Introduction
Asthma is a heterogeneous and chronic inflammatory
disease that is defined by a history of respiratory symptoms and
variable expiratory flow limitation (1). The dynamics of airway function are
influenced by a number of distinct smooth muscles (2). Ca2+ signaling serves an
important role in the cellular processes that are known to be
altered in the airway smooth muscle cells (ASMCs) of subjects with
asthma, including contractility, proliferation, migration and
secretion of inflammatory mediators (3).
Various Ca2+ influx pathways exist in the
plasma membranes of ASMCs, including voltage-gated Ca2+
channels, receptor-operated Ca2+ channels,
store-operated Ca2+ (SOC) channels and
Na+/Ca2+ exchangers (4). Experimental and clinical studies have
demonstrated that voltage-gated Ca2+ channel blockers
exert no obvious effects on the regulation of airway
hyperresponsiveness (5–7); by contrast, SOC channel blockers have
been demonstrated to be effective in attenuating airway
hyperresponsiveness in ovalbumin-sensitized guinea pigs (8). SOC entry (SOCE) serves an important
role in regulating Ca2+ signaling, and the cellular
responses and hyperplasia of ASMCs (9–11).
Ca2+ influx through calcium
release-activated channels (CRAC) via SOCE has been proposed to be
associated with the proliferation of ASMCs (9). The knockdown of stromal interaction
molecule 1 (STIM1) or calcium release-activated calcium modulator 1
(ORAI1) using small interfering RNA resulted in a decrease in SOCE
in response to store depletion by histamine or thapsigargin in
human ASMCs, indicating that STIM1 and ORAI1 are important
contributors to SOCE in ASMCs exhibiting hyperplasia (12,13).
Additionally, ORAI1 has been reported to interact with short
transient receptor potential channel member 1 (TRPC1) and forms a
ternary complex with STIM1 in the plasma membrane (14,15).
TRPC1, a molecular candidate component of SOC channels, has been
observed in proliferative porcine ASMCs (9). Therefore, Ca2+ influx
through SOCE appears to be important for the regulation of ASMC
proliferation.
Structural alterations induced by pathological
repair mechanisms, termed airway remodeling, are a consequence of
chronic inflammation and mechanical forces in airways exacerbated
by asthma (16). β1 integrin is
widely-expressed in the airway and the expression is altered in
asthma, particularly in ASMCs (17). β1 integrin is associated with cell
proliferation, airway and vascular remodeling, obstruction, and
hyperresponsiveness (18–20). An increase in β1 integrin
expression was observed to be associated with inflammation,
fibrosis and airway hyperresponsiveness (20–22).
Short hairpin (sh)RNA targeting β1 integrin markedly promoted
cellular apoptosis, and inhibited cell proliferation, migration,
and interleukin (IL)-6 and IL-8 secretion in vitro (19).
It was hypothesized that silencing of the β1
integrin gene may inhibit ASMC proliferation and allergic
inflammation by attenuating the transcription of SOCE-associated
genes. The present study assessed ASM thickness, and the expression
of six mRNAs and two inflammatory cytokines. The regulatory effect
of silencing the β1 integrin gene may increase the understanding of
the complex mechanism of airway remodeling and provide a basis for
novel treatments of asthma.
Materials and methods
Animal randomization and modeling
A total of 36 3–4-week-old female BALB/c mice were
purchased from the Guangdong Medical Laboratory Animal Center
(Foshan, China) and housed in 6 chambers (6 mice each) with a
temperature of 24±3°C, humidity 60±4%, free access to food and
water, and a 12-h light/dark cycle. Mice were divided into six
groups (6 mice/group) using a random number table: group C, control
group; group A, asthma group; group T, transfection group; group
BC, blank control group; group NC, negative control group; and
group PC, positive control group.
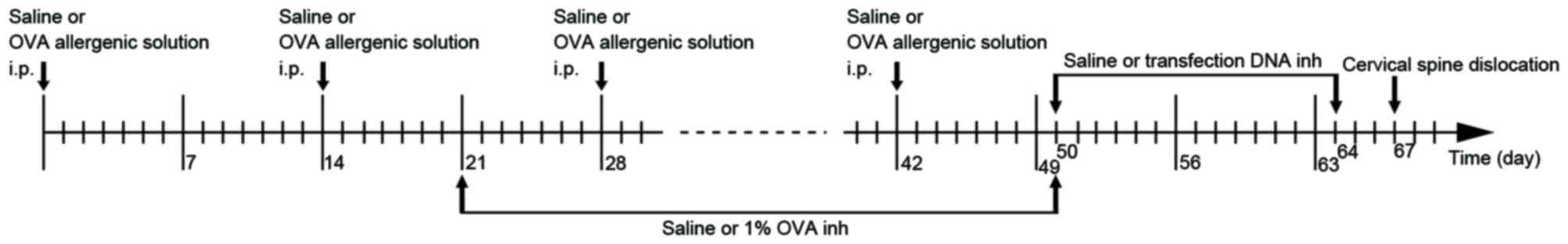
On days 0, 14, 28 and 42, each mouse in group C was
injected with 0.2 ml saline into the abdomen, and each mouse in
groups A, T, BC, NC and PC were injected with 0.2 ml allergenic
solution [10% ovalbumin (OVA; Sigma-Aldrich, Merck KGaA, Darmstadt,
Germany) +10% Al(OH)3]. Between days 21 and 50 (3
times/week for 4 weeks), atomized saline was administered to the
mice in group C and atomized 1% OVA was administered to the mice in
the other groups for 30 mins in a glass test container (30×15×20
cm; Fig. 1). Between days 50 and
64, the mice in group BC were administered 0.2 ml atomized saline,
and those in groups T, NC and PC were administered atomized
transfection liquid, for 15 mins in a glass container every 2 days
(Fig. 1). The atomized
transfection liquid consisted of 40 µg transfection DNA, 6 µl
transfection reagent (Polyplus-transfection SA, Illkirch, France)
and 5 ml 5% glucose solution. β1 integrin shRNA, missense chain and
GAPDH were used in groups T, NC and PC, respectively. All of the
mice were sacrificed by cervical dislocation on the 67th day. The
flowchart of the establishment of the mouse model is presented in
Fig. 1. The present study was
approved by the Ethics Committee of Shenzhen People's Hospital
(Shenzhen, China).
