Introduction
Pollen-induced allergic diseases, including rhinitis
(1,2), asthma (3) and atopic dermatitis (4), are significant health problems that
are often season-dependent. Numerous plants can release pollen,
including poplar, cypress and Platanus trees, and grasses,
which may cause serious allergic diseases.
Due to its high resistance to diseases, Platanus
acerifolia is widely planted worldwide (5); however, high concentrations of its
pollen are detected during the flowering season (6), and P. acerifolia is considered
an important source of allergenic pollen in numerous cities
(7). The reported prevalence of
sensitization to P. acerifolia pollen in Mediterranean
Europe ranges between 3 and 52% (8). P. acerifolia is also a major
cause of pollen-induced allergy in Spain; the prevalence ranges
between 52 and 56% in central Spain, between 8 and 9% in
north-western Spain, and 17% of the population is sensitive to
P. acerifolia pollen in south-western Spain (9).
Three major allergens have been identified in P.
acerifolia pollen. Pla a 3 is a non-specific lipid transfer
protein (10) and 45% of Spanish
patients with P. acerifolia pollen allergies were reported
to be sensitive to natural Pla a 3 (11). Pla a 2 is a 43-kDa glycoprotein
that displays polygalacturonase activity, and is associated with
the allergic responses of 84% of patients worldwide with
planetree-induced pollinosis (11,12).
Pla a 1 is an 18-kDa non-glycosylated protein that has sequence
homology to invertase inhibitory proteins (13–15)
and pectin methylesterase inhibitor proteins (16). In addition, 84% of patients with
Platanus allergies in Western European cities are sensitive
to Pla a 1 (8).
Previous studies have reported the expression and
purification of Pla a 1 (16,17).
Monoclonal antibodies (mAbs) that specifically target Pla a 1 may
be used in its quantification, as well as for the further
improvement of pollen allergy immunotherapy (18–20).
At present, to the best of our knowledge, there is no commercial
mAb against the Pla a 1 allergen. Therefore, the present study
produced and purified mAbs that specifically bound Pla a 1, which
may be used in the quantification of this allergen. In addition, an
indirect ELISA was developed with mAbs, which were produced against
recombinant Pla a 1.
Materials and methods
Patients and samples
A total of 6 patients (age, 14–34; 3 males and 3
females; recruited between January and May 2015) with allergic
rhinitis, with positive skin prick test (allergens supplied by
ALK-Abelló, A/S, Hørsholm, Denmark) and positive serum
immunoglobulin (Ig)E test to P. acerifolia pollen extract
(ImmunoCAP assay; Phadia AB; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA)], and 6 healthy controls (age, 19–45; 3 males and
3 females; recruited in May 2015) were recruited in the present
study. The study protocol was approved by the ethical committee of
the First Affiliated Hospital of Nanjing Medical University
(Nanjing, China). Written informed consent for the use of blood
samples was obtained from all participants prior to study entry,
according to the declaration of Helsinki. Serum was extracted from
whole blood samples (2 ml, collected three times from each
individual) by centrifugation at 6,000 × g and 4°C for 20 min.
Expression and purification of Pla a 1
in E. coli
The nucleotide acid and amino acid sequences of Pla
a 1 were obtained from the GenBank database (AJ427413.2; https://www.ncbi.nlm.nih.gov/nuccore/)
The open reading frame (ORF) of Pla a 1 comprises 540 bases pairs,
encoding 180 amino acids. This ORF contains a 24 amino acid signal
peptide. Mature Pla a 1 comprises 468 bases pairs, encoding 156
amino acids. The nucleic acid sequence of mature Pla a 1 was
synthesized by GenScript (Nanjing) Co., Ltd. (Nanjing, China) and
was subcloned into a pET-28a vector (Novagen; Merck KGaA,
Darmstadt, Germany) using EcoRI and XhoI sites, and
the clone was verified by Sanger DNA sequencing, as described
previously (21). The nucleotide
acid sequence of Pla a 1 is as follows: Gcc gat att gtt cag ggc aca
tgc aag aaa gtt gct cag aga agc cca aac gtg aac tac gat ttc tgc gtg
aaa tct ctt gga gca gat cct aag agc cac act g g at ctt caa gga ctt
ggg gtc atc tca gcg aat tta gcc ata cag cat gga tct aaa atcc aa aca
ttt att ggt cgc atc ttg aaa agt aaa gtg gac cca gct ctt aag aaa tac
ttg aat gat tgt gtg ggg ctt tac gct gat gcg aag tct tca gtt caa gag
gcc ata gct gac ttc aag tcc aag gac tac gca tca gct aat gtg aaa atg
agt gcg gct ttg gac gac tca gtg act tgt gaa gat ggg ttt aag gag aag
aaa ggt ata gta tca ccg gtg acgaag gag aac aag gat tat gta caa ctg
act gca ata tct ctt gca att acc aaa ctg ctt ggt gct tga. The
recombinant pET28a-Pla a 1 plasmid was transformed into the
ArcticExpress™ (DE3) RP Escherichia coli host strain
(Sigma-Aldrich; Merck KGaA). A total of 6 colonies were selected
and placed separately in 3 ml luria broth (LB)-kanamycin broth
induced by 0.5 mM Isopropyl-β-D-thiogalactopyranoside (IPTG) for 4
h at 37°C. After induction, 12% SDS-PAGE was performed and the
colony with an obvious band at 23 kDa was selected for inoculation
in 3 ml LB-kanamycin broth, and was incubated at 37°C overnight.
