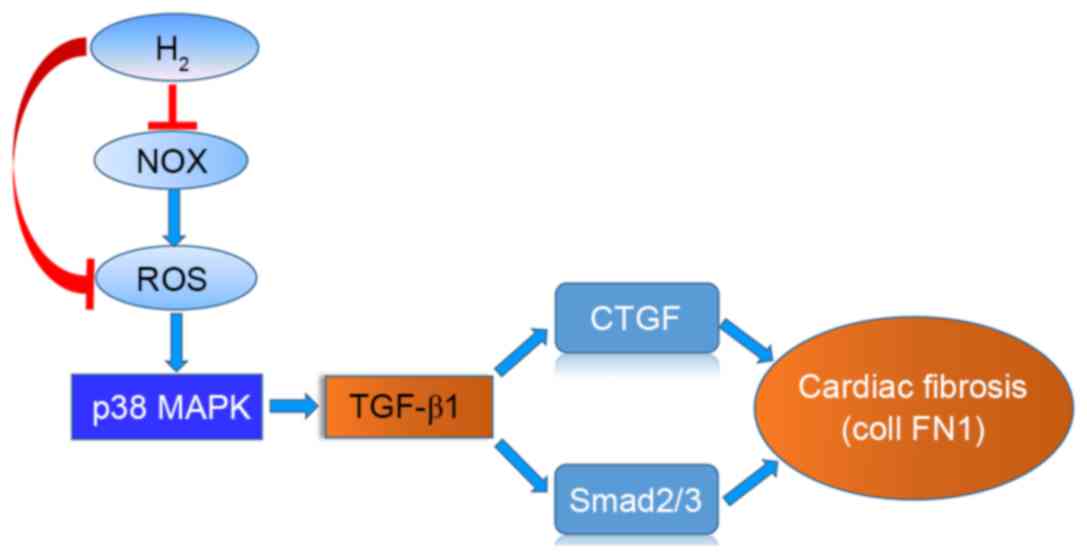
Introduction
Heart failure is the terminal stage of various
cardiovascular diseases and is a global problem with high
prevalence (1). Sustained pressure
overload associated with hypertension can induce cardiac fibrosis,
which is characterized by excess deposition of collagen and other
extracellular matrix (ECM) components in the interstitial and
peri-vascular regions of the myocardium that lead to myocardial
stiffness and ultimately, to heart failure (2). Therapeutic strategies that reduce
cardiac fibrosis may therefore block the progression of cardiac
fibrosis to heart failure in hypertensive heart diseases.
Transforming growth factor (TGF)-β1 serves an
essential role in cardiac fibrosis by inducing the proliferation of
cardiac fibroblasts and stimulating ECM deposition (3). It has been reported that TGF-β1
antagonists can prevent myocardial fibrosis and heart failure
(4). Smad2/3 is the important
downstream signaling of TGF-β1, and connective tissue growth factor
(CTGF) also serves as a critical downstream target gene of TGF-β1
to prompt fibrosis (5). The
generation of reactive oxygen species (ROS) by nicotinamide adenine
dinucleotide phosphate oxidases (NOX) is increased under pressure
overload, and induces other enzymes such as xanthine oxidase and
nitric oxide synthase to enhance ROS formation (6). NOX2 and NOX4 are notably relevant to
cardiac pathophysiology. Excessive ROS levels can directly affect
matrix protein expression and metabolism (2) in addition to cytokine and growth
factor signaling in cardiac fibrosis. For example, the upregulation
of TGF-β1 by angiotensin (Ang) II is mediated by NOX-induced ROS,
which activates downstream ROS-sensitive kinases such as p38
mitogen-activated protein kinase (MAPK) in cardiomyocytes and
fibroblasts (7). In addition,
enalaprilat, an AngII-converting enzyme inhibitor, suppresses
cardiac fibroblast proliferation induced by Ang II by blocking
NOX/ROS/p38 MAPK/TGF-β1 signaling (8).
Hydrogen (H2) has been proposed as a
medical gas in prophylactic and therapeutic applications with a
diverse physiological profile. Due to the fact that H2
was demonstrated to mitigate ischemia/reperfusion (I/R) injury in
the brain owing to its anti-oxidative properties (9), a number of studies have reported its
biological effects against oxidative stress and other aspects,
including anti-inflammatory (10),
anti-apoptotic (11),
anti-allergenic (12) and
metabolism-stimulating effects (13). In the cardiovascular system,
H2 treatment protects against myocardial I/R injury
(14,15), the development of atherosclerosis
(16) and radiation-induced
cellular damage (17) while
enhancing cardiac graft transplantation (18) and reducing left ventricular (LV)
hypertrophy (19). However, the
benefits of H2 for pressure overload-induced cardiac
fibrosis remain unclear. The present study investigated the effects
and underlying mechanisms of hydrogen-containing saline (HCS)
treatment in a rat model of interstitial fibrosis and cardiac
dysfunction induced by abdominal aortic constriction (AC).
Materials and methods
Animals and ethics
A total of 60 adult (6-week-old) male Wistar rats
(150–200 g) from the Laboratory Animal Center of the First
Affiliated Hospital of Harbin Medical University (Harbin, China)
were maintained in a temperature- and humidity-controlled room with
free access to food and water for 1 week prior to the operation.
Experimental procedures were in compliance with the Guide for the
Care and Use of Laboratory Animals of the National Research Council
of China (20) and were approved
by the Ethics Committee of the First Affiliated Hospital of Harbin
Medical University.
HCS preparation
HCS was produced by introducing H2 gas
(flow rate: 1 l/min) into 500 ml of 0.9% saline solution with
stirring for 10 min until reaching the point of saturation
(21). HCS was freshly prepared
every week and stored at 4°C under atmospheric pressure in an
aluminum bag with no dead volume. Hydrogen content in the saline
and in animal blood was confirmed by gas chromatography as
previously described (9).
Abdominal AC and HCS
administration
Rats were subjected to suprarenal abdominal AC as
previously described (22).
Briefly, rats were anaesthetized by intraperitoneal injection of
10% chloral hydrate (3 ml/kg), and a 4–0 suture was tied around the
suprarenal aorta with a 21-gauge needle placed alongside to yield
an internal diameter of 0.8 mm. A total of three days later,
surviving AC rats were randomly assigned to the AC + saline (AC;
n=7) and AC + HCS treatment (AC + H2; n=7) groups for 16
weeks, during which time 10 ml/kg of HCS or saline was administered
every morning to the animals via intraperitoneal injection.
Randomly selected normal rats served as the control group (Con;
n=8), and were administrated with the same dose of saline.