shRNA synthesis and vector
construction/verification
According to the gene information for β1 integrin in
GenBank (no. NM_16412; www.ncbi.nlm.nih.gov/genbank), the 425th nucleotide in
the gene encoding region was selected as the initial shRNA target
point. Target gene sequences with a GC content between 40 and 55%
were selected for potential optimization. Basic Local Alignment
Search Tool (www.ncbi.nlm.nih.gov/blast) retrieval was used in the
expressed sequence tag database in GenBank. The selected sequence
and the corresponding genome database were compared to eliminate
homology with other coding sequences and to determine specificity.
The efficiencies of sequences in inhibiting the mRNA expression of
β1 integrin were assessed using the reverse
transcription-quantitative polymerase chain reaction (RT-qPCR). The
missense chain was also selected based on β1 integrin gene. An
siRNA against GAPDH was included as a positive control to verify
transfection reliability, RNA extraction and gene expression
quantification. All shRNA sequences used were as follows: β1
integrin,
5′-CACCGCAGGGCCAAATTGTGGGTTTCAAGAGAACCCACAATTTGGCCCTGCTTTTTTG-3′;
missense chain,
5′-CACCGCAGGGCCAAATTGTGGGTTTCAAGAGAACCCACAATTTGGCCCTGCTTTTTTG-3′;
GAPDH,
5′-CACCGTATGACAACAGCCTCAAGTTCAAGAGACTTGAGGCTGTTGTCATACTTTTTTG-3′.
Subsequent to connecting the carrier to the shRNA
section, PCR and electrophoresis were performed. The recombinant
positive clone fragments were subjected to sequencing by Guangzhou
Genewiz Biotechnology (Guangzhou, China). The experiment also
included missense chain as a negative control, and GAPDH as a
positive control.
Specimen separation and
collection
The left lung was ligated following separation of
the trachea, bronchus and lung tissues. Tissue specimens were
frozen in liquid nitrogen for subsequent RNA extraction. The right
lung was perfused in 4% polyformaldehyde solution using a 24G
indwelling needle. The right middle lobe was separated, ligated and
preserved in 4% paraformaldehyde.
Hematoxylin and eosin staining and
measurement of ASM thickness
The tissue was embedded in paraffin and sectioned at
4 µm. Sections were deparaffinized by immersion in xylene and dyed
with hematoxylin for 3 mins at room temperature. Sections were
washed three times in ddH20 and placed in 85% acid ethanol for 2
mins. The sections were subsequently dyed with eosin for 5 mins and
dehydrated through graded alcohols (90, 80 and 70%). The tissues
were soaked in xylene, dried and the morphological alterations in
the stained sections were examined.
A total of four different cross-sectional airway
sections from each specimen were randomly selected to analyze under
light microscopy (magnification, ×200). ASM thickness was observed
and measured. The ASM thickness of each specimen was calculated
from the mean of the four airway cross-sections.
RNA extraction and RT-qPCR
analysis
Total RNA was extracted from tissue specimens using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), according to the manufacturer's protocol. cDNA
was generated using the PrimeScript™ RT reagent kit (DRR037A;
Takara Biotechnology Co., Ltd., Dalian, China), and amplified first
by PCR using the TaKaRa Ex Taq kit (RR001A; Takara Biotechnology
Co., Ltd.) to screen the primers, which were designed using Primer
3 software, version 0.4.0 (23),
based on data from Uniprot (www.uniprot.org). The thermocycling conditions for the
PCR were 95° for 30 sec to activate the DNA polymerase, followed by
40 cycles of 95°C for 5 sec, 55°C for 30 sec and 72°C for 30 sec.
Melting curve analysis was performed to verify a single product
without primer-dimers. The thermocycling conditions of qPCR were
the same as above. qPCR was performed on an iQ5 system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) using a SYBR Premix Ex Taq™
II kit (DRR081A; Takara Biotechnology Co., Ltd.). qPCR results were
analyzed using the 2−ΔΔCq method (24) and were normalized to β-actin.
Primer sequences are presented in Table I.
 | Table I.Primers used in the present
study. |
Table I.
