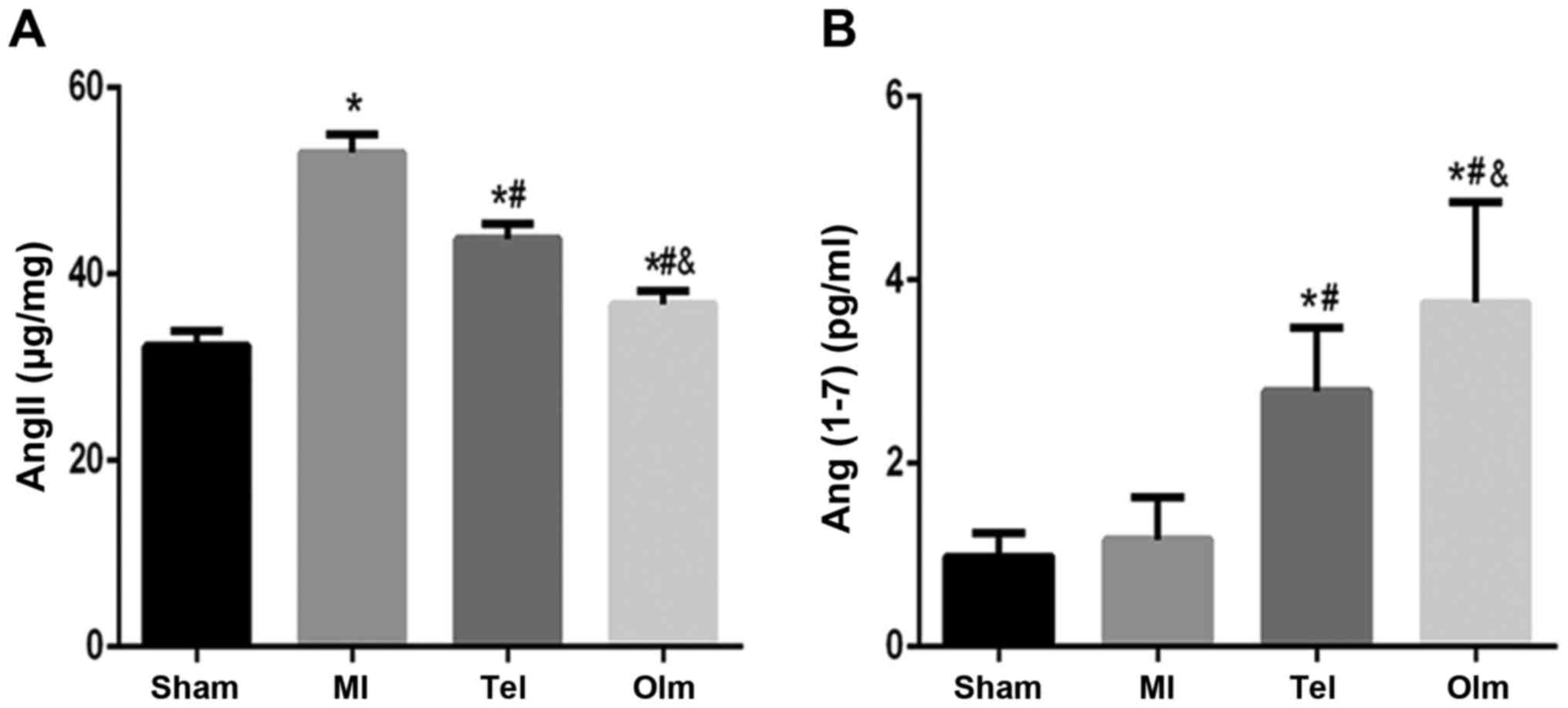
Ang (1–7) and
Ang II level
The expression of Ang II in the plasma of the MI
group increased compared with that of the sham group (P<0.05;
Fig. 2A), meanwhile the
expressions of Ang (1–7) increased (P>0.05; Fig. 2B). Following intervention with Tel
and Olm, compared with the MI group, the content of plasma Ang
(1–7) significantly increased in the Tel
group (P<0.05; Fig. 2B),
whereas Ang II expression decreased in the Tel group (P<0.05;
Fig. 2A), and a more obvious
effect was observed in the Olm group (P<0.01; Fig. 2A and B).
