Introduction
Gastric cancer has one of the highest
cancer-associated mortalities worldwide and patients have a
particularly high susceptibility to lymph node metastasis (1,2). The
incidence of gastric carcinoma has recently increased, which may be
due to various environmental and social factors (3), including H. pylori infection,
low socioeconomic status and perhaps dietary factors such as low
consumption of fruits and vegetables and a high intake of salty and
smoked food (4). Although higher
overall survival rates for patients with gastric carcinoma are
currently observed due to improved early cancer detection and
increased use of radical surgery, gastric carcinoma remains the
fourth most common cancer and is considered to be the second major
cause of cancer-associated deaths globally (5). It is of note that effective methods
for early diagnosis, monitoring for metastasis and prognosis are
remain to be established for gastric cancer (6). Therefore, the identification of novel
therapeutic targets for gastric cancer is required.
Transforming growth factor-β-activated kinase 1
(TAK1) regulates the nuclear factor-κB (NF-κB) and
mitogen-activated protein kinase (MAPK) signaling pathways, which
have important roles in various biological processes, including
development, cell survival, immune responses, metabolism and
carcinogenesis (7). Previous
studies demonstrated that TAK1 functions as a tumor promoter in
various tissues, including breast and thyroid cancer (8,9).
TAK1 inhibition has also been reported to induce cancer cell death
(10,11), indicating that targeting TAK1 may
be useful in the development of treatments for gastric cancer.
Therefore, in order to develop cancer therapies that target TAK1,
it is important to determine the regulation and role of TAK1 in the
pathogenesis (12). To the best of
our knowledge, the role of TAK1 in gastric cancer has not
previously been investigated. 5Z-7-Oxozeaenol, a natural product of
fungal origin, was reported to be a TAK1 specific inhibitor
(13). Therefore, the present
study investigated the expression of TAK1 in gastric cancer and its
clinical significance, and further investigated the function of
TAK1 in the development and progression of gastric cancer in
vitro using 5Z-7-oxozeaenol (OZ), which is a selective TAK1
inhibitor (14).
Materials and methods
Patients
Gastric cancer samples and adjacent normal tissue
samples used in the present study were obtained from 139 patients
with gastric cancer that underwent resection at The Third
Affiliated Hospital, Nanjing University of Traditional Chinese
Medicine (Nanjing, China) and Wuxi XiShan People's Hospital (Wuxi,
China) between January 2005 and August 2010. Normal gastric mucosa
tissue (≤5 cm) adjacent to the tumor was excised and confirmed to
be tumor-free following pathological analysis. Every resection
specimen was examined by the Department of Pathology, The Third
Affiliated Hospital, Nanjing University of Traditional Chinese
Medicine (Nanjing, China), to confirm their histology features. The
patients consisted of 81 males and 58 females, aged between 30 and
75 years (median, 50 years). The following inclusion criteria were
sued for the present study: i) Complete surgical R0 resection of
the primary tumor; ii) pathologically confirmed diagnosis of
gastric adenocarcinoma; iii) no chemotherapy or radiotherapy
administered; and iv) absence of secondary malignancies. All
patients provided a signed agreement for participation in the study
and the protocol was approved by the Ethics Committees of The Third
Affiliated Hospital of Nanjing University of Traditional Chinese
Medicine and Wuxi XiShan People's Hospital. Written informed
consent was obtained from all patients involved in the present
study. The epidemiological, clinical and pathological features of
patients included in the present study are summarized in Table I. The clinical outcome of the
patients was followed for 1–60 months, from the date of surgery to
either the date of mortality or August 30, 2015.
 | Table I.Clinicopathological parameters and
patients with positive expression of TAK1 in gastric cancer. |
Table I.
Clinicopathological parameters and
patients with positive expression of TAK1 in gastric cancer.
|
|
| TAK1
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
parameters | Total | Positive | Negative | P-value (Chi-square
test) |
|---|
| Gender |
|
|
| 0.96 |
|
Male | 81 | 57 | 24 |
|
|
Female | 58 | 41 | 17 |
|
| Age, years |
|
|
| 0.41 |
|
<60 | 62 | 42 | 20 |
|
|
≥60 | 77 | 56 | 21 |
|
| Tumor size, cm |
|
|
| 0.52 |
|
<5 | 60 | 44 | 16 |
|
| ≥5 | 79 | 54 | 25 |
|
| Neural/vascular
invasion |
|
|
| 0.51 |
|
Yes | 42 | 28 | 14 |
|
| No | 97 | 70 | 27 |
|
| Tumor grade |
|
|
| 0.26 |
| I and
II | 61 | 40 | 21 |
|
|
III | 78 | 58 | 20 |
|
| T stage |
|
|
| 0.60 |
| T1 and
T2 | 24 | 18 | 6 |
|
| T3 and
T4 | 115 | 80 | 35 |
|
| N stage |
|
|
| <0.001 |
| N0 | 28 | 11 | 17 |
|
|
N1-N3 | 111 | 87 | 24 |
|
| M stage |
|
|
| 0.77 |
| M0 | 131 | 92 | 39 |
|
| M1 | 8 | 6 | 2 |
|
| Pathological
stage |
|
|
| <0.001 |
| I and
II | 47 | 18 | 29 |
|
| III and
IV | 92 | 80 | 12 |
|
Immunohistochemistry and scoring
The tissues were fixed for 24 h in 4%
paraformaldehyde and embedded in paraffin. The paraffin-embedded
tissues were cut into 4 µm sections. Then, tissue sections were
deparaffinized in xylene and rehydrated through graded ethanol.
