Introduction
Cancer is among the primary causes of mortality in
developing and developed countries, and is therefore considered a
global concern. An increasing number of cases of cancer and
cancer-associated mortality are projected to occur worldwide. An
analysis of the available anticancer drugs revealed that the
majority of approved anticancer drugs are unmodified natural
products or their semisynthetic derivatives, or molecules
synthesized based on natural product compound pharmacophores
(1). Natural products are
considered a promising source for future drugs (2). It has previously been demonstrated
that aloperine, oxymatrine, sophoridine and cytisine exert
antitumor effects, with some having important roles in cancer
treatment (3). Aloperine has
previously been demonstrated to exhibit anti-inflammatory
properties in vitro and in vivo (4). In addition, aloperine was able to
significantly induce apoptosis of SW480 and HCT116 human colon
cancer cells (5). Previous studies
have indicated that oxymatrine exhibits activity against hepatic
fibrosis (6). Furthermore,
sophoridine exerts various pharmacological activities, including
anticancer effects, and selectively induces apoptotic cell death of
various human cancer cell types in vitro and in vivo
(7). The effects of sophoridine
have also been determined on the induction of apoptosis of human
glioma U87MG cells, revealing that sophoridine increased apoptosis
in these cells (8). Cytisine is a
naturally occurring quinolizidine alkaloid with antitumor
activities (9,10). Cytisine has been used as a nicotine
receptor partial agonist that may improve the success of smoking
cessation by maintaining moderate levels of dopamine, which
counteracts withdrawal symptoms, and reducing the satisfaction
associated with smoking (11).
However, although the application of cytisine increased the
likelihood of an individual quitting smoking, absolute quit rates
were modest. Nicotinic acetylcholine receptors (nAChRs) are
pharmacological targets that are thought to be involved in the
reinforcing effects associated with various drugs of abuse
(12). Cytisine is a nAChR partial
agonist that is currently in clinical use as a smoking cessation
aid. Previous studies have conducted radio-ligand displacement
experiments to characterize cytisine with regards to its binding
affinity for heteromeric nAChRs, using membrane preparations from
cells stably expressing human nAChRs (13–15).
Cytisine has also been used for the treatment of central nervous
system diseases; it has previously been demonstrated that cytisine
may significantly inhibit N-methyl-D aspartate receptor
exposure-induced neuronal apoptosis by reversing intracellular
Ca2+ overload and balancing the expression levels of
B-cell lymphoma 2 (Bcl-2) and Bcl-2-associated X protein (16). Furthermore, novel pharmacological
and antitumor activities of cytisine have previously been
identified. Cytisine has been demonstrated to inhibit the
proliferation of A549, HepG2, Ec109, K562, HL-60 and U937 cells
(3). In K562 cells and the
esophageal carcinoma cell line Ec109, as in other tumor cell lines,
marked cytisine-induced inhibition of proliferation was detected;
however, the specific mechanism of action remains to be elucidated
(17).
Hepatocellular carcinoma (HCC) is considered to be
the third leading cause of all cancer-associated mortalities and
the fifth most common cancer type worldwide. There are a number of
different treatment strategies for HCC, including liver
transplantation, ablation, resection, chemotherapy and
embolization, however, patient prognosis remains poor. It is
therefore necessary to develop specific drugs and treatment
strategies for HCC (18). The
present study investigated the molecular mechanisms underlying
cytisine-induced apoptosis of human HepG2 cells. In addition, the
mitochondrial proapoptotic effects of cytisine on HepG2 cells were
examined, with regards to loss of mitochondrial membrane potential,
cytochrome c (Cyt-c) release into the cytosol and altered
expression of components of the caspase cascade.
Materials and methods
Instruments and reagents
Cytisine was purchased from Shaanxi River
Pharmaceutical Co., Ltd. (Xi'an, China). 10-hydroxycamptothecin
(HCPT) was purchased from Aladdin Reagent Co., Ltd (Shanghai,
China). The HepG2 human hepatocellular carcinoma cell line was
obtained from the Center of Research and Development on Life
Sciences and Environmental Sciences, Harbin University of Commerce
(Harbin, China). MTT was purchased from Beijing Solarbio Science
& Technology Co., Ltd. (Beijing, China). RPMI-1640 medium was
purchased from Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA,
USA). Fetal calf serum (FCS) was obtained from Hyclone; GE
Healthcare Life Sciences (Logan, UT, USA). Hoechst 33258 stain and
propidium iodide (PI) were obtained from Beyotime Institute of
Biotechnology (Haimen, China). Cell lysis buffer for western
blotting and immunoprecipitation (IP) was also purchased from
Beyotime Institute of Biotechnology (cat. no. P0013). β-actin,
Cyt-c and caspase-3 monoclonal antibodies were purchased from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). The EPICS XL-MCL™ flow
cytometer was purchased from Beckman Coulter, Inc. (Brea, CA, USA)
and the fluorescence microscope was obtained from Olympus
Corporation (Tokyo, Japan).
