Introduction
Sweat glands are major skin structures distributed
across human skin that are vital for temperature regulation and
electrolyte balance (1,2). An increasing number of patients with
large area burns suffer from sweat gland defects, which severely
affect their quality of life (3).
Additionally, human eccrine sweat gland cells (HESGCs) have been
reported to be capable of repair of wounded human skin (4,5).
Therefore, increasing the availability of therapeutic sweat glands
is required for future clinical studies. However, HESGCs have a
limited proliferation capacity in the currently used culture
conditions (6,7). Previous studies have determined that
bone marrow mesenchymal and hair follicle stem cells may be induced
to differentiate into sweat gland cells in order to produce more
HESGCs in vitro (8,9). However, previous studies have not
compared the efficacy of different basal media for the culture of
sweat gland cells. Therefore, it is necessary to improve knowledge
of the current culture media to be used in future studies.
As presented in Table
I, there are two major categories of media presently used to
cultivate HESGCs, media with and without serum (10–18).
Sweat gland (SG) medium was the most commonly used medium,
containing fetal bovine serum (FBS). Similarly, keratinocyte growth
medium-2 (KGM-2) is a commonly used serum-free medium.
 | Table I.Supplements for media with or without
serum. |
Table I.
Supplements for media with or without
serum.
|
| Media with serum | Media without
serum |
|---|
|
|
|
|
|---|
| Factor | SG | FAD | Williums E | Rheinwald and
green | KFSM | EpiLife | KGM-1/2 |
|---|
| FBS | + | + | + | + | − | − | − |
| BPE | − | − | − | − | + | + | + |
| hEGF | + | + | + | + | + | + | + |
| Insulin | − | + | − | + | + | + | + |
| Hydrocortisone | + | + | + | + | + | + | + |
| Transferrin | − | − | − | − | + | + | + |
| Epinephrine | − | − | − | − | + | − | + |
| GA-1000 | − | − | − | − | − | + | + |
| Triiodothyronine | + | + | − | + | − | − | − |
|
Insulin-transferrin-selenium | + | − | + | − | − | − | − |
| Adenine | − | + | − | + | − | − | − |
| Cholera toxin | − | + | − | + | − | − | − |
| Reference | (10) | (11) | (12,13) | (14) | (15,16) | (17) | (18) |
The present study revealed that HESGCs cultured in
SG medium maintained the biological characteristics of HESGCs;
however, they demonstrated slow cell growth. HESGCs cultured in
KGM-2 medium demonstrated increased proliferation rates; however,
the cells gradually lost the biological characteristics of sweat
gland cells. The aforementioned findings of the present study
implied that competitive culture conditions are required for
HESGCs.
The present study aimed to mix the two media (SG and
KGM-2) in order to increase cell growth and maintain the biological
characteristics of HESGCs. The efficiency of the three media types
was compared and the mixed medium (SG:KGM-2 medium 1:1) was
identified to provide the most suitable HESGC culture conditions on
the basis of proliferation ability and maintenance of sweat gland
cell biological characteristics. Additionally, the present study
identified that the loss of the sweat gland cell biological
characteristics in serum-free medium occurred as the sweat gland
cells rapidly differentiated into keratinocytes without serum.
Materials and methods
Human skin tissues
A total of 12 infant polydactyl skin samples were
collected between January 2015 and January 2016 from male children
aged 1–3 years old who had a supernumerary sixth finger. The
samples are used for present study with the written informed
consent of patients' parents and the written approval was obtained
from the ethical review board of Children's Hospital Affiliated
with Soochow University (Suzhou, China). These skin samples were
used for the isolation of sweat gland cells. All methods were
performed in accordance with the approved guidelines. All
experimental protocols were approved by Soochow University. Copies
of the written consent provided by the subjects along with the
written approval from the review board were kept in the Children's
Hospital Affiliated with Soochow University ethical review board
office. All experimental procedures using skin samples in the
present study were reviewed and approved by the ethics
committee.
Isolation and culture of primary human
sweat gland cells
Following the removal of subcutaneous tissue, the
skin samples were minced into smaller pieces with sharp scissors in
phosphate buffered saline (PBS), then epidermal and dermal tissues
were separated using dispase II (1 mg/ml; Roche Applied Science,
Penzberg, Germany) at 4°C for ~18 h. The dermal tissue was digested
with collagenase type IV (2.5 mg/ml; Sigma-Aldrich, Merck
Millipore, Darmstadt, Germany) in an incubator at 37°C for 1 h.
Sweat gland tissues became accessible following digestion and were
collected using a Transferpettor under an ultraviolet-sterilized
phase-contrast inverted microscope at magnification ×40.