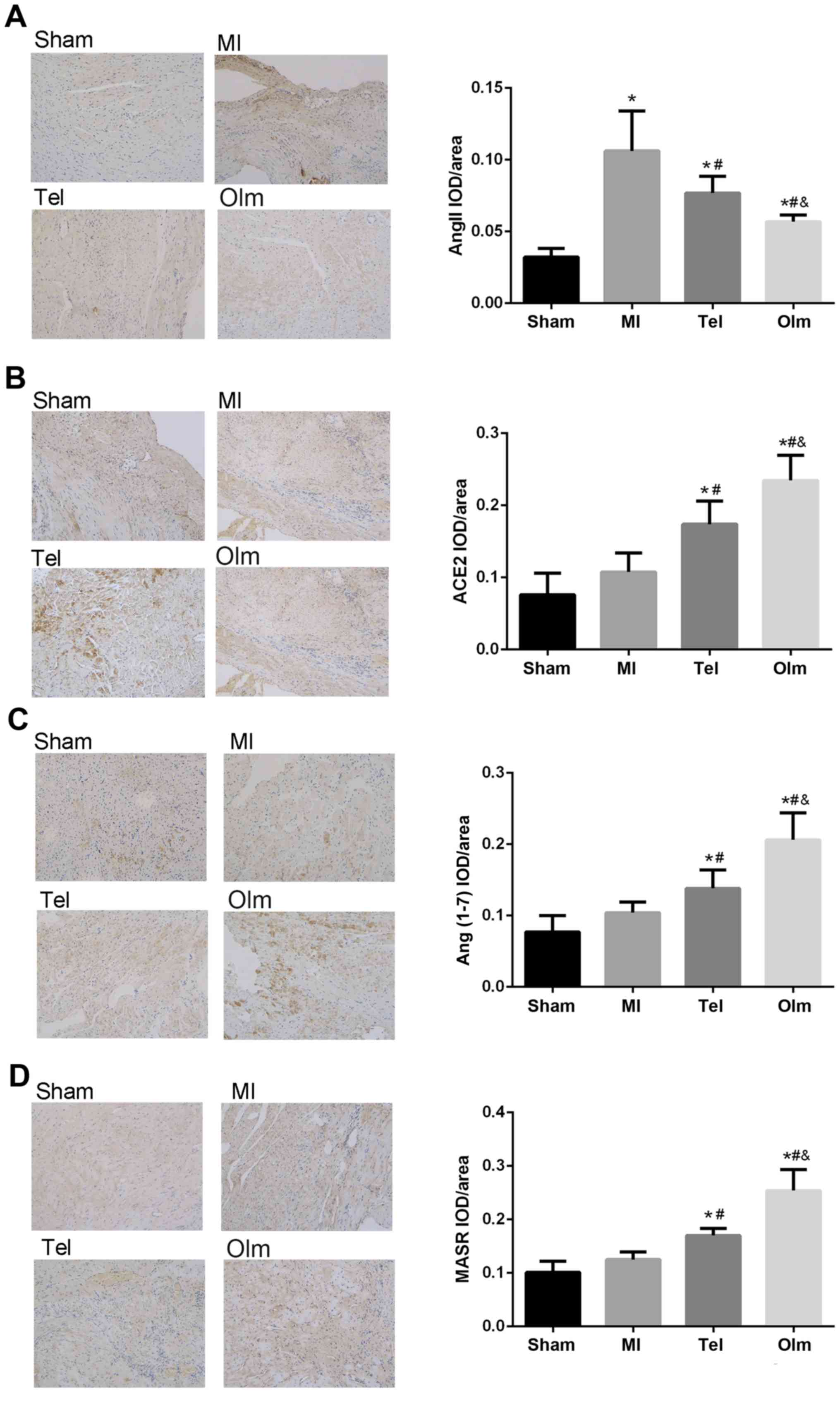
Immunohistochemical determination of
myocardial ACE-2, Ang (1–7), MasR and Ang II
In sham rats, the expressions of ACE2, Ang (1–7),
MasR and Ang II occurred in cardiomyocytes (Fig. 3A-D). Myocardial infarction
increased all immune staining simultaneously, while the expressions
of Ang II increased significantly (P<0.05). Following
intervention with Tel or Olm, compared with the MI group, the
expression of AngIIdecreased, conversely ACE2, Ang (1–7) and
MasR expression increased (P<0.05), and the effect of the Olm
group is more marked than that of the Tel group (P<0.05).