MTT assay
The viability of HepG2 cells following treatment was
determined by measuring the reduction of soluble MTT to water
insoluble formazan (19,20). HepG2 cells were cultured in
RPMI-1640 with 12% FCS. HepG2 cells were seeded at a density of
5×104/ml/well in a volume of 100 µl in 96-well plates
and cultured at 37°C with 5% CO2 for 24 h prior to being
used in the cell viability assay. Cells were treated with cytisine
at the following concentrations: 1.5, 3, 6, 12 and 24
mmol·l−1, or with positive drug HCPT (Aladdin Reagent
Co., Ltd.) at 0.1, 1, 10 and 100 µmol·l−1 for 72 h at
37°C. Cytisine and HCPT belong to the same family of alkaloids,
thus HCPT was used as positive drug in the present study to verify
that the experimental method was correct. Subsequently, 20 µl MTT
was added to each well, and the cells were incubated for an
additional 3 h at 37°C. Dimethyl sulfoxide (100 µl) was then added
to each well to dissolve the formazan crystals and absorbance was
read at a wavelength of 570 nm using a microplate reader. The
inhibition (%) of cell viability was determined as follows:
Viability inhibition=[(optical density of control cells-optical
density of treated cells)/optical density of control cells] ×100.
All observations were validated by at least three independent
experiments.
Nuclear staining with Hoechst
33258
The nuclear morphology of cytisine-treated HepG2
cells was examined to determine the effect of cytisine on
apoptosis. Nuclear morphology was evaluated using
membrane-permeable blue Hoechst 33258. Briefly, HepG2 cells were
plated on a 6-well (1 ml·well−1) microplate at a density
of 3×105/ml and cultured at 37°C and 5% CO2.
Cytisine (2.5, 5 and 10 mmol·l−1) or 60
µmol·l−1 HCPT was added to the cells and the plate was
incubated at 37°C in a humidified atmosphere containing 5%
CO2 for 48 h. HepG2 cells were then fixed with 800 µl 4%
(w/v) paraformaldehyde in PBS for 1 h at 4°C. For staining, the
cells were washed twice with PBS and stained with Hoechst 33258 at
room temperature in the dark for 30 min. The cells were
subsequently examined and images were captured under a fluorescence
microscope (21–23).
Apoptosis assays
Quantitative analysis of apoptosis was performed by
flow cytometry with PI staining (24,25).
HepG2 cells were plated on a 6-well (1 ml·well−1)
microplate at a density of 3×105/ml and cultured for 24
h at 37°C and 5% CO2. HepG2 cells were exposed to
cytisine (2.5, 5 and 10 mmol·l−1) or 60
µmol·l−1 HCPT for 48 h in culture media at 37°C in an
atmosphere containing 5% CO2. Cells were gently
harvested following trypsin digestion and washed twice with PBS.
The cells were subsequently centrifuged at 833 × g for 10 min at
room temperature. Cells (1×106) were formed into a
single cell suspension with PBS solution and fixed with 70%
ice-cold ethanol at 4°C overnight. Subsequently, cells were washed
twice with PBS, then permeabilized and stained with a solution
containing 800 µl PI (PI staining solution: Sodium citrate 33.4 mg,
PI 5 mg, RNaseA 1 mg and Trtiton-X-100 0.5 ml) for 30 min in the
dark at room temperature. The stained cells were detected by flow
cytometry (EPICS XL-MCL; Beckman Coulter, Inc.) and analyzed by
MultiCycle for Windows 32-bit software (Beckman Coulter, Inc.)
(26). The aim of this process was
to reveal the apoptotic cells by analyzing the results of the PI
and Hochest 33258 staining collectively.
Measurement of mitochondrial membrane
potential
Cells were treated with cytisine (2.5, 5 and 10
mmol·l−1) or HCPT (60 µmol·l−1) for 24 h.
Subsequently, 3×105 cells were collected and suspended
in rhodamine 123 dye (10 µg·ml−1) at 37°C for 30 min in
the dark (27,28). The cells were washed with PBS
twice, and fluorescence intensity was determined by flow cytometry
(EPICS XL-MCL; Beckman Coulter, Inc.) at 525 nm emission
wavelength. The data was analyzed using EXPO32™ ADC
software (Beckman Coulter, Inc).