Subsequently, 0.5 ml of the culture was inoculated into 50 ml fresh
LB-kanamycin broth, and incubated at 37°C with agitation at 250
rpm, until the optical density (OD) at A600 nm reached 0.6–0.8.
IPTG was added to the final concentration of 0.5 mM and the culture
was incubated for a further 4 h at 37°C. The bacterial cells were
harvested by centrifugation at 6,000 × g for 10 min at 4°C, and
were lysed in lysis buffer containing 20 mM Tris-HCl and 100 mM
NaH2PO4 by sonication at 40 kHz (4 sec
pulse-on, 8 sec pulse-off). After sonication, proteins from
non-induced recombinant Pla a 1 whole cell lysate, IPTG-induced
recombinant Pla a 1 whole cell lysate, and supernatant and
precipitation (inclusion bodies) fractions after ultrasonication,
were boiled for 10 min, visualized with loading buffer [250 mM
Tris-Hcl; 10% SDS (w/v); 0.5% bromophenol blue (w/v); 50% glycerin
(v/v); and 5% 2-mercaptoethanol (v/v)] and were analyzed by 12%
SDS-PAGE (10 µg protein per lane). The BCA method was used to
quantify protein. The results demonstrated that the recombinant Pla
a 1 was mainly contained within inclusion bodies. So the inclusion
bodies were collected by centrifugation at 10,000 × g for 20 min at
4°C. Following solubilization of the inclusion bodies using 8 M
urea, the supernatant was loaded onto a Nickel column [GenScript
(Nanjing) Co., Ltd.], washed with running buffer containing 20 mM
Tris-HCl, 100 mM NaH2PO4, 10 mM imidazole and
8 M urea (pH 8.0), and eluted with elution buffer containing 20 mM
Tris-HCl, 100 mM NaH2PO4, 250 mM imidazole
and 8 M urea (pH 8.0). The eluted fractions were collected and
dialyzed with 6, 4, 2, 1 and 0 M urea at 4°C, each for 3 h. Eluates
were subsequently subjected to 12% SDS-PAGE (10 µg protein per
lane).
SDS-PAGE analysis of the expression
and purification of Pla a 1 in Escherichia coli
These proteins (10 µg per lane), including whole
cell lysate, fractions after ultrasonication and eluates were
analyzed by 12% SDS-PAGE. The gel was incubated in 100 ml solution
with 1.5 mM Coomassie Brilliant Blue at room temperature for 1 h.
Subsequently, the gel was washed in 100 ml solution containing 30
ml methyl alcohol (analytic reagent, >99.5%), 10 ml acetic acid
(analytic reagent, >99.5%) and 60 ml distilled water for 3
h.
Immunoreactivity of human sera with
recombinant Pla a 1
Immunoblotting for the detection of serum specific
IgE was performed with recombinant Pla a 1, as described previously
(14). Recombinant Pla a 1 (5 µg)
was separated by 12% SDS-PAGE under reducing conditions and was
then transferred to polyvinylidene difluoride (PVDF) membranes
(22). The PVDF membranes were
blocked in 5% skim milk at room temperature for 2 h and were then
incubated with a mixed serum sample from 6 patients with P.
acerifolia pollen allergies [diluted 1:40 in phosphate-buffered
saline (PBS)] as the primary antibody overnight at 4°C. Following
rinsing with PBS, the membranes were incubated with a horseradish
peroxidase (HRP)-conjugated goat anti-human IgE mAb (cat. no.
AHI0504; Thermo Fisher Scientific, Inc.; diluted 1:3,000 in
secondary antibody diluent) at room temperature for 1 h and then
detected by a ImageQuant LAS 4000 Mini Detection System (GE
Healthcare Life Sciences, Little Chalfont, UK) using Immobilon
Western Chemiluminescent HRP Substrate (EMD Millipore, Billerica,
MA, USA). A mixed serum sample from 6 healthy individuals diluted
in PBS (1:20) was used as negative serum control in this
experiment.
Generation, purification and
characterization of mAbs against recombinant Pla a 1
immunization
The purified recombinant Pla a 1 was used as an
antigen, which was diluted with PBS to a concentration of 1 mg/ml.
For the initial immunization, 5 female BALB/c mice (age, 6–8 weeks;
Beijing Vital River Laboratory Animal Technology Co., Ltd.,
Beijing, China) were immunized subcutaneously with 100 µg Pla a 1
emulsified with an equal volume of complete Freund's adjuvant
(23–25). Mice were kept at a temperature of
18–22°C, humidity of 40–70%, a 12-h light/dark cycle and food and
water was freely available. A total of 2 and 4 weeks after the
initial injection, booster injections were administered
subcutaneously, with the same quantity of Pla a 1 emulsified with
an equal volume of incomplete Freund's adjuvant (18,26).
