Introduction
Acute promyelocytic leukemia (APL) is a subtype of
acute myeloid leukemia accounting for 5–15% of all forms of this
disease (1). APL is characterized
by a chromosomal translocation [t(15;17)(q24;q21)] resulting in the
formation of a retinoic acid receptor α and promyelocytic leukemia
(PML-RARα) fusion protein (1–3).
All-trans retinoic acid (ATRA) and arsenic trioxide (ATO) are the
most important treatments of APL patients and induce a high cure
rate. ATRA promotes differentiation and ATO induces apoptosis.
Although ATRA and ATO have made APL highly curable, some patients
may develop severe side effects. In addition, some APL patients are
not sensitive to these compounds (1,4–7).
Hence, it is necessary to develop new therapeutic strategies for
this disease.
Shikonin, an active component of the Chinese medical
herb Zi Cao has been used to treat burns, carbuncles, macular
eruptions, measles and sore throats (8,9). In
addition to the anti-bacterial and anti-inflammatory activities of
this medicine, shikonin inhibits proliferation and induces
apoptosis in different cancer cell lines including prostate cancer
(10), oral squamous cell
carcinoma (11), chronic
myelogenous leukemia (12),
hepatocellular carcinoma (13) and
thyroid cancer (14). Substantial
evidence indicates that shikonin induces apoptosis partly through
the mitogen-activated protein kinase (MAPK) pathway (12,15).
The MAPK family, that includes extracellular
signal-regulated kinase (ERK), p38 MAPK and c-Jun N-terminal kinase
(JNK), serves an important role in cell proliferation, cell cycle
progression, differentiation, survival and apoptosis (16,17).
The ERK pathway is primarily activated by growth factors and
mitogens, and is important in cell growth and differentiation
(18). p38 MAPK and JNK are mainly
responsive to stress signals and inflammatory cytokines, and are
associated with apoptosis (19,20).
Accumulating evidence indicates that shikonin exerts
antitumor activity in different cancer cells. However, the effects
and related mechanism of shikonin on human leukemia NB4 cells are
not known. In the present study, the authors investigated the
influence of shikonin on the proliferation and apoptosis of NB4
cells and explored the potential mechanisms. The results may be
beneficial for developing improved therapies for APL.
Materials and methods
Reagents
Shikonin and dimethylsulfoxide were purchased from
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The purity of
shikonin was >98%. Antibodies against caspase-3, poly ADP-ribose
polymerase (PARP), c-Myc, p-ERK1/2, ERK1/2, p38 MAPK, p-JNK and JNK
were purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA). The antibody against p-p38 MAPK was purchased from Merck
KGaA. Goat anti-rabbit, goat anti-mouse and β-actin antibodies were
purchased from Beijing Zhongshan Golden Bridge Biotechnology Co.,
Ltd. (Beijing, China).
Cell lines and culture
NB4 cells were obtained from the Shanghai Institute
for Biological Science, Chinese Academy of Sciences (Shanghai,
China) and maintained at 37°C under 5% CO2 in RPMI-1640
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) medium
containing 10% fetal calf serum (Gibco; Thermo Fisher Scientific,
Inc.).
Cell proliferation assays
Cell viability was detected by the Cell Counting Kit
(CCK)-8 (Sevenseas Futai Biotechnology Co., Ltd., Shanghai, China)
assay. Cells (1×104) were seeded in 96-well plates and
treated with increasing concentrations of shikonin for 12, 24 and
36 h. A total of 10 µl CCK-8 solution was added to each well at the
end of each culture period. Cells were then incubated for 2 h at
37°C and absorbance of the medium was measured at 450 nm using a
spectrophotometer.
Nucleus morphological changes examined
by Hochest 33342 staining
Cells (5×105) were seeded in six-well
plates and treated with shikonin (0, 0.3 µmol/l) for 24 h. Then,
cells were washed with PBS three times, fixed with cold methanol
overnight. Cells were washed with PBS three further times and
stained with Hoechst 33342 (Beyotime Institute of Biotechnology,
Beijing, China) for 5 min in the dark. Following three washes, the
cells were observed using fluorescence microscopy.
Flow cytometry analysis
NB4 cells were treated with 0.3 µmol/l shikonin for
24 h. Treated and control cells were harvested by centrifugation at
1,000 × g for 5 min then washed and re-suspended with cold PBS.
Cells were then stained with Annexin V and propidium iodide for
5–15 min at room temperature using the Annexin V/PI Apoptosis
Detection kit (KeyGene, Wageningen, The Netherlands). Apoptosis was
analyzed on a flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA). The experiment was repeated at least three times.
