Introduction
Stress is a well-known environmental factor that can
affect human and animals, resulting in behavioral alterations and
various diseases (1). In addition,
it is considered a physiological or psychological response induced
by adverse stimuli, which may disturb the functioning of an
organism (2). It has previously
been reported that cognitive impairments are common in
stress-associated mental disorders, and chronic stress was able to
impair learning and memory (3).
Furthermore, exposure to chronic stress results in atrophy and
functional impairment in several key brain regions, including the
frontal cortex and hippocampus (4). The hippocampus, which is a limbic
region important in learning and memory, is particularly
susceptible to chronic stress (5,6).
Chronic restraint stress (CRS) is a specific and
classic method that can be used to simultaneously induce
psychological and physiological stress. Previous studies have
demonstrated that CRS is able to impair cognitive
hippocampus-dependent functions, particularly learning and memory
(7,8). Brain-derived neurotrophic factor
(BDNF) serves an important role in hippocampal learning and memory,
and is affected by CRS; this has been hypothesized to be one of the
main mechanisms underlying the impaired cognitive function
(9,10). Although previous studies have
focused on this mechanism (9,10),
at present, few studies have investigated the effectiveness of
therapeutic agents for the treatment of chronic stress-induced
cognitive deficits.
Resveratrol is a natural polyphenolic compound
present in grapes and red wine, which exhibits numerous beneficial
properties, including anticancer, antioxidant, anti-aging,
anti-inflammatory and antiallergenic activities (11). Previous studies have demonstrated
that resveratrol exerts powerful neuroprotective effects (12,13).
Notably, it has been demonstrated that resveratrol protects against
cognitive impairment in animal models of Alzheimer's disease,
depression or vascular dementia (14–16).
The underlying protective mechanisms of resveratrol are associated
with the upregulation of cAMP response element-binding protein-BDNF
or the reduction of oxidative stress. A recent study revealed that
chronic resveratrol administration exhibited beneficial effects on
spatial memory retention following global cerebral ischemia
(17). However, the protective
effects of resveratrol on CRS-induced cognitive deficits and the
underlying molecular mechanisms remain unclear. Therefore, the
present study aimed to explore the neuroprotective effects of
resveratrol on CRS-induced cognitive dysfunction and its molecular
mechanisms.
Materials and methods
Animals
A total of 50 adult male Wistar rats (age, 8 weeks;
weight, 180–200 g) were purchased from Laboratory Animal Center,
Shandong University (Jinan, China). The rats were maintained under
standard laboratory conditions (temperature, 20±2°C; 12-h
light/dark cycle) and had free access to food and water. Animals
were acclimated to laboratory conditions for 1 week prior to
experimentation. The experimental protocol was approved by the
Ethics Committee of Shandong University.
Drug administration and experimental
groups
Resveratrol was purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany), and was dissolved in absolute ethanol
before being diluted to the desired concentration. Resveratrol was
administered intraperitoneally at a dose of 80 mg/kg to rats daily
for 21 days. Fluoxetine was purchased from Lilly S.A. (Madrid,
Spain). Fluoxetine (10 mg/kg), freshly suspended in saline, was
administered intragastrically daily for 21 days.
The rats were divided into five groups: The control,
resveratrol, CRS, CRS + resveratrol, and CRS + fluoxetine groups.
All drugs were administered 30 min prior to exposure to CRS for 21
days.
CRS procedure
The restrainers were comprised of transparent
plastic tubes (height, 5 cm; width, 5.5 cm; length, 22 cm). The
rats were subjected to 6 h of restraint stress daily for 21 days,
during which they were deprived of food and water, without physical
suppression.
Open field test
On day 22, an open field test was conducted.
Locomotor activity was measured in an open field, which consisted
of a 90×90 cm gray wooden floor divided into 36 equal squares by
red lines, surrounded by 45 cm high boundary walls. Each rat was
placed in the center of the floor and behavioral parameters,
including crossing, rearing and grooming, were counted for 5
min.
Morris water maze (MWM) test
On days 23–28 a MWM test was conducted. Spatial
learning and memory performance was determined using the MWM test,
as previously described with minor modifications (18). The MWM consisted of a black
cylindrical tank (diameter, 120 cm) with a circular escape platform
(diameter, 10 cm). The tank was filled with water (21–24°C) that
was dyed with the addition of an atoxic acrylic black dye. The maze
was divided into four equal quadrants and the platform was
submerged 2 cm beneath the surface of the water. A tracking system
was used to measure the performance of the rats (SMART polyvalent
video-tracking system; Panlab, Barcelona, Spain). Each rat was
subjected to 5 consecutive acquisition trials to find the hidden
platform. The rats were allowed 60 sec to locate the hidden
platform. The time taken to escape onto the hidden platform (escape
latency) was recorded by the tracking software. A total of 24 h
following the navigation test, the probe test was performed to
assess reference memory, during which the platform was withdrawn.