Tissues were placed in 0.01 M citrate buffer and incubated at 100°C
for 20 min for antigen retrieval. Tissues were blocked with a 3%
hydrogen peroxide solution to inhibit endogenous peroxidase
activity and washed with PBS, Subsequently, sections were incubated
with 5% normal rabbit serum (Abcam, Cambridge, UK) for 30 min at
room temperature to block non-specific binding sites. The slides
were subsequently incubated with a TAK1 antibody (1:50; catalog no.
sc-7162; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C
overnight, followed by incubation with a horseradish peroxidase
(HRP)-conjugated secondary antibody (dilution 1:1,000; catalog no.
ab6721; Abcam) for 60 min at room temperature. The sections were
then developed in 0.05% diaminobenzidine and counterstained with
0.1% hematoxylin and eosin (H&E) for 5 min at room temperature
prior to dehydration and mounting. Evaluation of immunostaining in
tumor cells was objectively performed by two pathologists under a
light microscope at high magnification (×400). TAK1 staining was
determined semi-quantitatively according to the intensity observed
(0=no staining; 1=weak staining; 2=moderate staining; and 3=strong
staining) and the percentage of positive cells (0, none or <5%;
1, 5–20%; 2, 21–40%; and 3, >40%). Scores of 0–2 were considered
to be negative expression and scores of 3–6 were considered to be
positive expression. Cells were counted in at least three randomly
selected fields (at ×400 magnification) in the tumor areas.
Cell culture
The MGC803 human gastric cancer cell line was
purchased from Cell Bank of Type Culture Collection of Chinese
Academy of Sciences (Shanghai, China). MGC803 cells were cultured
at 37°C and 5% CO2 and saturation humidity in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc.), with 10% fetal
bovine serum, 100 U/ml penicillin (Thermo Fisher Scientific), and
100 mg/ml streptomycin (Thermo Fisher Scientific, Inc.). The cells
adhered to the flask wall and grew into a single-cell monolayer and
were passaged every 2–3 days. Cells in the exponential growth phase
were harvested for subsequent experiments. There were four
experimental groups: Control (without any intervention); vehicle
treatment [1% dimethylsulfoxide (DMSO)]; low-dose OZ (3 µM); and
high-dose OZ (6 µM). TAK1 kinase inhibitor, OZ was purchased from
Tocris Bioscience, Bristol, UK (catalog no. 3604).
MTT assay
Cells were plated in 96-well plates at a density of
5×103/well and treated with 6 µM OZ or vehicle for 24,
48 and 72 h at 37°C. At the aforementioned time points, 20 µl MTT
substrate (5 mg/ml) was added to the cells and incubated at 37°C
for 4 h. The resulting colored product was made soluble in 200 µl
DMSO. Spectrometric absorbance at 490 nm was quantified using a
microplate reader. Each cell line was established in quadruplicate
wells and repeated three times.
Invasion assay
Cell invasion activity was determined using a BD
BioCoat Matrigel Invasion Chamber (8-µm; BD Biosciences, Franklin
Lakes, NJ, USA). Briefly, gastric cancer cells were harvested and
added to the upper chamber at a cell density of 2×105
cells/ml in RPMI-1640 medium without FBS and treated with 6 µM OZ
or DMSO. RPMI-1640 medium with 10% FBS was added to the lower
chamber. The chambers were incubated for 48 h at 37°C and 5%
CO2. At the end of the incubation period, cells that had
invaded through the membrane were subsequently fixed with 4%
paraformaldehyde for 15 min and stained with 0.5% crystal violet
for 30 min at room temperature. Cells were observed under ×40
magnification with a ZEISS light microscope and counted. Each
experiment was performed in triplicate and repeated three
times.
Flow cytometry analysis
Annexin V-fluorescein isothiocyanate (FITC)
apoptosis detection kit was used to analyze the apoptosis rate
according to the manufacturer's protocol (Nanjing KeyGen Biotech
Co., Ltd., Nanjing, China). MGC803 cells were seeded in six-well
plates (1×106 cells/well) at 37°C and treated with 6 µM
OZ or vehicle for 24, 48 and 72 h. Cells were dissociated using
trypsin, then centrifuged at 400 × g for 5 min. Next, cells were
washed twice with PBS and centrifuged at 400 × g for 5 min. For
apoptosis analysis, the cell pellet was resuspended in 500 µl
binding buffer. Then, 5 µl Annexin V-FITC and 5 µl propidiumiodide
(PI) was added to the cell suspension, which was gently mixed and
incubated at room temperature, and was protected from light, for 15
min. Within 1 h, the cells were analyzed via flow cytometry using a
BD FACSCanto II instrument (BD Biosciences, San Jose, CA, USA), and
FlowJo software version 9.5.3 (Tree Star, Inc., Ashland, OR,
USA).
Western blot analysis
MGC803 cells were treated with 6 µM OZ for 48 h at
37°C. Then, washed twice with PBS and centrifuged at 12,000 × g for
15 min at 4°C. A total cellular protein extraction kit (Beyotime
Institute of Biotechnology, Haimen, China) was used to extract the
total protein, and the nucleoprotein extraction kit (Beyotime
Institute of Biotechnology) was used to extract nucleoprotein,
according to the manufacturer's protocol. Isolation of
mitochondrial and cytosolic proteins was performed using the
Mitochondria/Cytosol Fractionation kit (Bi Yuntian Biological
Technology Institution). Protein concentrations were determined
using a BCA protein assay (Beyotime Institute of Biotechnology).
Cell lysate was boiled for 12 min, and samples (40 µg protein per
lane) were separated on 5–20% gradient SDS-PAGE gels. Proteins were
subsequently transferred to polyvinylidene difluoride membranes
(EMD Millipore, Billerica, MA, USA), which were blocked overnight
in 5% non-fat milk at 4°C. The membranes were incubated with the
following primary antibodies at a dilution of 1:1,000: (p)-TAK1
(Thr187) (catalog no. 4536), pro-caspase 3 (catalog no. 9665), cyt
c (catalog no. 11940), cyclin D1 (catalog no. 2978), Bcl-2
apoptosis regulator (Bcl-2; catalog no. 2827), voltage-dependent
anion channel (catalog no. 4661), cleaved caspase 3 (catalog no.
9654), matrix metallopeptidase (MMP) 9 (catalog no. 13667), p65
(catalog no. 4764), histone 3 (catalog no. 4499) and β-actin
(catalog no. 8457) at 4°C overnight. All primary antibodies were
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA).