The collected sweat gland tissues were seeded into a
6-well plate (Corning, Inc., Corning, NY, USA) in SG medium at a
density of ~120 tissues/well. The SG medium contained Dulbecco's
modified Eagle's medium-F12 (DMEM-F12, Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with 4.5 g/l glucose,
supplemented with 10% FBS (Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA), 1% penicillin streptomycin (Gibco; Thermo Fisher
Scientific, Inc.), 2 mM L-glutamine (Gibco; Thermo Fisher
Scientific, Inc.), 10x insulin-transferrin sodium selenite solution
(ITS, Gibco; Thermo Fisher Scientific, Inc.), 2 nM triiodothyronine
(Sigma-Aldrich, Merck Millipore), 0.4 mg/ml hydrocortisone
(Sigma-Aldrich, Merck Millipore), and 10 ng/ml human recombinant
epidermal growth factor (R&D Systems, Inc., Minneapolis, MN,
USA) (19). When the sweat gland
cells migrated from the tissues after ~5 days in the SG medium,
three different culture media [SG, KGM-2 (Lonza Group, Ltd., Basel,
Switzerland) and SG:KGM-2 (1:1)] were added in order to observe
cellular proliferation and phenotypes.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the HESGCs cultured in
the different media using the RNeasy Mini extraction kit (Qiagen,
Inc., Valencia, CA, USA) according to the manufacturer's protocol.
A total of 1 µg RNA was used for reverse transcription (Qiagen,
Inc., Valencia, CA, USA). For the qPCR assays, the primer mixes
were loaded in duplicate wells in 96-well plates and then reactions
were performed following the addition of SYBR Green PCR Master mix
(Thermo Scientific, Inc., Waltham, MA, USA) and 1 µg (final) cDNA.
The experiment was repeated for three times. After pre-denaturation
at 95°C for 5 min, 30 sec at 95°C cooling to 65°C for 45 sec for 40
cycles (Bio-Rad Laboratories, Inc., Hercules, CA, USA). As an
internal control, GAPDH levels were quantified with target genes in
parallel. Normalization and fold-changes were calculated using the
2−∆ΔCq method (20),
and the primers used are presented in Table II.
 | Table II.Primers for reverse
transcription-quantitative polymerase chain reaction. |
Table II.
Primers for reverse
transcription-quantitative polymerase chain reaction.
| Gene name | Amplicon length
(bp) | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| α-SMA | 122 |
CTATGAGGGCTATGCCTTGCC |
GCTCAGCAGTAGTAACGAAGGA |
| K77 | 105 |
TCTCAGTCCGCGTTTAGTTCA |
TCGAGCATAACACACAGAACC |
| CEA | 138 |
AAGAAATGACGCAAGAGCCTATG |
CCCGAAAGGTAAGACGAGTCTG |
| K8 | 150 |
TCCTCAGGCAGCTATATGAAGAG |
GGTTGGCAATATCCTCGTACTGT |
| K18 | 171 |
TCGCAAATACTGTGGACAATGC |
GCAGTCGTGTGATATTGGTGT |
| EDA | 155 |
AGATGGCCCAGTTAAAAACAAGA |
CAGGTGGTCCCATAACAGTTG |
| EDAR | 121 |
CAGCCCGAGCGGAATACTC |
CCGTAGCCACAGGACAGGTA |
| c-Myc | 216 |
TCAAGAGGCGAACACACAACGTCT |
GTTCTCGTCGTTTCCGCAACAAGT |
| Klf-4 | 139 |
CGGACATCAACGACGTGAG |
GACGCCTTCAGCACGAACT |
| Oct-4 | 164 |
CTTGAATCCCGAATGGAAAGGG |
GTGTATATCCCAGGGTGATCCTC |
| K5 | 75 |
ATCTCTGAGATGAACCGGATGATC |
CAGATTGGCGCACTGTTTCTT |
| K14 | 80 |
GGCCTGCTGAGATCAAAGACTAC |
CACTGTGGCTGTGAGAATCTTGTT |
| GAPDH | 197 |
GGAGCGAGATCCCTCCAAAAT |
GGCTGTTGTCATACTTCTCATGG |
Three-dimensional culture of sweat
gland cells derived from different media
Collagen I from bovine tendon (Gibco; Thermo Fisher
Scientific, Inc.) and Matrigel (BD Biosciences, Franklin Lakes, NJ,
USA) were used to establish a dermal layer on a polycarbonate
membrane. The acellular collagen matrix layers were allowed to
stand at room temperature for ~20 min and solidify, subsequently a
cellular collagen matrix layer mixed with 3.3×104
fibroblasts and 1×105 sweat gland cells was added. The
mixture was incubated for 1 h at 37°C in order to allow it to
solidify. When the mixtures were solidified, EpiLife medium (Gibco;
Thermo Fisher Scientific, Inc.) was used to cover the surface of
the gels and this medium was changed every two days. The gels were
subsequently used for experiments after 21 days of culture in an
incubator at 37°C.
Sweat gland-like structure counting
standards
A protocol to quantify the number of sweat
gland-like structures that formed in the gels was designed. A total
of 3 sections were selected randomly with an interval of at least
100 µm to avoid the same structure being counted repeatedly. The
counting standards were as follows: Sweat gland-like structures
formed by sweat gland cells in different culture media were defined
as structures composed of 6–15 cells in one region, which is
similar to the transverse section of normal sweat gland tissue. The
number of structures was confirmed under an optical microscope
(magnification, ×10 and ×40).