The activity of the rats was monitored for 60 sec. The latency to
enter the target quadrant and the total time spent in the target
quadrant were recorded.
Novel object recognition task
(NORT)
On days 29–31 the NORT experiment was conducted. The
basic design of the NORT was similar to that described by Torner
et al (19). The apparatus
was a Plexiglas cage (60×40×40 cm) with an exchangeable floor. A
total of 2 days prior to testing the rats were allowed to explore
for 15 min. The objects to be discriminated were water-filled
plastic bottles and their weight was such that the rats could not
displace them. The objects and the apparatus were cleaned to remove
the olfactory cues after each trial.
Each session consisted of two trials: Acquisition
phase and retrieval phase. In the acquisition phase, two different
objects were placed in the box and the animals were allowed to
explore for 10 min. The inter-trial interval was 60 min. During the
retrieval phase, one object from the acquisition phase was replaced
with a duplicate object, and the rats were allowed to explore for a
further 10 min. The objects and their positions were
counterbalanced within each session.
The measure was the time spent by rats exploring the
objects. A ratio reflecting the discrimination between the novel
and the familiar object was calculated, as follows: Novel/(novel +
familiar time).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Following the NORT, rats were sacrificed and their
hippocampi were dissected. Total RNA was isolated from the rat
hippocampus using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Total RNA (2 µg) was then
reverse-transcribed into cDNA using a commercial RT-PCR kit
(Fermentas; Thermo Fisher Scientific, Inc.) according to