Echocardiography
Transthoracic echocardiography was performed prior
and subsequent to HCS administration with an ultrasound apparatus
(SONOS 7500; Philips, Rotterdam, The Netherlands) equipped with a
12-MHz transducer. LV end-diastolic diameter (LVDd), LV
end-systolic diameter (LVSd), interventricular septum thickness
(IVSd) and LV posterior wall thickness (LVPWd) were measured. LV
ejection fraction (EF) and fractional shortening (FS) were
calculated from M-mode recordings. Measurements from three
consecutive cardiac cycles were averaged and data were analyzed by
a blinded observer.
Morphometric and histopathological
analysis
Following echocardiographic measurements and blood
sample collection, the heart was dissected and weighed. The LV was
separated from the atria, right ventricle and the large vessels
then was weighed, immersion-fixed in 4% buffered paraformaldehyde
and flash frozen in liquid nitrogen until use. Heart weight (HW)
and LV weight (LVW) were used to calculate the HW/body weight (BW)
and LVW/BW ratios. Paraffin-embedded tissue was cut into 4 µm
serial sections that were stained with Masson's trichrome to
evaluate ECM deposition. Digital images of 10 fields were randomly
selected from each section of the LV by a light microscope (Olympus
Corporation, Tokyo, Japan) and the percent of fibrosis area was
analyzed with Image-Pro Plus 6.0 image analysis software (Media
Cybernetics, Inc., Rockville, MD, USA).
Measurement of ROS
Intracellular ROS production in the LV tissues was
detected by the use of 2,7-dichlorofluorescin diacetate
(CM-H2DCFDA) (Shanghai Genmed, Gene Pharmaceutical Technology Co.,
Ltd., Shanghai, China) fluorescent dye. Briefly, frozen heart
tissues were incubated with 500 µl CM-H2DCFDA and dissolved in
Reagent C (500:1, Shanghai Genmed, Gene Pharmaceutical Technology
Co., Ltd.) at 37°C for 20 min. Fluorescence was detected by
confocal laser scanning microscopy with red light and measuring the
intensity of red fluorescence, where the ROS content increased in
proportion to the intensity of red fluorescence.
Measurement of malondialdehyde (MDA)
concentration and superoxide dismutase (SOD) activity and
hydroxyproline content
MDA is a marker of oxidant-mediated lipid
peroxidation, whereas SOD is an antioxidant enzyme. Hydroxyproline,
the decomposition product of collagen, is considered to be an index
of collagen quantity. MDA concentration, SOD activity and
hydroxyproline content were measured in LV tissue homogenates using
commercial kits (Nanjing Jiancheng Bioengineering Research
Institute, Nanjing, China) according to the manufacturer's
protocols. For the MDA and SOD assay, frozen tissues were
homogenized in a saline buffer and centrifuged at 1,082 × g for 10
min at room temperature to obtain supernatant, and then were
determined its protein concentration. For detecting hydroxyproline,
frozen tissues were weighed, hydrolyzed with alkali and centrifuged
at 2,121 × g for 10 min at room temperature to obtain the
supernatant. MDA concentration, SOD activity and hydroxyproline
content were measured by spectrophotometer colorimetry (722G;
Shanghai Jingke Scientific Instrument Co., Ltd., Shanghai, China)
at 532, 450 and 550 nm, respectively. The final results of MDA
concentration and SOD activity were corrected for protein content,
and hydroxyproline content was expressed as µg/g wet weight.
Western blot analysis
Frozen LV tissue was mechanically lysed using RIPA
buffer (Beyotime Institute of Biotechnology, Shanghai, China), then
centrifuged at 12,000 × g and 4°C for 10 min. Protein concentration
was estimated with the Bicinchoninic Acid Protein Assay kit
(Beyotime Insitute of Biotechnology). Protein samples (60 µg per
lane) were separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred to a polyvinylidene difluoride
membrane (EMD Millipore, Billerica, MA, USA), which was blocked
with 5% skimmed milk for 2 h at room temperature and then probed
overnight at 4°C with rabbit polyclonal antibodies against TGF-β1
(1:1,000; GTX110630; GeneTex, Irvine, CA, USA) and NOX4 (1:500,
sc-30141, Santa Cruz Biotechnology, Inc., Dallas, TX, USA), rabbit
monoclonal antibodies against NOX2 (1:1,000; ab129068; Abcam,
Cambridge, MA, USA), phosphorylated-Smad2/3 (p-Smad2/3; 1:1,000;
no. 8828; Cell Signaling Technology, Inc., Danvers, MA, USA),
Smad2/3 (1:1,000; no. 8685; Cell Signaling Technology, Inc.), p38
MAPK (D13E1) (1:1,000; no. 8690; Cell Signaling Technology, Inc.),
p-p38 MAPK (Thr180/Tyr182) (D3F9) (1:1,000; no. 4511; Cell
Signaling Technology, Inc.) and mouse monoclonal antibodies against
CTGF (E-5) (1:50; sc-365970; Santa Cruz Biotechnology, Inc.) and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:5,000; KC-5G4;
KangChen Bio-tech, Inc., Shanghai, China). The membrane was washed
three times for 10 min with phosphate-buffered saline containing
0.5% Tween-20, then incubated for 2 h with the secondary goat
antibodies against rabbit (ZDR-5306) or mouse (ZDR-5307) IgG
conjugated to horseradish peroxidase (1:2,000; OriGene
Technologies, Beijing, China). Protein bands were visualized by
enhanced chemiluminescence using western blot detection reagents
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) and exposed to
X-ray film, which was digitized with a scanner (LiED 110; Canon,
Inc., Tokyo, Japan). The band intensity (area × optical density) in
each group was measured using ImageJ 1.45s software (National
Institutes of Health, Bethesda, MD, USA) and normalized to that of
GAPDH.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from LV tissue samples using
RNAiso Plus (Takara Bio, Inc., Otsu, Japan) and 1 µg was reverse
transcribed into cDNA, using the PrimeScript RT Reagent kit with
gDNA Eraser (Takara Bio, Inc.) and M-MLV Reverse Transcriptase
(Takara Biotechnology Co., Ltd., Dalian, China), according to the
manufacturer's protocol. RT-qPCR analysis was conducted with
SYBR-Green (Roche Diagnostics GmbH, Mannheim, Germany) on an ABI
7500 RT-PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) in order to determine the mRNA levels of collagen I (Col I),
fibronectin 1 (FN1) and GAPDH. The primer sequences were as
follows: Col I, 5′-GACTGGCAACCTCAAGAAGG-3′ (forward) and
5′-GACTGTCTTGCCCCAAGTTC-3′ (reverse); FN1,
5′-TACACGGTTTCCCATTACGC-3′ (forward) and 5′-CCTTTCCATTCCCGAGACAT-3′
(reverse); GAPDH, 5′-GGAAAGCTGTGGCGTGAT-3′ (forward) and
5′-AAGGTGGAAGAATGGGAGTT-3′ (reverse). Among them, GAPDH was used as
an internal standard. The qPCR reaction mixture consisted of 10 µl
SYBR-Green, 0.2 µl forward primer, 0.2 µl reverse primer, 2 µl cDNA
and 7.6 µl sterile water. Following the thermal treatment of 15 min
at 95°C, the reactions were conducted using 45 cycles of 15 sec at
95°C, 30 sec at 55°C, 30 sec at 72°C, followed by a final extension
for 7 min at 72°C. The expression of target genes was quantified
relative to the level of GAPDH using the ΔΔCq method (23).