The membranes were then washed with 1x TBS containing 0.1%
Tween-20, incubated with anti-rabbit IgG conjugated to HRP
(dilution, 1:1,000; Cell Signaling Technology, Inc. catalog no.
7074) for 1 h at room temperature, and washed with 1xTBS containing
0.1% Tween-20 three times for 10 min each. Proteins were visualized
using an Enhanced Chemiluminescence reagent (Pierce; Thermo Fisher
Scientific, Inc., catalog no. 32106). The experiments were repeated
at least 3 times. Densitometry analysis was performed using ImageJ
software version 1.48 (National Institutes of Health).
Statistical analysis
Data are expressed as mean ± standard deviation.
SPSS version 19.0 (IBM, Armonk, NY, USA) was used for statistical
analysis. Count data were analyzed with a χ2 test.
Survival curves of the patients were compared using the
Kaplan-Meier method and analyzed by the log-rank test. One-way
analysis of variance followed by the Tukey post test was used to
analyze differences between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
TAK1 protein expression in gastric
cancer tissue and the association with clinical pathology
All gastric cancer tissue specimens and the adjacent
normal tissue specimens used in the current study were verified
using hematoxylin and eosin staining (Fig. 1). Immunohistochemistry revealed
that the cytoplasm of gastric cancer cells appeared yellow or brown
in a diffuse pattern, indicating high TAK1 expression; however,
TAK1 expression was reduced in the adjacent normal tissues
(Fig. 1).
To investigate the biological significance of TAK1
expression in gastric cancer, the patients were divided into two
groups according to TAK1 immunostaining: the TAK1 negative group
and the TRAF6 positive group. TAK1 positive expression was
quantified as 70.5% in gastric cancer tissue samples and 25.9% in
the adjacent normal tissues (P<0.001; Table II). Furthermore, TAK1 expression
was positively associated with advanced N stage and pathological
stage, indicating that TAK1 protein expression level may be
elevated during gastric cancer progression. No significant
association was identified between TAK1 protein expression level
and gender, age, tumor size, tumor grade, neural or vascular
invasion, T stage or M stage (P>0.05; Table I). Kaplan-Meier survival analysis
(Fig. 2) demonstrated that the
median 5-year survival was 21 months in patients with positive TAK1
expression, which was significantly lower compared with patients
with negative TAK1 expression (41 months; P=0.009).
 | Table II.Analysis of TAK1 expression level in
gastric cancer tissues and adjacent normal tissues. |
Table II.
Analysis of TAK1 expression level in
gastric cancer tissues and adjacent normal tissues.
|
| TAK1
expression |
|
|---|
|
|
|
|
|---|
| Tissue | Positive (%) | Negative (%) | P-value |
|---|
| Gastric cancer | 98 (70.5) | 41 (29.5) | <0.001 |
| Adjacent
normal | 36 (25.9) | 103 (74.1) |
|
Effect of OZ, the TAK1 inhibitor, on
apoptosis
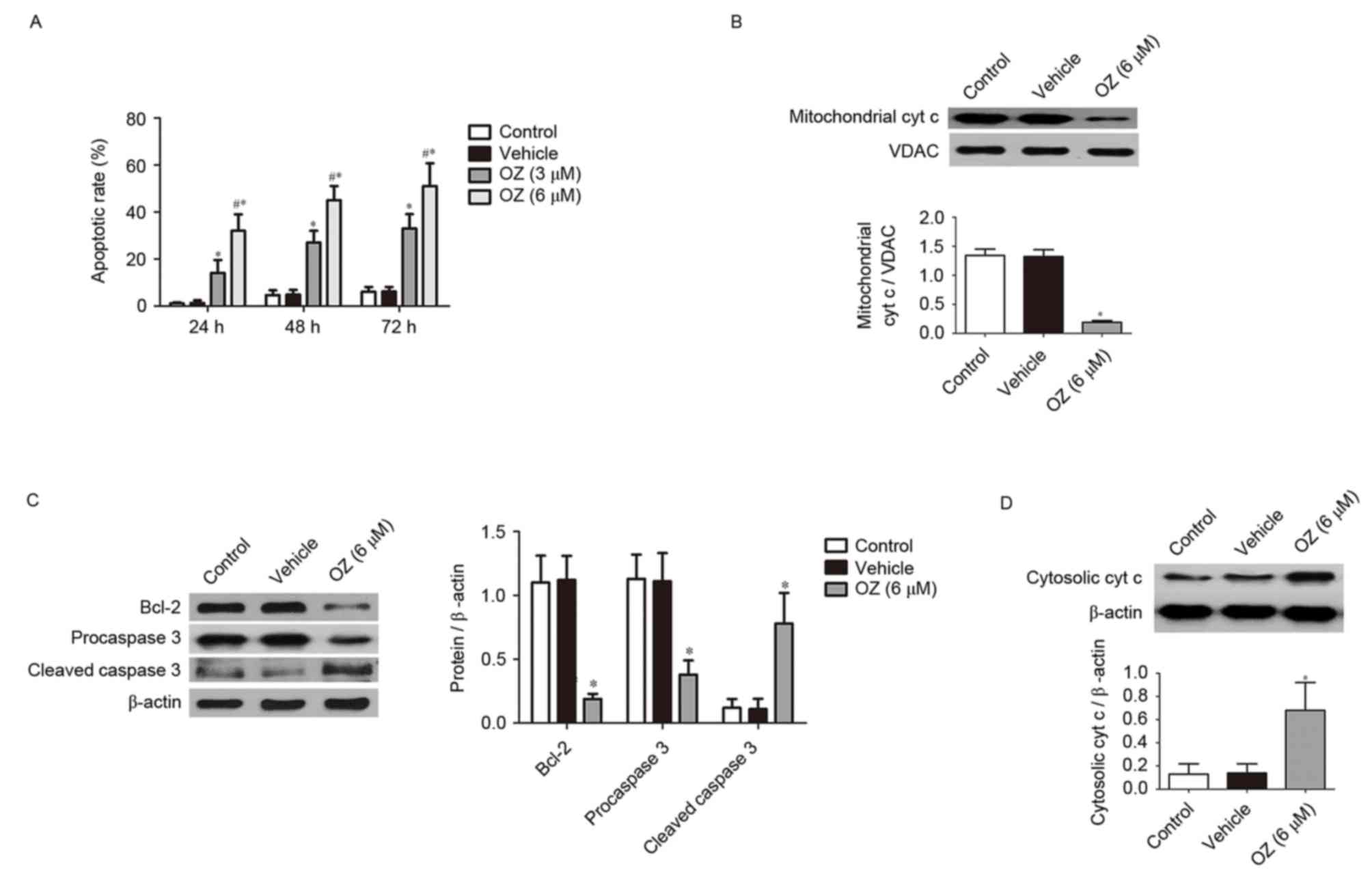
To evaluate the effects of OZ, the TAK1 inhibitor,
on MGC803 cells, the present study examined the apoptotic
properties of MGC803 cells incubated with OZ. The Annexin V and
propidium iodide dual staining revealed that MGC803 cells from the
TAK1 inhibitor treatment groups (3 and 6 µM) had a significantly
greater percentage of apoptotic cells compared with the
vehicle-treated group (P<0.05; Fig.