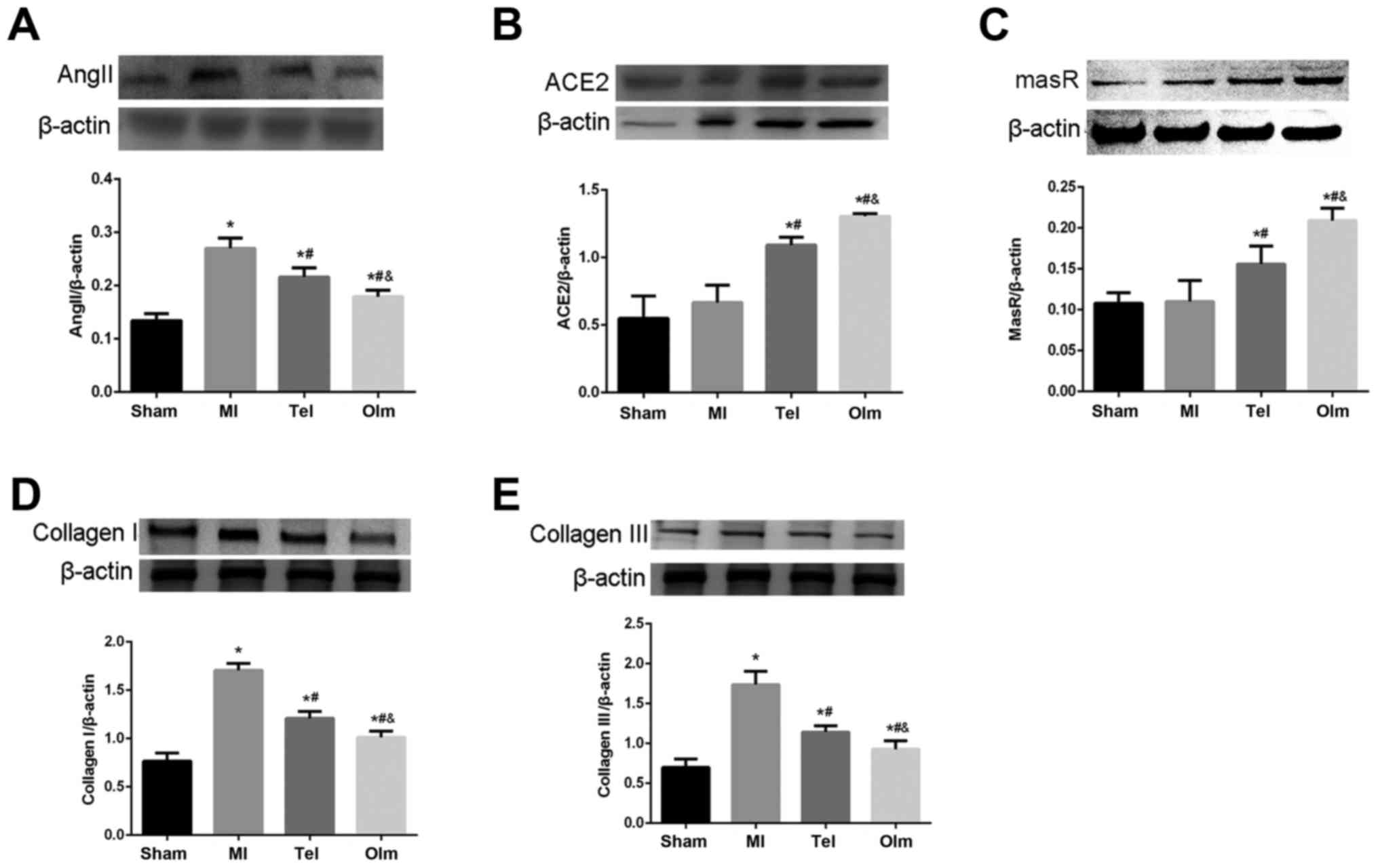
Protein expression of ACE2, MasR, Ang
II, Collagen I and CollagenIII in myocardial tissue
To investigate the protein expression of ACE2, MasR,
Ang II, Collagen I and Collagen III, the authors extracted the
total protein from myocardial tissue. Consistent with mRNA level,
Ang II, Collagen I and Collagen III protein expression
significantly increased in the MI group compared with sham group
following surgery for 4 weeks (P<0.05; Fig. 4A, D and E). ACE2 and MasR protein
expression increased slightly in the MI group (Fig. 4B and C). Following treatment with
Tel and Olm, Ang II, Collagen I and Collagen III protein expression
notably decreased, meanwhile, ACE2 and MasR protein expression
increased, and more obviously in the Olm group (P<0.05; Fig. 4A-E).