manufacturer's protocol. A total of 20 µl cDNA was subjected to
qPCR amplification using the UltraSYBR mixture (Cowin Bioscience
Co., Ltd., Beijing, China) according to the manufacturer's
protocol. The thermocycling protocol was as follows: Initial
denaturation at 95°C for 10 min, followed by 40 cycles at 95°C for
10 sec, and at 58°C for 30 sec. At the end of the PCR reaction, a
melting curve was obtained by holding the temperature at 95°C for
15 sec, cooling to 60°C for 1 min, and heating slowly at a rate of
0.5°C/s up to 95°C. The primer sequences were as follows: BDNF
forward, 5′-AGCTGAGCGTGTGTGACAGT-3′ and reverse,
5′-ACCCATGGGATTACACTTGG-3′; and β-actin forward,
5′-TGGAATCCTGTGGCATCCATGAAAC-3′ and reverse,
5′-TAAAACGCAGCTCAGTAACAGTCCG-3′. Relative expression levels of BDNF
were analyzed using the 2−ΔΔCq method and normalized to
the expression of GAPDH (20).
Determination of BDNF expression
BDNF protein expression was measured in hippocampal
tissue using an ELISA kit (cat no. EK0309; Wuhan Boster Biological
Technology, Ltd., Wuhan, China), according to the manufacturer's
protocol. The samples and protein standards were added to a 96-well
plate. After incubation and washing, the detection antibody was
added to the wells, followed by the addition of a horseradish
peroxidase conjugate. After incubation and washing, the stop
solution was added. The optical density was measured at 450 nm
using a microplate reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Statistical analysis
Statistical analysis was performed using SPSS
software version 19.0 (IBM Corp., Armonk, NY, USA). Data are
presented as the mean ± standard deviation of 4–6 independent
experiments. Data from the MWM test were analyzed with repeated