3A). To further investigate the cellular basis of the apoptotic
response observed in the MGC803 cell line, the expression of
apoptosis-associated proteins was investigated using western
blotting. The aforementioned experiments confirmed that the high
dose OZ (6 µM) effectively promoted apoptosis in MGC803 cells, 6 µM
OZ was used for the subsequent experiments investigating the
apoptotic mechanism. As demonstrated in Fig. 3B-D, OZ treatment significantly
reduced the expression of mitochondrial cyt c (P<0.05;
Fig. 3B), Bcl-2 (P<0.05;
Fig. 3C) and procaspase 3
(P<0.05; Fig. 3C) compared with
the vehicle treatment group. Conversely, cleaved caspase 3
(P<0.05; Fig. 3C) and cytosolic
cyt c (P<0.05; Fig. 3D)
expression levels were significantly greater in the OZ-treated
group compared with the vehicle group.
Effect of OZ treatment on cell
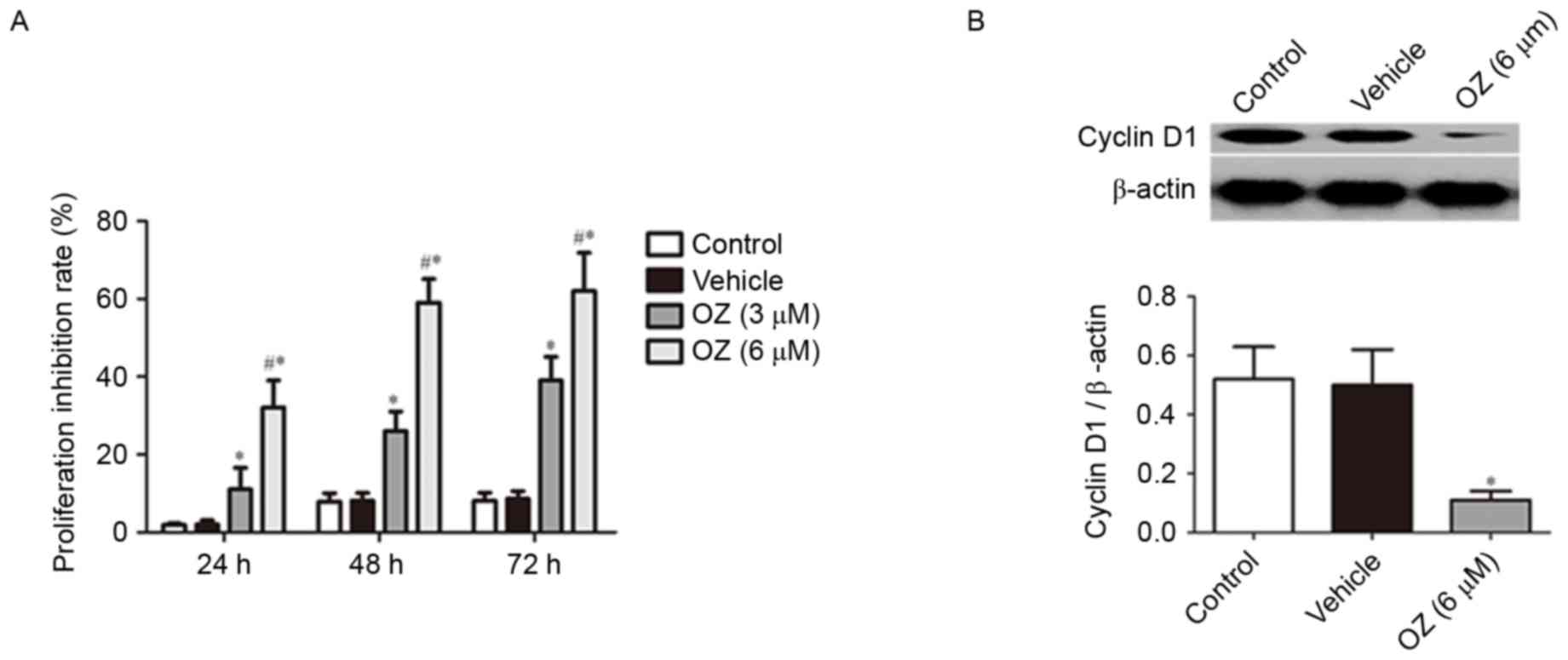
proliferation
To verify the effect of OZ treatment on tumor growth
in MGC803 cells, cell proliferation was examined using an MTT
assay. It was revealed that OZ treatment significantly inhibited
the growth of MGC803 cells in a time-dependent manner (P<0.05;
Fig. 4A) compared with
vehicle-treated cells. As cyclin D1 has previously been reported to
have an important role in gastric cancer proliferation (15,16),
the cyclin D1 protein expression level was examined by western blot
analysis. The findings indicated that cyclin D1 expression was
significantly downregulated in the OZ treatment group compared with
the vehicle-treated group (P<0.05; Fig. 4B).