Primers used in the present
study.
| Gene name | Gene ID | Forward primer | Reverse primer | Product size
(bp) | E, % |
|---|
| β actin | A1E281 |
AAGAGCTATGAGCTGCCTGA |
GTTGAAGGTAGTTTCGTGGA | 159 | 98 |
| β1 integrin | P09055 |
TTCAGACTTCCGCATTGGCT |
TGGAAAACACCAGCAGTCGT | 302 | 104 |
| α-SMA | P62737 |
CTCTGCCTCTAGCACACAACT |
ACGCTCTCAAATACCCCGTTT | 333 | 96 |
| STIM1 | P70302 |
GGTGGAGAAACTGCCTGACA |
CAACTGGAGATGGCGTGTCT | 188 | 102 |
| ORAI1 | Q8BWG9 |
CCACAACCTCAACTCGGTCA |
AACTGCCGGTCCGTCTTATG | 351 | 89 |
| TRPC1 | Q61056 |
AGTCCTTCGTTGGAGCTGTG |
TGCCTTTCGAGGTATGCGAG | 276 | 103 |
| NFAT2 | Q60591 |
ACCTGGCTTGGTAACACCAC |
GGGCTGTCTTTCGAGACTTG | 135 | 96 |
ELISA analysis of IL-4 and interferon
(IFN)-γ levels in serum
Blood samples were placed in serum separator tubes,
maintained at room temperature for 30 min and centrifuged for 15
min with 1,400 × g at 25°C. Serum was transferred into a 1.5-ml
centrifuge tube and stored at 4°C. The levels of IFN-γ and IL-4 in
the serum samples were determined using mouse IFN-γ kit DKW 12–2000
and IL-4 ELISA kit DKW12-2040 (Dakewe Biotech Co., Ltd., Shenzhen,
China), respectively, in accordance with the manufacturers'
protocol.
Statistical analysis
All data are presented as the mean ± standard error
of the mean. P<0.05 was considered to indicate as statistically
significant difference. All data represented the average of six
replicate experiments. The ASM thickness, RT-qPCR results, and IL-4
and IFN-γ levels were analyzed using one-way analysis of variance
followed by Student-Newman-Keuls post hoc tests. Statistical
analysis was performed using GraphPad Prism (version 6.02; GraphPad
Software, Inc., La Jolla, CA, USA).
Results
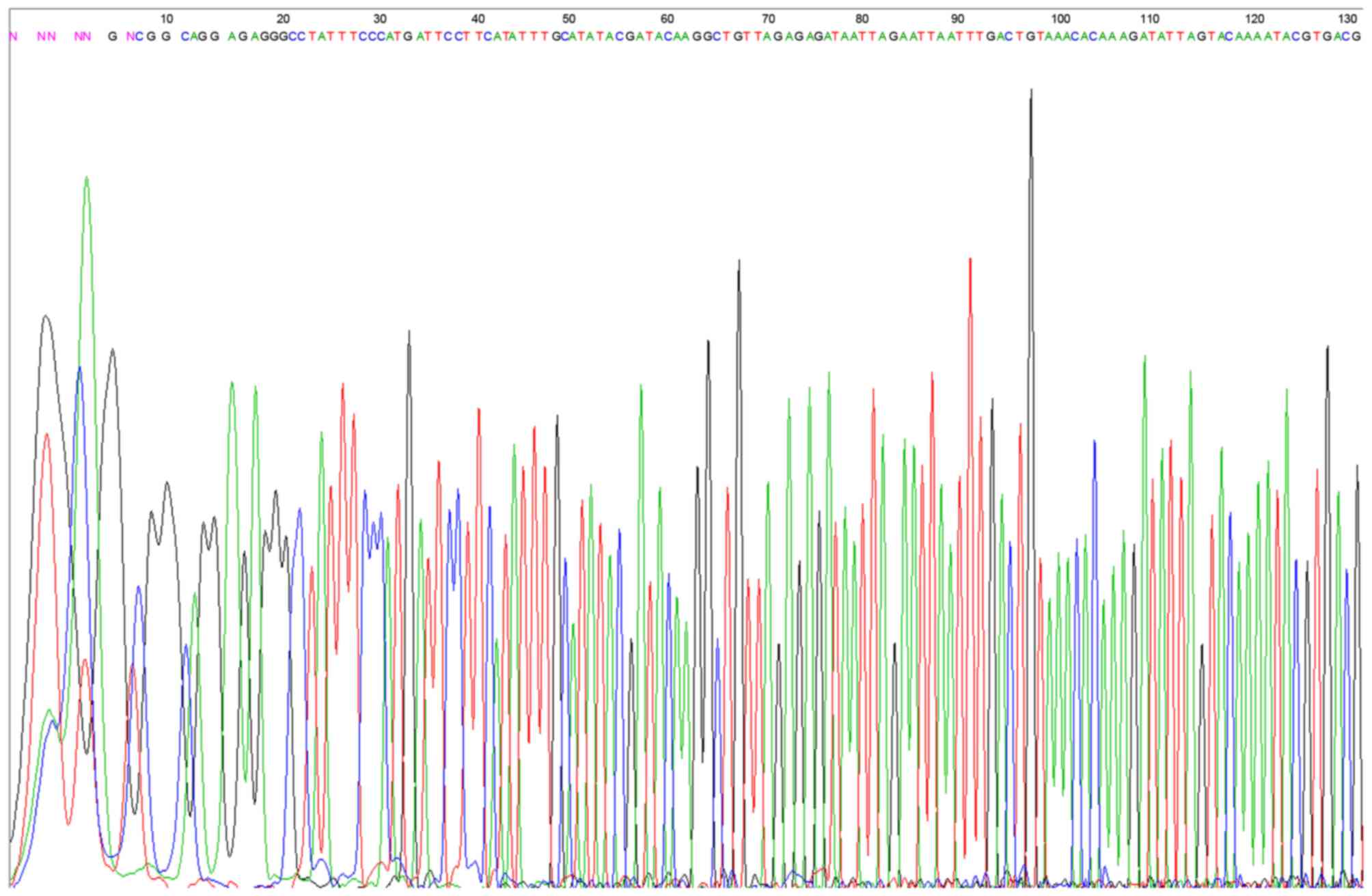
Identification of β1 integrin shRNA
vector
The results demonstrated that the restructured RNA
interference vector fragments were all consistent with the
synthesized target chain, which confirmed that the synthesized DNA
oligo had been successfully inserted into the carrier for the
construction of the shRNA vector (Fig.
