Introduction
Previous studies have demonstrated that mesenchymal
stem cells (MSCs) may be isolated from muscle, adipose and
endometrial tissues, as well as umbilical cord blood, and are able
to be induced to undergo osteogenic and adipogenic differentiation
(1–4). The primary function of MSCs is
considered to be the maintenance of local tissue remodeling
(1–4). Following observations that MSCs are
weakly immunogenic and exhibit immunosuppressive effects on the
adaptive and innate immune systems, MSCs have become a particular
interest to immunologists (5).
The invasion of trophoblasts induces the
differentiation of endometrial stromal cells to form the decidua,
which is a highly specialized structure consisting of stromal
cells, glandular cells and leukocytes (6,7). The
primary function of the human decidua is to ensure optimal
conditions for implantation of the embryo and placentation
(8). In addition, the decidua is
thought to function as an active immune regulation partner for the
immune tolerance microenviroment at the maternal-fetal interface.
It has been proposed that a population of stem cells exists within
the human decidua, which may be responsible for mediating cell
proliferation during embryonic implantation and the formation of
the placenta (9). The maintenance
of pregnancy involves maternal tolerance to the fetal allograft,
which is associated with decidual natural killer cell (dNK)
tolerance phenotype (10) and a
T-helper (Th) 2 cell bias at the maternal-fetal interface (11). Spaggiari and Moretta (12) reviewed what is known regarding the
interactions between human MSCs and NK cells. It was demonstrated
that the function and proliferation of NK cells is inhibited by
MSCs (12). However, decidual MSCs
(DMSCs) are not well characterized, and, to the best of the
author's knowledge, the effect of DMSCs on the phenotype and
function of dNK cells has not been previously investigated. In the
present study, DMSCs were isolated from early human decidua and
were first confirmed to be MSCs by examining the expression of
specific cell surface markers. These cells were then employed to
investigate the ability of DMSCs to regulate the phenotype and
biological function of dNK cells.
Materials and methods
Human decidual tissue collection
All procedures involving study participants were
approved by the Human Research Ethics Committee of Binzhou Medical
University (Yantai, China), and written informed consent was
received from all subjects for the collection and use of their
tissue samples for the purposes of the study. Human decidual
tissues were obtained from 3 women that had undergone elective
vaginal termination of first-trimester pregnancies (gestational
age, 6–9 weeks; age, 24–26 years) for non-medical reasons between
October and November 2014 in Yuhuangding Hospital (Yantai, China).
All tissues were immediately collected and stored in ice-cold
Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), transported to the laboratory
within 60 min following surgery and washed in DMEM containing 100
U/ml penicillin and 100 mg/ml streptomycin (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China).
Isolation and culture of DMSCs and dNK
cells
Decidual tissues were minced into >1
mm3 pieces. Following an additional wash with PBS (pH
7.4), the pieces were cultured in complete DMEM/F12 medium
supplemented with 10% fetal bovine serum (FBS) (both from Gibco;
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin in 5% CO2 at 37°C. Following 5 days of
culture, large sections were removed and adherent cells were
maintained in culture with medium replenishment every third day.
Cells were visualized under a microscope and identified using
MSC-specific markers with flow cytometry as described below, and
cells at passages 3–10 were employed for downstream
experiments.
Decidual tissues were minced into 1 mm3
pieces and enzymatically digested for 20 min at 37°C, under
vigorous agitation, with 1.5 mg DNase type I and 24 mg collagenase
type IV (Sigma-Aldrich; Merck KGaA; Darmstadt, Germany) in 15 ml
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.). Following
digestion, decidual immune cells (DICs) were isolated and purified
by discontinuous Percoll gradient centrifugation, using the methods
described previously (13). The
concentration of DICs obtained ranged between 1.062 and 1.077 g/ml,
and were subsequently collected and cultured in complete RPMI-1640
medium supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin in 5% CO2 at 37°C. Following primary
culture for 30 min at 37°C in 5% CO2, the adherent
decidual stromal cells were removed, leaving DICs that were 98%
pure. A total of 4×107 dNK cells were purified with
microbeads conjugated to the anti-human CD56 monoclonal antibody,
using the CD56 MultiSort kit (cat no. 130-055-401; Miltenyi Biotec
GmbH, Bergisch Gladbach, Germany). Separation was performed using
the autoMACS® Pro separator with Volumes software
(version 2.0.1.5; Miltenyi Biotec GmbH), as previously described
(14).