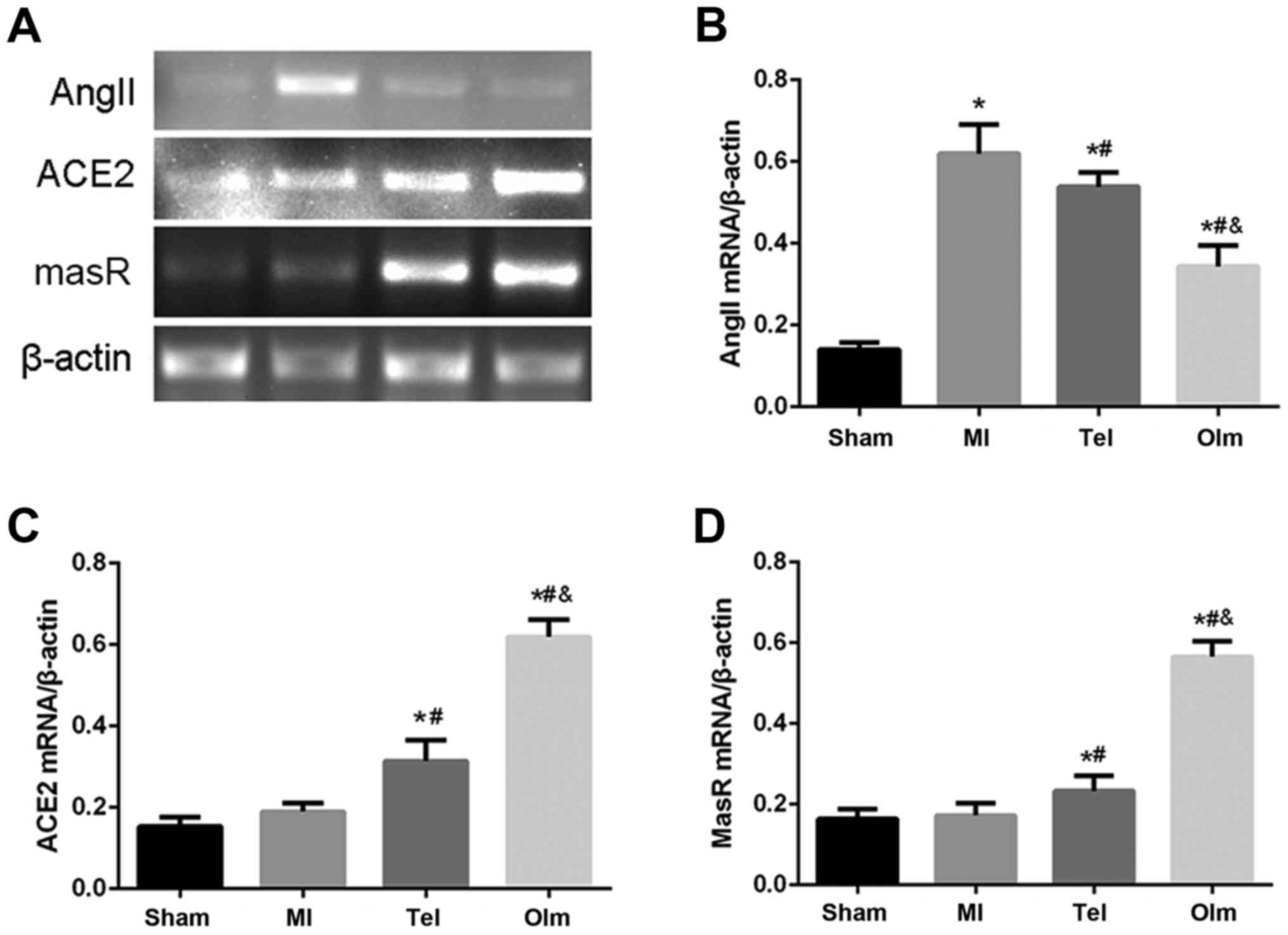
The mRNA expression of ACE2, MasR and
Ang II in myocardial tissue
To test mRNA level of ACE2-Ang (1–7)-Mas,
the authors extracted RNA from myocardial tissue and conducted
RT-qPCR. The results indicated that ACE2 and MasR mRNA expression
are slightly increased in the MI group (P<0.05; Fig. 5A, C and D). Following treatment
with Tel and Olm, the expression of ACE2 and MasR mRNA was
increased (P<0.05; Fig. 5A, C and
D). Compared with the sham group, Ang II mRNA level
significantly increased in the MI group (P<0.05), while its
expression decreased following drug-treatment intervention
(Fig. 5A and B). The effect of Olm
is more significantly than Tel (P<0.05; Fig. 5A-D).