Statistical analysis
Data are expressed as the mean ± standard deviation.
One-way analysis of variance followed by Tukey's post hoc test was
conducted to determine differences among groups using SPSS
software, version 20.0 (IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
H2 treatment improves
cardiac function induced by pressure overload
The effect of H2 on cardiac function was
assessed by echocardiography (Table
I). Chronic pressure overload resulted in a decline in EF and
FS in the AC as compared with the Con group (all P<0.001),
indicating impaired cardiac function. Treatment with HCS improved
both parameters as compared with the AC group (all P<0.001).
Chronic pressure overload also caused the dilation of the heart, as
evidenced by increases in LVDd and LVSd in the AC relative to the
Con group (all P<0.001). HCS treatment attenuated the increase
in LV dimension (all P<0.001). However, there were no
differences in IVSd and LVPWd across groups (all P>0.05).
 | Table I.Effects of H2 on
echocardiographic and morphometric parameters. |
Table I.
Effects of H2 on
echocardiographic and morphometric parameters.
| Parameter | Con (n=8) | AC (n=7) | AC + H2
(n=7) |
|---|
| IVSd (mm) |
1.64±0.07 | 1.76±0.10 | 1.72±0.09 |
| LVPWd (mm) |
1.63±0.07 | 1.71±0.08 | 1.67±0.06 |
| BW (g) | 381.86±15.03 | 390.29±13.87 | 375.29±10.81 |
| LVW (g) |
0.85±0.08 |
1.02±0.07a | 0.94±0.06 |
| LVW/BW (mg/g) |
2.23±0.12 |
2.60±0.09b | 2.49±0.10 |
| HW/BW (mg/g) |
2.88±0.12 |
3.34±0.13b | 3.23 ±0.10 |
| LVDd (mm) |
6.00±0.17 |
7.05±0.19b |
6.52±0.12c |
| LVSd (mm) |
3.51±0.15 |
5.63±0.24b |
4.82±0.09c |
| FS (%) | 41.56±1.79 |
20.11±1.44b |
26.13±1.04c |
| EF (%) | 79.99±1.86 |
48.96±2.77b |
59.67±1.69c |
In addition, the morphometric parameters of the
heart were also examined (Table
I). BW was similar across groups. LVW, LVW/BW and HW/BW were
increased in the AC as compared with the Con group (all P<0.01).
However, HCS treatment didn't suppress the increase in LVW, LVW/BW
and HW/BW as compared with the AC group (all P>0.05). These
results indicate that HCS treatment improved cardiac function
however had no effects on cardiac hypertrophy induced by sustained
pressure overload.
H2 treatment reduces
interstitial fibrosis induced by pressure overload
ECM deposition was assessed by Masson's trichrome
staining (Fig. 1A and B). The
interstitial fibrotic area was greater in the AC as compared with
the Con group (P<0.001). Treatment with HCS reduced ECM
deposition (AC + H2 vs. AC; P<0.001). Consistent with
the above results, the LV hydroxyproline content (Fig. 1C) was increased in the AC as
compared with the Con group (P<0.001), whereas the content was