Western blot analysis
HepG2 cells were harvested following treatment with
cytisine (2.5, 5 and 10 mmol·l−1) or HCPT (60
µmol·l−1) for 24 h at 37°C and 5% CO2. Whole
cellular proteins were extracted and cytosolic fractions were
prepared, according to the procedure described by the manufacturer
of cell lysis buffer for western blotting and IP (cat. no. P0013;
Beyotime Institute of Biotechnology). Protein concentrations were
quantified using the bicinchoninic acid method. After boiling for
10 min, 50 µg protein was loaded onto a 15% SDS-PAGE gel and run at
80 V for 30 min and 120 V for 1 h. Proteins were transferred onto
nitrocellulose membranes. After incubation for 1 h in blocking
solution (5% nonfat dry milk in 20 mM of TBS with 0.1% Tween) at
room temperature, the membrane was incubated for 24 h with Cyt-c
(1:200; cat. no. SC-13156, ZSGB Bio Co., Ltd.), caspase-3 (1:500;
cat. no. SC-7148; ZSGB Bio Co., Ltd., Beijing, China),
pro-caspase-3 (1:500; cat. no. TA336455; ZSGB Bio Co., Ltd.) and
β-actin (1:1,000; cat. no. TA-09; ZSGB Bio Co., Ltd.) primary
antibodies at 4°C. The secondary antibodies, such as
Peroxidase-Conjugated AffiniPure Goat Anti-Rabbit IgG [heavy +
light chain (H+L); 1:5,000; cat. no. ZB-2301; ZSGB Bio Co., Ltd.]
and Peroxidase-Conjugated AffiniPure Goat Anti-Mouse IgG (H+L;
1:5,000; cat. no. ZB-2305; ZSGB Bio Co., Ltd.) were added at a
1:5,000 dilution and the membranes were incubated at room
temperature for 2 h. Membranes were visualized with
3,3′-diaminobenzidine (cat. no. K145911; ZSGB Bio Co., Ltd.).
Images were captured using a GIS-2019 Tanon Gel Imaging system
(Tanon Science & Technology Co., Ltd., Shanghai, China) and
hybrid bands were semi-quantitatively analyzed using Gel-Pro
Analyzer 3.1 (Tannon gel imaging system GIS-2019; Tanon Science
& Technology Co., Ltd.) density analysis software (29).
Statistical analysis
The results are presented as the mean ± standard
deviation and three experimental repeats were conducted.
Differences among groups were analyzed using one-way analysis of
variance and multiple comparisons were performed using the
Student-Newman-Keuls method. Statistical analysis was performed
using SPSS 19.0 software (IBM SPSS, Armonk, NY, USA). P<0.01 was
considered to indicate a statistically significant difference.
Results
Cytisine inhibits cell viability and