The effects of shikonin on cell cycle distribution
were detected with a cell cycle kit according to the manufacturer's
instructions. Cells treated with 0.3 µmol/l shikonin for 24 h and
control cells were collected by centrifugation, followed by
washing, fixation and propidium iodide staining. The cell cycle
distribution was examined by flow cytometry (BD Biosciences).
Western blot analyses
Cells were lysed with cold radioimmunoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology) containing
protease inhibitor cocktail for 10 min. Cell proteins were
collected by centrifuging at 13,000 × g for 30 min and used for
immunoblotting analyses. Cell proteins (60 µg) were separated by
SDS-PAGE and transferred to polyvinylidene fluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were blocked with 5%
non-fat milk dissolved in TBS with 20% Tween-20, and then incubated
with primary antibodies against caspase-3 (no. 9665; 1:1,000), PARP
(no. 9532; 1:1,000), c-Myc (no. 5605; 1:1,000), p-ERK1/2 (no. 4370;
1:1,000), ERK1/2 (no. 4695; 1:1,000), p-p38 MAPK (no. 09-272;
1:1,000), p38 MAPK (no. 9218; 1:1,000), p-JNK (no. 4668; 1:1,000),
JNK (no. 9252; 1:1,000), β-actin (no. BM0627; 1:4,000) at 4°C
overnight. Following three washes, the membranes were incubated
with goat anti-rabbit (no. ZB-2301; 1:4,000) or goat anti-mouse
(no. ZB-2305; 1:4,000) IgG horseradish peroxidase-linked secondary
antibodies, at room temperature for 1 h. The bands were visualized
using Immobilon Western Chemiluminescent HRP Substrate (EMD
Millipore). Semi-quantification was performed using Cool Imager
software (version. 4.0.1; Viagen Biotech Inc., Los Angeles, CA,
USA).
Statistical analysis
All experiments were repeated at least three times.
Data are presented as means ± standard deviation. Statistical
analysis was performed with SPSS software (version, 17.0; SPSS,
Inc., Chicago, IL, USA). One-way analysis of variance and Student's
t-test were used for comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
Shikonin inhibits the proliferation of
NB4 cells
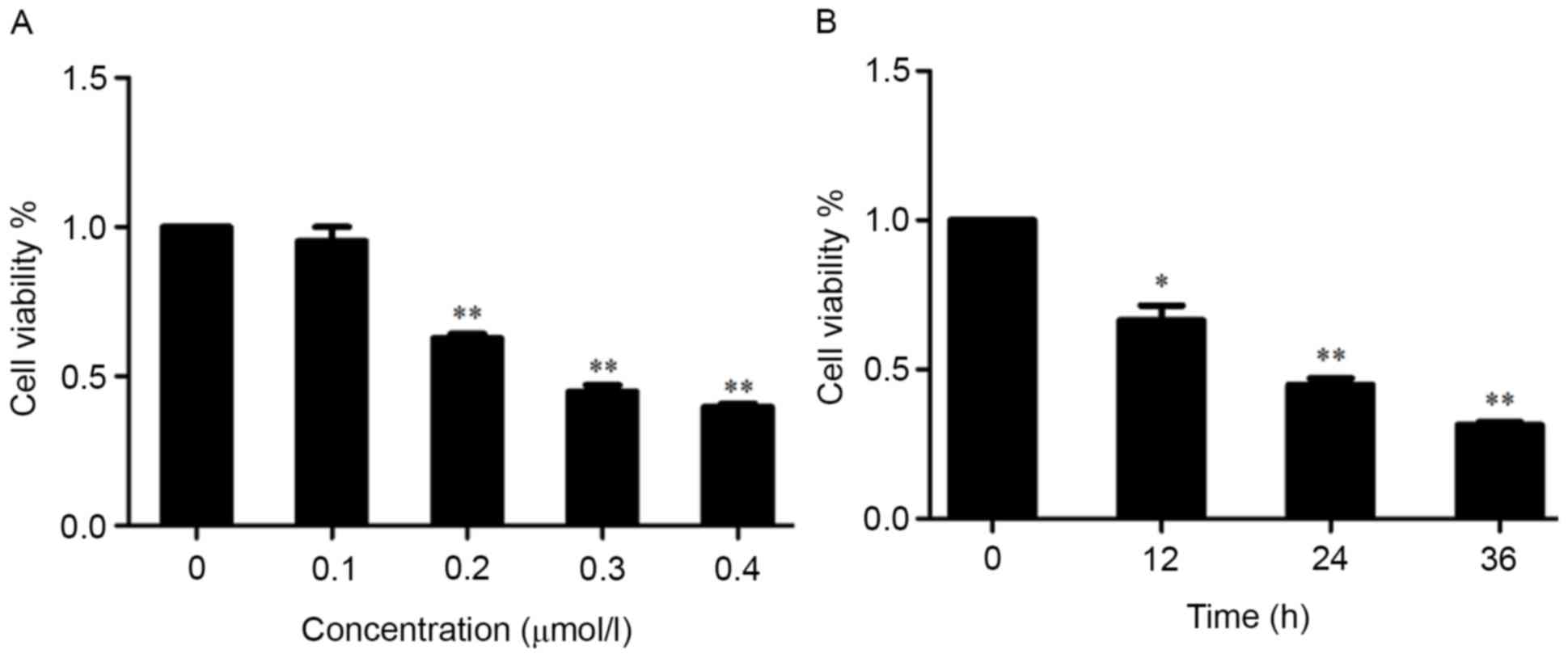
NB4 cells were treated with increasing
concentrations of shikonin for 24 h and viability assessed using
the CCK-8 assay. There was no significant difference between the
control and shikonin 0.1 µmol/l group in cell viability (Fig. 1A). However, cell viability was
reduced significantly by treatment with shikonin concentrations of
0.2 µmol/l or more. The IC50 value was 0.3 µmol/l. NB4
cells were also treated with 0.3 µmol/l shikonin for 0–36 h. The
CCK-8 results demonstrated that this treatment reduced cell
viability significantly from 12–36 h (Fig. 1B). These results indicated that
shikonin inhibited the viability of NB4 cells in a concentration-
and time-dependent manner.
Nucleus morphological changes observed
by fluorescence micsroscopy
The cell nuclei of untreated NB4 cells stained by
Hoechst 33342 were round and uniform, while shikonin treatment
resulted in chromatin agglutination, karyopyknosis and nuclear
fragmentation (Fig. 2).
Shikonin-induced cell cycle
changes
NB4 cells were treated with 0.3 µmol/l shikonin for
24 h and the cell cycle was detected by flow cytometry. Compared
with the control group, shikonin-treated cells were arrested at the