decreased in HCS-treated rats (AC + H2 vs. AC;
P<0.01). In the AC group, the mRNA levels of Col I and FN1, two
ECM components, were upregulated by approximately 2-fold and
6-fold, respectively, as compared with control rats (all
P<0.001; Fig. 1D and E); HCS
treatment inhibited the expression of both transcripts to 68% for
Col I and 65% for FN1 of the levels in the AC group (all
P<0.01).
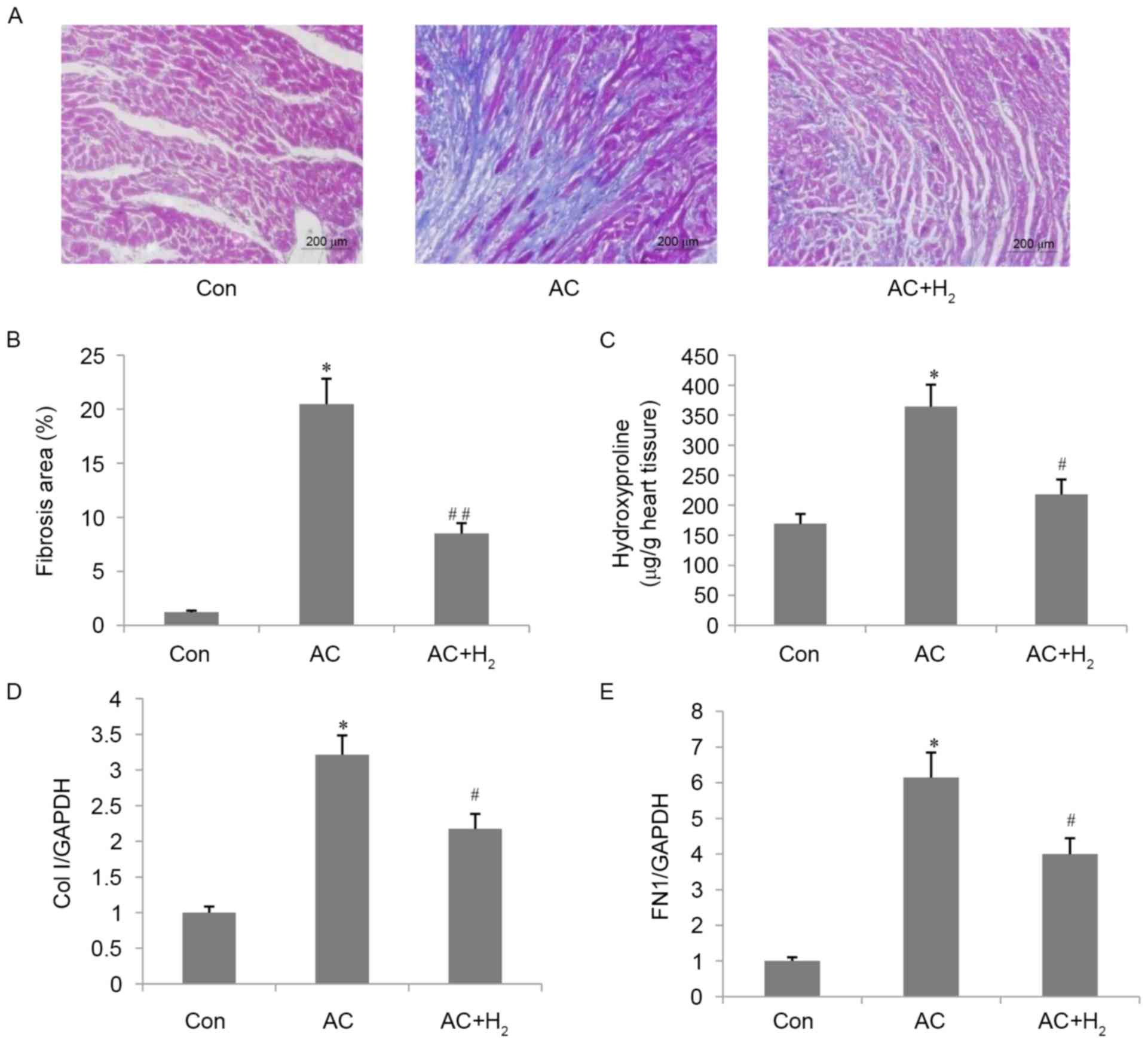 | Figure 1.Effect of H2 on cardiac
interstitial fibrosis in pressure-overloaded hearts. (A)
Representative image (magnification, ×200) of heart sections
stained by Masson's trichrome. (B) Quantitative analysis of
fibrotic area. (C) Hydroxyproline content in the LV. mRNA
expression level of (D) Col I and (E) FN1 in the LV. Data are
presented as the mean ± standard deviation (n=4 for B, n=3 for C,
D, E). *P<0.001 vs. Con group; #P<0.01,
##P<0.001 vs. AC group. LV, left ventricle; Col
I, collagen I; FN1, fibronectin 1; Con, control; AC,
aortic constriction. |
H2 abolishes the increase
in TGF-β1 and its downstream genes induced by pressure
overload
TGF-β1 serves a key role in the development of
cardiac fibrosis. AC resulted in an increase in TGF-β1 protein
level compared with control rats (P<0.001; Fig. 2A and B). HCS treatment abolished
the AC-induced upregulation of TGF-β1 (P<0.001). The
phosphorylation of Smad2/3 and CTGF protein level were upregulated
following the tendency of TGF-β1 in AC group when compared with the
Con group (all P<0.01; Fig. 2A, C
and D), however significantly decreased in the AC +
H2 group (AC + H2 vs. AC; P<0.05). These
results indicate that hydrogen inhibits TGF-β1 and its downstream
signaling.
 | Figure 2.Effect of H2 on TGF-β1 and
its downstream signals in pressure-overloaded hearts. (A) TGF-β1,
p-Smad2/3, Smad2/3 and CTGF protein levels, as determined by
western blotting; GAPDH was used as the loading control.
Quantitative analysis of protein levels of (B) TGF-β1, (C) the
ratio of p-Smad2/3/Smad2/3 and (D) the protein levels of CTGF. Data
are presented as the mean ± standard deviation (n=3). *P<0.01,
**P<0.001 vs. Con group; #P<0.05,
##P<0.001 vs. AC group. TGF-β1, transforming growth
factor β1; p-, phosphorylated-; CTGF, connective tissue growth
factor; Con, control; AC, aortic constriction. |
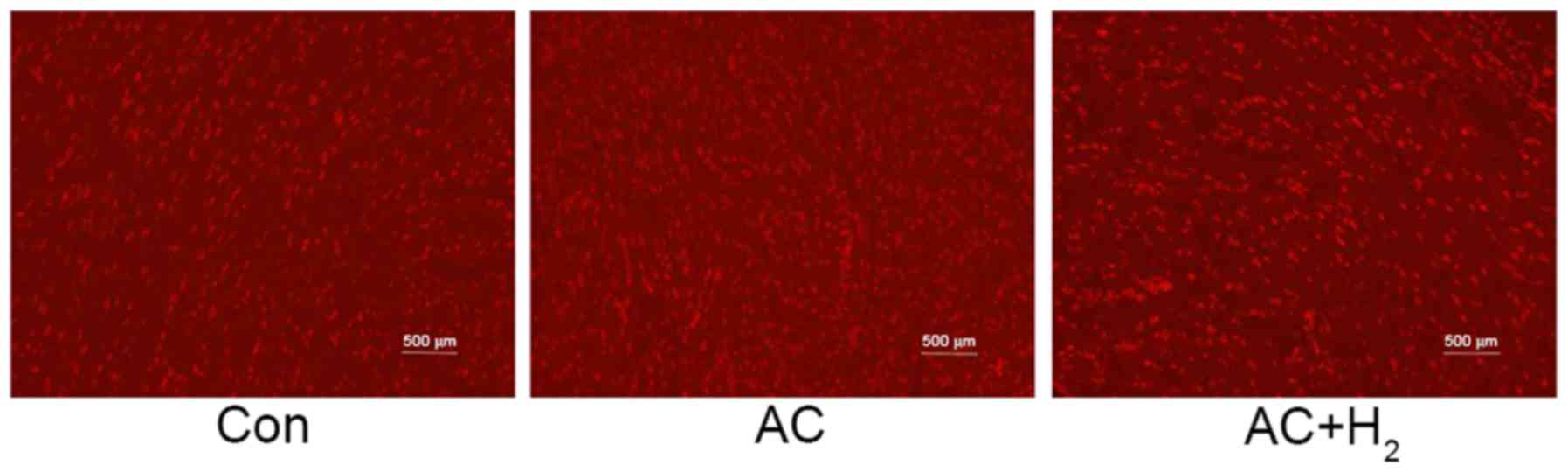
H2 reduced ROS formation in
the myocardium
ROS production in the LV tissues was detected by
CM-H2DCFDA fluorescence (Fig. 3).
The ROS content, indicating a higher concentration of red
fluorescent particles, was significantly increased in the AC group,
compared with the Con group. However, HCS treatment decreased the
ROS level of the AC group.