Osteogenic and adipogenic
differentiation of DMSCs
The osteogenic differentiation of DMSCs was
performed using OriCell™ Mesenchymal Stem Cell Osteogenic
Differentiation Medium (cat no. GUXMX-90021; Cyagen Biosciences,
Inc., Santa Clara, CA, USA). DMSCs at passage 4 were detached
following treatment with a solution containing 0.05% trypsin and 1
mM EDTA, washed in PBS and then seeded at a final density of
1×104 cells/cm2 in 24-well plates in
triplicate. At 80–90% confluency, the growth medium was carefully
aspirated from each well, and 1 ml osteogenic differentiation
medium was added. Cells were cultured at 37°C and 5%
CO2, and the medium was refreshed every 3 days. The
presence of calcium-containing osteocytes was determined following
21 days of exposure to osteogenic differentiation medium by
staining the cells with alizarin red S. To achieve this, the
osteogenic differentiation medium was first removed and cells were
rinsed with PBS and fixed with 4% formaldehyde solution at 20°C for
30 min. The cells were subsequently stained with a working solution
of 1% alizarin red S at 20°C for 3–5 min, followed by washing with
distilled water to remove any unbound dye.
The adipogenic differentiation of DMSCs was
performed using OriCell™ Mesenchymal Stem Cell Adipogenic
Differentiation Medium (cat no. GUXMX-90031; Cyagen Biosciences,
Inc.). DMSCs at passage 4 were seeded at a concentration of
1×104 cells/cm2 in 24-well plates in
triplicate. When the cells were 100% confluent, the growth medium
was aspirated from the wells, and 0.5 ml OriCell™ Mesenchymal Stem
Cell Adipogenic Differentiation Medium was added. Following 3 days
in culture, the medium was replaced with adipogenic differentiation
medium B, and cells were incubated for a further 24 h. Following 4
cycles of induction and maintenance, the cells were cultured in
adipogenic differentiation medium B for an additional 7 days, and
subsequently stained with oil red O. To achieve this, the
adipogenic differentiation medium was first removed, the cells were
rinsed with PBS and then fixed with 4% formaldehyde solution at
20°C for 30 min. The cells were subsequently stained with a 0.6%
oil red O solution at 20°C for 30 min, followed by washing with
distilled water to remove the unbound dye. Control cells from the
same passage were cultured in DMEM supplemented with 10% FBS and
stained with oil red O according to the same protocol.
Prolyl-4-hydroxylase (P4H) short
hairpin RNA (shRNA) plasmid transfection
Collagen molecules are trimeric polypeptide chains
that form a triple helix. Hydroxylation of proline residues present
in the polypeptides is catalyzed by collagen P4H and is essential
for triple helix formation and stability (15). In the present study,
5×104 DMSCs were seeded in 24-well plates with DMEM
supplemented with 10% FBS. When cells had reached 85% confluence, 2
µl Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), 50 µl Opti-MEM™ (Gibco; Thermo Fisher
Scientific, Inc.) and 20 pmol pScoR-GFP-hP4H shRNA plasmid
(GeneChem Co., Ltd., Shanghai, China) were mixed and incubated at
20°C for 20 min, and subsequently added to the cells according to
the manufacturer's protocol (Lipofectamine® 2000;
Invitrogen; Thermo Fisher Scientific, Inc.). The vector-only
pScoR-GFP plasmid (Shanghai GeneChem Co., Ltd.) was used as a
negative control. Non-transfected DMSCs were treated as the blank
controls. Following 6 h of incubation at 37°C, the plasmid
vector-containing medium was replaced with DMEM/F12 supplemented
with 10% FBS and cells were cultured in 5% CO2 at
37°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The effect of transfection on P4H expression in
DMSCs was determined by RT-qPCR, using the 2−∆∆Cq method
for quantification of P4H expression (16). Total RNA was extracted from DMSCs
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. Total
RNA (1 µg) was denatured, and reverse transcribed into cDNA for 1 h
at 42°C with 0.5 µg oligo(dT) 18, 1.0 mM 4dNTP, 20 U RNasin RNase
inhibitor (Promega Corporation, Madison, WI, USA), 200 U Moloney
virus-reverse transcriptase (SuperScript II; Thermo Fisher
Scientific, Inc.), and 5X reaction buffer, in a reaction volume of
20 µl. Amplification was performed with SYBR-Green PCR Master Mix
(PerkinElmer, Inc., Waltham, MA, USA), 0.8 mM specific sense and
antisense primers, and 5 µl cDNA in a 50 µl reaction volume in a
T100™ thermal cycler (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The primers used for the detection of P4H were as follows:
Forward, 5′-CTGCGGGACCTGACTAGATT-3′ and reverse,
5′-TGCTCCACCTTCTCATAGCC-3′; and for β-actin were: Forward,
5′-CCCTGGACTTCGAGCAAGAG-3′ and reverse, 5′-TCTCCTTCTGCATCCTGTCG-3′.
After a 5-min pre-cycle at 95°C, the reaction was followed by 30
cycles at 94°C for 1 min, at 55°C for 1 min, and at 72°C for 1 min.
A final extension step at 72°C for 15 min was performed. Relative
gene expression was normalized to β-actin. All experiments were
repeated three times.