2).
Assessment of the model and
measurement of ASM thickness
Mice in group C did not exhibit tachypnea or other
asthma symptoms. No pathological alterations in the structure of
the airway wall were observed in the tissue sections from group C
(Fig. 3A). Conversely, mice in
group A exhibited tachypnea and mild cyanosis symptoms during
OVA-induced asthma. Following continuous OVA-induced asthma, The
fur of mice was lackluster in group A. The tissue sections
demonstrated epithelial denudation with goblet cell metaplasia,
increased thickness of ASM and angiogenesis (Fig. 3B). Compared with group C, ASM
thickness was increased in groups A, T, BC, NC and PC (Fig. 3C).
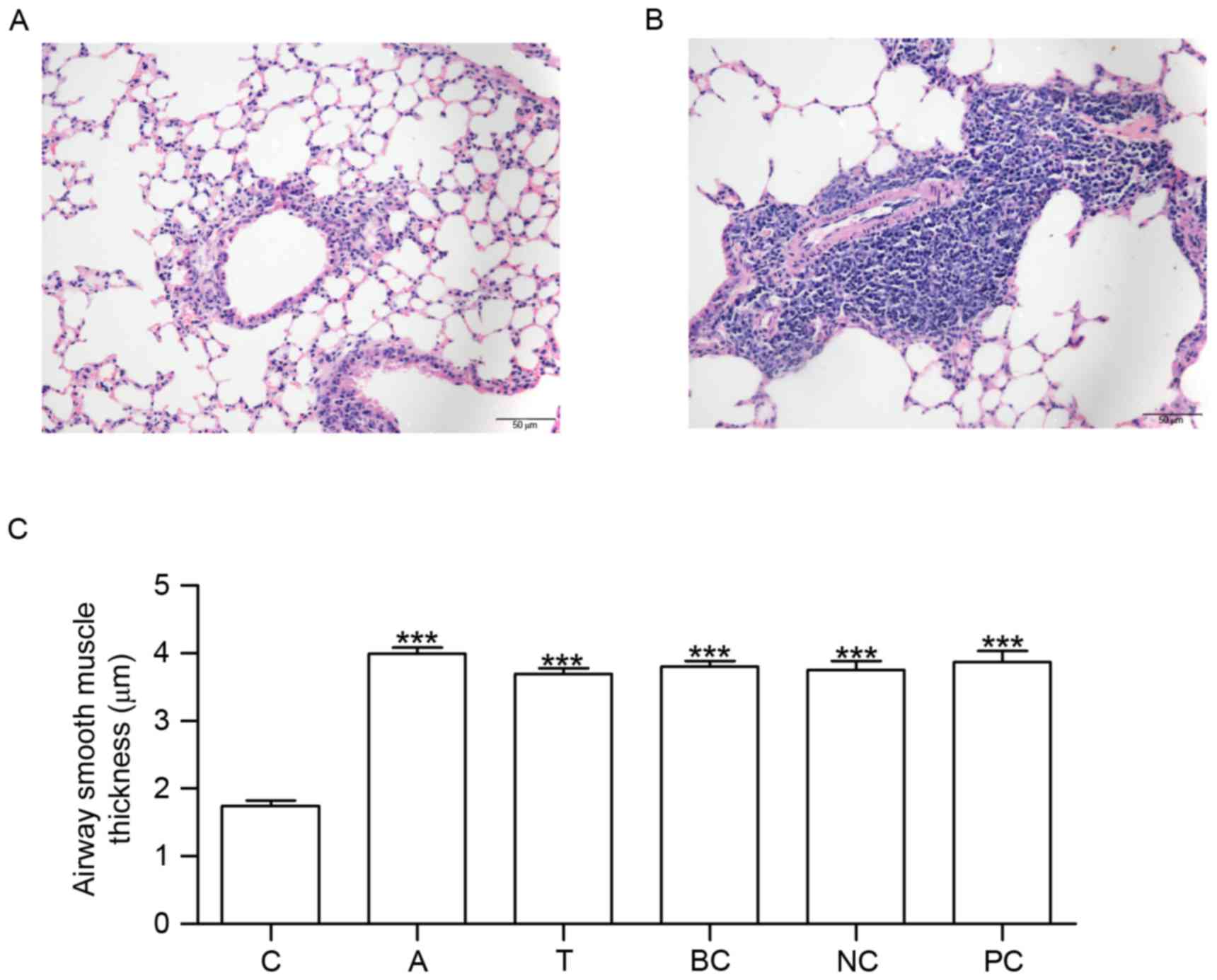 | Figure 3.HE staining and measurement of airway
smooth muscle thickness. Lung tissue sections were stained using
HE. The morphological alterations in the stained sections were
examined under microscopy. (A) HE staining in C. (B) HE staining in
A. (C) Histogram exhibiting the increased airway smooth muscle
thickness which occurred in A, T, BC, NC and PC. n=6. ***P<0.001
vs. C. C, control group; A, asthma group; T, transfection group;
BC, blank control group; NC, negative control group; PC, positive
control group; HE, hematoxylin and eosin. |
Silencing of β1 integrin gene
regulates the gene expression of SOCE-associated genes and nuclear
factor of activated T-cells cytoplasmic 1 (NFAT2)
The mRNA expression of six genes was measured in all
of the groups, including β1 integrin, α-smooth muscle actin (SMA),
three SOCE-associated genes (STIM1, ORAI1 and TRPC1) and NFAT2.