Effect of OZ treatment on cell
invasion
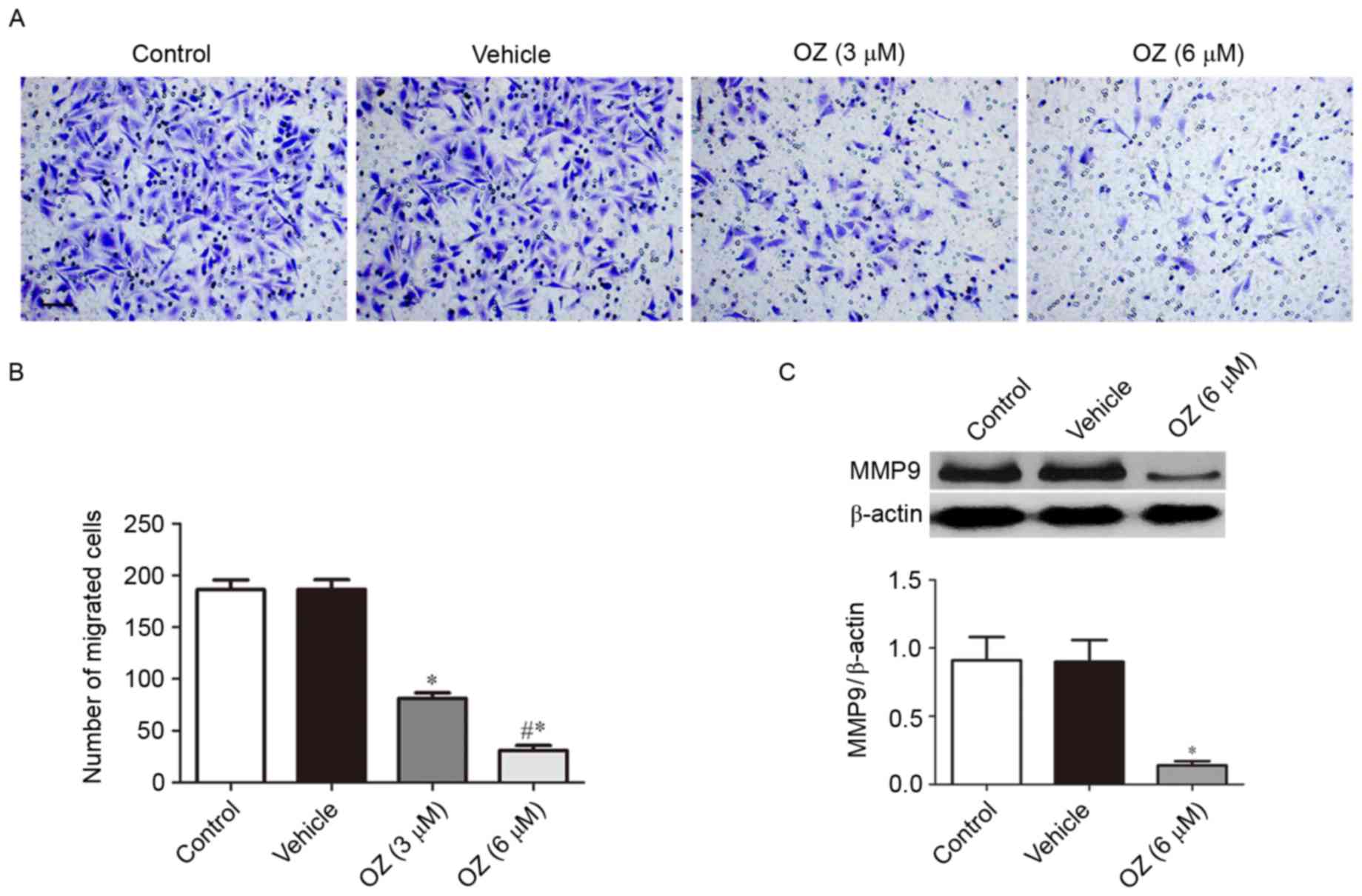
The present study further investigated the effect of
OZ treatment on the invasive behavior of MGC803 cells. As presented
in Fig. 5A and B, OZ treatment
significantly reduced the invasive ability of MGC803 cells compared
with the vehicle-treated group (P<0.05). As MMP9 has been
previously reported to have an important role in gastric cancer
invasion (17), the present study
also investigated the expression level of MMP9 protein by western
blot analysis and demonstrated that MMP9 expression was
significantly downregulated in the OZ treatment group compared with
the vehicle treatment group (P<0.05; Fig. 5C).