measures analysis of covariance (ANCOVA) followed by a post hoc
Tukey-Kramer test. The other data were analyzed by one-way analysis
of variance followed by a post hoc Tukey honest significant
difference test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of CRS and resveratrol on
locomotor activity in the open field test
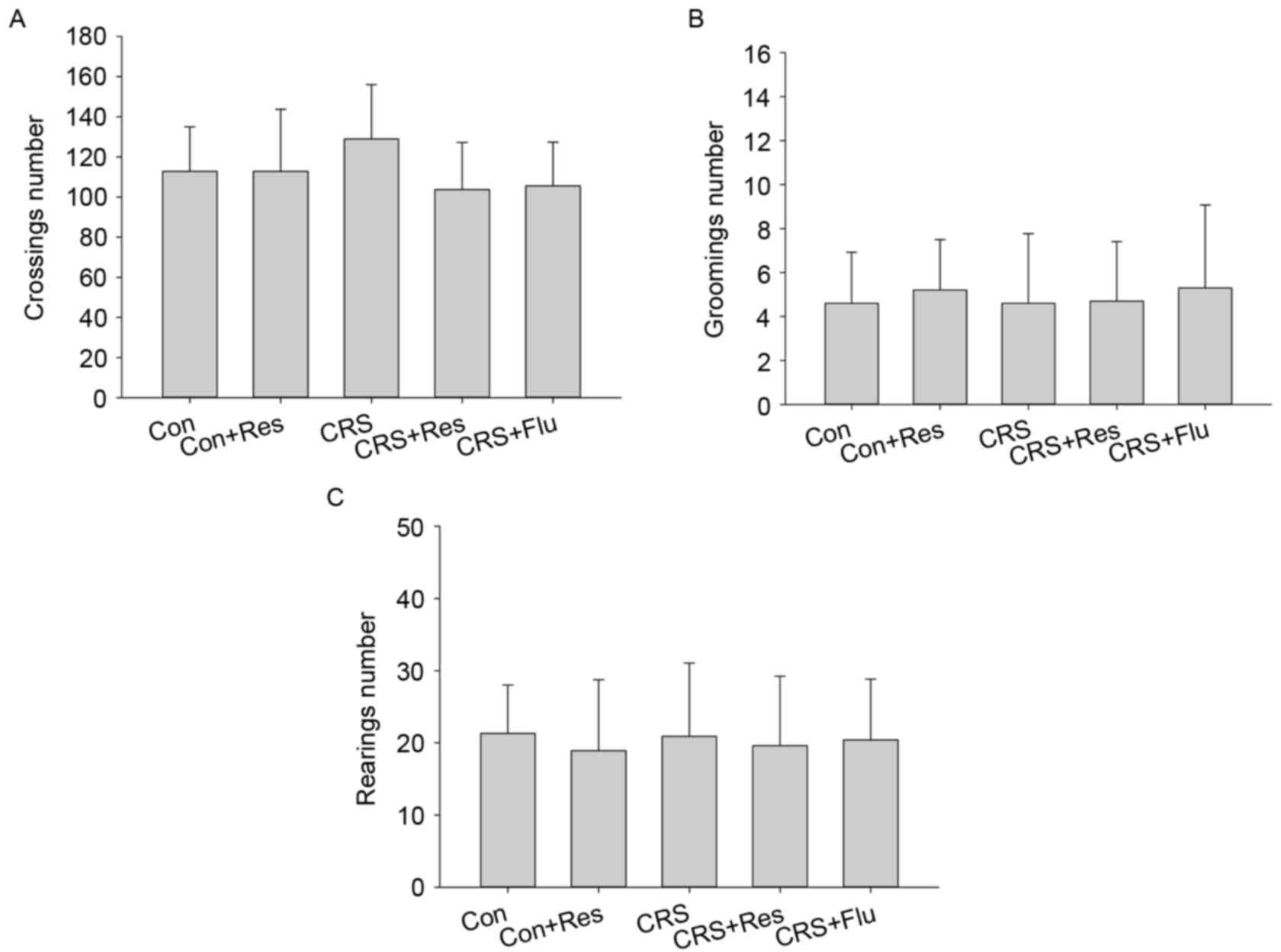
As presented in Fig.
1, analysis of the results obtained in the open field test
yielded no statistically significant differences in the number of
crossings [F(4,49)=1.535, P>0.05], and grooming [F(4,49)=0.138,
P>0.05] and rearing behaviors [F(4, 49)=0.116, P>0.05]
between the groups.
Effects of CRS and resveratrol on
learning and memory in MWM test
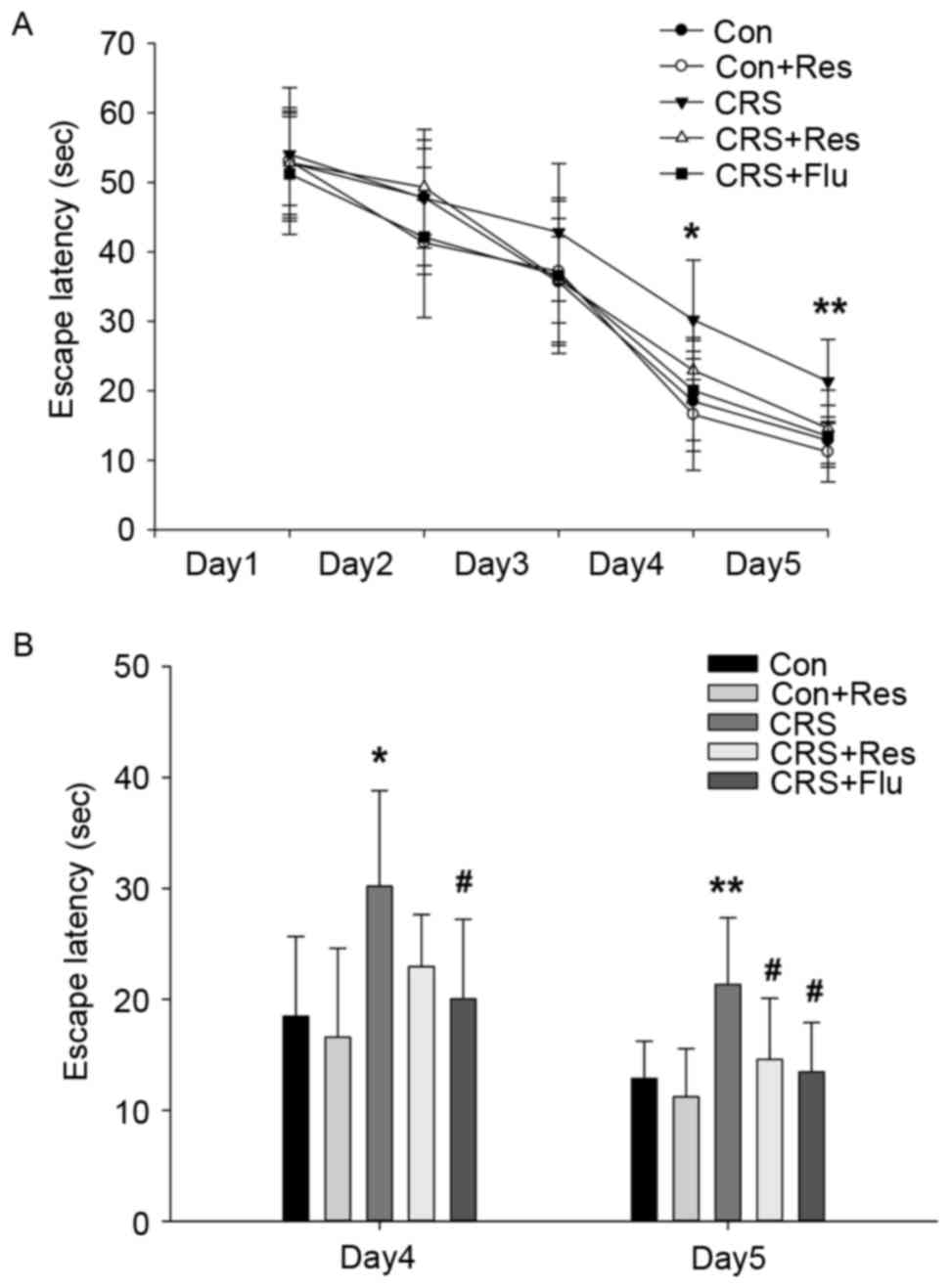
As presented in Fig.
2A, with regards to learning, a repeated measures ANCOVA of
escape latency revealed a significant improvement during the 5 days
of training [F(4,49) =210.343, P<0.001], however there was no
association between treatments and days [F(4,49=0.998, P>0.05],
suggesting that animals in each group behaved similarly regardless
of the previous treatments. In addition, the stressed rats reached
the platform significantly later than the control rats on days 4
(P<0.05) and 5 (P<0.01). Treatment with resveratrol and
fluoxetine significantly decreased the latency compared with the
CRS group on day 5 (P<0.05, Fig.
2B).
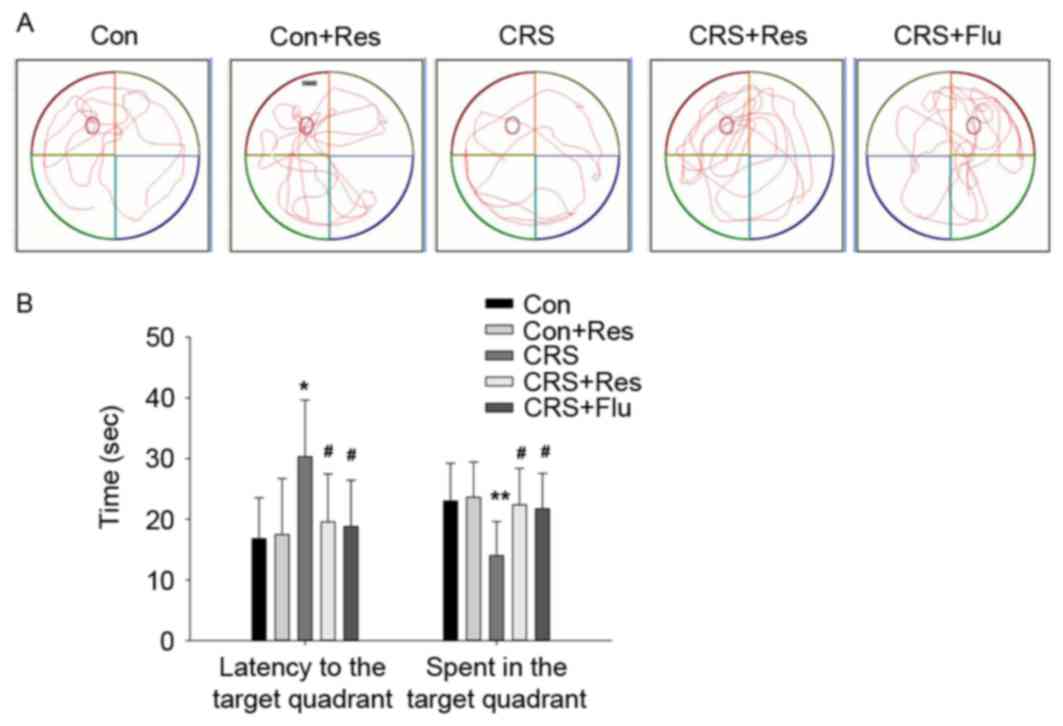
As shown in Fig. 3,
rats exposed to CRS spent more time reaching the platform compared
with the control group (P<0.05); however, treatment with
resveratrol or fluoxetine significantly decreased the latency to
reach the platform compared with the rats in the CRS group
(P<0.05).