increases HepG2 cell death
HepG2 cells were exposed to various concentrations
of cytisine or HCPT for 72 h and cytotoxicity was determined by MTT
assays. As presented in Table I
cytisine reduced HepG2 cell viability in vitro in a
dose-dependent manner. The half maximal inhibitory concentration
(IC50) of cytisine was 5.36 mmol·l−1, whereas
the IC50 value of HCPT was 8.56 µmol·l−1.
 | Table I.Inhibition of HepG2 cell viability by
cytisine, as determined by MTT assay. |
Table I.
Inhibition of HepG2 cell viability by
cytisine, as determined by MTT assay.
| Group | Optical
density | IR (%) |
IC50 |
|---|
| Control | 1.77±0.07 | NA | NA |
| Cytisine,
mmol·l−1 |
|
| 5.36
mmol·l−1 |
|
1.5 |
1.42±0.07a | 19.77 |
|
|
3.0 |
1.23±0.08a | 30.51 |
|
|
6.0 |
0.83±0.04a | 53.11 |
|
|
12.0 |
0.43±0.04a | 75.71 |
|
|
24.0 |
0.29±0.04a | 84.18 |
|
| HCPT,
µmol·l−1 |
|
| 8.56
µmol·l−1 |
|
0.1 |
1.36±0.05a | 23.60 |
|
| 1 |
1.14±0.12a | 37.08 |
|
|
10.0 |
0.88±0.04a | 48.31 |
|
|
100.0 |
0.75±0.03a | 57.87 |
|
Morphological investigation
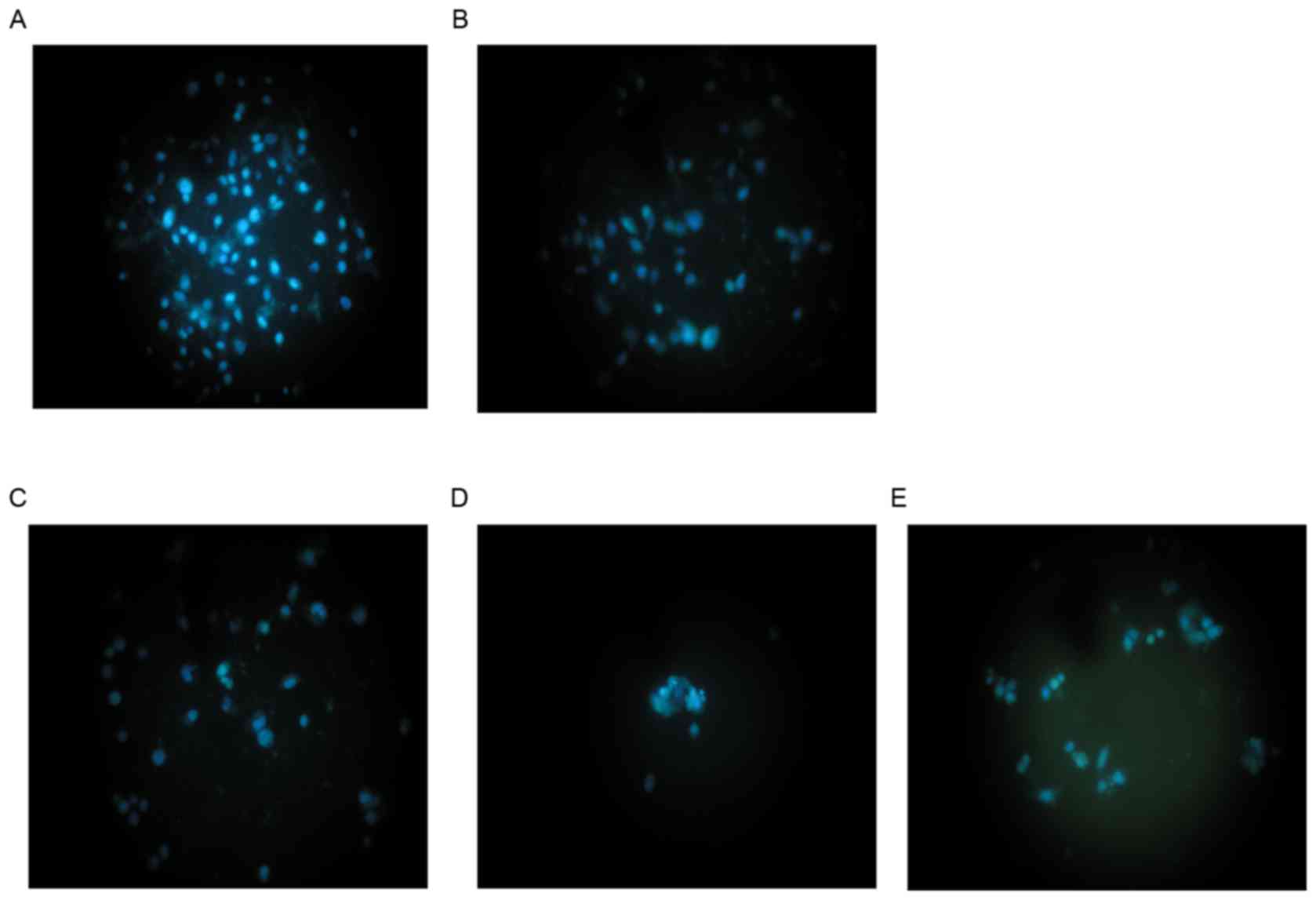
As shown in Fig. 1,
Hoechst 33258 fluorescence photomicrographs of cultured HepG2 cells
treated with cytisine or HCPT, and control cells, were captured. In
control cultures, the nuclei of the HepG2 cells were round, large
in size and exhibited regular contours (Fig. 1A). The number of HepG2 cells with
smaller nuclei and condensed chromatin was limited. Conversely, the
majority of nuclei in cytisine-treated HepG2 cells (Fig. 1B-D) were hypercondensed (brightly
stained). The number of apoptotic nuclei containing condensed
chromatin increased as the result of increased concentration. Some
treated cells exhibited formation of apoptotic bodies, which were
visible as round or oval masses of cytoplasm with multifragmented
nuclei. When HepG2 cells were treated with 60 µmol/l HCPT, a number
of apoptosis-associated morphologies were detected including
chromatin condensation, marginalization of apoptotic cells and
nuclear membrane lysis. In addition, chromatin was divided into
blocks and typical apoptotic bodies (Fig. 1E).
Apoptotic analysis
Apoptotic rates were analyzed using PI staining and
flow cytometric analysis. The results demonstrated that cytisine is
capable of inducing apoptosis at concentrations of 2.5 and 5
mmol·l−1 (17.14±0.49 and 73.32±4.42%, respectively).