Discussion
Inhibiting the RAS is an important strategy for
cardioprotection, not only by controlling blood pressure, but also
by reducing target-organ damage. Indeed, treatment with RAS
blockers showed clear benefits in distinct stages of cardiovascular
disease. These treatments reduce cardiovascular events in patients
with subclinical lesions, and prolong survival in patients with
clinical disease or organ dysfunction (2,18).
The left ventricular remodeling progression
increased in the rat of myocardial infarction by ligating the left
coronary artery, the expression of Ang II, ACE2, MasR and collagen
deposition increased more than sham group. Treatment with Tel and
Olm could improve cardiac function, alleviate collagen deposition,
antagonize Ang II and increase ACE2, MasR, Ang (1–7)
expression in myocardial tissue, and the effect of Olm was more
remarkable than that of Tel. These findings indicated that Olm
treatment inhibits cardiac remodeling and fibrosis not only through
its original Ang II receptor 1 (AT1R) blockade, but partly through
enhancement of the ACE2-Ang (1–7)-MasR
axis pathway.
As expected, myocardial infarction involves cardiac
dysfunction and cardiac remodeling caused by an increase in Ang II
through the AT1R signaling. Consistent with this, the present study
demonstrated that cardiac dysfunction and cardiac remodeling
occurred in the MI group, as demonstrated by echocardiography and
histological assessments. Consistent with these results, the
authors demonstrated that the expression of Ang II was higher in
the MI group, and that the decrease of Ang II was induced by
treatment with Ang II receptor blockers (ARBs) Tel and Olm. Olm was
more effective at reducing Ang II levels. Furthermore, these
results are supported by the previous report, stating that Olm can
downregulate Ang II levels both in cardiomyocytes in vivo
and in vitro (19,20).
The extracellular matrix (ECM) is a living network
of proteins that maintains the structural integrity of the
myocardium, collagen is an important mediator for cardiac fibrosis
induced by Ang II, so cardiac remodeling and fibrosis are closely
linked each other (21,22). Lindsey et al (23) reported that collagen Iα1
matricryptin p1158/59 facilitates LV remodeling in post-MI by
regulating scar formation through targeted ECM generation and
stimulation of angiogenesis. In the present study, the expression
of collagen genes was increased in the MI group, while the increase
observed in the ARB-drug group was inhibited by Olm or Tel
treatment. Under the same concentration (5 mg/kg), the effect of
Olm is more interesting. These findings suggested that Olm can
block collagen expression and deposition via reducing Ang II
levels.
Accumulating evidence suggests that the ACE2-Ang
(1–7)-Mas pathway has a crucial role in
cardio protection (24). ACE2 gene
expression is upregulated in the infarct zone and survival
myocardial tissue from mice with myocardial infarction (25). Consistent with the previous
studies, these data demonstrated that the mRNA and protein
expression of ACE2 in myocardial tissues was increased slightly in
MI rats, while administration of ARB significantly upregulated the
expression of ACE2 to protect the heart. It has been reported that
blockage of RAS by an ACE inhibitor or ARB increases cardiac Ang
(1–7), ACE2 mRNA and ACE2 activity.