Cell co-culture unit
Control DMSCs and those transfected with empty
vector or pScoR-GFP-hP4H shRNA were seeded in 24-well plates at a
density of 1×105 cells/well. In this co-culture unit,
dNK cells were subsequently directly added to the wells at the same
density following 12 h of co-culture. DMSCs and dNK cells
(1×105 cells/well) were cultured alone as controls. A
total volume of 1 ml DMEM/F-12 supplemented with 10% FBS was added
to each well. A total of 6 h prior to the addition of dNK cells, 10
µg/ml leukocyte-associated immunoglobulin-like receptor 2 (LAIR-2;
cat no. ab182705; Abcam, Cambridge, UK) was added to specific
co-culture wells. Floating dNK cells were collected for flow
cytometry analysis following 48 h of co-culture.
Flow cytometry
Monoclonal antibodies targeting the following
proteins: CD44 (cat no. 555478), CD73 (cat no. 561254), CD105 (cat
no. 561443), CD34 (cat no. 555821), CD31/platelet endothelial cell
adhesion molecule (PECAM-1; cat no. 555445), CD14 (cat no. 555397),
CD45 (cat no. 555482), CD305/LAIR-1 (cat no. 550,811), CD56 (cat
no. 555516) and CD3 (cat no. 561809) were obtained from BD
Biosciences (Franklin Lakes, NJ, USA); monoclonal antibodies
targeting CD90 (cat no. A15761), CD11b (cat no. 11-0113-42), and
human leukocyte antigen-antigen D related (HLA-DR; cat no.
11-9952-42) were purchased from Thermo Fisher Scientific, Inc.
Multi-color flow cytometric analysis was performed using a
FACSCalibur flow cytometer (BD Biosciences). dNK cells were
resuspended in PBS at a density of 1×107/ml, then
aliquoted (100 µl/tube) to Falcon round bottom polystyrene tubes
(BD Biosciences). The expression of surface molecules on NK cells
was detected by triplicate labeling with fluorescein isothiocyanate
(FITC)-conjugated anti-human CD56, phycoerythrin (PE)-conjugated
anti-human CD3 and PE-cyanine 5.5-conjugated anti-human LAIR-1,
Alexa Fluor 647-conjugated anti-natural cytotoxicity triggering
receptor 3 (NKp30; cat no. 558408; BD Biosciences) and
allophycocyanin (APC)-conjugated anti-killer cell
immunoglobulin-like receptor 2DL1 (KIR2DL1; cat no. 564319; BD
Biosciences) or their corresponding isotype controls: FITC mouse
immunoglobulin (Ig) G1, κ isotype control (cat no. 556649) and PE
mouse IgG1, κ isotype control (cat no. 551436) (both from BD
Biosciences). For intracellular molecule detection,
CD3−CD56+ NK cells were gated and the
expression of perforin, interferon (IFN)-γ, tumor necrosis factor
(TNF)-α, interleukin (IL)-4 and IL-10 was determined using
APC-conjugated anti-perforin (cat no. 563576), anti-IFN-γ (cat no.
563495) anti-TNF-α (cat no. 551384), anti-IL-4 (cat no. 561233) or
anti-IL-10 (cat no. 564372) antibodies (BD Biosciences). Cells were
fixed and permeabilized at 4°C for 20 min with
Fixation/Permeabilization Solution kit (cat no. 554715; BD
Cytofix/Cytoperm™; BD Biosciences) according to the manufacturer's
protocol. All antibodies were incubated with DMSCs or dNK cells at
4°C for 30 min with the recommended volumes at a 1:10 dilution. The
levels of each molecule were subsequently analyzed using a
FACSCalibur Flow Cytometer (BD Biosciences) and BD CellQuest
software version 5.1 (BD Biosciences). Post-acquisition
fluorescence-activated cell sorting results were analyzed using
FlowJo software version 9.9.5 (FlowJo LLC, Inc., Ashland, OR,
USA).
Collagen assay
DMSCs were plated in a 24-well plate at a density of
1×105 cells/well overnight in DMEM supplemented with 10%
FBS; P4H shRNA or the control plasmid was added to the medium
according to the protocols listed above. Following 48 h, the cells
were centrifuged at 1,000 × g for 10 min at 20°C. Collagen IV
levels in sample supernatants were quantified using an
enzyme-linked immunosorbent assay analysis using commercial kit
(cat no. SEA180Hu; Wuhan USCN Business Co., Ltd., Wuhan, China)
according to the manufacturer's instructions. The absorbance of
each well was measured using a DigiScan microplate reader (Tecan
Group Ltd., Männedorf, Switzerland) at a wavelength of 450 nm.
Total collagen produced by DMSCs was analyzed using
an amino acid analyzer (Biochrom, Ltd., Cambridge, UK). Briefly,
DMSCs were centrifuged at 1,000 × g for 10 min at 20°C and the
supernatant was hydrolyzed in 6 M HCl at 110°C for 22 h. The
samples were subsequently cooled, dried, filtered and analyzed on a
Biochrom 30+ Amino Acid Analyzer according to the manufacturer's
instructions (Biochrom, Ltd.). Hydroxyproline was identified
against an amino acid standard, and the total quantity of collagen
in each sample was calculated.