Furthermore, compared with the control group, rats
in the CRS group spent less time in the target quadrant
(P<0.01), whereas administration of resveratrol or fluoxetine
significantly increased the time spent in the target quadrant
compared with the CRS group (P<0.05, Fig. 3B).
Effects of CRS and resveratrol
administration on NORT
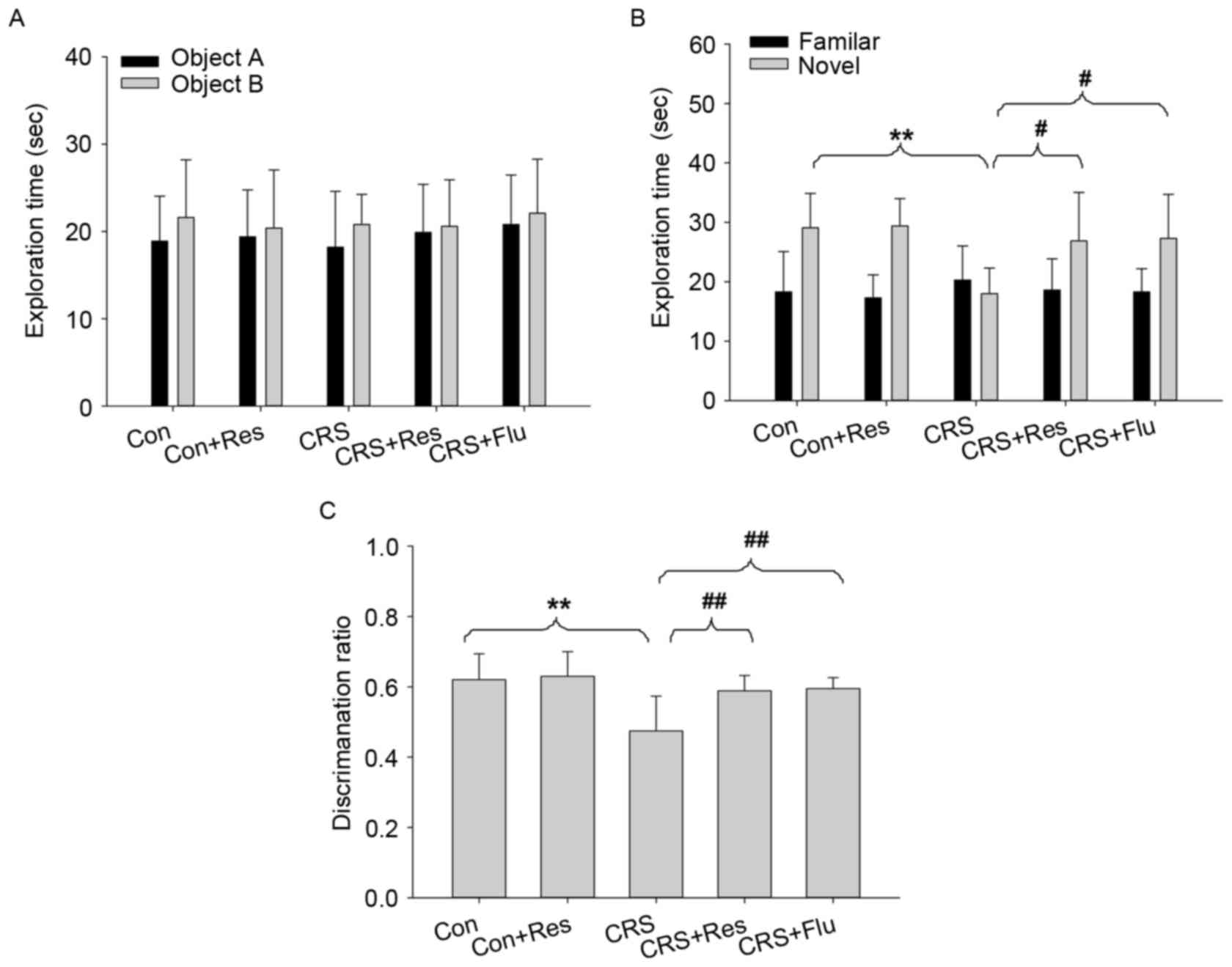
As presented in Fig.