Compared with group C, the transcription of β1 integrin, all of the
SOCE-associated genes and α-SMA was upregulated in groups A, BC, NC
and PC (Fig. 4). Additionally, the
transcription of α-SMA and STIM1 was upregulated in group T, in
contrast with group C. A total of four genes did not exhibit
significantly altered expression between groups T and C, including
β1 integrin, ORAI1, TRPC1 and NFAT2 (Fig. 4).
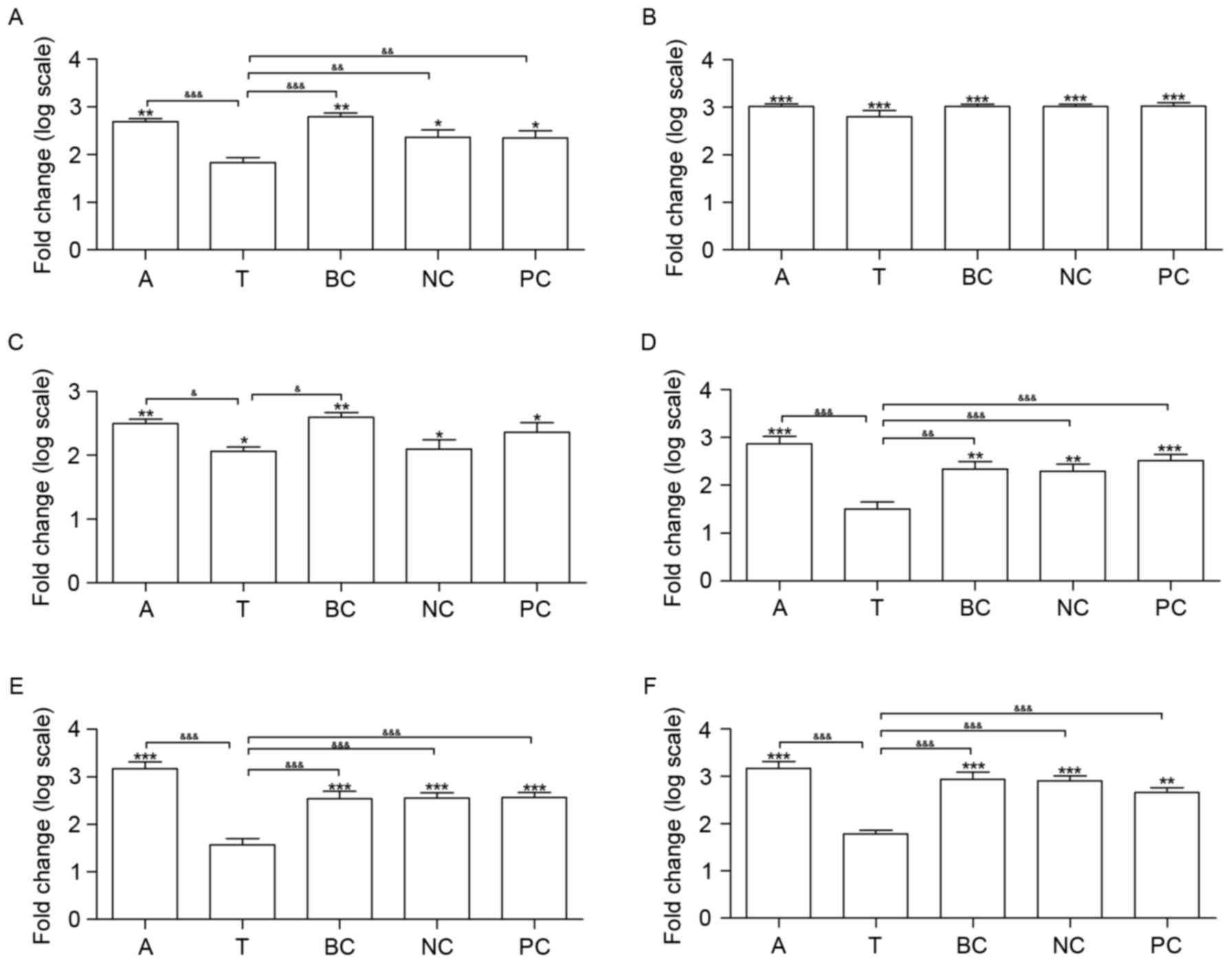 | Figure 4.Effects of β1 integrin short hairpin
RNA on the expression of β1 integrin, α-SMA and SOCE signaling
pathway genes in the lungs of mice. Silencing β1 integrin affected
six SOCE signaling pathway genes at the transcriptional level. The
mRNA expression of (A) β1 integrin, (B) α-SMA, (C) STIM1, (D)
ORAI1, (E) TRPC1 and (F) NFAT2 was measured. n=6. *P<0.05,
**P<0.01 and ***P<0.001 vs. C. &P<0.05,
&&P<0.01 and
&&&P<0.001. C, control group; A, asthma
group; T, transfection group; BC, blank control group; NC, negative
control group; PC, positive control group; SMA, smooth muscle
actin; SOCE, store-operated Ca2+ entry; STIM1, stromal
interaction molecule 1; ORAI1, calcium release-activated calcium
modulator 1; TRPC1, short transient receptor potential channel
member 1; NFAT2, nuclear factor of activated T-cells cytoplasmic
1. |
Silencing the β1 integrin gene
regulates IL-4 and IFN-γ expression levels
Cytokine IL-4 was increased in groups A, BC, NC and
PC compared with group C. Compared with group C, IFN-γ was
downregulated in group A (Fig. 5).