G1 phase. The percentage of cells in the G1 phase increased from
37.3 to 51.8% (Fig. 3). This
result indicated that shikonin induced cell cycle arrest of NB4
cells.
Shikonin induced apoptosis of NB4
cells
The effect of 0.3 µmol/l shikonin on NB4 cell
apoptosis was detected by flow cytometry at 24 h. The percentage of
apoptosis cell was increased significantly by shikonin treatment
(Fig. 4).
Shikonin increased the expression
levels of cleaved PARP and caspase-3
Western blotting was used to detect the
apoptosis-related proteins PARP and caspase-3. The expression of
cleaved PARP and caspase-3 was increased following treatment with
0.3 µmol/l shikonin for 24 h, as compared with the control group
(Fig. 5), supporting the induction
of apoptosis by this treatment.
Shikonin regulated MAPKs and
downregulated c-Myc in NB4 cells
To investigate possible mechanisms for
shikonin-induced apoptosis in NB4 cells, the authors assessed the
levels of MAPKs and the expression of c-Myc. Shikonin increased the
phosphorylation of p38 MAPK and JNK significantly, and inhibited
ERK phosphorylation (Fig. 6).
However, the expression of total ERK, p38 MAPK and JNK was not
affected by shikonin. Meanwhile, the expression of c-Myc was
significantly decreased by treatment with shikonin.
Discussion
Shikonin, a natural product derived from the Chinese
medical herb Zi Cao, has been used for treating wounds and burns,
and has anti-inflammatory and anti-viral properties (8,9).
Previously, evidence has indicated that shikonin exerts antitumor
activity by inhibiting cell proliferation and inducing apoptosis in
different tumor cell lines (10–14).
However, little is known about the effects of shikonin on human
leukemia NB4 cells.
In the present study, the authors firstly
investigated the effects of shikonin on proliferation and apoptosis
in NB4 cells. The results demonstrated that shikonin inhibited the
proliferation of NB4 cells in a time- and concentration-dependent
manner and induced cell cycle arrest in the G1 phase. The
percentage of apoptotic cells was increased significantly following
shikonin treatment. Shikonin treatment led to nucleus morphological
changes such as chromatin agglutination, karyopyknosis and nuclear
fragmentation. These results indicated that shikonin could inhibit
the proliferation and induce apoptosis in NB4 cells. The study used
NB4 cells because it is the primary cell type with the APL
containing PML-RARα fusion protein. In addition, the effects of
shikonin on normal healthy cells were not explored because of the
difficulty of culturing normal blood cells. Western blotting
analyses indicated that shikonin treatment increased the cleaved
caspase-3 and PARP, two apoptosis-related proteins. Furthermore,
shikonin can generate reactive oxygen species and active caspases
to induce apoptosis in human colorectal carcinoma cells (21). Apoptosis signal transduction
involves the death receptor and mitochondrial pathways (22). In order to explore the molecular
mechanism underlying the antitumor activity of shikonin, we
measured its effects on cell proliferation and apoptosis signaling
pathways. Studies reported that MAPK signaling pathway closely
related to proliferation and apoptosis (16,17).