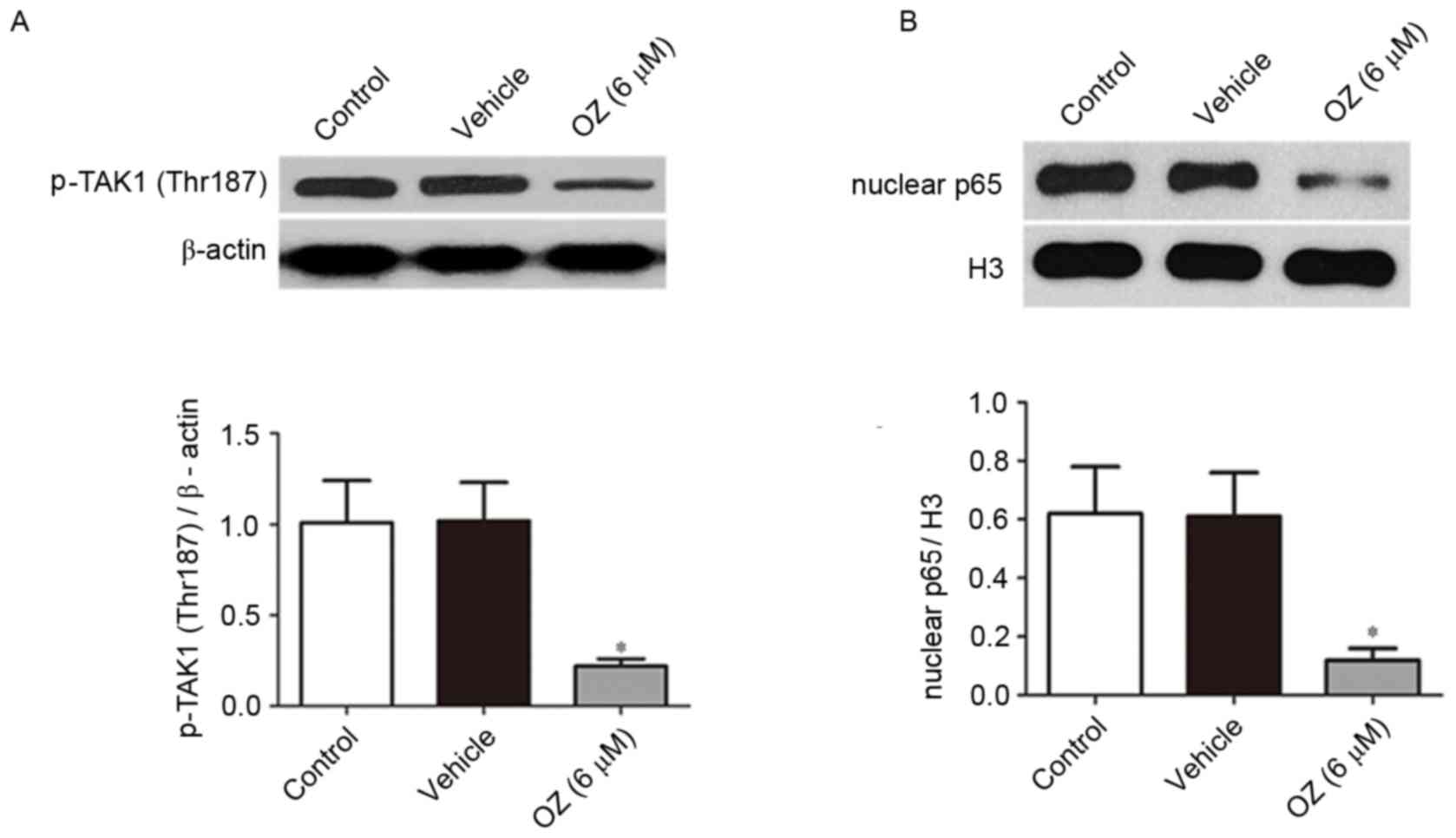
Effect of OZ treatment on the
TAK1/NF-κB signaling pathway
Following treatment with OZ for 48 h, the expression
levels of p-TAK1 (Thr187) and nuclear p65 protein were detected by
western blot analysis. As presented in Fig. 6, OZ treatment significantly reduced
p-TAK1 (Thr187) and nuclear p65 expression levels compared with the
vehicle treatment group (both P<0.05).
Discussion
TAK1 is a serine/threonine protein kinase, which
belongs to the family of MAPK kinases. TAK1 is a key kinase in the
signal pathway of toll-like receptors and the interleukin-1
receptor. Previous reports have demonstrated that the TAK1-mediated
signal transduction pathway is a key regulator in signal
transduction and the chain reaction of stress responses,
inflammation immunity and the occurrence and development of tumors
(7,12). In addition, previous studies have
demonstrated a high expression of TAK1 in a variety of tumor
tissues such as thyroid cancer, non-small cell lung carcinoma and
breast cancer, and an association with tumor occurrence,
development and invasion (8,9,18).
TAK1 regulates the activation of the MAPK and NF-κB signaling
pathways (7). Furthermore,
abnormal activation of the MAPK signaling pathway is a hallmark of
gastric cancer tissues and inhibition of MAPK activity may
significantly inhibit the proliferation and invasion of gastric
cancer cells, thus promoting apoptosis (19). In addition, the high expression of
NF-κB in gastric cancer tissues was significantly associated with
poor prognosis of patients with gastric cancer (20,21).
Inhibition of the NF-κB signaling pathway may also inhibit the
proliferation and invasion of gastric cancer cells, subsequently
promoting apoptosis (22).
Comprehensive analysis of previous research indicated that TAK1 may
also have an important role in the occurrence and development of
gastric cancer. The findings of the current study demonstrated that
25.9% of normal (non-neoplastic) gastric mucosae tissue samples
exhibited positive TAK1 expression; therefore, it is possible that
this regulation occurs at a transcriptional level as TAK1 has a key
role in signal transduction in normal tissues (12). TAK1 protein expression was
significantly increased in gastric cancer tissues compared with the
normal tissues, which was consistent with the findings of a
previous study (23). Furthermore,
the findings of the present study also demonstrated that TAK1
expression was associated with the advanced N stage and the
pathological stage of gastric carcinoma. However, no significant
association was identified in terms of gender, age, tumor size,
tumor grade, neural or vascular invasion, T and M stage. In
addition, the 5-year survival rate of patients with positive TAK1
expression was significantly lower compared with patients with
negative TAK1 expression. Therefore, postoperative detection of
TAK1 in gastric cancer tumor specimens may be used for a prognosis
of the patient.
OZ is a selective inhibitor of TAK1 (13). Several recent studies demonstrated
that OZ inhibited the proliferation and invasion of a variety of
tumor cells, and promoted apoptosis (8,24–26).
Therefore, after confirming high expression of TAK1 in gastric
cancer tissues, the present study further investigated the effects
of TAK1 on the invasion and apoptosis of gastric cancer cells, and
the potential underlying mechanisms using an in vitro
culture of MGC803 human gastric carcinoma cells. The present study
used a previously reported dose of OZ (8,24,26,27),
and the findings indicated that OZ treatment significantly
inhibited the invasion and proliferation of gastric cancer cells,
whilst promoting apoptosis. A previous report demonstrated that the
phosphorylation of threonine 187 at the loci of TAK1 protein kinase
was important for the activation of this kinase (28). Previous studies have demonstrated
that OZ inhibited the expression of p-TAK1 (Thr187) (29) and significantly reduced the
expression of p65 in the nucleus (30,31).