Subsequently, the serum of each mouse was collected 2 weeks after
each immunization by centrifugation of 100 µl blood at 6,000 × g
and 4°C for 20 min. Each serum titer was determined by indirect
ELISA, as previously described (27). Finally, the mice with the highest
serum titers were administered intraperitoneal injections of Pla a
1 without adjuvant 2 days prior to fusion (26,28).
The mouse with the highest serum titer was selected for hybridoma
production (29,30).
Cultivation of mouse myeloma
cells
Mouse myeloma cells (SP2/0) were cultivated in
RPMI-1640 medium (Thermo Fisher Scientific, Inc.) supplemented with
10% fetal bovine serum (Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin in a humidified atmosphere containing 5%
CO2 at 37°C. Cells in the exponential growth phase were
grown to concentrations of 4×105 cells/ml prior to cell
fusion (20,31).
Fusion and selection of hybridoma
cells
Following sacrifice, spleen cells harvested from the
immunized mouse with the highest serum titer were fused with SP2/0
cells at a ratio of 10:1 using 50% polyethylene glycol (PEG). The
hybridoma cells were selected using
hypoxanthine-aminopterin-thymidine (HAT) medium, as previously
described (32,33). After 10–14 days of fusion, the
supernatants of the harvested spleen cells were screened by
indirect ELISA for antibodies against Pla a 1. Hybridoma cells from
positive wells were cloned by limiting dilution and were repeatedly
subcloned to obtain stable cell lines secreting antibodies
(31).
Large-scale preparation of mAbs
Pla a 1-specific mAbs were prepared as previously
described (34). Briefly,
following intraperitoneal injection of hybridoma cells
(5×105; stable cell lines secreting antibodies) in three
mice, ascites fluid was produced in all three BALB/c mice within
7–14 days. Purified mAbs were obtained from the ascites fluid by
affinity chromatography using protein A-agarose (Bio-Rad
Laboratories GmbH, München, Germany), as previously described
(35), and were analyzed by
SDS-PAGE.
SDS-PAGE of purified mAbs
The BCA method was used to quantify purified mAbs
and the purified mAbs (10 µg per lane) were analyzed by SDS-PAGE
(gel concentration of 12%). The gel was incubated in 100 ml
solution with 1.5 mM Coomassie Brilliant Blue at room temperature
for 1 h. The gel was washed in 100 ml solution containing 30 ml
methyl alcohol (analytic reagent, >99.5%), 10 ml acetic acid
(analytic reagent, >99.5%) and 60 ml distilled water for 3
h.
ELISA
ELISA for the determination of serum titer was
conducted, as previously described (13,36).
Briefly, microwell plates were coated with 100 µl 5 µg/ml Pla a 1
and incubated at 4°C for 24 h. Subsequently, coated wells were
blocked with 200 µl PBS containing 1% bovine serum albumin
(Sigma-Aldrich; Merck KGaA) and were incubated with 100 µl diluted
serum (1:500) from the mouse with the highest serum titer at 4°C
for 1 h. Following incubation with 100 µl HRP-conjugated goat
anti-mouse IgG antibody (1:4,000; cat. no. M6898; Sigma-Aldrich;
Merck KGaA) at 4°C for 1 h. Peroxidase activity was measured by
adding 100 µl 3,3′,5,5′-O-tetramethylbenzidine solution as a
substrate and the reaction was terminated by adding 50 µl 3 M
H2SO4. Subsequently, the optical density was
measured at 450 nm (14).
Results
Expression and purification of Pla a 1
in E. coli
The P. acerifolia pollen Pla a 1 was
subcloned into a pET-28a vector and transformed into the Arctic
Express™ (DE3) RP E. coli host strain. The results
demonstrated that Pla a 1 was predominantly expressed within
inclusion bodies (Fig. 1A). The
Pla a 1-containing inclusion bodies were purified using Ni columns.
Following the successful renaturation of purified Pla a 1, ~1.4 mg
recombinant Pla a 1 was obtained from 500 ml cell culture. The
purity of the purified Pla a 1 was identified by SDS-PAGE as a
single band with an apparent molecular weight of 20 kDa (Fig. 1B).