Overexpression of ACE2 can attenuate ventricular fibrosis and
improved ventricular remodeling in a rat model of diabetic
cardiomyopathy (26). ACE2
deficiency worsened epicardial adipose tissue inflammation and
cardiac dysfunction in response to diet-induced obesity (27). Clinical studies have also
demonstrated that expression of the ACE2 gene had a positive
correlation with cardiac remodeling. Serum ACE2 activity rises in
relation to infarct size, left ventricular systolic dysfunction and
is associated with the occurrence of left ventricular remodeling in
patient with myocardial infarction (28). Increased ACE/ACE2 ratios may induce
Ang II overactivation and accelerate cardiac remodeling in patients
with moderate to severe heart failure (29). In the present study, the cardiac
remodeling and fibrosis were attenuated by ARB treatment, and also
associated with an ACE2 increase. Moreover, the increase of ACE2 in
the Olm group was more remarkable than in the Tel group. In
addition, ACE2 overexpression significantly inhibits the
progression of atherosclerosis (30), oxidative stress and inflammatory
cytokine expression (31), SNPs in
the ACE2 gene are also associated with LVH, impaired cardiac
function and HT (32,33). Therefore, the ACE2 gene is
considered as a functional candidate for CVD (34), and also a potential therapy target
of Olm.
Still, Ang (1–7) is a
cardiovascular protective peptide in RAS against cardiovascular
toxic effects of Ang II. Like other peptides, Ang (1–7) has
a physiological role when it binds to the specific MasR. MasR is
involved in the homeostasis of the heart, as well as in the
establishment and progression of cardiac diseases (35). There is accumulating interest in
viewing MasR as a crucial modulator and pharmacological target in
cardiovascular disease research (36). Studies have confirmed that Ang
(1–7) reduces the growth of cardiomyocytes
through activation of the MasR (37). Ang (1–7)
promotes angiogenesis via stimulating the expression of cardiac
vascular endothelial growth factor-D and matrix
metalloproteinase-9, thus facilitating cardiac repair and
ventricular function (38). The
cardioprotective effects of Ang (1–7) are
mediated by MasR to different signaling pathways involving
mitogen-associated protein kinase, phosphoinositide3-kinase/protein
kinase B and NADPH oxidase (15,16).
Consistently, the current study demonstrated that the expression of
Ang (1–7) and MasR was slightly increased in the
MI group, while upregulated following Tel and Ole intervention,
suggesting a protective role of MasR in the development of the
heart.
In conclusion, the current study presents that the
ACE2-Ang (1–7)-MasR axis is closely related with the
development of MI-induced cardiac remodeling of rats. The levels of
ACE2 and Ang (1–7) increased both in plasma and myocardial
tissue from MI group, as well as MasR level. Under the same
concentrations of Olm and Tel, Olm was more effective on modulating
the activities of components of the Ang (1–7)-ACE2-MasR axis in plasma and myocardial
tissue. Their expression levels were significantly upregulated when
treated with Olm. Moreover, Olm reduced the collagen deposition,
delay the left ventricular remodeling and eventually improved the
cardiac function in MI rats. Taken together, Olm can inhibit
myocardial fibrosis of MI rats through activating the ACE2-Ang
(1–7)-MasR signaling pathway. Research on the
contribution of the ACE2-Ang (1–7)-MasR
axis to cardiovascular disease will lead to the development of new
pharmacological approaches resulting in the design of molecular or
genetic means to targeting modulate the expression of ACE2, Ang
(1–7) or MasR, which will provide new targets
for drug design, drug combination therapy and gene therapy for
cardiovascular diseases.
Acknowledgements
The present work was partly supported by funds of
research projects of the Xinjiang Uyghur Autonomous Region Project
of Science and Technology (grant no. 2016E02075) and the National
Natural Science Foundation (grant no. 81560689).
References
|
1
|
Mendis S, Davis S and Norrving B:
Organizational update: The world health organization global status
report on noncommunicable diseases 2014; one more landmark step in
the combat against stroke and vascular disease. Stroke.
46:e121–e122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Houston Miller N: Cardiovascular risk
reduction with Renin-Angiotensin aldosterone system blockade. Nurs
Res Pract 2010. 1017492010.
|
|
3
|
Schmieder RE, Hilgers KF, Schlaich MP and
Schmidt BM: Renin-angiotensin system and cardiovascular risk.
Lancet. 369:1208–1219. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferrario CM and Strawn WB: Role of the
renin-angiotensin-aldosterone system and proinflammatory mediators
in cardiovascular disease. Am J Cardiol. 98:121–128. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mehta PK and Griendling KK: Angiotensin II
cell signaling: Physiological and pathological effects in the
cardiovascular system. Am J Physiol Cell Physiol. 292:C82–C97.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chappell MC: Nonclassical
renin-angiotensin system and renal function. Compr Physiol.