4A, there were no significant differences in the exploration
time between the groups during the acquisition phase
[F(4,49)=0.306, P>0.05]. However, the results of an ANOVA
revealed significant alterations in the exploration time between
the groups in the retrieval phase [F(4,49)=5.563, P<0.01]. Post
hoc analysis demonstrated that rats exposed to CRS exhibited a
shorter exploration time compared with the control group
(P<0.01). Furthermore, rats treated with resveratrol or
fluoxetine spent longer exploring the novel object during the
retrieval phase compared with the CRS group (P<0.05, Fig. 4B). As shown in Fig. 4C, rats in the CRS group exhibited a
decreased discrimination ratio compared with the control group
(P<0.01), whereas treatment with resveratrol or fluoxetine
significantly increased the discrimination ratio (P<0.01).
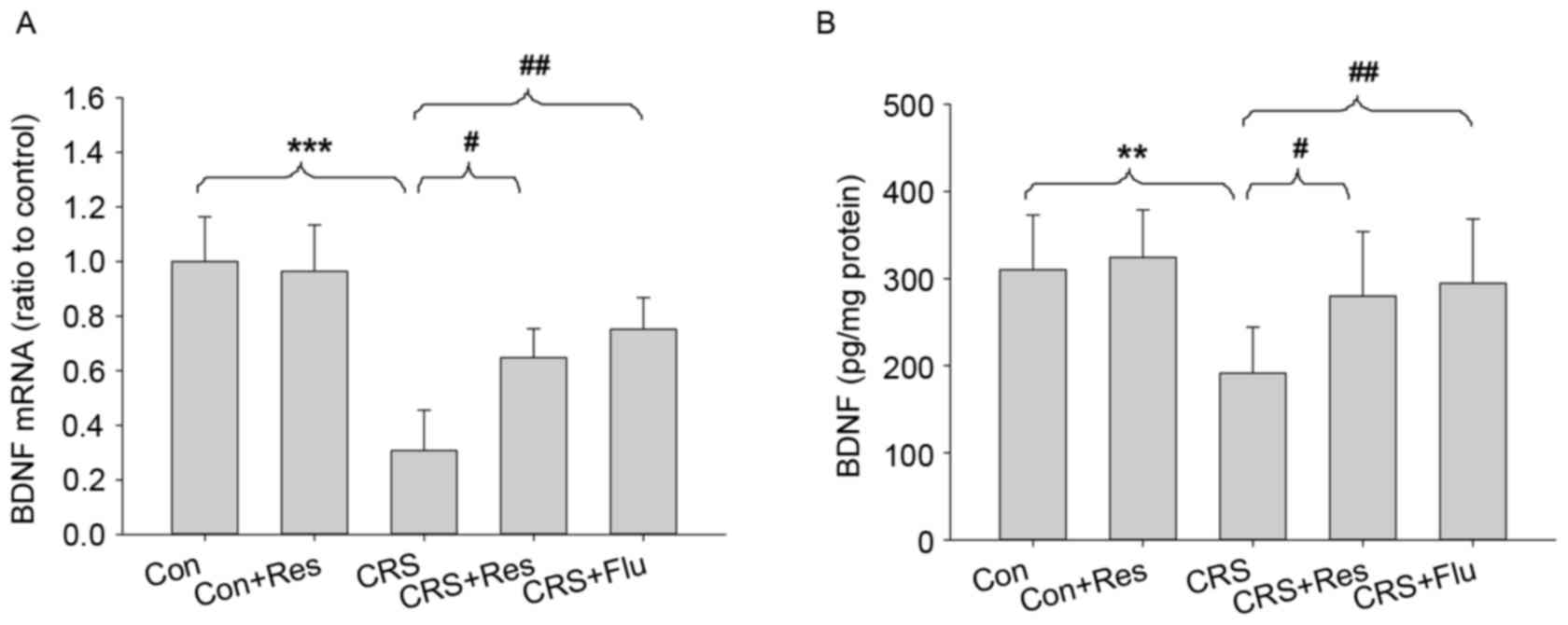
Effects of CRS and resveratrol on BDNF
expression
As presented in Fig.
5, there were significant alterations in the BDNF mRNA [F(4,
49)=15.299, P<0.001] and protein [F(4,49)=6.234, P<0.001]
expression levels between the groups in the hippocampus. CRS
significantly decreased BDNF mRNA (P<0.001, Fig. 5A) and protein levels in the
hippocampus (P<0.01, Fig. 5B)
compared with the control rats. In addition, administration of
resveratrol or fluoxetine markedly reversed CRS-induced reductions
in BDNF mRNA and protein expression (Fig. 5).
Discussion
The present study demonstrated that treatment with
resveratrol prevented CRS-induced cognitive impairment in rats. In
addition, resveratrol increased the mRNA and protein expression
levels of BDNF in the hippocampus of CRS-exposed rats. These
results were similar to those observed in rats treated with
fluoxetine.