Immunoreactivity to IgE of Pla a
1
In order to determine the allergenicity of Pla a 1,
the ability of Pla a 1 to bind IgE in the serum of patients with
P. acerifolia pollen allergies was determined by western
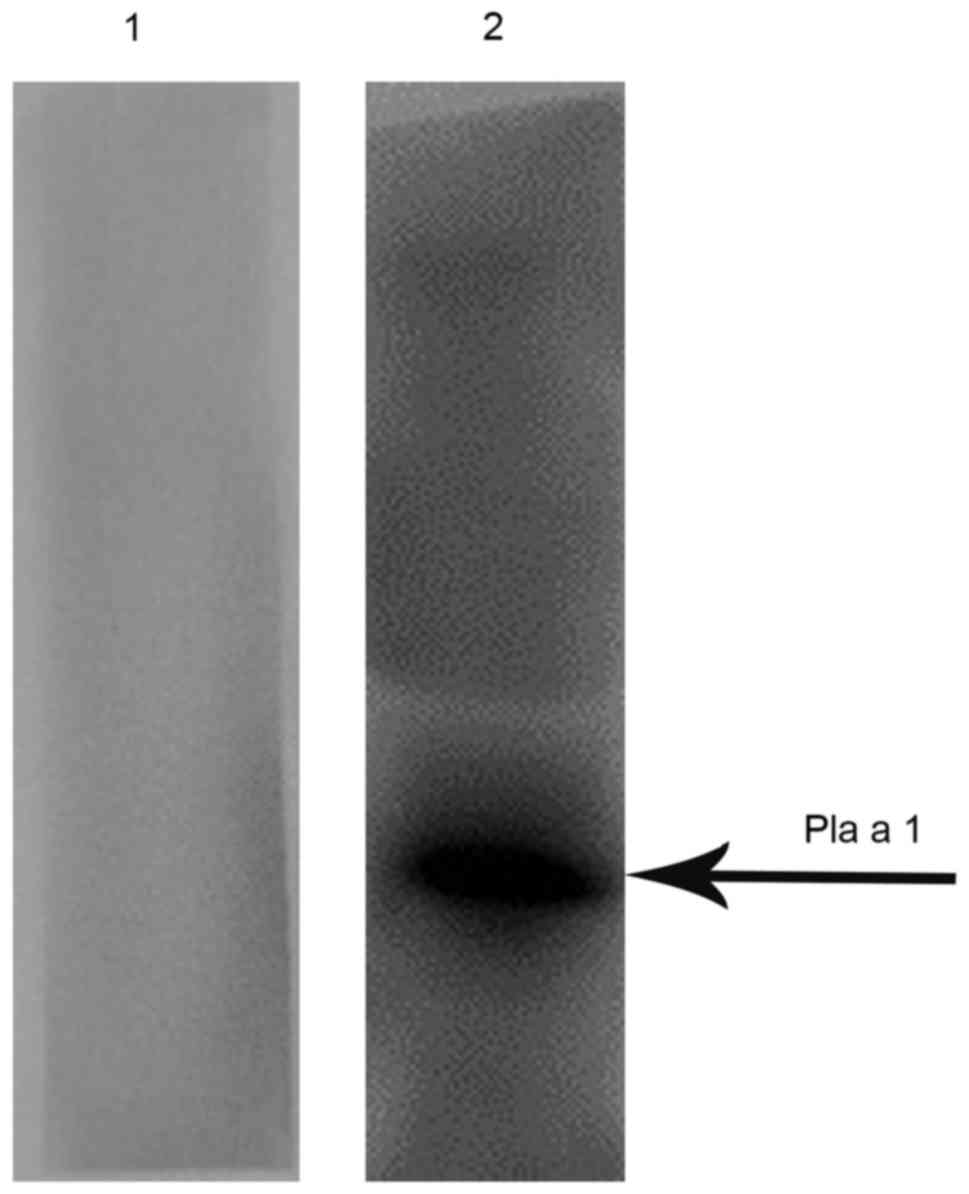
blotting. As presented in Fig. 2,
mixed serum from patients with P. acerifolia pollen
allergies exhibited positive IgE reactivity to Pla a 1, whereas
mixed serum from healthy controls failed to do so.
Generation, purification and
characterization of mAbs against recombinant Pla a 1
In the present study, BALB/c mice were immunized
four times with the purified Pla a 1 together with an adjuvant,
after which splenocytes were collected and fused with SP2/0 using
50% PEG. The fused cells were selected in HAT medium. Positive
cells were screened with ELISA and subcloned by limiting dilution
at least three times, in order to obtain stable cell lines
secreting mAbs. The titers of the hybridoma culture supernatants
were determined with indirect ELISA based on purified Pla a 1
(18). In this assay, the
supernatant of SP2/0 myeloma cells was used as the negative
control. A total of 11 hybridoma cell lines stably secreting mAbs
were screened; the cell lines were named as follows: 6D12, 6E1,
6F10, 6F12, 6H2, 10C9, 10D9, 10E9, 10F9, 11D5 and 11F5. An OD
analysis of the supernatant from each of the 11 hybridoma cell
lines revealed that the optimal hybridoma was 6D12 (Table I). When the ratio of the sample
OD/blank OD is >2.1, the highest dilution degree used (that has
a sample OD/blank OD >2.1) is considered to indicate the titer
of a mAb. The results of the indirect ELISA indicated that the
titer of mAbs purified from the 6D12 hybridoma was >512,000
(Table II). Therefore, 6D12 was
used for further cloning using the limiting dilution method.
Resurgent cells, cells that exhibited normal activity and good
condition after being thawed, were used following liquid nitrogen
frozen storage. After three repeats of the limiting dilution
method, the positive rate was determined, which is presented in
Table III. The monoclonal cell
positive rate following the second repeat was 95%, whereas after
the third repeat the monoclonal cell positive rate was 100%. These
data indicated that, following frozen storage and recovery,
hybridoma cells can secrete specific antibodies against Pla a 1,
the cell line was named Pla a 1-mAb-6D12.
 | Table I.Optical density of 11 hybridomas. |
Table I.