Previous studies have demonstrated that cleaved caspase 3 has an
important role in the apoptosis of gastric cancer cells. High
protein expression level of cleaved caspase 3 significantly
promoted the apoptosis of gastric cancer cells, whereas
downregulation of Bcl-2 promoted cyt c release and induced
the apoptosis of gastric cancer cells (32–35).
Importantly, cleaved caspase 3 and Bcl-2 were both regulated by
NF-κB signaling pathways (36–38).
A previous study also demonstrated that inhibition of TAK1 was
associated with the release of cyt c from the mitochondria,
which served as an important initial step for apoptosis (39). The findings of the present study
indicated that OZ treatment significantly increased cleaved
caspase-3 and cytosolic cyt c expression and inhibited the
expression of Bcl-2 in gastric cancer cells. These findings may
elucidated the underlying mechanism whereby OZ functions as a tumor
suppressor by inducing apoptosis. In addition, previous reports
indicated that MMP9, which is regulated by NF-κB (40), had an important role in the
invasion of gastric cancer cells (17,41).
The current study demonstrated that OZ significantly inhibited the
expression of MMP9, which may be one of the molecular mechanisms by
which OZ inhibited the invasion of gastric cancer cells. NF-κB has
also been reported to stimulate the transcription of cyclin D1
(42), which is a key regulator of
gastric cancer proliferation (15,16).
In the current study, OZ treatment significantly reduced the cyclin
D1 expression level. This may be a potential method by which OZ
inhibited gastric cancer cell proliferation.
As only one gastric cancer cell line was utilized
for mechanistic studies in the current study, the results may be
limited and a variety of gastric cancer cell lines are required for
future investigation of the relevant mechanisms. Previous studies
have revealed that TAK1 may have a biphasic role in tumorigenesis
and promote tumor growth during the early development of a tumor
and delay metastasis in advanced tumor stages (43,44).
Lam et al (43)
hypothesized that this discrepancy may be due the influence of
other surrounding cell types, such as cancer-associated
fibroblasts. Based on these observations, the role of TAK1 in
gastric cancer development may require further investigation using
in vivo experiments.
In conclusion, the present study demonstrated that
TAK1 expression was elevated in gastric carcinoma tissues and was
associated with the poor prognosis of patients with gastric cancer.
OZ, the specific inhibitor of TAK1, significantly inhibited the
proliferation and invasion of gastric cancer cells and promoted
cell apoptosis, indicating that TAK1 may be a novel target for the
treatment of gastric cancer and that OZ may have the potential to
be developed as a novel drug for the treatment of gastric
cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation (grant no. 81470866).
References
|
1
|
Digklia A and Wagner AD: Advanced gastric
cancer: Current treatment landscape and future perspectives. World
J Gastroenterol. 22:2403–2414. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lordick F and Janjigian YY: Clinical
impact of tumour biology in the management of gastroesophageal
cancer. Nat Rev Clin Oncol. 13:348–360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tomasello G, Ghidini M, Liguigli W, Ratti
M, Toppo L and Passalacqua R: Targeted therapies in gastric cancer
treatment: Where we are and where we are going. Invest New Drugs.
34:378–393. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karimi P, Islami F, Anandasabapathy S,
Freedman ND and Kamangar F: Gastric cancer: Descriptive
epidemiology, risk factors, screening and prevention. Cancer
Epidemiol Biomarkers Prev. 23:700–713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu X and Meltzer SJ: Gastric cancer in
the era of precision medicine. Cell Mol Gastroenterol Hepatol.
3:348–358. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee SY and Oh SC: Changing strategies for
target therapy in gastric cancer. World J Gastroenterol.
22:1179–1189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakurai H: Targeting of TAK1 in
inflammatory disorders and cancer. Trends Pharmacol Sci.
33:522–530. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang HL, Chiang CH, Hung WC and Hou MF:
Targeting of TGF-β-activated protein kinase 1 inhibits chemokine
(C-C motif) receptor 7 expression, tumor growth and metastasis in
breast cancer. Oncotarget. 6:995–1007. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin P, Niu W, Peng C, Zhang Z and Niu J:
The role of TAK1 expression in thyroid cancer. Int J Clin Exp
Pathol. 8:14449–14456. 2015.PubMed/NCBI
|
|
10
|
Wu M, Shi L, Cimic A, Romero L, Sui G,
Lees CJ, Cline JM, Seals DF, Sirintrapun JS, McCoy TP, et al:
Suppression of Tak1 promotes prostate tumorigenesis. Cancer Res.
72:2833–2843. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bosman MC, Schepers H, Jaques J,
Brouwers-Vos AZ, Quax WJ, Schuringa JJ and Vellenga E: The
TAK1-NF-κB axis as therapeutic target for AML. Blood.