2:2733–2752. 2012.PubMed/NCBI
|
|
7
|
McKinney CA, Fattah C, Loughrey CM,
Milligan G and Nicklin SA: Angiotensin-(1–7) and angiotensin-(1–9):
Function in cardiac and vascular remodelling. Clin Sci (Lond).
126:815–827. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Varagic J, Ahmad S, Nagata S and Ferrario
CM: ACE2: Angiotensin II/angiotensin-(1–7) balance in cardiac and
renal injury. Curr Hypertens Rep. 16:4202014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ocaranza MP, Michea L, Chiong M, Lagos CF,
Lavandero S and Jalil JE: Recent insights and therapeutic
perspectives of angiotensin-(1–9) in the cardiovascular system.
Clin Sci (Lond). 127:549–557. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moritani T, Iwai M, Kanno H, Nakaoka H,
Iwanami J, Higaki T, Ishii E and Horiuchi M: ACE2 deficiency
induced perivascular fibrosis and cardiac hypertrophy during
postnatal development in mice. J Am Soc Hypertens. 7:259–266. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iwanami J, Mogi M, Tsukuda K, Wang XL,
Nakaoka H, Ohshima K, Chisaka T, Bai HY, Kanno H, Min LJ and
Horiuchi M: Role of angiotensin-converting enzyme
2/angiotensin-(1–7)/Mas axis in the hypotensive effect of
azilsartan. Hypertens Res. 37:616–620. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Z, Chen L, Zhong J, Gao P and Oudit
GY: ACE2/Ang-(1–7) signaling and vascular remodeling. Sci China
Life Sci. 57:802–808. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Li B, Wang B, Zhang J, Wu J and
Morgan T: Alteration of cardiac ACE2/Mas expression and cardiac
remodelling in rats with aortic constriction. Chin J Physiol.
57:335–342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang B, Li Y, Han Z, Xue J, Zhang Y, Jia
S and Wang C: ACE2-Ang (1–7) axis is induced in pressure overloaded
rat model. Int J Clin Exp Pathol. 8:1443–1450. 2015.PubMed/NCBI
|
|
15
|
Nemoto W, Ogata Y, Nakagawasai O, Yaoita
F, Tadano T and Tan-No K: Angiotensin (1–7) prevents angiotensin
II-induced nociceptive behaviour via inhibition of p38 MAPK
phosphorylation mediated through spinal Mas receptors in mice. Eur
J Pain. 18:1471–1479. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng J, Li G, Chen S, Bihl J, Buck J, Zhu
Y, Xia H, Lazartigues E, Chen Y and Olson JE: Activation of the
ACE2/Ang-(1–7)/Mas pathway reduces oxygen-glucose
deprivation-induced tissue swelling, ROS production, and cell death
in mouse brain with angiotensin II overproduction. Neuroscience.
273:39–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de la Sierra A: Renin-angiotensin system
blockade and reduction of cardiovascular risk: Future perspectives.
Expert Rev Cardiovasc Ther. 9:1585–1591. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang X, Ye Y, Gong H, Wu J, Yuan J, Wang
S, Yin P, Ding Z, Kang L, Jiang Q, et al: The effects of different
angiotensin II type 1 receptor blockers on the regulation of the
ACE-AngII-AT1 and ACE2-Ang(1–7)-Mas axes in pressure
overload-induced cardiac remodeling in male mice. J Mol Cell
Cardiol. 97:180–190. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanno T, Tomita H, Narita I, Kinjo T,
Nishizaki K, Ichikawa H, Kimura Y, Tanaka M, Osanai T and Okumura
K: Olmesartan inhibits cardiac hypertrophy in mice overexpressing
renin independently of blood pressure: Its beneficial effects on
ACE2/Ang(1–7)/Mas Axis and NADPH oxidase expression. J Cardiovasc
Pharmacol. 67:503–509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Segura AM, Frazier OH and Buja LM:
Fibrosis and heart failure. Heart Fail Rev. 19:173–185. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schnee JM and Hsueh WA: Angiotensin II,
adhesion, and cardiac fibrosis. Cardiovasc Res. 46:264–268. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lindsey ML, Iyer RP, Zamilpa R,
Yabluchanskiy A, DeLeon-Pennell KY, Hall ME, Kaplan A, Zouein FA,
Bratton D, Flynn ER, et al: A novel collagen matricryptin reduces
left ventricular dilation post-myocardial infarction by promoting
scar formation and angiogenesis. J Am Coll Cardiol. 66:1364–1374.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chamsi-Pasha MA, Shao Z and Tang WH:
Angiotensin-converting enzyme 2 as a therapeutic target for heart
failure. Curr Heart Fail Rep. 11:58–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Burrell LM, Risvanis J, Kubota E, Dean RG,
MacDonald PS, Lu S, Tikellis C, Grant SL, Lew RA, Smith AI, et al:
Myocardial infarction increases ACE2 expression in rat and humans.