Statistical analysis
Statistical analysis was performed by one-way or
two-way analysis of variance. The post hoc Dunnett's test was used
to compare significance levels between the control and various
treatment groups. Statistical analysis was performed using SPSS
software version 13.0 (SPSS, Inc., Chicago, IL, USA). The results
are presented as the mean + standard error of three replicates.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Microscopically-defined morphology of
DMSCs
Light microscopy was employed to examine the
morphology of fibroblast-like cells from deciduas following 5 days
of culture and prior to the removal of decidua pieces (Fig. 1A). Following 5 days of culture, the
decidua tissue sections were removed and MSC-like cells were
observed (Fig. 1B). These cells
exhibited a characteristic spindle-shape and were adherent to the
plastic culture vessel (Fig. 1B).
The MSC-like cells proliferated to form a morphologically
homogenous and adherent layer of cells following ~10 days (Fig 1C). At this point the cells were
referred to as DMSCs (Fig.
1C).
Identification of DMSCs by
immunophenotypes and multilineage capacity
DMSCs at passage 5 were analyzed by flow cytometry
for the expression of specific cell lineage markers. DMSCs were
negative for hematopoietic and endothelial antigens CD45, CD34,
CD14, CD31, CD11b and HLA-DR, while they were positive for the MSCs
markers CD44, CD90, CD73 and CD105 (Fig. 2) (1,2,5). It
has been previously demonstrated that MSCs may be induced to
differentiate along the adipogenic and osteogenic lineages using
specific culture media (17). In
the present study, DMSCs were cultured in adipogenic or osteogenic
differentiation medium, and stained with oil red O (Fig. 3A) and alizarin red S (Fig. 3B) to confirm successful
differentiation into adipocytes and osteocytes, respectively.
Therefore, the isolated DMSCs met the essential criteria used to
define MSCs.
DMSCs regulate dNK cells via collagen
secretion
The present study investigated whether dNK cells may
be regulated by DMSCs. The expression of CD56 and LAIR-1 on dNK
cells purified with microbeads was first analyzed by flow
cytometry. The results demonstrated that the percentage of CD56-
and LAIR-1-positive cells was >98% (Fig. 4A). dNK cells were then co-cultured
with DMSCs for 2 days, before the floating cells were harvested for
analysis of NK cell phenotype and the expression of intracellular
cytokines by flow cytometry. The expression of NKp30 and perforin
was significantly decreased, while KIR2DL1 expression was
significantly increased in dNK cells co-cultured with DMSCs when
compared with dNK cells alone (Fig.
4B-D). In addition, the intracellular cytokine expression
profile of TNF-α and IL-4 was significantly altered following
co-culture of dNK cells with DMSCs, whereas IFN-γ levels were not
(Fig. 4E-G). As expected, the
observed effects of DMSCs on dNK cells were abrogated by LAIR-2
pretreatment, indicating that DMSCs may regulate dNK cells via the
interaction between collagen and LAIR-1.
 | Figure 4.DMSCs modulate the phenotype and
cytokine production of dNK cells via collagen secretion. (A)
Purified dNK cells were identified by flow cytometry analysis. dNKs
were cultured alone or with DMSCs, and in the presence or absence
of recombinant human LAIR-2. Following culture for 48 h, cells were
harvested and labeled with fluorescence-conjugated antibodies. Flow
cytometry was performed to detect the expression of (B) KIR2DL1 and
(C) NKp30 cell surface molecules, as well as (D) perforin, (E)
IFN-γ, (F) TNF-α and (G) IL-4 intracellular molecules in dNK cells.
The results are presented as the mean + standard error of the mean
(n=3) using different decidua samples. *P<0.05 vs. control dNKs
cultured in medium only; #P<0.05 vs. control dNKs
co-cultured with non-pretreated DMSCs. DMSCs, decidual mesenchymal
stem cells; dNK, decidual natural killer cells; LAIR-2,
leukocyte-associated immunoglobulin-like receptor 2; KIR2DL1,
killer cell immunoglobulin-like receptor 2DL1; NKp30, natural
cytotoxicity triggering receptor 3; IFN, interferon; TNF, tumor
necrosis factor; IL, interleukin. |
Knockdown of collagen expression in
DMSCs affects the phenotype and cytokine expression profile of dNK
cells
To confirm whether collagen produced by DMSCs was
responsible for the observed effects of DMSCs on dNK cells, the
expression of collagen in DMSCs was silenced by transfection with
an shRNA specific to the β-subunit of P4H. RT-qPCR analysis
demonstrated that P4H mRNA levels were reduced by >70% in
P4H-shRNA-transfected cells when compared with empty vector
controls (Fig. 5A). In addition,
secretion of total collagen and collagen IV was significantly
reduced in P4H-shRNA-transfected cells when compared with control
cells (Fig. 5B and C).