To investigate whether resveratrol can alleviate
CRS-induced impaired cognitive function, MWM test and NORT were
used to explore learning and memory ability. In the MWM test, rats
treated with resveratrol took less time to reach the platform
compared with the rats in the CRS group. In addition, treatment
with resveratrol increased the time spent in the target quadrant
compared with the rats in the CRS group. These data indicated that
resveratrol was able to prevent CRS-induced learning and memory
impairment in rats. In the open field test, the results revealed
that there was no statistical difference among the experimental
groups, indicating that CRS and drug administration had no effect
on locomotor activity, which would not interfere with performance
in the MWM test. Similar results were also obtained in the NORT and
these data were comparable to those in the rats treated with
fluoxetine. These findings suggested that resveratrol may exhibit a
protective role in alleviating CRS-induced cognitive
impairment.
The hippocampus is one of the most important regions
in the brain, and is a primary structure for learning and memory
(21). BDNF, which is an important
member of the neurotrophin family of growth factors, is widely
distributed in the hippocampus and cerebral cortex (22). BDNF helps to support the survival
of existing neurons, encourages the growth and differentiation of
neurons and synapses (23), and
promotes the formation of long-term memory (24). Previous studies have demonstrated
that CRS decreases the expression of BDNF in the hippocampus and
induces impaired cognitive function (25,26).
Recently, resveratrol has been reported to increase the expression
of BDNF in various in vivo and in vitro models
(27–29). In addition, it has been indicated
that resveratrol is able to reverse stress-induced impaired
cognitive function (15,30). Consistent with these findings, the
results of the present study demonstrated that resveratrol was able
to prevent CRS-induced cognitive deficit in rats, as evidenced by
the MWM test and NORT. Furthermore, the CRS-induced decrease in
BDNF mRNA and protein expression was reversed by treatment with
resveratrol. These results suggested that the molecular mechanism
underlying the protective effects of resveratrol may be associated
with upregulation of hippocampal BDNF expression in rats.
In conclusion, the present study demonstrated that
resveratrol may serve a crucial role in preventing CRS-induced
cognitive deficits in rats. It may be hypothesized that these
neuroprotective effects may be due to resveratrol-mediated
alterations in BDNF expression in the hippocampus. These results
indicated the possibility of using resveratrol as a therapeutic
agent to prevent stress-induced cognitive impairment.
Acknowledgements
The present study was supported by funding from the
National Natural Science Foundation of China (grant no. 81200879)
and the Fundamental Research Funds of Shandong University (grant
no. 2015JC008).
References
|
1
|
Dayi A, Cetin F, Sisman AR, Aksu I, Tas A,
Gönenc S and Uysal N: The effects of oxytocin on cognitive defect
caused by chronic restraint stress applied to adolescent rats and
on hippocampal VEGF and BDNF levels. Med Sci Monit. 21:69–75. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johnson EO, Kamilaris TC, Chrousos GP and
Gold PW: Mechanisms of stress: A dynamic overview of hormonal and
behavioral homeostasis. Neurosci Biobehav Rev. 16:115–130. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mohammadi HS, Goudarzi I, Lashkarbolouki
T, Abrari K and Salmani M Elahdadi: Chronic administration of
quercetin prevent spatial learning and memory deficits provoked by
chronic stress in rats. Behav Brain Res. 270:196–205. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Y, Kan H, Yin Y, Wu W, Hu W, Wang M
and Li W and Li W: Protective effects of ginsenoside Rg1 on chronic
restraint stress induced learning and memory impairments in male
mice. Pharmacol Biochem Behav. 120:73–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagata K, Nakashima-Kamimura N, Mikami T,
Ohsawa I and Ohta S: Consumption of molecular hydrogen prevents the
stress-induced impairments in hippocampus-dependent learning tasks
during chronic physical restraint in mice. Neuropsychopharmacology.
34:501–508. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aggleton JP, Vann SD, Oswald CJ and Good
M: Identifying cortical inputs to the rat hippocampus that subserve
allocentric spatial processes: A simple problem with a complex
answer. Hippocampus. 10:466–474. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ghadrdoost B, Vafaei AA, Rashidy-Pour A,
Hajisoltani R, Bandegi AR, Motamedi F, Haghighi S, Sameni HR and
Pahlvan S: Protective effects of saffron extract and its active
constituent crocin against oxidative stress and spatial learning
and memory deficits induced by chronic stress in rats. Eur J
Pharmacol. 667:222–229. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang RR, Hu W, Yin YY, Wang YC, Li WP and
Li WZ: Chronic restraint stress promotes learning and memory
impairment due to enhanced neuronal endoplasmic reticulum stress in
the frontal cortex and hippocampus in male mice. Int J Mol Med.