Isolation and culture of primary human
fibroblasts
The skin samples obtained from the patients'
mentioned earlier were minced into smaller pieces with sharp
scissors in phosphate buffered saline (PBS), then epidermal and
dermal tissues were separated using dispase II (1 mg/ml; Roche
Diagnostics GmbH, Mannheim, Germany) at 4°C for ~18 h. The dermal
tissue was digested with trypsin (0.25% trypsin, 0.1% EDTA;
Beyotime Institute of Biotechnology, JiangSu, China) in an
incubator at 37°C for 10 min. Fibroblasts were cultured in DMEM
(Thermo Fisher Scientific, Inc.) with 4.5 g/l glucose, supplemented
with 10% FBS (Hyclone; GE Healthcare Life Sciences), 1% penicillin
streptomycin (Gibco; Thermo Fisher Scientific, Inc.), 2 mM
L-glutamine (Gibco; Thermo Fisher Scientific, Inc.). Approximately
95% of the non-adherent cells were removed after 24 h, and the
culture media was replaced every day. Cells were passaged by
trypsinization (0.25% trypsin, 0.1% EDTA) and expanded serially
with a split ratio of 1:3 at 70% confluence in 2 or 3 days.
Three-dimensional air-liquid interface
tissue culture
Collagen I from bovine tendon, populated with
fibroblasts, was used for establishing successive layers of
acellular and cellular collagen on a polycarbonate membrane. The
collagen I was prepared as aforementioned. After ~7 days,
1×105 sweat gland cells from the three different media
cultures were added to the center of the collagen gel and incubated
for 1 h at 37°C to allow the sweat gland cells to fully adhere.
Cells were exposed to the air-liquid interface for 8 days in
EpiLife medium.
Histochemistry analysis
Cells were fixed with 4% paraformaldehyde,
dehydrated, and embedded in paraffin for hematoxylin & eosin
(H&E) staining. The samples were observed under an optical
microscope, where 10 different views, selected at random, were
examined at magnifications ×10, ×20 and ×40 (Leica Microsystems,
Inc., Buffalo Grove, IL, USA).
Statistical analysis
Data are presented as the mean ± standard deviation
and were acquired following at least 3 independent experiments
prior to being analyzed using Prism 5 software (Graph Pad Software,
San Diego, CA, USA). The qPCR data are presented as the fold-change
in gene expression normalized to GAPDH (used as a reference gene)
using the 2−∆∆Cq method. Differences between each group
were analyzed using one-way analysis of variance followed by a
Tukey's HSD post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Biological characteristics of sweat
gland cells cultured in different media
Different cell morphologies resulted from sweat
gland cell culture in different media. HESGCs cultured in SG medium
and SG:KGM-2 (1:1) medium maintained an HESGC-like morphology. A
higher number of fibroblasts survived in the SG medium compared
with the other media. HESGCs cultured in KGM-2 medium had a clear
boundary, significant cell interval and typical keratinocyte-like
morphology (Fig. 1A) as previously
described (21). The cells were
subcultured three times in succession, at passage 0, 1 and 2.
HESGCs that were cultured in SG:KGM-2 (1:1) medium had higher
proliferation compared with those cultured in SG medium or KGM-2
medium alone (Fig. 1B). RT-qPCR
was used to analyze the mRNA expression levels of marker genes of
HESGCs, including α smooth muscle actin (α-SMA), keratin (K)77,
carcinoembryonic antigen (CEA), K8, K18 and ectodysplasin A (EDA)
and stem cell markers, such as c-Myc, Kruppel-like factor 4 (Klf-4)
and octamer-binding transcription factor 4 (Oct-4). The cells
cultured in SG:KGM-2 (1:1) medium had higher mRNA expression levels
of HESGCs gland markers (α-SMA, CEA, K8, K18 and EDA) and stem cell
markers (c-Myc, Klf-4 and Oct-4) compared with cells cultured in SG
medium or KGM-2 medium only. The expression levels of the markers
(CEA, K8, K18, EDA, c-Myc, and Oct-4) in the SG:KGM-2 (1:1)
medium-cultured cells did not differ significantly from the
expression levels of the markers in normal sweat gland tissue.
However, the expression level of K77, a marker of the sweat gland
duct, was very low in the three medium groups. The lack of K77
expression suggested that the majority of the sweat gland cells
cultured in vitro in the present study were derived from the
secretory portion of the gland. These findings indicate that the
SG:KGM-2 (1:1) medium is more suitable for culturing HESGCs
compared with SG or KGM-2 medium primarily due to the improved
maintenance of the HESGCs morphology (Fig. 1C).