Optical density of 11 hybridomas.
| Hybridoma | Optical
density |
|---|
| 6D12 | 3.353 |
| 6E1 | 3.239 |
| 6F10 | 3.312 |
| 6F12 | 3.274 |
| 6H2 | 3.353 |
| 10C9 | 3.353 |
| 10D9 | 3.197 |
| 10E9 | 3.201 |
| 10F9 | 3.099 |
| 11D5 | 3.154 |
| 11F5 | 3.128 |
 | Table II.Titer of monoclonal antibodies
purified from hybridoma 6D12. |
Table II.
Titer of monoclonal antibodies
purified from hybridoma 6D12.
| Serum dilution
times | Optical
density |
|---|
| 500 | 2.979 |
| 1,000 | 2.941 |
| 2,000 | 2.483 |
| 4,000 | 2.015 |
| 8,000 | 1.840 |
| 16,000 | 1.632 |
| 32,000 | 1.337 |
| 64,000 | 1.196 |
| 128,000 | 0.845 |
| 256,000 | 0.725 |
| 512,000 | 0.426 |
| Blank | 0.074 |
| Titer | >512,000 |
 | Table III.Establishment of the monoclonal cell
line Pla a 1-mAb-6D12. |
Table III.
Establishment of the monoclonal cell
line Pla a 1-mAb-6D12.
| Dilution
number | Total number of
wells | Positive well | Positive rate
(%) |
|---|
| 1 | 59 | 31 | 52.54 |
| 2 | 40 | 38 | 95 |
| 3 | 70 | 70 | 100 |
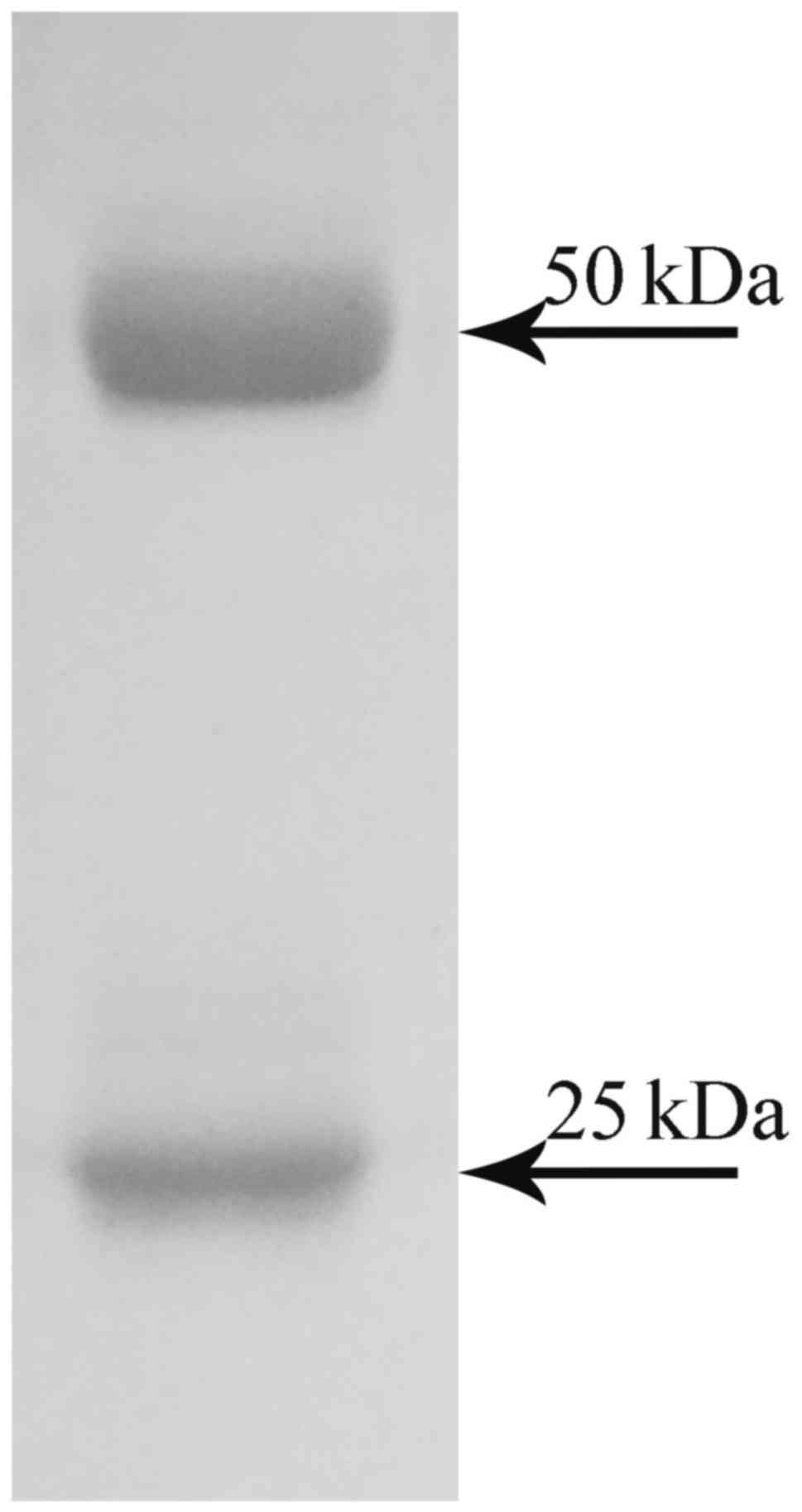
Finally, the purified mAbs were obtained by protein
A-agarose affinity chromatography. The purified antibody was
analyzed by SDS-PAGE. It contained a heavy chain of 50 kDa and a
light chain of 25 kDa (Fig.