Modulation of the p38 MAPK and JNK pathways often associated with
apoptosis, and the ERK pathway always related to cell survival
(23–25). Western blotting examined the p38
MAPK, JNK and ERK pathways. In addition, the expression of c-Myc,
an oncogene that serves an important role in cell proliferation,
differentiation and apoptosis (26), and is overexpressed in many cancer
cells (27), was examined. c-Myc
can promote the PARP-dependent DNA repair pathway resulting in
chemoresistance (28). In
addition, a previous study reported that shikonin induces apoptosis
by downregulating c-Myc in U937 cells (15). The current results are consistent
with previous findings. Total MAPKs were unaffected by shikonin
treatment. However, shikonin inhibited the expression of p-ERK and
increased levels of p-p38 MAPK and p-JNK. Meanwhile, c-Myc was
significantly downregulated by shikonin. These results indicated
that shikonin-induced apoptosis may be through the MAPK pathway and
downregulation of c-Myc in NB4 cells. However, further
investigations are needed to identify how these pathways are
regulated and their relationship to each other and to investigate
the effects of Shikonin in animal models of APL.
In conclusion, the current study indicated that
shikonin inhibited cell proliferation and induced cell cycle arrest
and apoptosis in NB4 cells. These effects involved modulation of
the MAPK pathway and downregulation of c-Myc. These results
suggested that shikonin may be a novel agent for treating APL.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81171658) and the
Natural Science Foundation Project of CQ CSTC (grant no.
2011BA5037).
References
|
1
|
Wang ZY and Chen Z: Acute promyelocytic
leukemia: From highly fatal to highly curable. Blood.
111:2505–2515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Albano F, Zagaria A, Anelli L, Coccaro N,
Tota G, Brunetti C, Minervini CF, Impera L, Minervini A, Cellamare
A, et al: Absolute quantification of the pretreatment PML-RARA
transcript defines the relapse risk in acute promyelocytic
leukemia. Oncotarget. 6:13269–13277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Powell BL: Arsenic trioxide in acute
promyelocytic leukemia: Potion not poison. Expert Rev Anticancer
Ther. 11:1317–1319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang K, Li J, Meng W, Xing H and Yang Y:
Tanshinone IIA inhibits acute promyelocytic leukemia cell
proliferation and induces their apoptosis in vivo. Blood Cells Mol
Dis. 56:46–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li L, Song H, Zhong L, Yang R, Yang XQ,
Jiang KL and Liu BZ: Lithium chloride promotes apoptosis in human
leukemia NB4 cells by inhibiting glycogen synthase kinase-3 beta.
Int J Med Sci. 12:805–810. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iland HJ, Bradstock K, Supple SG, Catalano
A, Collins M, Hertzberg M, Browett P, Grigg A, Firkin F, Hugman A,
et al: All-trans-retinoic acid, idarubicin and IV arsenic trioxide
as initial therapy in acute promyelocytic leukemia (APML4). Blood.
120:1570–1580. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ghavamzadeh A, Alimoghaddam K, Rostami S,
Ghaffari SH, Jahani M, Iravani M, Mousavi SA, Bahar B and Jalili M:
Phase II study of single-agent arsenic trioxide for the front-line
therapy of acute promyelocytic leukemia. J Clin Oncol.
29:2753–2757. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Papathanasiou K, Papageorgiou C, Panidis D
and Mantalenakis S: Our experience in laparoscopic diagnosis and
management in women with chronic pelvic pain. Clin Exp Obstet
Gynecol. 26:190–192. 1999.PubMed/NCBI
|
|
9
|
Chen X, Yang L, Oppenheim JJ and Howard
MZ: Cellular pharmacology studies of shikonin derivatives.
Phytother Res. 16:199–209. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gaddipati JP, Mani H, Shefali, Raj K,
Mathad VT, Bhaduri AP and Maheshwari RK: Inhibition of growth and
regulation of IGFs and VEGF in human prostate cancer cell lines by
shikonin analogue 93/637 (SA). Anticancer Res. 20:2547–2552.