 | Figure 1.(A) Morphology of sweat gland cells
cultured in three different media. Cells cultured in KGM-2 medium
had typical epithelial morphology, whereas cells cultured in
SG:KGM-2 (1:1) medium exhibited the same morphology as cells
cultured in normal SG medium. The red dotted line indicates the
separation between sweat gland cells and fibroblasts. (B) Following
subculture of sweat gland cells to passage 3, cells cultured in the
mixed medium (SG:KGM-2 1:1 medium) demonstrated a higher
proliferation rate compared with cells cultured in the remaining
two media. (C) mRNA expression levels of sweat gland cell markers
(α-SMA, K77, CEA, K8, K18 and EDA) and stem cell markers (c-Myc,
Klf-4 and Oct-4) were quantified in sweat gland cells cultured in
three different media. The sweat gland tissues were used as
positive control. Data are presented as the mean ± standard
deviation of three independent experiments. *P<0.05. Scale bar,
40 µm. SG, sweat gland medium; KGM-2, keratinocyte growth medium-2;
1, sweat gland tissue; 2, SG medium; 3, KGM-2 medium; 4, SG:KGM-2
(1:1) medium; α-SMA, α smooth muscle actin; K77/8/18, keratin
77/8/18; CEA, carcinoembryonic antigen; EDA, ectodysplasin A;
Klf-4, Kruppel-like factor 4; Oct-4, octamer-binding transcription
factor 4. |
HESGCs cultured in different media
form sweat gland-like structures
In order to determine whether HESGCs may
differentiate into sweat gland-like structures in vitro, the
present study compared the cell structures resulting from
three-dimensional culture conditions. The sweat gland cells
cultured in SG medium and SG:KGM-2 (1:1) medium formed sweat
gland-like structures, whereas sweat gland cells cultured in KGM-2
only medium did not differentiate into sweat gland-like structures
(Fig. 2A). In addition to the
formation of sweat gland-like structures, the number of sweat
gland-like structures formed during the culture in different media
was also monitored. HESGCs cultured in SG:KGM-2 (1:1) medium formed
a significantly greater number of sweat gland-like structures
compared with cells cultured in SG medium only (Fig. 2B). However, no sweat gland-like
structures were formed in the KGM-2 only medium. In summary, the
SG:KGM-2 (1:1) medium was suitable for the culture of sweat gland
cells and the formation of sweat gland-like structures.
 | Figure 2.HESGCs cultured in recommended medium
may differentiate into sweat gland-like structures in vitro.
(A) Hematoxylin and eosin staining images of sweat gland-like
structures formed by cells were cultured in normal SG, KGM-2 and
SG:KGM-2 (1:1) medium. HESGCs cultured in SG medium and SG:KGM-2
(1:1) medium were able to form sweat gland-like structures, whereas
cells cultured in KGM-2 medium did not. Blue dashed circle
indicates the shape of the tubule-like structure. Red dashed
circle, indicates the lumen of the tubule-like structure. (B)
Number of sweat gland-like structures derived from cells cultured
in three media. HESGCs cultured in the SG:KGM-2 (1:1) medium formed
more sweat gland-like structures compared with cells cultured in SG
medium and KGM-2 medium (n=4). 1, SG medium; 2, KGM-2 medium; 3,
SG:KGM-2 (1:1) medium. *P<0.05. Scale bar, 20 µm. SG, sweat
gland medium; KGM-2, keratinocyte growth medium-2; HESGCs, human
eccrine sweat gland cells. |
HESGCs cultured in KGM-2 medium
differentiate into epithelium
In order to determine why HESGCs cultured in KGM-2
medium did not differentiate into sweat gland-like structures, the
mRNA expression of the keratinocyte markers K14 and K5 was
determined. K14 and K5 expression levels in the HESGCs cultured in
KGM-2 only medium were higher compared with the remaining groups
(Fig. 3A). Subsequently, the
air-liquid interface system was used to culture the cells derived
from the three different media groups. After 8 days of air-liquid
differentiation, H&E staining revealed that cells cultured in