Furthermore, flow cytometry analysis demonstrated that interference
with collagen expression in DMSCs abrogated the effects of DMSCs on
dNK cells. As shown in Fig. 6A,
the expression levels of perforin, IFN-γ and TNF-α were increased
in dNKs, and the expression of IL-4 and IL-10 was decreased
following co-culture of dNKs with DMSCs transfected with P4H shRNA
for 2 days. In addition, the expression of NKp30 was significantly
increased, while KIR2DL1 was significantly reduced in dNKs
co-cultured with DMSCs transfected with P4H shRNA when compared
with the non-transfected co-culture group (Fig. 6B and C).
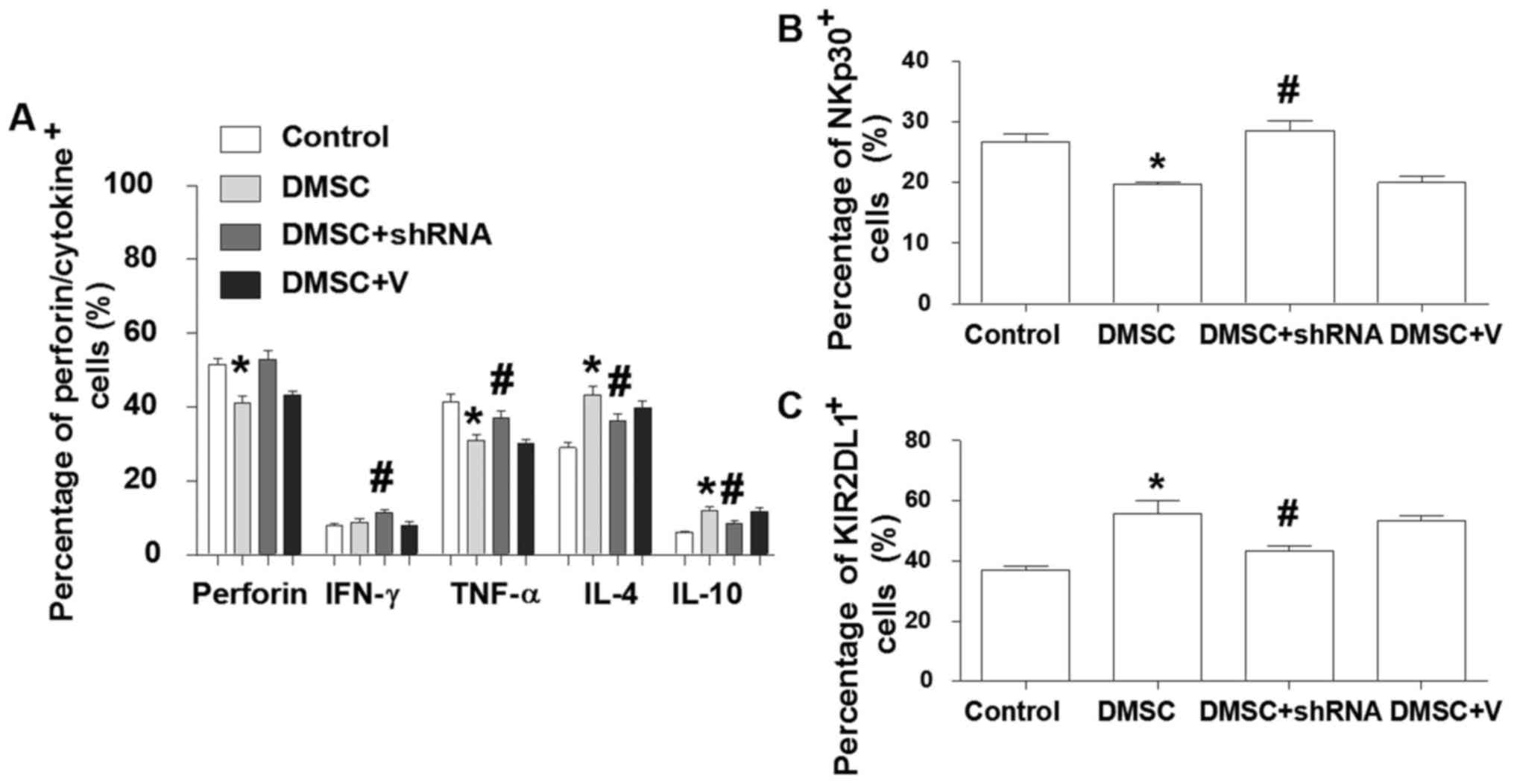 | Figure 6.Inhibition of collagen expression in
DMSCs abrogates DMSC-induced alterations in the phenotype and
cytokine production of dNK cells. Purified dNK cells were cultured
alone, with DMSCs or with DMSCs transfected with
prolyl-4-hydroxylase shRNA or empty vector controls. Following 48-h
of culture, floating dNK cells were harvested and labeled with
fluorescence-conjugated antibodies. Flow cytometry analysis was
employed to detect the expression of the (A) intracellular
molecules IFN-γ, TNF-α and IL-4, and the cell surface molecules (B)
KIR2DL1 and (C) NKp30 in dNK cells. The results are presented as
the mean + standard error of the mean (n=3) using different decidua
samples. *P<0.05 vs. control and #P<0.05 vs. DMSC
+ V. DMSCs, decidual mesenchymal stem cells; dNK, decidual natural
killer cells; shRNA, short hairpin RNA; KIR2DL1, killer cell
immunoglobulin-like receptor 2DL1; NKp30, natural cytotoxicity
triggering receptor 3; IFN, interferon; TNF, tumor necrosis factor;
IL, interleukin; V, empty vector plasmid. |
Discussion
The primary function of the decidua is to ensure
that optimal conditions are provided for embryonic implantation and
placentation. It has been hypothesized that MSCs originating from
human bone marrow are recruited via the circulation to the
endometrium, where they proliferate and differentiate under the
influence of the specific environment supported by reproductive
hormones and growth factors (8).