Eur Heart J. 26:369–375, 322–324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dong B, Yu QT, Dai HY, Gao YY, Zhou ZL,
Zhang L, Jiang H, Gao F, Li SY, Zhang YH, et al:
Angiotensin-converting enzyme-2 overexpression improves left
ventricular remodeling and function in a rat model of diabetic
cardiomyopathy. J Am Coll Cardiol. 59:739–747. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Montaigne D, Coisne A, Marechal X and
Staels B: Comment on Patel et al: ACE2 deficiency worsens
epicardial adipose tissue inflammation and cardiac dysfunction in
response to diet-induced obesity. Diabetes 2016;65:85-95. Diabetes.
65:e1–e2. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ortiz-Pérez JT, Riera M, Bosch X, de
Caralt TM, Perea RJ, Pascual J and Soler MJ: Role of circulating
angiotensin converting enzyme 2 in left ventricular remodeling
following myocardial infarction: A prospective controlled study.
PLoS One. 8:e616952013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang J, Li N, Gao F, Song R, Zhu S and
Geng Z: Balance between angiotensin converting enzyme and
angiotensin converting enzyme 2 in patients with chronic heart
failure. J Renin Angiotensin Aldosterone Syst. 16:553–558. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Tikellis C, Thomas MC and Golledge
J: Angiotensin converting enzyme 2 and atherosclerosis.
Atherosclerosis. 226:3–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Simões e Silva AC, Silveira KD, Ferreira
AJ and Teixeira MM: ACE2, angiotensin-(1–7) and Mas receptor axis
in inflammation and fibrosis. Br J Pharmacol. 169:477–492. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang M, Zhao J, Xing L and Shi L: The
association between angiotensin-converting enzyme 2 polymorphisms
and essential hypertension risk: A meta-analysis involving 14,122
patients. J Renin Angiotensin Aldosterone Syst. 16:1240–1244. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang SX, Tao T, Fu ZQ, Xie XZ, Wang H and
Wang YT: Polymorphisms of angiotensin-converting enzyme 2 gene
confer a risk to lone atrial fibrillation in Chinese male patients.
Chin Med J (Engl). 126:4608–4611. 2013.PubMed/NCBI
|
|
34
|
Burrell LM, Harrap SB, Velkoska E and
Patel SK: The ACE2 gene: Its potential as a functional candidate
for cardiovascular disease. Clin Sci (Lond). 124:65–76. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin L, Liu X, Xu J, Weng L, Ren J, Ge J
and Zou Y: Mas receptor mediates cardioprotection of
angiotensin-(1–7) against Angiotensin II-induced cardiomyocyte
autophagy and cardiac remodelling through inhibition of oxidative
stress. J Cell Mol Med. 20:48–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Singh K, Sharma K, Singh M and Sharma PL:
Possible mechanism of the cardio-renal protective effects of
AVE-0991, a non-peptide Mas-receptor agonist, in diabetic rats. J
Renin Angiotensin Aldosterone Syst. 13:334–340. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tallant EA, Ferrario CM and Gallagher PE:
Angiotensin-(1–7) inhibits growth of cardiac myocytes through
activation of the mas receptor. Am J Physiol Heart Circ Physiol.
289:H1560–H1566. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao W, Zhao T, Chen Y and Sun Y:
Angiotensin 1–7 promotes cardiac angiogenesis following infarction.
Curr Vasc Pharmacol. 13:37–42. 2015. View Article : Google Scholar : PubMed/NCBI
|