Neither IL-4 nor IFN-γ exhibited significantly altered expression
between groups T and C (Fig.
5).
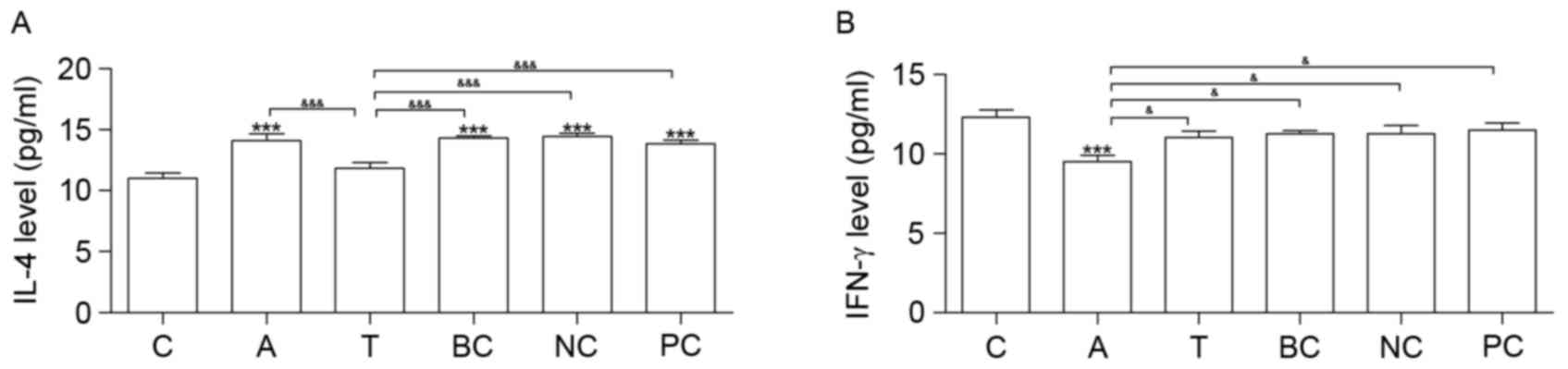 | Figure 5.ELISA analysis. Results of the ELISA
analysis of (A) IL-4 and (B) IFN-γ expression levels. n=6.
*P<0.05, **P<0.01 and ***P<0.001 vs. C.
&P<0.05, &&P<0.01 and
&&&P<0.001. C, control group; A, asthma
group; T, transfection group; BC, blank control group; NC, negative
control group; PC, positive control group; IL-4, interleukin-4;
IFN-γ, interferon-γ. |
Discussion
The accumulation of β1 integrin in ASM has been
demonstrated to be associated with the degree of airway fibrosis,
inflammation and hyperresponsiveness (19,20).
The present study demonstrated that silencing the β1 integrin gene
led to a downregulation of β1 integrin mRNA, without statistically
decreasing ASM thickness and α-SMA gene expression, in OVA
asthmatic mice. Additionally, silencing of the β1 integrin gene was
able to regulate the transcription of SOCE-associated genes at
normal levels, including ORAI1, TRPC1 and NFAT2. Silencing of β1
integrin was additionally able to maintain inflammatory cytokines
at normal levels in OVA asthmatic mice, including IL-4 and
IFN-γ.
β1 integrin shRNA was specifically combined with
target β1 integrin mRNA, causing enzymatic degradation of mRNA and
thereby decreasing the expression of β1 integrin in mice. The
results of the present study demonstrated that the expression level
of β1 integrin was not significantly different among groups NC, BC
and PC, indicating that the shRNA was able to silence β1 integrin
mRNA with high specificity. The present result may provide a
foundation for follow-up studies of β1 integrin-targeted
intervention in asthma.
Altered expression of calcium channel-associated
genes has been associated with airway remodeling in asthma
(10,11,25).
The results of the present study demonstrated an increase in STIM1,
ORAI1 and TRPC1 mRNA levels in the asthma group compared with the
control group. STIM1 and ORAI1 have been observed to be upregulated
in ASMCs from asthmatic mice (13,25).
STIM1/ORAI1-mediated SOCE has been observed to be associated with
ASMC proliferation (10). Further
studies are required to investigate the expression of STIM1, ORAI1
and TRPC1 in ASMCs from patients with asthma.