Cytisine induced the highest percentage of apoptosis at 10
mmol·l−1 (90.74±2.33%) compared with the control
(P<0.01; Fig. 2 and Table II). These findings indicated that
cytisine induced HepG2 cell apoptosis in a dose-dependent
manner.
 | Figure 2.Apoptotic rate of HepG2 cells, as
determined by flow cytometry. Cells were treated with 2.5, 5 and 10
mmol·l−1 cytisine or 60 µmol·l−1 HCPT for 48
h. The cells were subsequently stained with propidium iodide and
analyzed using an EPICS™ XL-MCL flow cytometer. HepG2 cells treated
with (A) RPMI-1640, (B) 2.5 mmol·l−1 cytisine, (C) 5
mmol·l−1 cytisine, (D) 10 mmol·l−1 cytisine
and (E) 60 µmol·l−1 HCPT. In each graph, the percentages
indicated by gates C-E were used to obtain the apoptotic rates
presented in Table II. HCPT,
10-hydroxycamptothecin; C gate, apoptosis cells; D gate,
G1 cells; E gate, G2/M cells. |
 | Table II.Apoptotic rate of HepG2 cells, as
determined by flow cytometry. |
Table II.
Apoptotic rate of HepG2 cells, as
determined by flow cytometry.
| Group | Number of
cells | Apoptotic rate,
% |
|---|
| Control |
1×105 | 1.39±0.62 |
| Cytisine,
mmol·l−1 |
|
|
|
2.5 |
1×105 |
17.14±0.49a |
|
5.0 |
1×105 |
73.32±4.42a |
|
10.0 |
1×105 |
90.74±2.33a |
| HCPT,
µmol·l−1 |
|
|
|
60.0 |
1×105 |
13.83±3.16a |
Effects of cytisine on mitochondrial
membrane potential
A decrease in fluorescence intensity was observed
following cytisine treatment in a dose-dependent manner, as
presented as in Fig. 3. These
findings indicated that cytisine reduced mitochondrial membrane
potential.
Effects of cytisine on the expression
of apoptosis-associated proteins
As presented in Fig.
4, the expression levels of cytosolic Cyt-c increased in a
dose-dependent manner following treatment with cytisine, as
compared with the untreated controls. Cytosolic Cyt-c protein
expression significantly increased in the HCPT group when compared
with the control group. Furthermore, cytisine significantly reduced
the levels of pro-caspase-3 and significantly increased caspase-3
expression compared with in the control cells (Fig. 5). In the HCPT group, the levels of
caspase-3 gradually increased with the increase in dose, and the
levels of pro-caspase-3 decreased, which indicated that caspase-3
expression may be upregulated by HCPT (Fig. 5).
Discussion
Induction of apoptosis is one potential mechanism
for the anticancer therapeutic effects of cytisine. MTT assays are
commonly used to evaluate cell proliferation and viability. The
reduction of MTT indicates cellular metabolic activity, and sites
of reduction include mitochondrial and cytosolic redox reactions
(30). The present study
investigated the inhibitory effect of cytisine on HepG2 cell
viability using the MTT assay. The results indicated that the
viability of HepG2 cells was dose-dependently inhibited following
exposure to cytisine.
Various types of cell death exist, including
necrosis, apoptosis and autophagic cell death. Apoptosis, which is
a form of programmed cell death, is a highly regulated process that
permits self-degradation of cells to remove dysfunctional cells.
There are two complex apoptotic pathways: The extrinsic death
receptor-mediated pathway and the intrinsic mitochondria-mediated
pathway (31,32). In the present study, fluorescence
microscopy revealed features that are characteristic of apoptosis
following treatment with cytisine, confirming the ability of
cytisine to induce apoptosis of HepG2 cells. Fluorescence
microscopy is a common method used for the observation of cell
ultrastructure (33). To determine
whether the cell membrane was disrupted, nuclear morphology was
also examined using Hoechst 33258. The results indicated that
cytisine induced characteristics associated with apoptosis,
including shrinkage of the cytoplasm; condensation of nuclear
chromatin, and its segregation into sharply delineated masses
against the nuclear membrane; karyorrhexis; and the occurrence of
apoptotic bodies.
Natural products continue to be an invaluable
resource for anticancer drug discovery (34). Cytisine is a natural product
isolated from plants and is a member of the quinolizidine alkaloid
family. It has been used medically to aid with smoking cessation
(35). However, to the best of our
knowledge, no previous studies have published data concerning the
effects of cytisine on hepatic carcinoma. The present study
investigated the antitumor effects of cytisine on HepG2 human
hepatocellular carcinoma cells and the potential mechanisms of
action in vitro. The results demonstrated that cytisine may
inhibit the viability and induce the apoptosis of HepG2 cells.