35:553–559. 2015.PubMed/NCBI
|
|
9
|
Ortiz JB, Mathewson CM, Hoffman AN,
Hanavan PD, Terwilliger EF and Conrad CD: Hippocampal brain-derived
neurotrophic factor mediates recovery from chronic stress-induced
spatial reference memory deficits. Eur J Neurosci. 40:3351–3362.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Radahmadi M, Alaei H, Sharifi MR and
Hosseini N: Effects of different timing of stress on
corticosterone, BDNF and memory in male rats. Physiol Behav.
139:459–467. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gambini J, Inglés M, Olaso G, Lopez-Grueso
R, Bonet-Costa V, Gimeno-Mallench L, Mas-Bargues C, Abdelaziz KM,
Gomez-Cabrera MC, Vina J and Borras C: Properties of resveratrol:
In vitro and in vivo studies about metabolism, bioavailability and
biological effects in animal models and humans. Oxid Med Cell
Longev. 2015:8370422015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsai SK, Hung LM, Fu YT, Cheng H, Nien MW,
Liu HY, Zhang FB and Huang SS: Resveratrol neuroprotective effects
during focal cerebral ischemia injury via nitric oxide mechanism in
rats. J Vasc Surg. 46:346–353. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kiziltepe U, Turan NN, Han U, Ulus AT and
Akar F: Resveratrol, a red wine polyphenol, protects spinal cord
from ischemia-reperfusion injury. J Vasc Surg. 40:138–145. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kumar A, Naidu PS, Seghal N and Padi SS:
Neuroprotective effects of resveratrol against
intracerebroventricular colchicine-induced cognitive impairment and
oxidative stress in rats. Pharmacology. 79:17–26. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu D, Zhang Q, Gu J, Wang X, Xie K, Xian
X, Wang J, Jiang H and Wang Z: Resveratrol prevents impaired
cognition induced by chronic unpredictable mild stress in rats.
Prog Neuropsychopharmacol Biol Psychiatry. 49:21–29. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma X, Sun Z, Liu Y, Jia Y, Zhang B and
Zhang J: Resveratrol improves cognition and reduces oxidative
stress in rats with vascular dementia. Neural Regen Res.
8:2050–2059. 2013.PubMed/NCBI
|
|
17
|
Girbovan C, Kent P, Merali Z and Plamondon
H: Dose-related effects of chronic resveratrol administration on
neurogenesis, angiogenesis, and corticosterone secretion are
associated with improved spatial memory retention following global
cerebral ischemia. Nutr Neurosci. 19:352–368. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morris R: Developments of a water-maze
procedure for studying spatial learning in the rat. J Neurosci
Methods. 11:47–60. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Torner L, Tinajero E, Lajud N,
Quintanar-Stéphano A and Olvera-Cortés E: Hyperprolactinemia
impairs object recognition without altering spatial learning in
male rats. Behav Brain Res. 252:32–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Estrada NM and Isokawa M: Metabolic demand
stimulates CREB signaling in the limbic cortex: Implication for the
induction of hippocampal synaptic plasticity by intrinsic stimulus
for survival. Front Syst Neurosci. 3:52009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamada K and Nabeshima T: Brain-derived
neurotrophic factor/TrkB signaling in memory processes. J Pharmacol
Sci. 91:267–270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang EJ and Reichardt LF: Neurotrophins:
Roles in neuronal development and function. Annu Rev Neurosci.
24:677–736. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bekinschtein P, Cammarota M, Katche C,
Slipczuk L, Rossato JI, Goldin A, Izquierdo I and Medina JH: BDNF
is essential to promote persistence of long-term memory storage.
Proc Natl Acad Sci USA. 105:2711–2716. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kwon DH, Kim BS, Chang H, Kim YI, Jo SA
and Leem YH: Exercise ameliorates cognition impairment due to
restraint stress-induced oxidative insult and reduced BDNF level.
Biochem Biophys Res Commun. 434:245–251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yan W, Zhang T, Jia W, Sun X and Liu X:
Chronic stress impairs learning and hippocampal cell proliferation
in senescence-accelerated prone mice. Neurosci Lett. 490:85–89.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ali SH, Madhana RMKVA, Kasala ER,
Bodduluru LN, Pitta S, Mahareddy JR and Lahkar M: Resveratrol
ameliorates depressive-like behavior in repeated
corticosterone-induced depression in mice. Steroids. 101:37–42.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song J, Cheon SY, Jung W, Lee WT and Lee
JE: Resveratrol induces the expression of interleukin-10 and
brain-derived neurotrophic factor in BV2 microglia under hypoxia.
Int J Mol Sci. 15:15512–15529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hurley LL, Akinfiresoye L, Kalejaiye O and
Tizabi Y: Antidepressant effects of resveratrol in an animal model
of depression. Behav Brain Res. 268:1–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yazir Y, Utkan T, Gacar N and Aricioglu F:
Resveratrol exerts anti-inflammatory and neuroprotective effects to
prevent memory deficits in rats exposed to chronic unpredictable
mild stress. Physiol Behav. 138:297–304. 2015. View Article : Google Scholar : PubMed/NCBI
|