KGM-2 medium exhibited a stratified epithelium with basal layer,
stratum spinosum, stratum granulosum and stratum corneum observed
as superposed layers of dead, squamous, enucleated cells. However,
HESGCs cultured in SG medium and SG:KGM-2 (1:1) medium did not form
completely stratified epithelia (Fig.
3B). These findings demonstrated that HESGCs cultured in KGM-2
medium were unable to differentiate into a sweat gland-like
structure; however, sweat gland cells cultured in KGM-2 only medium
rapidly differentiate into keratinocytes.
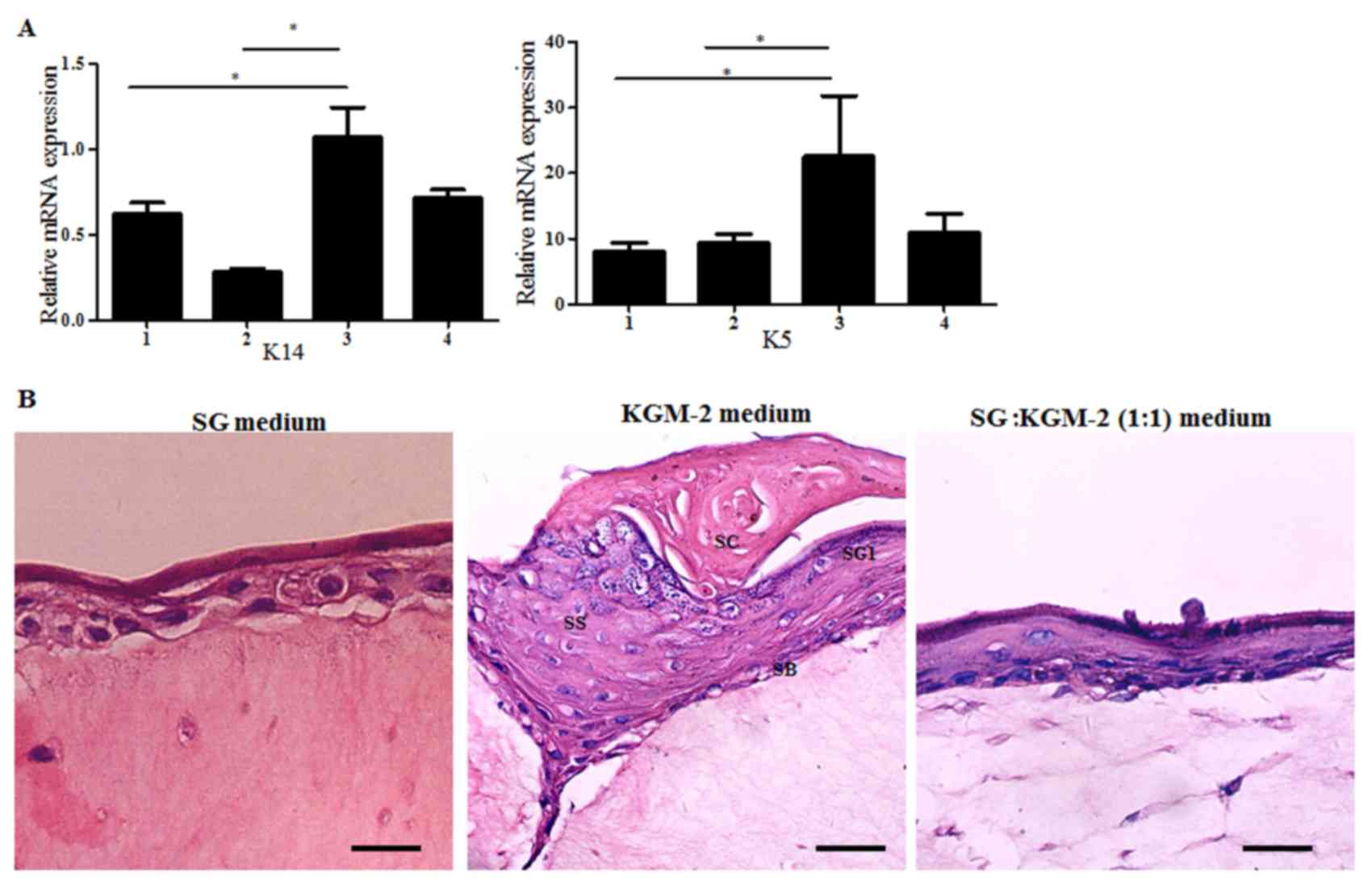 | Figure 3.Sweat gland cells cultured in KGM-2
medium differentiated into keratinocytes. (A) mRNA expression of
keratinocyte markers (K14, K5) in sweat gland cells cultured in
three different media. The sweat gland tissues were used as
negative controls. Cells cultured in KGM-2 medium had highest K5
mRNA expression among the cells cultured in the three media. Data
are presented as the mean ± standard deviation of three independent
experiments. *P<0.05. (B) Hematoxylin and eosin staining images
of epidermis constructed by sweat gland cells. Cells cultured in
KGM-2 medium displayed a well-organized, multilayer epithelium that
included all morphological layers (SC, SG1, SS, and SB); however,
human eccrine sweat gland cells cultured in SG medium and SG: KGM-2
(1:1) medium did not differentiate into epidermis. Scale bar, 20
µm. SG, sweat gland medium; KGM-2, keratinocyte growth medium-2; 1,
sweat gland tissue; 2, SG medium; 3, KGM-2 medium; 4, SG:KGM-2
(1:1) medium; SC, stratum corneum; SG1, stratum granulosum; SS,
stratum spinosum; SB, stratum basal; K5/14, keratin 5/14. |
Serum allowed the survival of
fibroblasts and the maintenance of HESGCs biological
characteristics
From the various components (data not shown) of the
media used in the present study, the presence or absence of serum
had the greatest impact (Table I).
In order to determine whether the serum was the primary factor
supporting the maintenance of the desired HESGC biological
characteristics and functions and to determine whether media with
or without serum would lead to sweat gland cells rapidly
differentiating into keratinocytes, HESGCs were cultured in four
different media: i) SG medium; ii) SG medium without FBS; iii)
KGM-2 medium; and iv) KGM-2 medium with FBS. Morphology of cells in
the different media at 2, 4 and 6 days are presented in Fig. 4A. Due to the presence of serum in
the SG medium and the KGM-2 medium with FBS, the sweat gland cells
were able to maintain their morphology. However, cells cultured in
the media without FBS, such as the SG medium without FBS and the
KGM-2 medium, differentiated into keratinocyte-like cells. In order
to determine whether these 4 groups of cells may form sweat
gland-like structures, they were transferred into three-dimensional
culture systems. H&E staining revealed that cells cultured in
the media without FBS did not have the ability to form sweat
gland-like structures. In addition, cells cultured in the media
with FBS were able to form sweat gland-like structures, this is
partly due to FBS allowing for the survival of fibroblasts, which
provide essential factors such as transforming growth factor-β
(TGF-β) and platelet-derived growth factor (PDGF) for maintaining
the desired cellular biological characteristics of HESGCs (Fig. 4B). The increased number of sweat
gland-like structures observed in HESGCs cultured in media with FBS
revealed that HESGCs had an increased proliferation capability
compared with those cultured in media without FBS (Fig. 4C). RT-qPCR revealed that HESGCs
cultured in media with FBS had higher expression levels of SG cell
markers (CEA, K8, EDA and EDAR), whereas cells cultured in media
without FBS expressed keratinocyte markers (K5, K14) at higher
levels compared with those cultured with FBS (Fig. 4D).