Furthermore, cytisine-induced HepG2 apoptosis was associated with
the mitochondrial pathway. The present study demonstrated by flow
cytometry that cytisine may be an important apoptosis inducer in
HepG2 cells, since an increase in the number of cells in the
apoptotic stages was observed in vitro. After 48 h of
treatment with 10 mmol·l−1 cytisine, the apoptotic rate
of HepG2 cells was 90.74±2.33%. The rate of apoptosis appears to be
dependent on the concentration of cytisine.
Cyt-c is one of the major signaling molecules
involved in cellular apoptosis, and it is usually located between
the inner and outer mitochondrial membranes (36). The intrinsic apoptotic pathway is a
mitochondria-dependent process where a number of proapoptotic
signals lead to the release of apoptogenic factors, such as Cyt-c,
from the intermembrane space of the mitochondria to the cytosol
(37). Once released, Cyt-c forms
a complex with apoptotic peptidase activating factor 1 and
deoxyadenosine triphosphate, the resulting complex is termed the
apoptosome, which subsequently activates an initiator caspase.
Cyt-c release leads to the activation of the caspase cascade and is
therefore critical to the activation of intracellular apoptotic
signals and subsequent apoptosis (38). In the present study, western blot
analysis was performed to analyze the expression of
apoptosis-associated proteins, in order to investigate the
mechanism by which cytisine may induce apoptosis. The proteins
involved in apoptosis include caspases, which are a family of
proteases. Of the caspases, a previous study has demonstrated that
caspase-3 is important in the process of apoptosis, particularly in
certain apoptotic signal transduction pathways (39). The protein expression of this
caspase was investigated in the present study. Caspase-3, a member
of the cell death protein 3 subfamily of the caspase family, has
been identified downstream of the cysteine protease and is a
mediator of apoptosis (40).
Cytisine has been demonstrated to improve the expression of the
caspase-3 protein, suggesting that cytisine activates caspase-3. It
is possible that within the cells, mitochondrial apoptosis is
induced by the release of Cyt-c, which in turn leads to the
activation of caspase-3. This may serve a key role in the signaling
pathways leading to cell apoptosis. The results of the present
study highlight the contribution of the cytosolic accumulation of
Cyt-c, induced by these apoptotic stimuli, in promoting the
cleavage and activity of caspase-3.
In conclusion, the results of the present study
indicated that cytisine induced apoptosis of HepG2 cells in a
dose-dependent manner. Therefore, cytisine may exert antitumor
effects. Cytisine may induce apoptosis of tumor cells through the
mitochondrial pathway, as indicated by the reduction in
mitochondrial membrane potential. Following treatment with
cytisine, mitochondrial permeability may increase, which may
subsequently lead to mitochondrial matrix expansion, outer membrane
rupture and the release of Cyt-c. Increased Cyt-c protein
expression was detected in the cytosol following cytisine treatment
in the present study. Increased Cyt-c release into the cytoplasm
causes activation of the caspase cascade, including caspase-3. In
addition, the present study reported increased levels of activated
caspase-3, and reduced levels of inactive pro-caspase-3, thus
indicating that cytisine may induce apoptosis via this pathway. The
results of the present study provide a good experimental and
theoretical basis for the further development and application of
cytisine in clinical treatments for liver cancer.
Acknowledgements
The present study was supported in part by the Open
Research Program for Key Laboratory of College of Heilongjiang
Province, China (grant no. CPAT-2012003), the Natural Science Item
of Department of Education of Heilongjiang Province, China (grant
no. 12541205), the Innovation Talents Item of Science and
Technology of Harbin city, China (grant no. 2014RFQXJ154), the
Doctoral Research Project of Harbin University of Commerce (grant
no. 12DL008), the Graduate Students Innovative Research Project of
Harbin University of Commerce (grant no. YJSCX2015-390HSD), the
2016 Harbin University of Commerce Youth Innovation Talent Support
Program (grant no. 2016QN057) and the Scientific Research Team
Program of Harbin University of Commerce (grant no. 2016TD002).
References
|
1
|
Rebecca L, Kimberly D and Ahmedin J:
Cancer statistics, 2016. A Can J Clin. 66:7–30. 2016. View Article : Google Scholar
|
|
2
|
Begnini KR, de Moura Leon PM, Thurow H,
Schultze E, Campos VF, Martins Rodrigues F, Borsuk S, Dellagostin
OA, Savegnago L, Roesch-Ely M, et al: Brazilian red propolis
induces apoptosis-like cell death and decreases migration potential
in bladder cancer cells. Evid Based Complement Alternat Med.
2014:6398562014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin Z, Huang CF, Liu XS and Jiang J: In
vitro anti-tumour activities of quinolizidine alkaloids derived
from Sophora flavescens Ait. Basic Clin Pharmacol Toxicol.