3).
Discussion
The development of high purity and hypoallergenic
preparations has become a particular focus of allergic research
worldwide in recent years. In addition, screening the main
allergens of pollen is a key step for the standardized preparation
of pollen allergen vaccines (37).
Therefore, the determination of Pla a 1 content is crucial for the
development of a P. acerifolia pollen allergen vaccine
(38,39). In the present study, Pla a 1 was
predominantly expressed in the inclusion bodies of E. coli.
Subsequently, purified Pla a 1 underwent western blot analysis and
the results revealed the Pla a 1 exerts immunological activities by
binding IgE in the sera from patients with P. acerifolia
pollen allergies. Furthermore, purified Pla a 1 was used as an
immunizing antigen to generate mAbs in mice; a total of 11
hybridoma cell lines stably secreting mAbs against the Pla a 1
protein were screened in the present study. The results of an
indirect ELISA confirmed that all 11 mAbs could specifically
recognize the recombinant Pla a 1 protein.
mAb-based immunoassays have been used to measure
allergen contents in the indoor environment (40). For example, mAbs against Der f 1 (a
major allergen of the house dust mite Dermatophagoides
farina) can be used for the detection of this allergen
(41). In addition, Der f 2 is a
major allergen from D. farina, and mAbs against Der f 2 can
be used to create a precise quantitative method to identify
allergen components in dust samples (35,42).
Der f 7 is another major allergen of house dust mites, and mAbs
against Der f 7 may be useful for environmental studies and for the
standardization of mite allergen extracts (43, 44). Standardization of allergenic
extracts is essential to improve their diagnostic and therapeutic
quality.
It is important to develop an effective tool to
monitor the concentration of allergen components in the outdoor
environment. The high titer, highly specific antibodies that have
been produced in the present study may be used to reduce the
potential for pollen allergens to cause allergy symptoms in
individuals that are treated with the antibodies. The antibodies
produced may be used as an immunotherapy for humans, which will
allow the body to identify antigens associated with the
administered antibodies and prevent allergic responses to these
antigens, thereby reducing the potential number of allergens in the
environment that an individual may be allergic to. In the present
study, 11 mAbs were identified that can specifically recognize the
Pla a 1 protein. These mAbs may be valuable for the rapid and
accurate detection of the Pla a 1 allergen.
Although a breakthrough has been made regarding the
use of mAbs in the accurate quantification of allergen levels
(45), there remain some
disadvantages to their use. A limitation is that mAbs are specific
to only one type of antigen. It is important that more mAbs against
P. acerifolia pollen allergens are prepared, which may
contribute toward the generation of specific immunotherapies, such
as a P. acerifolia pollen vaccine.
Acknowledgements
The present study was supported by grants from the
Special Fund for Forestry-Scientific Research in the Public
Interest (grant no. 201304103), the National Natural Science
Foundation of China (grant nos. 81571568, 31340073 and 81273274),
the Jiangsu Province's Key Provincial Talents Program (grant no.
RC201170), and the Priority Academic Program Development of Jiangsu
Higher Education Institutions (PAPD).
References
|
1
|
Desai MB, Gavrilova T, Liu J, Patel SA,
Kartan S, Greco SJ, Capitle E and Rameshwar P: Pollen-induced
antigen presentation by mesenchymal stem cells and T cells from
allergic rhinitis. Clin Transl Immunology. 2:e72013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dondi A, Tripodi S, Panetta V, Asero R,
Businco AD, Bianchi A, Carlucci A, Ricci G, Bellini F, Maiello N,
et al: Pollen-induced allergic rhinitis in 1360 Italian children:
Comorbidities and determinants of severity. Pediatr Allergy
Immunol. 24:742–751. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Subiza J, Cabrera M, Valdivieso R, Subiza
JL, Jerez M, Jiménez JA, Narganes MJ and Subiza E: Seasonal asthma
caused by airborne Platanus pollen. Clin Exp Allergy.
24:1123–1129. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sybilski AJ, Zalewska M, Furmańczyk K,
Lipiec A, Krzych-Fałta E and Samoliński B: The prevalence of
sensitization to inhalant allergens in children with atopic
dermatitis. Allergy Asthma Proc. 36:e81–e85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen Z, Yang Y, Chen X, Wu Z and Li S:
Characterization of two pollen allergens of the London plane tree
in Shanghai. Iran J Allergy Asthma Immunol. 14:139–148.
2015.PubMed/NCBI
|
|
6
|
Alcázar P, Cariñanos P, De Castro C,
Guerra F, Moreno C, Dominguez-Vilches E and Galán C: Airborne
plane-tree (Platanus hispanica) pollen distribution in the
city of Cordoba, South-western Spain and possible implications on
pollen allergy. J Investig Allergol Clin Immunol. 14:238–243.