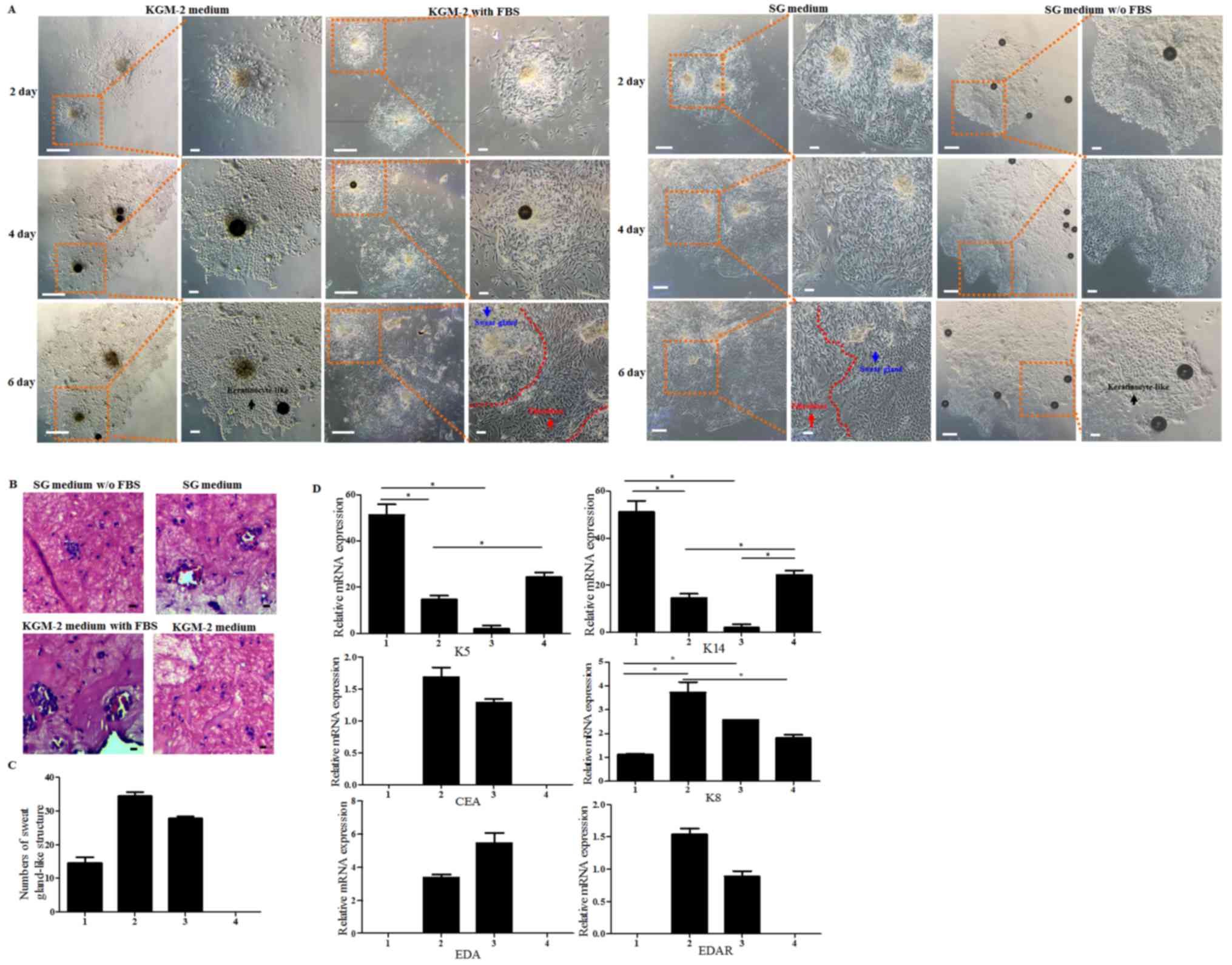 | Figure 4.Serum is an indispensable factor for
the maintenance of HESGCs morphology and biological
characteristics. (A) Representative images of HESGCs cultured in
four different media, including SG medium, SG medium without FBS,
KGM-2 medium, and KGM-2 medium with FBS, at days 2, 4, 6.
Fibroblasts continued to exist in the media with FBS, SG medium and
KGM-2 medium with FBS. Red arrow indicates fibroblasts. Blue arrow
indicates sweat gland cells. Black arrow indicates
keratinocyte-like cells. Sweat gland cells and fibroblasts were
distinguished by the red dotted line. (B) Hematoxylin and eosin
staining images of sweat gland-like structures formed in gels by
cells under four different culture conditions, including SG without
FBS, SG medium, KGM-2 with FBS, and KGM-2 medium. Cells cultured in
medium with FBS formed more sweat gland-like structures. (C)
Numbers of sweat gland-like structures derived from cells cultured
in four different media. Cells cultured in SG medium and KGM-2 with
FBS medium formed more sweat gland-like structures, whereas cells
cultured in KGM-2 medium did not form this type of structure due to
cornification (n=3). 1, SG medium without FBS; 2, SG medium; 3,
KGM-2 with FBS; 4, KGM-2 medium. (D) mRNA expression levels of
sweat gland cell markers (CEA, K8, EDA, and EDAR) and keratinocyte
markers (K5 and K14) in media without FBS (KGM-2 and SG medium
without FBS) and media with FBS (SG medium and KGM-2 medium with
FBS). 1, KGM-2 medium; 2, KGM-2 with FBS; 3, SG medium; 4, SG
medium without FBS. Data are presented as the mean ± standard
deviation of three independent experiments. *P<0.05. Scale bar,
10 µm. SG, sweat gland medium; KGM-2, keratinocyte growth medium-2;
K5/14/8, keratin 5/14/8; CEA, carcinoembryonic antigen; EDA,
ectodysplasin A; EDAR, EDA receptor. |
In conclusion, serum is the primary factor that
maintains the biological characteristics and functions of sweat
gland cells, and medium without serum leads to the rapid
differentiation of sweat gland cells into keratinocytes.