It has been previously reported that MSCs may inhibit NK cell
proliferation and their cytotoxic activities (18); however, the isolation of MSCs from
the decidua and the regulation of dNK cells by DMSCs has not been
previously investigated.
In the present study, DMSCs with a fibroblast-like
morphology, were cultured in vitro and exhibited sustained
growth for >10 passages. The DMSCs exhibited a phenotype that is
usually ascribed to cells of mesenchymal origin, as evidenced by
the positive expression of CD44, CD90, CD73 and CD105 markers and
the absence of hematopoietic cell markers. The results indicated
that co-culture of dNKs with DMSCs reduced the expression of NKp30
and increased the percentage of KIR2DL1-expressing dNK cells when
compared with dNKs cultured alone. In addition, co-culture with
DMSCs downregulated perforin, IFN-γ and TNF-α production, and
upregulated IL-4 and IL-10 expression by dNK cells.
It has been previously established that collagen is
important for generating a microenvironment that supports
trophoblast survival and migration (14). A previous study reported that,
during normal pregnancy, type IV collagen is detected in decidual
cells by pericellular immunostaining (19). However, upon spontaneous abortion,
a weak or complete absence of type IV collagen staining was
observed in the cells (19). In
addition, the decidual tissues from patients that had undergone a
spontaneous abortion exhibited reduced total collagen expression
(14). Although dNK cells utilize
collagen for migration and retention at the maternal-fetal
interface (20), the
immunoregulatory effects of collagen on DICs requires further
investigation. It has been previously reported that collagens
produced by tumor cell lines are capable of activating LAIR-1, and
may induce immune tolerance in the tumor microenviroment (21). However, whether DMSCs at the
maternal-fetal interface induce immune tolerance via collagen
remains unknown. LAIR-1 is a member of the immunoglobulin
superfamily and contains two immunoreceptor tyrosine-based
inhibition motifs in its intracellular domain that are required to
convey inhibitory signals to NK cells (22). LAIR-2 is a potent inhibitor of the
interaction between the LAIR-1 and collagen (23,24).
The results of the present study demonstrated that pretreatment of
dNK cells with LAIR-2 abrogated the effects of DMSCs on the
cytotoxic phenotype and proinflammatory cytokine secretion of dNK
cells.
Collagen triple helix integrity is dependent on
proline hydroxylation by P4H. It has been reported that LAIR-1 may
bind to the collagen triple helix peptides that contain multiple
glycine-proline-hydroxyproline repeats (25). In the present study, the
post-transcriptional modification of collagen was disrupted by
transfecting DMSCs with P4H shRNA, as this leads to insufficient
proline hydroxylation of collagen and subsequently affects the
ability of collagen to bind LAIR-1. Knockdown of P4H in DMSCs
induced alterations in the phenotype and cytokine expression
profile of dNKs, resulting in a proinflammatory milieu. The switch
from a Th2 to Th1 cytokine profile at the maternal-fetal interface
is harmful for the maintenance of successful pregnancy (13). The results of the present study
demonstrated that DMSCs may serve an important role in maintaining
a Th2 bias at the maternal-fetal interface, and that this may be
achieved via collagen. These results are consistent with a previous
report demonstrating that the collagen-specific CD4+
T-cell response is altered from a dominant Th0/Th1 response to a
Th2 phenotype in vivo (26).
In the majority of maternal DICs, various immune
inhibitory receptors are expressed that function to prevent
excessive immune responses to paternal alloantigens originating
from trophoblasts (14). However,
further studies regarding the mechanisms involved in maternal-fetal
immune tolerance during normal pregnancy are required. The activity
of NK cells depends on the balance between inhibitory and
activating receptors. It has been demonstrated that maternal
KIR2DL1 serves a protective role for the fetus (27,28).