2004.PubMed/NCBI
|
|
7
|
Alcázar P, García-Mozo H, Trigo Mdel M,
Ruiz L, González-Minero FJ, Hidalgo P, de la Díaz Guardia C and
Galán C: Platanus pollen season in Andalusia (southern
Spain): Trends and modeling. J Environ Monit. 13:2502–2510. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fernández-González D, González-Parrado Z,
Vega-Maray AM, Valencia-Barrera RM, Camazón-Izquierdo B, De Nuntiis
P and Mandrioli P: Platanus pollen allergen, Pla a 1:
Quantification in the atmosphere and influence on a sensitizing
population. Clin Exp Allergy. 40:1701–1708. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iglesias I, Rodriguez-Rajo FJ and Méndez
J: Behavior of Platanus hispanica pollen, an important
spring aeroallergen in northwestern Spain. J Investig Allergol Clin
Immunol. 17:145–156. 2007.PubMed/NCBI
|
|
10
|
Wangorsch A, Larsson H, Messmer M,
García-Moral A, Lauer I, Wolfheimer S, Schülke S, Bartra J, Vieths
S, Lidholm J and Scheurer S: Molecular cloning of plane pollen
allergen Pla a 3 and its utility as diagnostic marker for peach
associated plane pollen allergy. Clin Exp Allergy. 46:764–774.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lauer I, Miguel-Moncin MS, Abel T,
Foetisch K, Hartz C, Fortunato D, Cistero-Bahima A, Vieths S and
Scheurer S: Identification of a plane pollen lipid transfer protein
(Pla a 3) and its immunological relation to the peach
lipid-transfer protein, Pru p 3. Clin Exp Allergy. 37:261–269.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ibarrola I, Arilla MC, Martinez A and
Asturias JA: Identification of a polygalacturonase as a major
allergen (Pla a 2) from Platanus acerifolia pollen. J
Allergy Clin Immunol. 113:1185–1191. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Asturias JA, Ibarrola I, Eraso E, Arilla
MC and Martínez A: The major Platanus acerifolia pollen
allergen Pla a 1 has sequence homology to invertase inhibitors.
Clin Exp Allergy. 33:978–985. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arilla MC, Ibarrola I, Mir A, Monteseirin
J, Conde J, Martínez A and Asturias JA: Development of a
sandwich-type ELISA for measuring Pla a 1, the major allergen of
Platanus acerifolia pollen. Int Arch Allergy Immunol.
138:127–133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fernández-González M, Guedes A, Abreu I
and Rodríguez-Rajo FJ: Pla a_1 aeroallergen immunodetection related
to the airborne Platanus pollen content. Sci Total Environ.
463–464:855–860. 2013. View Article : Google Scholar
|
|
16
|
Asturias JA, Ibarrola I, Bartolomé B,
Ojeda I, Malet A and Martínez A: Purification and characterization
of Pla a 1, a major allergen from Platanus acerifolia
pollen. Allergy. 57:221–227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Y, Sun X, Wang G, Tao A, Wu Y, Li M,
Shi H and Xie M: Expression, purification and identification of Pla
a1 in a codon-optimized Platanus pollen allergen. Mol Med
Rep. 12:2197–2202. 2015.PubMed/NCBI
|
|
18
|
Geng S, Qian S, Pan Z, Sun L, Chen X and
Jiao X: Preparation of monoclonal antibodies against SpiC protein
secreted by T3SS-2 of salmonella spp. Monoclon Antib Immunodiagn
Immunother. 34:432–435. 2015.PubMed/NCBI
|
|
19
|
James LK: The cloning and expression of
human monoclonal antibodies: Implications for allergen
immunotherapy. Curr Allergy Asthma Rep. 16:152016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dietrich R and Märtlbauer E: Development
and application of monoclonal antibodies against the mycotoxin
mycophenolic acid. Mycotoxin Res. 31:185–190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shendure JA, Porreca GJ, Church GM,
Gardner AF, Hendrickson CL, Kieleczawa J and Slatko BE: Overview of
DNA sequencing strategies. Curr Protoc Mol Biol Chapter.
7:Unit7.12011.
|
|
22
|
Towbin H, Staehelin T and Gordon J:
Electrophoretic transfer of proteins from polyacrylamide gels to
nitrocellulose sheets: Procedure and some applications. Proc Natl
Acad Sci USA. 76:4350–4354. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smith KA, Favata MF and Oroszlan S:
Production and characterization of monoclonal antibodies to human
interleukin 2: Strategy and tactics. J Immunol. 131:1808–1815.
1983.PubMed/NCBI
|
|
24
|
Zhang C, Jin K, Xiao Y, Cheng Y, Huang Z,
Wang S and Lu S: Potent monoclonal antibodies against Clostridium
difficile toxin A elicited by DNA immunization. Hum Vaccin
Immunother. 9:2157–2164. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoshida R, Igarashi M, Ozaki H, Kishida N,
Tomabechi D, Kida H, Ito K and Takada A: Cross-protective potential
of a novel monoclonal antibody directed against antigenic site B of
the hemagglutinin of influenza A viruses. PLoS Pathog.