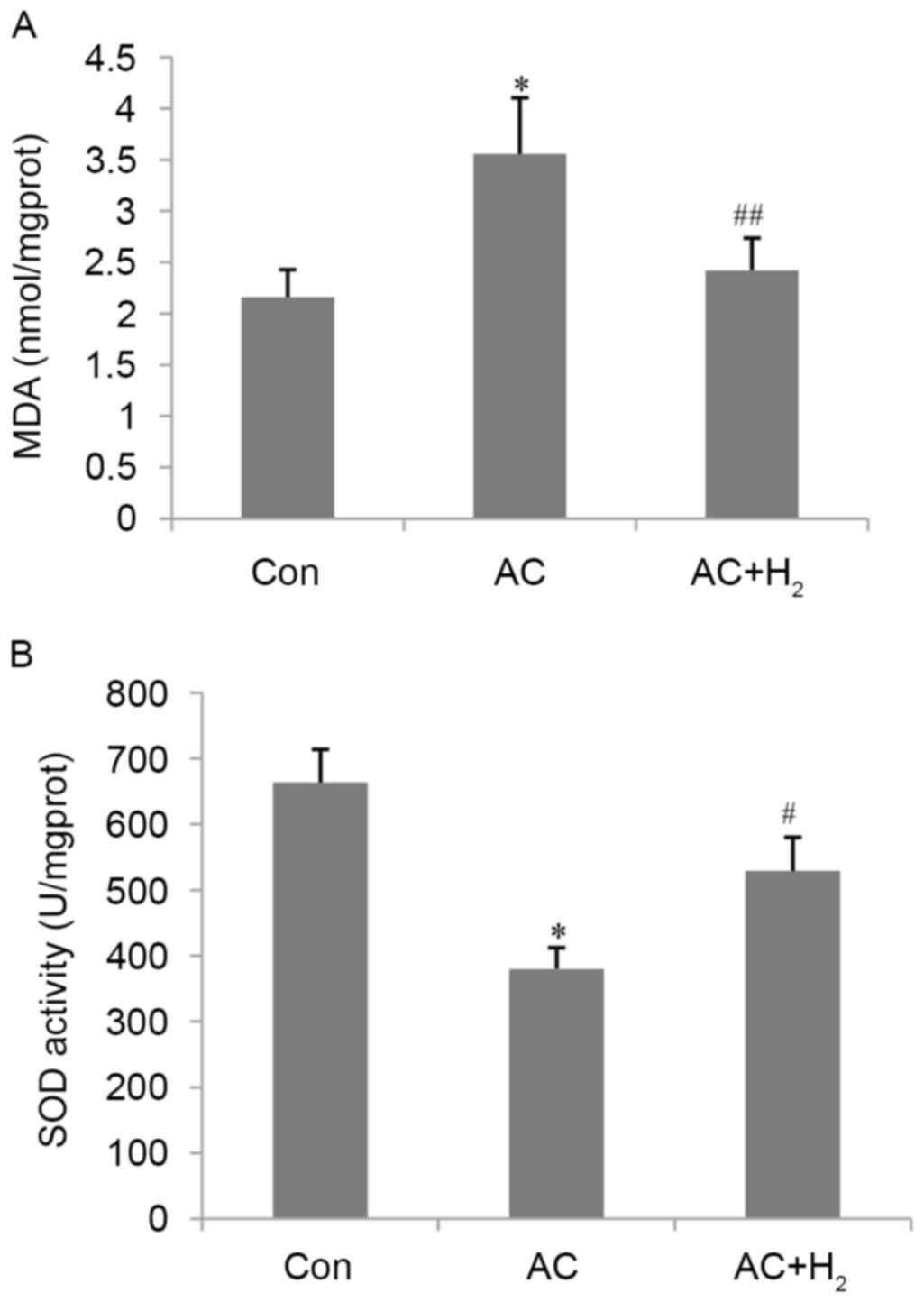
H2 restores MDA levels and
SOD activity in the myocardium
MDA levels and SOD activity are reliable indicators
of oxidative stress and antioxidant capacity, respectively. AC
resulted in a 65% increase in MDA content and a 43% decrease in SOD
activity (all P<0.01) compared with the Con group (Fig. 4). HCS treatment abrogated these
effects, reducing MDA levels and increasing SOD activity (all
P<0.05) as compared with the AC group. These results indicate
that hydrogen an antioxidant effect on the pressure-overloaded
heart.
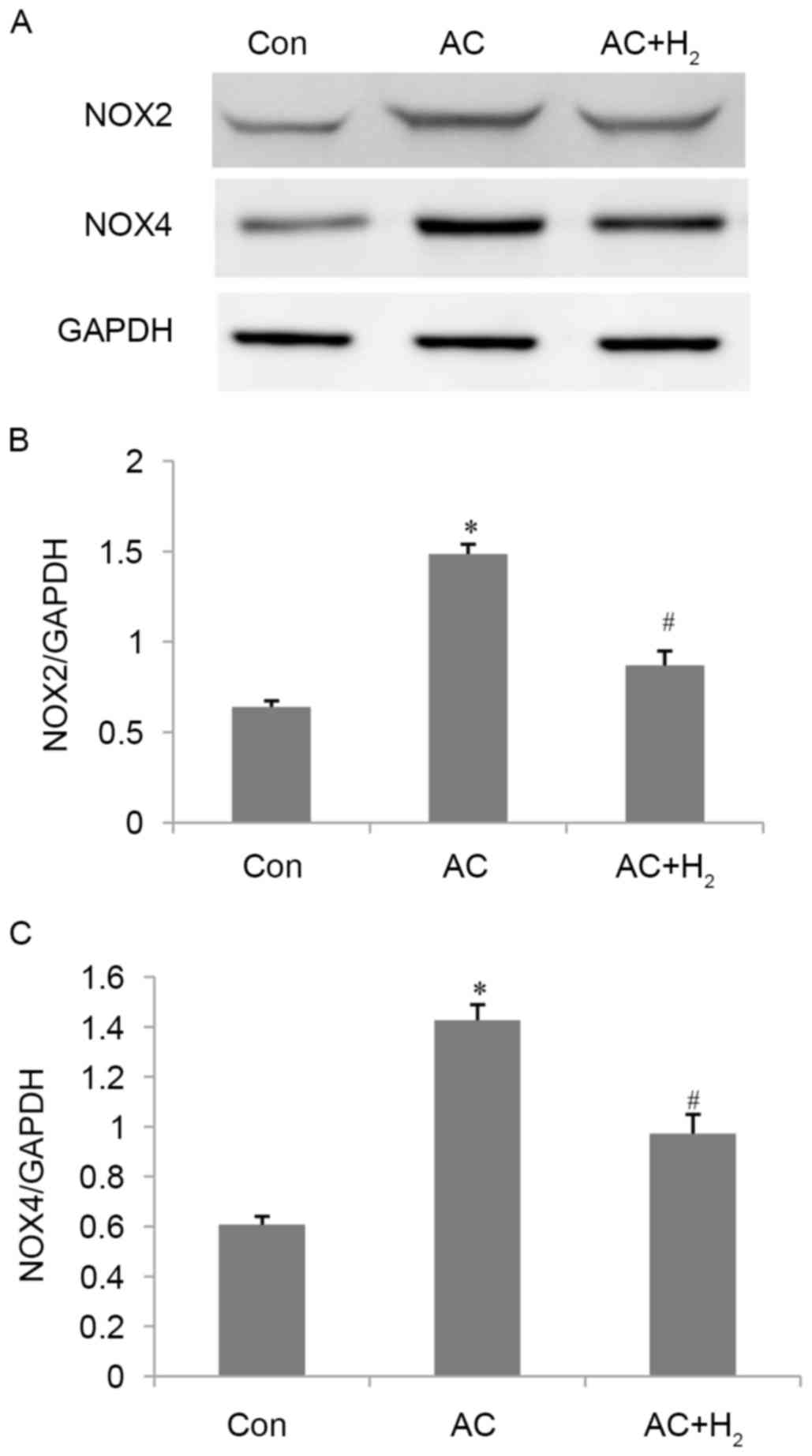
H2 inhibits NOX2 and NOX4
expression induced by pressure overload
The protein levels of NOX2 and NOX4 were illustrated
in Fig. 5. Their expression of
NOX2 and NOX4 were increased in the AC compared with the Con group
(all P<0.001). Subsequent to treatment with HCS, expression was
significantly decreased compared with the AC group (all
P<0.001). These results indicate that HCS treatment could
attenuate oxidative stress by partially inhibiting the expression
of NOX2 and NOX4.