108:304–309. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou CC, Gao HB, Sun XB, Shi HB, Liu W,
Yuan HN and Wang ZX: Anti-inflammatory and anti-allergic action of
aloperine. Zhongguo Yao Li Xue Bao. 10:360–365. 1989.PubMed/NCBI
|
|
5
|
Zhang L, Zheng Y, Deng H, Liang L and Peng
J: Aloperine induces G2/M phase cell cycle arrest and apoptosis in
HCT116 human colon cancer cells. Int J Mol Med. 33:1613–1620.
2014.PubMed/NCBI
|
|
6
|
Du M, Zhang J, Xu D, Li W, Liu J and Liu
F: Inhibition of pro-collagen I expression by oxymatrine in hepatic
stellate cells is mediated via nuclear translocation of Y-box
binding protein 1. Mol Med Rep. 12:8101–8106. 2015.PubMed/NCBI
|
|
7
|
Liang L, Wang XY, Zhang XH, Ji B, Yan HC,
Deng HZ and Wu XR: Sophoridine exerts an anti-colorectal carcinoma
effect through apoptosis induction in vitro and in vivo. Life Sci.
91:1295–1303. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang WX, Sun ZH, Chen HM, Xu BN and Wang
FY: Role and mechanism of Sophoridine on proliferation inhibition
in human glioma U87MG cell line. Int J Clin Exp Med. 8:464–471.
2015.PubMed/NCBI
|
|
9
|
Zhu YY, Huang HY and Wu YL: Anticancer and
apoptotic activities of oleanolic acid are mediated through cell
cycle arrest and disruption of mitochondrial membrane potential in
HepG2 human hepatocellular carcinoma cells. Mol Med Rep.
12:5012–5018. 2015.PubMed/NCBI
|
|
10
|
Luo X, Budihardjo I, Zou H, Slaughter C
and Wang X: Bid, a Bcl2 interacting protein, mediates cytochrome c
release from mitochondria in response to activation of cell surface
death receptors. Cell. 94:481–490. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marcaurelle LA, Johannes C, Yohannes D,
Tillotson BP and Mann D: Diversity-oriented synthesis of a
cytisine-inspired pyridone library leading to the discovery of
novel inhibitors of Bcl-2. Bioorg Med Chem Lett. 19:2500–2503.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chellappan SK, Xiao Y, Tueckmantel W,
Kellar KJ and Kozikowski AP: Synthesis and pharmacological
evaluation of novel 9- and 10-substituted cytisine derivatives.
Nicotinic ligands of enhanced subtype selectivity. J Med Chem.
49:2673–2676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Muslim NS, Ng KW, Itam A, Nassa ZD, Ismail
Z and Majid AMS Abdul: Evaluation of cytotoxic, anti-angiogenic and
antioxidant properties of standardized extracts of strobilanthes
crispus leaves. Int J Pharmacol. 6:591–599. 2010. View Article : Google Scholar
|
|
14
|
Harpsøe K, Hald H, Timmermann DB, Jensen
ML, Dyhring T, Nielsen EØ, Peters D, Balle T, Gajhede M, Kastrup JS
and Ahring PK: Molecular determinants of subtype-selective
efficacies of cytisine and the novel compound NS3861 at heteromeric
nicotinic acetylcholine receptors. J Biol Chem. 288:2559–2570.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang H, Yang S, Zhou H, Sun M, Du L, Wei
M, Luo M, Huang J, Deng H, Feng Y, et al: Aloperine executes
antitumor effects against multiple myeloma through dual apoptotic
mechanisms. J Hematol Oncol. 8:262015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sciamanna MA, Griesmann GE, Williams CL
and Lennon VA: Nicotinic acetylcholine receptors of muscle and
neuronal (alpha7) types coexpressed in a small cell lung carcinoma.
J Neurochem. 69:2302–2311. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Fu XQ, Lei WL, Wang T, Sheng AL
and Luo ZG: Nuclear factor kappa в controls acetylcholine receptor
clustering at the neuromuscular junction. J Neurosci.
30:11104–11113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chacko S and Samanta S: Hepatocellular
carcinoma: A life-threatening disease. Biomed Pharmacother.
84:1679–1688. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dickinson BC and Chang CJ: Chemistry and
biology of reactive oxygen species in signaling or stress
responses. Nat Chem Biol. 7:504–511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Muchmore SW, Sattler M, Liang H, Meadows
RP, Harlan JE, Yoon HS, Nettesheim D, Chang BS, Thompson CB, Wong
SL, et al: X-ray and NMR structure of human Bcl-xL, an inhibitor of
programmed cell death. Nature. 381:335–341. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carmichael J, DeGraff WG, Gazdar AF, Minna
JD and Mitchell JB: Evaluation of a tetrazolium-based semiautomated
colorimetric assay: Assessment of chemosensitivity testing. Cancer
Res. 47:936–942. 1987.PubMed/NCBI
|
|
22
|
Araki T, Yamamoto A and Yamada M: Accurate
determination of DNA content in single cell nuclei stained with
Hoechst 33258 fluorochrome at high salt concentration.