5:e10003502009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Treanor JJ, Tierney EL, Zebedee SL, Lamb
RA and Murphy BR: Passively transferred monoclonal antibody to the
M2 protein inhibits influenza A virus replication in mice. J Virol.
64:1375–1377. 1990.PubMed/NCBI
|
|
27
|
Lin AV: Indirect ELISA. Methods Mol Biol.
1318:51–59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Smith KA: Commentary: Production and
characterization of monoclonal antibodies to human interleukin 2:
Strategy and tactics. Front Immunol. 6:4542015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen X, Ou Z, Xie XL, Xu ZZ and Jiao XA:
Preparation of monoclonal antibodies against Mycobacterium
tuberculosis TB10.4 antigen. Monoclon Antib Immunodiagn Immunother.
33:444–447. 2014.PubMed/NCBI
|
|
30
|
Geng S, Qian S, Pan Z, Sun L, Chen X and
Jiao X: Preparation of monoclonal antibody against SpiC protein
secreted by T3SS-2 of Salmonella spp. Monoclon Antib
Immunodiagn Immunother. 34:432–435. 2015.PubMed/NCBI
|
|
31
|
Zhang Y, Bao H, Miao F, Peng Y, Shen Y, Gu
W, Meng Q, Wang W and Zhang J: Production and application of
polyclonal and monoclonal antibodies against Spiroplasma
eriocheiris. Sci Rep. 5:178712015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Köhler G and Milstein C: Derivation of
specific antibody-producing tissue culture and tumor lines by cell
fusion. Eur J Immunol. 6:511–519. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de StGroth SF and Scheidegger D:
Production of monoclonal antibodies: Strategy and tactics. J
Immunol Methods. 35:1–21. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Arilla MC, Asturias JA, Gómez-Bayón N,
Martínez A, Martínez J and Palacios R: Production and
characterization of profilin monoclonal antibodies. Allergol
Immunopathol (Madr). 25:145–151. 1997.PubMed/NCBI
|
|
35
|
Chen H, Zhang K, Wang S, Xu C, Zou Z and
Tao A: Generation and purification of monoclonal antibodies against
Der f 2, a major allergen from Dermatophagoides farinae.
Drug Discov Ther. 10:103–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kida H, Brown LE and Webster RG:
Biological activity of monoclonal antibodies to operationally
defined antigenic regions on the hemagglutinin molecule of
A/Seal/Massachusetts/1/80 (H7N7) influenza virus. Virology.
122:38–47. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Boluda L, Alonso C and Fernández-Caldas E:
Purification, characterization and partial sequencing of two new
allergens of Olea europaea. J Allergy Clin Immunol.
101:210–216. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Asero R, Mistrello G, Amato S and Villalta
D: Monosensitization to a novel plane pollen allergen. Eur Ann
Allergy Clin Immunol. 44:167–169. 2012.PubMed/NCBI
|
|
39
|
Enrique E, Alonso R, Bartolomé B,
Miguel-Moncín M San, Bartra J, Fernández-Parra B, Tella R, Asturias
JA, Ibarrola I, Martínez A and Cisteró-Bahíma A: IgE reactivity to
profilin in Platanus acerifolia pollen-sensitized subjects
with plant-derived food allergy. J Investig Allergol Clin Immunol.
14:335–342. 2004.PubMed/NCBI
|
|
40
|
Ovsyannikova IG, Vailes LD, Li Y, Heymann
PW and Chapman MD: Monoclonal antibodies to group II
Dermatophagoides spp. allergens: Murine immune response,
epitope analysis and development of a two-site ELISA. J Allergy
Clin Immunol. 94:537–546. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chapman MD, Heymann PW, Wilkins SR, Brown
MJ and Platts-Mills TA: Monoclonal immunoassays for major dust mite
(Dermatophagoides) allergens, Der p I and Der f I and
quantitative analysis of the allergen content of mite and house
dust extracts. J Allergy Clin Immunol. 80:184–194. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jeong KY, Jin HS, Oh SH, Hong CS, Lee IY,
Ree HI and Yong TS: Monoclonal antibodies to recombinant Der f 2
and development of a two-site ELISA sensitive to major Der f 2
isoallergen in Korea. Allergy. 57:29–34. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yong TS, Lee SM, Park GM, Lee IY, Ree HI,
Kim KS, Oh SH, Park JW and Hong CS: Monoclonal antibodies to
recombinant Der p 2, a major house dust mite allergen: Specificity,
epitope analysis and development of two-site capture ELISA. Korean
J Parasitol. 37:163–169. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shen HD, Lin WL, Tsai LC, Tam MF, Chua KY,
Chen HL, Hsieh KH, Li CS and Thomas WR: Characterization of the
allergen Der f 7 from house dust mite extracts by species-specific
and crossreactive monoclonal antibodies. Clin Exp Allergy.
27:824–832. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wootla B, Denic A and Rodriguez M:
Polyclonal and monoclonal antibodies in clinic. Methods Mol Biol.
1060:79–110. 2014. View Article : Google Scholar : PubMed/NCBI
|