Discussion
The sweat gland is one of the important skin
structures and its primary function is to regulate body temperature
(22). The sweat gland cell is a
somatic cell; therefore, its tolerance for in vitro
amplification and subcultivation is low (23). The establishment of a novel culture
method, which allows for the maintenance of the desired biological
characteristics and improves sweat gland cell proliferation ability
would be indispensable for future studies. The findings of the
present study revealed that the two different types of media
previously reported to be used for the culture of HESGCs had
several shortcomings (10,18). A serum-free medium, such as the
KGM-2 medium, frequently led to sweat gland cells differentiating
into keratinocytes, whereas a medium with serum, such as SG medium,
reduced the HESGC proliferation ability. Based on the findings of
the current study, a mix of SG medium and KGM-2 medium (SG:KGM 1:1
medium) may provide improved culture conditions for HESGCs.
The present study determined that HESGCs cultured in
media without serum led to HESGCs rapidly differentiating into
keratinocytes. HESGCs cultured in the mixed medium formed more
sweat gland-like structures compared with cells cultured in the SG
medium only. HESGCs cultured in the mixed medium also maintained
the phenotypic characteristics of sweat gland cells, whereas cells
cultured in KGM-2 medium did not. Overall, HESGCs cultured in the
mixed medium maintained their biological characteristics and had
increased proliferation ability. A previous study reported that
HESGCs have the potential to generate epidermal keratinocytes that
migrate toward local wounds in the epidermis (10). The findings of the present study
were also consistent with the findings of previous studies which
stated that when sweat gland cells were cultured in serum-free
media, they rapidly lost their biological characteristics and
differentiated into keratinocytes (5,14).
Furthermore, the current findings suggested that
media with serum allow for fibroblast survival and thus the
maintenance of the HESGC biological characteristics; however, an
excessive fibroblast population would have a competitive effect on
HESGCs as fibroblasts have a higher proliferation ability. When the
HESGCs were cultured in the SG medium only, a number of fibroblasts
survived due to the high serum concentration, which led to the
sweat gland cells being deprived of growth space. However, when the
HESGCs were cultured in SG:KGM-2 (1:1) medium, moderate fibroblast
growth allowed enough growth space for the sweat glands cells.
Additionally, fibroblasts have the ability to secrete different
types of factors, including basic fibroblast growth factor (bFGF),
that stimulate cell growth (24),
which is necessary for HESGCs and appropriate numbers of
fibroblasts to coexist. The findings of the present study supported
the hypothesis that media containing serum allowed for fibroblast
survival and a fibroblast population may maintain the morphology
and function of sweat gland cells. Several issues remain unsolved;
including, if the fibroblasts are removed, it is uncertain whether
or not the serum alone may directly maintain the characteristics of
the sweat gland cells. It is also uncertain if the removal of the
serum and the fibroblasts from the culture would allow the sweat
gland cells to maintain their biological characteristics if the
necessary factors such as TGF-β, PDGF, bFGF were added
independently. Whether the serum directly maintained the biological
characteristics of the sweat gland cells or allowed the fibroblasts
and the HESGCs to coexist remains to be elucidated as some of the
factors secreted by the fibroblasts that maintained the HESGC
biological characteristics have not been identified.
In conclusion, the present study suggested that the
coexistence and development of HESGCs with an appropriate number of
fibroblasts may be considered in future HESGCs culture conditions.
The use of mixed medium containing KGM-2 medium and SG medium may
provide vital information for future sweat gland research. The
mixed medium is a valuable compound and should be considered a
future substantial supplemental medium.
Acknowledgements
We are grateful to Mr. YongPing Gu for histology.
Funding for the present study was provided by the 973 Plan Research
Special Subject Program (grant no. 2012CB22302), the National
Natural Science Foundation (grant no. 81402584), the Natural
Science Foundation of Jiangsu Province (grant no. BK20140360) and
China National Textile and Apparel Council (grant no. J201405).
References
|
1
|
Cui CY and Schlessinger D: Eccrine sweat
gland development and sweat secretion. Exp Dermatol. 24:644–650.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dobson RL and Sato K: The secretion of
salt and water by the eccrine weat gland. Arch Dermatol.
105:366–370. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gallico GG III, O'Connor NE, Compton CC,
Kehinde O and Green H: Permanent coverage of large burn wounds with
autologous cultured human epithelium. N Engl J Med. 311:448–451.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Danner S, Kremer M, Petschnik AE, Nagel S,
Zhang Z, Hopfner U, ReckhenrichG AK, Weber C, Schenck TL, Becker T,
et al: The use of human sweat gland-derived stem cells for
enhancing vascularization during dermal regeneration. J Invest
Dermatol. 132:1707–1716. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pontiggia L, Biedermann T,
Böttcher-Haberzeth S, Oliveira C, Braziulis E, Klar AS,
Meuli-Simmen C, Meuli M and Reichmann E: De novo epidermal
regeneration using human eccrine sweat gland cells: Higher
competence of secretory over absorptive cells. J Invest Dermatol.