Histochemistry. 87:331–338. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Holmquist G: Hoechst 33258 fluorescent
staining of Drosophila chromosomes. Chromosoma. 49:333–356. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wyllie AH, Morris RG, Smith AL and Dunlop
D: Chromatin cleavage in apoptosis: Association with condensed
chromatin morphology and dependence on macromolecular synthesis. J
Pathol. 142:67–77. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gong J, Traganos F and Darzynkiewicz Z: A
selective procedure for DNA extraction from apoptotic cells
applicable for gel electrophoresis and flow cytometry. Anal
Biochem. 218:314–319. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nicoletti I, Migliorati G, Pagliacci MC,
Grignani F and Riccardi C: A rapid simple method for measuring
thymocyte apoptosis by propidium iodide staining and flow
cytometry. J Immunol Methods. 139:271–279. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Basiji DA, Ortyn WE, Liang L,
Venkatachalam V and Morrissey P: Cellular image analysis and
imaging by flow cytometry. Clin Lab Med. 27:653–670, viii. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scaduto RC Jr and Grotyohann LW:
Measurement of mitochondrial membrane potential using fluorescent
rhodamine derivatives. Biophys J. 76:469–477. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ashley RL, Militoni J, Lee F, Nahmias A
and Corey L: Comparison of Western blot (immunoblot) and
glycoprotein G-specific immunodot enzyme assay for detecting
antibodies to herpes simplex virus types 1 and 2 in human sera. J
Clin Microbiol. 26:662–667. 1988.PubMed/NCBI
|
|
30
|
Zhang H, Xiong Z, Wang J, Zhang SS, Lei L,
Yang L and Zhang Z: Glucagon-like peptide-1 protects cardiomyocytes
from advanced oxidation protein product-induced apoptosis via the
PI3K/Akt/Bad signaling pathway. Mol Med Rep. 13:1593–1601.
2016.PubMed/NCBI
|
|
31
|
Kim KN, Ham YM, Moon JY, Kim MJ, Kim DS,
Lee WJ, Lee NH and Hyun CG: In vitro cytotoxic activity of
sargassum thunbergii and dictyopteris divaricata (Jeju Seaweeds) on
the HL-60 tumour cell line. Int J Pharmacol. 5:298–306. 2009.
View Article : Google Scholar
|
|
32
|
Oh HL, Lee DK, Lim H and Lee CH: HY253, a
novel decahydrofluorene analog, from Aralia continentalis, induces
cell cycle arrest at the G1 phase and Cyt-C-mediated apoptosis in
human lung cancer A549 cells. J Ethnopharmacol. 129:135–139. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Darzynkiewicz Z, Juan G, Li X, Gorczyca W,
Murakami T and Traganos F: Cytometry in cell necrobiology: Analysis
of apoptosis and accidental cell death (necrosis). Cytom. 27:1–20.
1997. View Article : Google Scholar
|
|
34
|
Hwang MW and Kim BJ: Apoptotic effects and
involvement of TRPM7 channels of the traditional herbal medicine,
dangkwisoo-san in gastric cancer cells. Int J Pharmacol.
10:398–405. 2014. View Article : Google Scholar
|
|
35
|
Gazaliev AM, Zhurinov MZ and Tuleuov BI:
Isolation, analysis, biosynthesis, and modification of the alkaloid
cytisine. Chem Nat Com. 27:259–269. 1991. View Article : Google Scholar
|
|
36
|
Scorrano L: Opening the doors to
cytochrome c: Changes in mitochondrial shape and apoptosis. Int J
Biochem Cell Biol. 41:1875–1883. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rouhollahi E, Moghadamtousi S Zorofchian,
Paydar M, Fadaeinasab M, Zahedifard M, Hajrezaie M, Hamdi OA Ahmed,
Looi CY, Abdulla MA, Awang K and Mohamed Z: Inhibitory effect of
Curcuma purpurascens BI. rhizome on HT-29 colon cancer cells
through mitochondrial-dependent apoptosis pathway. BMC Complement
Altern Med. 15:152015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Philchenkov AA: Caspases as regulators of
apoptosis and other cell functions. Biochem (Mosc). 68:365–376.
2003. View Article : Google Scholar
|
|
39
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Bio.
5:897–907. 2004. View Article : Google Scholar
|
|
40
|
Alnemri ES, Livingston DJ, Nicholson DW,
Salvesen G, Thornberry NA, Wong WW and Yuan J: Human ICE/CED-3
protease nomenclature. Cell. 87:1711996. View Article : Google Scholar : PubMed/NCBI
|