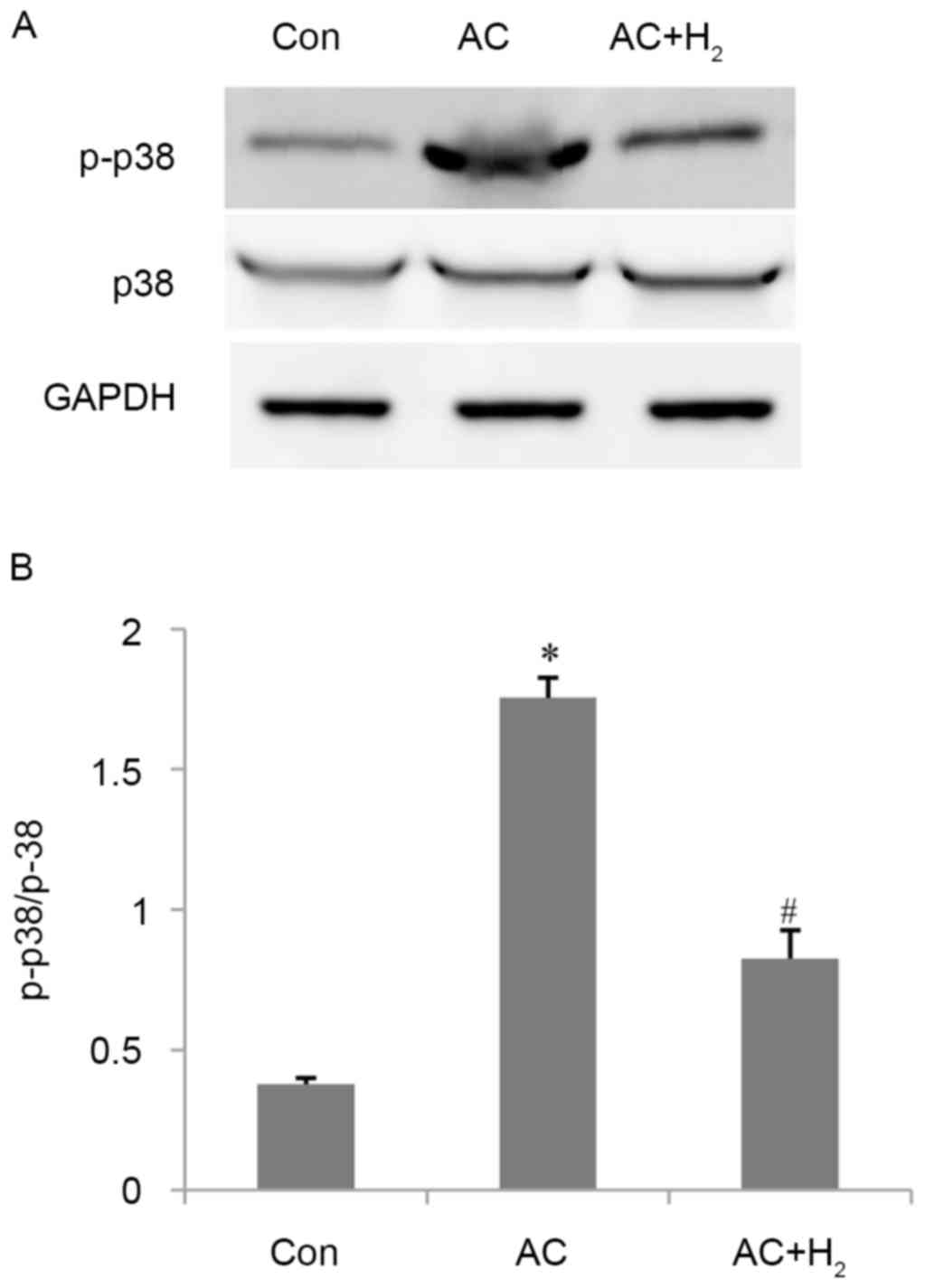
H2 inhibits p38 MAPK
activation induced by pressure overload
AC increased the percentage of phosphorylation of
p38 MAPK by 3.6-fold as compared with control animals (P<0.001)
(Fig. 6). HCS treatment decreased
the ratio of p-p38 MAPK/p38 MAPK by 53% compared with the AC group
(P<0.001).
Discussion
H2 has been widely used in clinical
applications owing to its capacity to specifically neutralize
cytotoxic oxygen radicals (24).
Previously studies have demonstrated that H2
administration has protective effects against cerebral reperfusion
injury, neurodegeneration and metabolic syndromes for patients by
inhalation of H2 gas, intake of H2-dissolved
water, or injection of H2-dissolved saline (25), among which injection of
H2-dissolved saline ensures more accurate delivery of
H2 (24). H2
has a protective effect on the cardiovascular system (14,18,19,26–28);
however, the role of H2 in pressure overload-induced
cardiac fibrosis has not, to the best of our knowledge, been
previously investigated. The present study demonstrated that HCS
treatment prevents interstitial fibrosis and the progression of
heart failure induced by pressure overload in rats. These effects
were accompanied by changes in the levels of oxidative stress
markers and the expression of pro-fibrotic factors. The results
indicate that HCS may be effective for treating cardiac fibrosis
and heart failure caused by hypertension-induced pressure
overload.