134:1735–1742. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li HH, Zhou G, Fu XB and Zhang L: Antigen
expression of human eccrine sweat glands. J Cutan Pathol.
36:318–324. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li HH, Fu XB, Zhang L and Zhou G:
Comparison of proliferating cells between human adult and fetal
eccrine sweat glands. Arch Dermatol Res. 300:173–176. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hagmann S, Moradi B, Frank S, Dreher T,
Kämmerer PW, Richter W and Gotterbarm T: Different culture media
affect growth characteristics, surface marker distribution and
chondrogenic differentiation of human bone marrow-derived
mesenchymal stromal cells. BMC Musculoskelet Disord. 14:2232013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Liu ZY, Zhao Q, Sun TZ, Ma K and
Fu XB: Future application of hair follicle stem cells: Capable in
differentiation into sweat gland cells. Chin Med J (Engl).
126:3545–3552. 2013.PubMed/NCBI
|
|
10
|
Böttcher-Haberzeth S, Biedermann T,
Pontiggia L, Braziulis E, Schiestl C, Hendriks B, Eichhoff OM,
Widmer DS, Meuli-Simmen C, Meuli M and Reichmann E: Human eccrine
sweat gland cells turn into melanin-uptaking keratinocytes in
dermo-epidermal skin substitutes. J Invest Dermatol. 133:316–324.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schön M, Benwood J, O'Connell-Willstaedt T
and Rheinwald JG: Human sweat gland myoepithelial cells express a
unique set of cytokeratins and reveal the potential for alternative
epithelial and mesenchymal differentiation states in culture. J
Cell Sci. 112:1925–1936. 1999.PubMed/NCBI
|
|
12
|
Czifra G, Szöllősi AG, Tóth BI, Demaude J,
Bouez C, Breton L and Bíró T: Endocannabinoids regulate growth and
survival of human eccrine sweat gland-derived epithelial cells. J
Invest Dermatol. 132:1967–1976. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee CM, Carpenter F, Coaker T and Kealey
T: The primary culture of epithelia from the secretory coil and
collecting duct of normal human and cystic fibrotic eccrine sweat
glands. J Cell Sci. 83:103–118. 1986.PubMed/NCBI
|
|
14
|
Biedermann T, Pontiggia L,
Böttcher-Haberzeth S, Tharakan S, Braziulis E, Schiestl C, Meuli M
and Reichmann E: Human eccrine sweat gland cells can reconstitute a
stratified epidermis. J Invest Dermatol. 130:1996–2009. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lei X, Liu B, Wu J, Lu Y and Yang Y:
Matrigel-induced tubular morphogenesis of human eccrine sweat gland
epithelial cells. Anat Rec (Hoboken). 294:1525–1531. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tao R, Han Y, Chai J, Li D and Sun T:
Isolation, culture, and verification of human sweat gland
epithelial cells. Cytotechnology. 62:489–495. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lei X, Wu J, Lu Y and Zhu T: Effects of
acetylcholine chloride on intracellular calcium concentration of
cultured sweat gland epithelial cells. Arch Dermatol Res.
300:335–341. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Uchida N, Oura H, Nakanishi H, Urano Y and
Arase S: Dispersed cell culture of human sweat duct cells under
serum-free conditions. J Dermatol. 20:684–690. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Collie G, Buchwald M, Harper P and Riordan
JR: Culture of sweat gland epithelial cells from normal individuals
and patients with cystic fibrosis. In vitro Cell Dev Boil.
21:597–602. 1985. View Article : Google Scholar
|
|
20
|
Guenou H, Nissan X, Larcher F, Feteira J,
Lemaitre G, Saidani M, Del Rio M, Barrault CC, Bernard FX,
Peschanski M, et al: Human embryonic stem-cell derivatives for full
reconstruction of the pluristratified epidermis: a preclinical
study. Lancet. 374:1745–1753. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun Q, Li F, Li H, Chen RH, Gu YZ, Chen Y,
Liang HS, You XR, Ding SS, Gao L, et al: Amniotic fluid stem cells
provide considerable advantages in epidermal regeneration: B7H4
creates a moderate inflammation microenvironment to promote wound
repair. Sci Rep. 5:115602015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu C and Fuchs E: Sweat gland progenitors
in development, homeostasis, and wound repair. Cold Spring Harb
Perspect Med. 4:pii: a015222. 2014. View Article : Google Scholar
|
|
23
|
Morimoto Y and Saga K: Proliferating cells
in human eccrine and apocrine sweat glands. J Histochem Cytochem.
43:1217–1221. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hainfellner JA, Voigtländer T, Ströbel T,
Mazal PR, Maddalena AS, Aguzzi A and Budka H: Fibroblasts can
express glial fibrillary acidic protein (GFAP) in vivo. J
Neuropathol Exp Neurol. 60:449–461. 2001. View Article : Google Scholar : PubMed/NCBI
|


