Transcriptional modulation of SOC channel-associated
genes may represent an important mechanism underlying airway
remodeling. The knockdown of ORAI1 expression in synthetic rat
ASMCs has been demonstrated to attenuate ASMC proliferation and
migration (25). Zou et al
(10) observed that suppressing
the mRNA expression of STIM1 or ORAI1 with specific shRNA resulted
in a decrease in SOCE and ASMC proliferation. The mRNA expression
of TRPC1 was observed to be increased in proliferative ASMCs
compared with growth-arrested cells by Sweeney et al
(9). The results of the present
study demonstrated that silencing β1 integrin led to the
downregulation of the SOCE-associated genes ORAI1 and TRPC1.
Therefore, attenuating the proliferation of ASMCs by silencing β1
integrin may be a promising therapeutic approach for the treatment
of airway remodeling.
NFAT2 regulates the transcription of
pro-inflammatory T cell cytokines (26). T and B cells from ORAI1 knockout
mice have been demonstrated to exhibit impaired SOCE and CRAC
function, resulting in decreased expression of cytokines IL-4 and
IFN-γ in CD4+ and CD8+ T cells (27). In the present study, upregulated
expression of NFAT2 and IL-4, and downregulation of IFN-γ
expression, was observed in the asthma group compared with the
control group.
CRAC signaling via SOCE activates NFAT transcription
factors in addition to NFAT-promoted gene expression (28,29).
Inhibition of the CRAC channel was demonstrated to attenuate
allergic inflammation in rats, and airway lymphocyte cytokine
production in cells from patients with asthma, by Kaur et al
(30). ORAI1-knockout mice were
demonstrated to exhibit decreased T cell cytokine production by
McCarl et al (27). In
addition, Ca2+-signaling of T cells is mediated by the
induction of [Ca2+]i by β1 integrin through
increased Ca2+-influx (31). In the present study, it was noted
that the silencing of β1 integrin maintained the expression of
NFAT2 and inflammatory cytokines IL-4 and IFN-γ at normal levels.
It may be hypothesized that silencing β1 integrin may inhibit
allergic inflammation by attenuating the transcription of
SOCE-associated genes.
Bronchial hyperresponsiveness has been demonstrated
to be associated with airway wall thickness in asthma (16). In addition, the expression of α-SMA
has been hypothesized to be an important indicator of airway
remodeling in asthma (2). The
present study demonstrated that ASM thickness and α-SMA gene
expression were increased in Group T, in contrast with Group C. The
results of the present study indicated that silencing β1 integrin
was insufficient to maintain normal ASM thickness and α-SMA gene
expression in asthmatic mice. Previous studies have reported that a
number of factors may influence ASM thickness (32,33).
For example, Hou et al (32) reported that the anti-inflammatory
factor high-mobility group box protein 1 decreased smooth muscle
thickness in OVA asthmatic mice. It is hypothesized that silencing
β1 integrin may delay the ASMC proliferation and prolong the time
of airway remodeling, without altering the final outcome. Numerous
signaling pathways may be simultaneously associated with the
regulation of ASMC proliferation.
In conclusion, the results of the present study
demonstrated that β1 integrin serves a role in ASMC proliferation
and airway remodeling. Therefore, silencing β1 integrin may
represent a novel target for drug design to attenuate airway
remodeling in chronic asthma.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (Beijing, China; grant no.
81270074).
References
|
1
|
Bateman ED, Hurd SS, Barnes PJ, Bousquet
J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O'Byrne P, Pedersen
SE, et al: Global strategy for asthma management and prevention:
GINA executive summary. Eur Respir J. 31:143–178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Slats AM, Janssen K, van Schadewijk A, van
der Plas DT, Schot R, van den Aardweg JG, de Jongste JC, Hiemstra
PS, Mauad T, Rabe KF and Sterk PJ: Expression of smooth muscle and
extracellular matrix proteins in relation to airway function in
asthma. J Allergy Clin Immunol. 121:1196–1202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mahn K, Ojo OO, Chadwick G, Aaronson PI,
Ward JP and Lee TH: Ca(2+) homeostasis and structural and
functional remodelling of airway smooth muscle in asthma. Thorax.
65:547–552. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Helli PB and Janssen LJ: Properties of a
store-operated nonselective cation channel in airway smooth muscle.
Eur Respir J. 32:1529–1539. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hendeles L, Hill M, Harman E, Moore P and
Pieper J: Dose-response of inhaled diltiazem on airway reactivity
to methacholine and exercise in subjects with mild asthma. Clin
Pharmacol Ther. 43:387–392. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hoppe M, Harman E and Hendeles L: The
effect of inhaled gallopamil, a potent calcium channel blocker, on
the late-phase response in subjects with allergic asthma. J Allergy
Clin Immunol. 89:688–695. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Twiss M Ann, Harman E, Chesrown S and
Hendeles L: Efficacy of calcium channel blockers as maintenance
therapy for asthma. Br J Clin Pharmacol. 53:243–249. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohga K, Takezawa R, Yoshino T, Yamada T,
Shimizu Y and Ishikawa J: The suppressive effects of
YM-58483/BTP-2, a store-operated Ca2+ entry blocker, on
inflammatory mediator release in vitro and airway responses in
vivo. Pulm Pharmacol Ther. 21:360–369. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sweeney M, McDaniel SS, Platoshyn O, Zhang
S, Yu Y, Lapp BR, Zhao Y, Thistlethwaite PA and Yuan JX: Role of
capacitative Ca2+ entry in bronchial contraction and
remodeling. J Appl Physiol (1985). 92:1594–1602. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zou JJ, Gao YD, Geng S and Yang J: Role of
STIM1/Orai1-mediated store-operated Ca2+ entry in airway
smooth muscle cell proliferation. J Appl Physiol (1985).