Hypertension affects approximately 1 billion people
worldwide and is a major public health challenge (29). Pressure overload induces early
cardiac hypertrophy, fibrosis and diastolic dysfunction; when these
persist, the heart becomes decompensated and dilated, eventually
leading to systolic dysfunction (2). A previous study identified that
fibrosis can promote the transition from left ventricular
hypertrophy to heart failure (30). As such, reversing or preventing
cardiac fibrosis is an important goal for improving the prognosis
of patients with hypertensive heart disease. In the present study,
cardiac fibrosis, LV dilation and systolic dysfunction were
observed in rats in which pressure overload was induced by AC after
16 weeks. However, HCS treatment shortened LVDd and LVSd, reduced
interstitial fibrotic area, and increased EF and FS, but did not
decrease IVSd, LVPWd, LVW/BW or HW/BW ratios. The results indicated
that initial cardiac hypertrophy progressed to cardiac fibrosis and
heart failure (22), which were
mitigated by H2. A previous study reported that HCS
treatment attenuates left ventricular hypertrophy in spontaneously
hypertensive rats, which differed from the results of the present
study, potentially due to the fact that different time points were
examined (19).
Oxidative stress serves a critical role in heart
failure resulting from sustained pressure overload (31). The expression of oxidative stress
markers is upregulated in the advent of heart failure (32), in addition to during the transition
from hypertrophy to heart failure (33). It was demonstrated that the levels
of ROS and MDA, an index of lipid peroxidation, were increased by
AC, an effect that was reversed by HCS treatment. It was previously
observed that HCS treatment protected against doxorubicin-induced
heart failure, which was accompanied by a downregulation of MDA
levels (34). In addition,
antioxidant capacity, as reflected by SOD, was impaired under
pressure overload, and this was also ameliorated by HCS
treatment.
ROS levels are increased in various types of heart
diseases, including cardiac hypertrophy, fibrosis and heart failure
(35,36). ROS have been reported to activate
TGF-β1 to enhance ECM deposition in the interstitium (37). TGF-β1 is an important fibrogenic
factor that mediates the proliferation of fibroblast and conversion
to myofibroblasts in addition to the production of ECM components
including fibrillar collagen and fibronectin (2). CTGF is induced by TGF-β1 through
signaling cascades such as Smad2/3, and is essential for
TGF-β1-induced collagen synthesis (5). In the present study, Col I and
FN1 mRNA levels and hydroxyproline content were upregulated
under pressure overload. CTGF protein expression and the ratio of
p-Smad2/3 to Smad2/3 were additionally upregulated following
TGF-β1. Administration of HCS abolished these effects and reduced
ECM deposition. These results are consistent with that of a
previous study demonstrating that TGF-β1 expression was induced by
pressure overload, and that blocking its activity prevented
myocardial fibrosis and cardiac dysfunction (38).
NOX are the main sources of ROS in the
cardiovascular system, and among them, NOX2 and NOX4 serve an
important role in the development of cardiac hypertrophy and
interstitial fibrosis. p38 MAPK serves a key role in intracellular
signal transduction in cardiac development and pathology (39). For example, p38 MAPK mediates the
upregulation of TGF-β1 induced by AngII, which is also dependent on
NOX-induced ROS (7). In addition,
ROS/p38 MAPK/TGF-β1 signaling is essential for cardiac fibroblast
proliferation (8). It was observed
that pressure overload increased oxidative stress, p38 MAPK
phosphorylation and TGF-β1 expression; these were abrogated by HCS
treatment, suggesting that H2 can inhibit ROS/p38
MAPK/TGF-β1 signaling to improve interstitial fibrosis. However,
intracellular ROS production should be directly measured and the
effect of inhibiting NOX activity on TGF-β1 expression must be
analyzed in a further study.
H2 has been previously reported to
suppress the production of cytotoxic oxygen radicals, including •OH
and ONOO− (9); it also
regulates gene expression and the phosphorylation of signaling
proteins. H2 was demonstrated to inhibit the expression
of matrix metalloproteinase (MMP)-2 and −9 (40) in addition to the phosphorylation of
p38MAPK, exerting anti-allergenic and anti-inflammatory effects
(12,41). However, the primary molecular
target of H2 remains unclear, and further studies are
required to clarify the mechanism by which H2 regulates
TGF-β1 expression and to determine whether it directly attenuates
cardiac fibrosis by negatively regulating MMP2 and −9 levels.
In conclusion, HCS treatment reduced interstitial
fibrosis and improved cardiac function in pressure-overloaded rats,
which was likely mediated by its anti-oxidative properties and via
inhibition of p38 MAPK phosphorylation and TGF-β1 downstream
signaling. A schematic of the action of H2 is presented
in Fig. 7. These data may provide
a potential therapeutic application of H2 in preventing
cardiac fibrosis and the progression of heart failure.
Acknowledgements
The authors would like to thank International
Science Editing for the English language editing of the
manuscript.
References
|
1
|
Braunwald E: The war against heart
failure: The Lancet lecture. Lancet. 385:812–824. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kong P, Christia P and Frangogiannis NG:
The pathogenesis of cardiac fibrosis. Cell Mol Life Sci.
71:549–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dobaczewski M, Chen W and Frangogiannis
NG: Transforming growth factor (TGF)-β signaling in cardiac
remodeling. J Mol Cell Cardiol. 51:600–606. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lim H and Zhu YZ: Role of transforming
growth factor-beta in the progression of heart failure. Cell Mol
Life Sci. 63:2584–2596. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Creemers EE and Pinto YM: Molecular
mechanisms that control interstitial fibrosis in the
pressure-overloaded heart. Cardiovasc Res. 89:265–272. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murdoch CE, Zhang M, Cave AC and Shah AM:
NADPH oxidase-dependent redox signalling in cardiac hypertrophy,
remodelling and failure. Cardiovasc Res. 71:208–215. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rosenkranz S: TGF-beta1 and angiotensin
networking in cardiac remodeling. Cardiovasc Res. 63:423–432. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu M, Zheng Y, Sun HX and Yu DJ:
Inhibitory effects of enalaprilat on rat cardiac fibroblast
proliferation via ROS/P38MAPK/TGF-β1 signaling pathway. Molecules.
17:2738–2751. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ohsawa I, Ishikawa M, Takahashi K,
Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S
and Ohta S: Hydrogen acts as a therapeutic antioxidant by
selectively reducing cytotoxic oxygen radicals. Nat Med.
13:688–694. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie K, Yu Y, Pei Y, Hou L, Chen S, Xiong L
and Wang G: Protective effects of hydrogen gas on murine
polymicrobial sepsis via reducing oxidative stress and HMGB1
release. Shock. 34:90–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cai J, Kang Z, Liu WW, Luo X, Qiang S,
Zhang JH, Ohta S, Sun X, Xu W, Tao H and Li R: Hydrogen therapy
reduces apoptosis in neonatal hypoxia-ischemia rat model. Neurosci
Lett. 441:167–172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Itoh T, Fujita Y and Ito M, Masuda A, Ohno
K, Ichihara M, Kojima T, Nozawa Y and Ito M: Molecular hydrogen
suppresses FcepsilonRI-mediated signal transduction and prevents
degranulation of mast cells. Biochem Biophys Res Commun.