The results of the current study demonstrated that DMSCs may induce
the expression of KIR2DL1 via collagen, and that the activity of
dNKs may be affected when a dominant KIR2DL1-mediated inhibitory
signal is not received due to reduced collagen expression at the
maternal-fetal interface. Natural cytotoxicity receptors (NCRs) are
markers that regulate NK cell cytotoxicity and cytokine production.
It has been previously reported that an increase in the percentage
of NKp30+NK cells is observed in the decidual tissue of
patients that exhibit embryonic implantation failure and recurrent
miscarriages (29). Conversely, in
patients with successful pregnancies, the expression of NKp30 is
significantly reduced (30). In
addition, NCRs and NK1-derived cytokines have been demonstrated to
be downregulated in normal fertile women (29). The results of the current study are
consistent with a previous study demonstrating that NKp30 mediates
the secretion of IFN-γ and TNF-α in the decidua during early
pregnancy (31). When combined
with the observed decrease of perforin in dNKs co-cultured with
DMSCs, these results indicate that DMSCs may contribute to the
reduced cytotoxicity of dNKs via collagen at the maternal-fetal
interface.
In conclusion, the present study reports a novel
method to isolate multipotent DMSCs from first-trimester human
decidua tissues. The results demonstrated that DMSCs exhibit
clonogenic properties, are able to be cultured in vitro for
prolonged periods, differentiate into different cell lineages and
express cell surface markers specific to MSCs. In addition, when
co-cultured with dNKs, DMSCs exhibited LAIR-1-mediated inhibition
of TNF-α and perforin secretion and NKp30 expression, thus
suggesting a LAIR-1-mediated tolerance phenotype of dNK cells via
collagen, which may contribute to the induction of an
immune-tolerant microenvironment at the maternal-fetal
interface.
Acknowledgements
The present study was supported by the Nature
Science Foundation of China (grant nos. 81370730, 81571512,
81273200, 31370905 and 31300751), the Nature Science Foundation of
Shandong Province (grant nos. ZR2015JL027 and ZR2011HQ006) and the
Science and Technology Plan of Binzhou Medical University (grant
no. BY2007KYQD02).
References
|
1
|
Reyes M, Lund T, Lenvik T, Aguiar D,
Koodie L and Verfaillie CM: Purification and ex vivo expansion of
postnatal human marrow mesodermal progenitor cells. Blood.
98:2615–2625. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zvaifler NJ, Marinova-Mutafchieva L, Adams
G, Edwards CJ, Moss J, Burger JA and Maini RN: Mesenchymal
precursor cells in the blood of normal individuals. Arthritis Res.
2:477–488. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zuk PA, Zhu M, Ashjian P, De Ugarte DA,
Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P and Hedrick
MH: Human adipose tissue is a source of multipotent stem cells. Mol
Biol Cell. 13:4279–4295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schwab KE and Gargett CE: Co-expression of
two perivascular cell markers isolates mesenchymal stem-like cells
from human endometrium. Hum Reprod. 22:2903–2911. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma S, Xie N, Li W, Yuan B, Shi Y and Wang
Y: Immunobiology of mesenchymal stem cells. Cell Death Differ.
21:216–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kammerer U, von Wolff M and Markert UR:
Immunology of human endometrium. Immunobiology. 209:569–574. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Poehlmann TG, Schaumann A, Busch S,
Fitzgerald JS, Aguerre-Girr M, Le Bouteiller P, Schleussner E and
Markert UR: Inhibition of term decidual NK cell cytotoxicity by
soluble HLA-G1. Am J Reprod Immunol. 56:275–285. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dimitrov R, Kyurkchiev D, Timeva T,
Yunakova M, Stamenova M, Shterev A and Kyurkchiev S:
First-trimester human decidua contains a population of mesenchymal
stem cells. Fertil Steril. 93:210–219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo C, Zhu H, Huang W, Li S, Qu W, Liu Y
and Tan A: Side population cells in the human decidua of early
pregnancy exhibit stem/progenitor cell-like characteristics. Reprod
Biomed Online. 21:783–793. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakashima A, Shima T, Inada K, Ito M and
Saito S: The balance of the immune system between T cells and NK
cells in miscarriage. Am J Reprod Immunol. 67:304–310. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu XY, Zhou YH, Wang MY, Jin LP, Yuan MM
and Li DJ: Blockade of CD86 signaling facilitates a Th2 bias at the
maternal-fetal interface and expands peripheral CD4+
CD25+ regulatory T cells to rescue abortion-prone
fetuses. Biol Reprod. 72:338–345. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spaggiari GM and Moretta L: Cellular and
molecular interactions of mesenchymal stem cells in innate
immunity. Immunol Cell Biol. 91:27–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Piao HL, Tao Y, Zhu R, Wang SC, Tang CL,
Fu Q, Du MR and Li DJ: The CXCL12/CXCR4 axis is involved in the
maintenance of Th2 bias at the maternal/fetal interface in early
human pregnancy. Cell Mol Immunol. 9:423–430. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fu Q, Tao Y, Piao H, Du MR and Li DJ:
Trophoblasts and decidual stromal cells regulate decidual NK cell
functions via interaction between collagen and LAIR-1. Am J Reprod
Immunol. 71:368–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qi HH, Ongusaha PP, Myllyharju J, Cheng D,
Pakkanen O and Shi Y, Lee SW, Peng J and Shi Y: Prolyl
4-hydroxylation regulates Argonaute 2 stability. Nature.