2000.PubMed/NCBI
|
|
11
|
Min R, Tong J, Wenjun Y, Wenhu D, Xiaojian
Z, Jiacai H, Jian Z, Wantao C and Chenping Z: Growth inhibition and
induction of apoptosis in human oral squamous cell carcinoma
Tca-8113 cell lines by shikonin was partly through the inactivation
of NF-kappaB Pathway. Phytother Res. 22:407–415. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mao X, Yu CR, Li WH and Li WX: Induction
of apoptosis by shikonin through a ROS/JNK mediated process in
Bcr/Abl-positive chronic myelogenous leukemia (CML) cells. Cell
Res. 18:879–888. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gong K and Li W: Shikonin, a Chinese
plant-derived naphthoquinone, induces apoptosis in hepatocellular
carcinoma cells through reactive oxygen species: A potential new
treatment for hepatocellular carcinoma. Free Radic Biol Med.
51:2259–2271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang Q, Ji M, Guan H, Shi B and Hou P:
Shikonin inhibits thyroid cancer cell growth and invasiveness
through targeting major signaling pathways. J Clin Endocrinol
Metab. 98:E1909–E1917. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao Q, Assimopoulou AN, Klauck SM,
Damianakos H, Chinou I, Kretschmer N, Rios JL, Papageorgiou VP,
Bauer R and Efferth T: Inhibition of c-MYC with involvement of
ERK/JNK/MAPK and AKT pathways as a novel mechanism for shikonin and
its derivatives in killing leukemia cells. Oncotarget.
6:38934–38951. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang W and Liu HT: MAPK signal pathways
in the regulation of cell proliferation in mammalian cells. Cell
Res. 12:9–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cobb MH: MAP kinase pathways. Prog Biophys
Mol Biol. 71:479–500. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dent P and Grant S: Pharmacologic
interruption of the mitogen-activated extracellularregulated
kinase/mitogen-activated protein kinase signal transduction
pathway: Potential role in promoting. Clin Cancer Res. 7:775–783.
2001.PubMed/NCBI
|
|
19
|
Fan M and Chambers TC: Role of
mitogen-activated protein kinases in the response of tumor cells to
chemotherapy. Drug Resist Updat. 4:253–267. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dent P and Grant S: Pharmacologic
interruption of the mitogen-activated extracellular-regulated
kinase/mitogen-activated protein kinase signal transduction
pathway: Potential role in promoting cytotoxic drug action. Clin
Cancer Res. 7:775–783. 2001.PubMed/NCBI
|
|
21
|
Hsu PC, Huang YT, Tsai ML, Wang YJ, Lin JK
and Pan MH: Induction of apoptosis by shikonin through coordinative
modulation of the Bcl-2 family, p27, and p53, release of cytochrome
c, and sequential activation of caspases in human colorectal
carcinoma cells. J Agric Food Chem. 52:6330–6337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Engeland M, Ramaekers FC, Schutte B
and Reutelingsperger CP: A novel assay to measure loss of plasma
membrane asymmetry during apoptosis of adherent cells in culture.
Cytometry. 24:131–139. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moon DO, Kim MO, Choi YH, Kim ND, Chang JH
and Kim GY: Bcl-2 overexpression attenuates SP600125-induced
apoptosis in human leukemia U937 cells. Cancer Lett. 264:316–325.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cross TG, Scheel-Toellner D, Henriquez NV,
Deacon E, Salmon M and Lord JM: Serine/threonine protein kinases
and apoptosis. Exp Cell Res. 256:34–41. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park HS, Hwang HJ, Kim GY, Cha HJ, Kim WJ,
Kim ND, Yoo YH and Choi YH: Induction of apoptosis by fucoidan in
human leukemia U937 cells through activation of p38 MAPK and
modulation of Bcl-2 family. Mar Drugs. 11:2347–2364. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pan XN, Chen JJ, Wang LX, Xiao RZ, Liu LL,
Fang ZG, Liu Q, Long ZJ and Lin DJ: Inhibition of c-Myc overcomes
cytotoxic drug resistance in acute myeloid leukemia cells by
promoting differentiation. PLoS One. 9:e1053812014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu JJ, Meng LH, Shankavaram UT, Zhu CH,
Tong LJ, Chen G, Lin LP, Weinstein JN and Ding J:
Dihydroartemisinin accelerates c-MYC oncoprotein degradation and
induces apoptosis in c-MYC-overexpressing tumor cells. Biochem
Pharmacol. 80:22–30. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ganesan S: MYC, PARP1, and
chemoresistance: BIN there, done that? Sci Signal. 4:pe152011.
View Article : Google Scholar : PubMed/NCBI
|