110:1256–1263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Spinelli AM and Trebak M: Orai
channel-mediated Ca2+ signals in vascular and airway
smooth muscle. Am J Physiol Cell Physiol. 310:C402–C413. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peel SE, Liu B and Hall IP: A key role for
STIM1 in store operated calcium channel activation in airway smooth
muscle. Respir Res. 7:1192006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peel SE, Liu B and Hall IP: ORAI and
store-operated calcium influx in human airway smooth muscle cells.
Am J Respir Cell Mol Biol. 38:744–749. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ong HL, Cheng KT, Liu X, Bandyopadhyay BC,
Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, et al:
Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved
in store-operated calcium influx. Evidence for similarities in
store-operated and calcium release-activated calcium channel
components. J Biol Chem. 282:9105–9116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng KT, Liu X, Ong HL and Ambudkar IS:
Functional requirement for Orai1 in store-operated TRPC1-STIM1
channels. J Biol Chem. 283:12935–12940. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Manuyakorn W: Airway remodelling in
asthma: Role for mechanical forces. Asia Pac Allergy. 4:19–24.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Teoh CM, Tam JK and Tran T: Integrin and
GPCR crosstalk in the regulation of ASM contraction signaling in
asthma. J Allergy (Cairo). 2012:3412822012.PubMed/NCBI
|
|
18
|
Lei L, Liu D, Huang Y, Jovin I, Shai SY,
Kyriakides T, Ross RS and Giordano FJ: Endothelial expression of
beta1 integrin is required for embryonic vascular patterning and
postnatal vascular remodeling. Mol Cell Biol. 28:794–802. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi F, Qiu C, Qi H and Peng W: shRNA
targeting β1-integrin suppressed proliferative aspects and
migratory properties of airway smooth muscle cells. Mol Cell
Biochem. 361:111–121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bazan-Perkins B, Sánchez-Guerrero E,
Vargas MH, Martínez-Cordero E, Ramos-Ramírez P, Alvarez-Santos M,
Hiriart G, Gaxiola M and Hernandez-Pando R: Beta1-integrins
shedding in a guinea-pig model of chronic asthma with remodelled
airways. Clin Exp Allergy. 39:740–751. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Black JL, Panettieri RA Jr, Banerjee A and
Berger P: Airway smooth muscle in asthma: Just a target for
bronchodilation? Clin Chest Med. 33:543–558. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fernandes DJ, Bonacci JV and Stewart AG:
Extracellular matrix, integrins, and mesenchymal cell function in
the airways. Curr Drug Targets. 7:567–577. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rozen S and Skaletsky H: Primer3 on the
WWW for general users and for biologist programmers. Methods Mol
Biol. 132:365–386. 2000.PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Spinelli AM, González-Cobos JC, Zhang X,
Motiani RK, Rowan S, Zhang W, Garrett J, Vincent PA, Matrougui K,
Singer HA and Trebak M: Airway smooth muscle STIM1 and Orai1 are
upregulated in asthmatic mice and mediate PDGF-activated SOCE, CRAC
currents, proliferation and migration. Pflugers Arch. 464:481–492.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Macian F: NFAT proteins: key regulators of
T-cell development and function. Nat Rev Immunol. 5:472–484. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McCarl CA, Khalil S, Ma J, Oh-hora M,
Yamashita M, Roether J, Kawasaki T, Jairaman A, Sasaki Y, Prakriya
M and Feske S: Store-operated Ca2+ entry through ORAI1
is critical for T cell-mediated autoimmunity and allograft
rejection. J Immunol. 185:5845–5858. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Samanta K, Bakowski D and Parekh AB: Key
role for store-operated Ca2+ channels in activating gene
expression in human airway bronchial epithelial cells. PLoS One.
9:e1055862014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gwack Y, Srikanth S, Feske S,
Cruz-Guilloty F, Oh-hora M, Neems DS, Hogan PG and Rao A:
Biochemical and functional characterization of Orai proteins. J
Biol Chem. 282:16232–16243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kaur M, Birrell MA, Dekkak B, Reynolds S,
Wong S, De Alba J, Raemdonck K, Hall S, Simpson K, Begg M, et al:
The role of CRAC channel in asthma. Pulm Pharmacol Ther. 35:67–74.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schottelndreier H, Mayr GW and Guse AH:
Beta1-integrins mediate Ca2+-signalling and T cell
spreading via divergent pathways. Cell Signal. 11:611–619. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hou C, Kong J, Liang Y, Huang H, Wen H,
Zheng X, Wu L and Chen Y: HMGB1 contributes to allergen-induced
airway remodeling in a murine model of chronic asthma by modulating
airway inflammation and activating lung fibroblasts. Cell Mol
Immunol. 12:409–423. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park CS, Bang BR, Kwon HS, Moon KA, Kim
TB, Lee KY, Moon HB and Cho YS: Metformin reduces airway
inflammation and remodeling via activation of AMP-activated protein
kinase. Biochem Pharmacol. 84:1660–1670. 2012. View Article : Google Scholar : PubMed/NCBI
|