389:651–656. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kamimura N, Nishimaki K, Ohsawa I and Ohta
S: Molecular hydrogen improves obesity and diabetes by inducing
hepatic FGF21 and stimulating energy metabolism in db/db mice.
Obesity (Silver Spring). 19:1396–1403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshida A, Asanuma H, Sasaki H, Sanada S,
Yamazaki S, Asano Y, Shinozaki Y, Mori H, Shimouchi A, Sano M, et
al: H(2) mediates cardioprotection via involvements of K(ATP)
channels and permeability transition pores of mitochondria in dogs.
Cardiovasc Drugs Ther. 26:217–226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun Q, Kang Z, Cai J, Liu W, Liu Y, Zhang
JH, Denoble PJ, Tao H and Sun X: Hydrogen-rich saline protects
myocardium against ischemia/reperfusion injury in rats. Exp Biol
Med (Maywood). 234:1212–1219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ohsawa I, Nishimaki K, Yamagata K,
Ishikawa M and Ohta S: Consumption of hydrogen water prevents
atherosclerosis in apolipoprotein E knockout mice. Biochem Biophys
Res Commun. 377:1195–1198. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qian L, Cao F, Cui J, Wang Y, Huang Y,
Chuai Y, Zaho L, Jiang H and Cai J: The potential cardioprotective
effects of hydrogen in irradiated mice. J Radiat Res. 51:741–747.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Noda K, Shigemura N, Tanaka Y, Kawamura T,
Lim S Hyun, Kokubo K, Billiar TR, Bermudez CA, Kobayashi H and
Nakao A: A novel method of preserving cardiac grafts using a
hydrogen-rich water bath. J Hear Lung Transplant. 32:241–250. 2013.
View Article : Google Scholar
|
|
19
|
Yu YS and Zheng H: Chronic hydrogen-rich
saline treatment reduces oxidative stress and attenuates left
ventricular hypertrophy in spontaneous hypertensive rats. Mol Cell
Biochem. 365:233–242. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Regulations of People's Republic of China
for Administration of Laboratory Animals. State Science and
Technology Commission; Beijing: 1988
|
|
21
|
Oharazawa H, Igarashi T, Yokota T, Fujii
H, Suzuki H, Machide M, Takahashi H, Ohta S and Ohsawa I:
Protection of the retina by rapid diffusion of hydrogen:
Administration of hydrogen-loaded eye drops in retinal
ischemia-reperfusion injury. Investig Ophthalmol Vis Sci.
51:487–492. 2010. View Article : Google Scholar
|
|
22
|
Ma Y, Chen Y, Yang Y, Chen B, Liu D, Xiong
Z, Zhang C and Dong Y: Proteasome inhibition attenuates heart
failure during the late stages of pressure overload through
alterations in collagen expression. Biochem Pharmacol. 85:223–233.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ohta S: Recent progress toward hydrogen
medicine: Potential of molecular hydrogen for preventive and
therapeutic applications. Curr Pharm Des. 17:2241–2252. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ohta S: Molecular hydrogen as a preventive
and therapeutic medical gas: Initiation, development and potential
of hydrogen medicine. Pharmacol Ther. 144:1–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hayashida K, Sano M, Ohsawa I, Shinmura K,
Tamaki K, Kimura K, Endo J, Katayama T, Kawamura A, Kohsaka S, et
al: Inhalation of hydrogen gas reduces infarct size in the rat
model of myocardial ischemia-reperfusion injury. Biochem Biophys
Res Commun. 373:30–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hayashida K, Sano M, Kamimura N, Yokota T,
Suzuki M, Maekawa Y, Kawamura A, Abe T, Ohta S, Fukuda K and Hori
S: H(2) gas improves functional outcome after cardiac arrest to an
extent comparable to therapeutic hypothermia in a rat model. J Am
Heart Assoc. 1:e0034592012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hayashi T, Yoshioka T, Hasegawa K,
Miyamura M, Mori T, Ukimura A, Matsumura Y and Ishizaka N:
Inhalation of hydrogen gas attenuates left ventricular remodeling
induced by intermittent hypoxia in mice. Am J Physiol Heart Circ
Physiol. 301:H1062–H1069. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kearney PM, Whelton M, Reynolds K, Muntner
P, Whelton PK and He J: Global burden of hypertension: Analysis of
worldwide data. Lancet. 365:217–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lazzeroni D, Rimoldi O and Camici PG: From
left ventricular hypertrophy to dysfunction and failure. Circ J.
80:555–564. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sawyer DB, Siwik DA, Xiao L, Pimentel DR,
Singh K and Colucci WS: Role of oxidative stress in myocardial
hypertrophy and failure. J Mol Cell Cardiol. 34:379–388. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Van Kimmenade RR and Januzzi JL Jr:
Emerging biomarkers in heart failure. Clin Chem. 58:127–138. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dhalla AK, Hill MF and Singal PK: Role of
oxidative stress in transition of hypertrophy to heart failure. J
Am Coll Cardiol. 28:506–514. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu S, Zhu L, Yang J, Fan Z, Dong Y, Luan
R, Cai J and Fu L: Hydrogen-containing saline attenuates
doxorubicin-induced heart failure in rats. Pharmazie. 69:633–636.
2014.PubMed/NCBI
|
|
35
|
Takimoto E and Kass DA: Role of oxidative
stress in cardiac hypertrophy and remodeling. Hypertension.
49:241–248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsutsui H, Kinugawa S and Matsushima S:
Oxidative stress and heart failure. Am J Physiol Heart Circ
Physiol. 301:H2181–H2190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Barcellos-Hoff MH and Dix TA:
Redox-mediated activation of latent transforming growth factor-beta
1. Mol Endocrinol. 10:1077–1083. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kuwahara F, Kai H, Tokuda K, Kai M,
Takeshita A, Egashira K and Imaizumi T: Transforming growth
factor-beta function blocking prevents myocardial fibrosis and
diastolic dysfunction in pressure-overloaded rats. Circulation.
106:130–135. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Y: Mitogen-activated protein kinases
in heart development and diseases. Circulation. 116:1413–1423.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen CH, Manaenko A, Zhan Y, Liu WW,
Ostrowki RP, Tang J and Zhang JH: Hydrogen gas reduced acute
hyperglycemia-enhanced hemorrhagic transformation in a focal
ischemia rat model. Neuroscience. 169:402–414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cardinal JS, Zhan J, Wang Y, Sugimoto R,
Tsung A, McCurry KR, Billiar TR and Nakao A: Oral hydrogen water
prevents chronic allograft nephropathy in rats. Kidney Int.
77:101–109. 2010. View Article : Google Scholar : PubMed/NCBI
|