455:421–424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bae YJ, Kwon YR, Kim HJ, Lee S and Kim YJ:
Enhanced differentiation of mesenchymal stromal cells by
three-dimensional culture and azacitidine. Blood Res. 52:18–24.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pradier A, Passweg J, Villard J and
Kindler V: Human bone marrow stromal cells and skin fibroblasts
inhibit natural killer cell proliferation and cytotoxic activity.
Cell Transplant. 20:681–691. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iwahashi M, Muragaki Y, Ooshima A and
Nakano R: Decreased type IV collagen expression by human decidual
tissues in spontaneous abortion. J Clin Endocrinol Metab.
81:2925–2929. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Burrows TD, King A and Loke YW: The role
of integrins in adhesion of decidual NK cells to extracellular
matrix and decidual stromal cells. Cell Immunol. 166:53–61. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rygiel TP, Stolte EH, de Ruiter T, van de
Weijer ML and Meyaard L: Tumor-expressed collagens can modulate
immune cell function through the inhibitory collagen receptor
LAIR-1. Mol Immunol. 49:402–406. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meyaard L, Adema GJ, Chang C, Woollatt E,
Sutherland GR, Lanier LL and Phillips JH: LAIR-1, a novel
inhibitory receptor expressed on human mononuclear leukocytes.
Immunity. 7:283–290. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lebbink RJ, van den Berg MC, de Ruiter T,
Raynal N, van Roon JA, Lenting PJ, Jin B and Meyaard L: The soluble
leukocyte-associated Ig-like receptor (LAIR)-2 antagonizes the
collagen/LAIR-1 inhibitory immune interaction. J Immunol.
180:1662–1669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lenting PJ, Westerlaken GH, Denis CV,
Akkerman JW and Meyaard L: Efficient inhibition of collagen-induced
platelet activation and adhesion by LAIR-2, a soluble Ig-like
receptor family member. PLoS One. 5:e121742010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lebbink RJ, Raynal N, de Ruiter T, Bihan
DG, Farndale RW and Meyaard L: Identification of multiple potent
binding sites for human leukocyte associated Ig-like receptor LAIR
on collagens II and III. Matrix Biol. 28:202–210. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Doncarli A, Stasiuk LM, Fournier C and
Abehsira-Amar O: Conversion in vivo from an early dominant Th0/Th1
response to a Th2 phenotype during the development of
collagen-induced arthritis. Eur J Immunol. 27:1451–1458. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kusnierczyk P: Killer cell
immunoglobulin-like receptor gene associations with autoimmune and
allergic diseases, recurrent spontaneous abortion, and neoplasms.
Front Immunol. 4:82013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Flores AC, Marcos CY, Paladino N, Arruvito
L, Williams F, Middleton D and Fainboim L: KIR receptors and HLA-C
in the maintenance of pregnancy. Tissue Antigens. 69:(Suppl 1).
S112–S113. 2007. View Article : Google Scholar
|
|
29
|
Fukui A, Ntrivalas E, Fukuhara R, Fujii S,
Mizunuma H, Gilman-Sachs A, Beaman K and Kwak-Kim J: Correlation
between natural cytotoxicity receptors and intracellular cytokine
expression of peripheral blood NK cells in women with recurrent
pregnancy losses and implantation failures. Am J Reprod Immunol.
62:371–380. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Marlin R, Duriez M, Berkane N, de Truchis
C, Madec Y, Rey-Cuille MA, Cummings JS, Cannou C, Quillay H,
Barré-Sinoussi F, et al: Dynamic shift from CD85j/ILT-2 to NKG2D NK
receptor expression pattern on human decidual NK during the first
trimester of pregnancy. PLoS One. 7:e300172012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
El Costa H, Casemayou A, Aguerre-Girr M,
Rabot M, Berrebi A, Parant O, Clouet-Delannoy M, Lombardelli L,
Jabrane-Ferrat N, Rukavina D, et al: Critical and differential
roles of NKp46-and NKp30-activating receptors expressed by uterine
NK cells in early pregnancy. J Immunol. 181:3009–3017. 2008.
View Article : Google Scholar : PubMed/NCBI
|




