124:3130–3140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kilty I and Jones LH: TAK1 selective
inhibition: State of the art and future opportunities. Future Med
Chem. 7:23–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu J, Powell F, Larsen NA, Lai Z, Byth KF,
Read J, Gu RF, Roth M, Toader D, Saeh JC and Chen H: Mechanism and
in vitro pharmacology of TAK1 inhibition by (5Z)-7-Oxozeaenol. ACS
Chem Biol. 8:643–650. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fakhouri L, El-Elimat T, Hurst DP, Reggio
PH, Pearce CJ, Oberlies NH and Croatt MP: Isolation, semisynthesis,
covalent docking and transforming growth factor beta-activated
kinase 1 (TAK1)-inhibitory activities of (5Z)-7-oxozeaenol
analogues. Bioorg Med Chem. 23:6993–6999. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arici DS, Tuncer E, Ozer H, Simek G and
Koyuncu A: Expression of retinoblastoma and cyclin D1 in gastric
carcinoma. Neoplasma. 56:63–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seo JH, Jeong ES and Choi YK: Therapeutic
effects of lentivirus-mediated shRNA targeting of cyclin D1 in
human gastric cancer. BMC Cancer. 14:1752014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Akter H, Park M, Kwon OS, Song EJ, Park WS
and Kang MJ: Activation of matrix metalloproteinase-9 (MMP-9) by
neurotensin promotes cell invasion and migration through ERK
pathway in gastric cancer. Tumour Biol. 36:6053–6062. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu J, Li Q, He JT and Liu GY: Expression
of TAK1/TAB1 expression in non-small cell lung carcinoma and
adjacent normal tissues and their clinical significance. Int J Clin
Exp Pathol. 8:15801–15807. 2015.PubMed/NCBI
|
|
19
|
Yang M and Huang CZ: Mitogen-activated
protein kinase signaling pathway and invasion and metastasis of
gastric cancer. World J Gastroenterol. 21:11673–11679. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li X, Tu J, Zhang D, Xu Z, Yang G, Gong L
and Yu M: The clinical significance of HER-2 and NF-KB expression
in gastric cancer. Hepatogastroenterology. 60:1519–1523.
2013.PubMed/NCBI
|
|
21
|
Li Q, Yu YY, Zhu ZG, Ji YB, Zhang Y, Liu
BY, Chen XH and Lin YZ: Effect of NF-kappaB constitutive activation
on proliferation and apoptosis of gastric cancer cell lines. Eur
Surg Res. 37:105–110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Uetsuka H, Haisa M, Kimura M, Gunduz M,
Kaneda Y, Ohkawa T, Takaoka M, Murata T, Nobuhisa T, Yamatsuji T,
et al: Inhibition of inducible NF-kappaB activity reduces
chemoresistance to 5-fluorouracil in human stomach cancer cell
line. Exp Cell Res. 289:27–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pak KH, Kim DH, Kim H, Lee DH and Cheong
JH: Differences in TGF-b1 signaling and clinicopathologic
characteristics of histologic subtypes of gastric cancer. BMC
Cancer. 16:602015. View Article : Google Scholar
|
|
24
|
Zhang J, Li B, Wu H, Ou J, Wei R, Liu J,
Cai W, Liu X, Zhao S, Yang J, et al: Synergistic action of
5Z-7-oxozeaenol and bortezomib in inducing apoptosis of Burkitt
lymphoma cell line Daudi. Tumour Biol. 37:531–539. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hrabe JE, O'Leary BR, Fath MA, Rodman SN,
Button AM, Domann FE, Spitz DR and Mezhir JJ: Disruption of
thioredoxin metabolism enhances the toxicity of transforming growth
factor β-activated kinase 1 (TAK1) inhibition in KRAS-mutated colon
cancer cells. Redox Biol. 5:319–327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cai PC, Shi L, Liu VW, Tang HW, Liu IJ,
Leung TH, Chan KK, Yam JW, Yao KM, Ngan HY and Chan DW: Elevated
TAK1 augments tumor growth and metastatic capacities of ovarian
cancer cells through activation of NF-κB signaling. Oncotarget.
5:7549–7562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fan Y, Cheng J, Vasudevan SA, Patel RH,
Liang L, Xu X, Zhao Y, Jia W, Lu F, Zhang H, et al: TAK1 inhibitor
5Z-7-oxozeaenol sensitizes neuroblastoma to chemotherapy.
Apoptosis. 18:1224–1234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Singhirunnusorn P, Suzuki S, Kawasaki N,
Saiki I and Sakurai H: Critical roles of threonine 187
phosphorylation in cellular stress-induced rapid and transient
activation of transforming growth factor-beta-activated kinase 1
(TAK1) in a signaling complex containing TAK1-binding protein TAB1
and TAB2. J Biol Chem. 280:7359–7368. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choo MK, Kawasaki N, Singhirunnusorn P,
Koizumi K, Sato S, Akira S, Saiki I and Sakurai H: Blockade of
transforming growth factor-beta-activated kinase 1 activity
enhances TRAIL-induced apoptosis through activation of a caspase
cascade. Mol Cancer Ther. 5:2970–2976. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cao H, Lu J, Du J, Xia F, Wei S, Liu X,
Liu T, Liu Y and Xiang M: TAK1 inhibition prevents the development
of autoimmune diabetes in NOD mice. Sci Rep. 5:145932015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song Z, Zhu X, Jin R, Wang C, Yan J, Zheng
Q, Nanda A, Granger DN and Li G: Roles of the kinase TAK1 in
CD40-mediated effects on vascular oxidative stress and neointima
formation after vascular injury. PloS One. 9:e1016712014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo JQ, Li SJ and Guo GX: Long noncoding
RNA AFAP1-AS1 promotes cell proliferation and apoptosis of gastric
cancer cells via PTEN/p-AKT pathway. Dig Dis Sci. Apr 27–2017.(Epub
ahead of print). View Article : Google Scholar
|
|
33
|
Tong K, Xin C and Chen W: Isoimperatorin
induces apoptosis of the SGC-7901 human gastric cancer cell line
via the mitochondria-mediated pathway. Oncol Lett. 13:518–524.
2017.PubMed/NCBI
|
|
34
|
Wang D, Li Y, Cui P, Zhao Q, Tan BB, Zhang
ZD, Liu Y and Jia N: Zerumbone induces gastric cancer cells
apoptosis: Involving cyclophilin A. Biomed Pharmacother.
83:740–745. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shen X, Si Y, Wang Z, Wang J, Guo Y and
Zhang X: Quercetin inhibits the growth of human gastric cancer stem
cells by inducing mitochondrial-dependent apoptosis through the
inhibition of PI3K/Akt signaling. Int J Mol Med. 38:619–626.
2016.PubMed/NCBI
|
|
36
|
Yang LQ, Fang DC, Wang RQ and Yang SM:
Effect of NF-kappaB, survivin, Bcl-2 and Caspase3 on apoptosis of
gastric cancer cells induced by tumor necrosis factor related
apoptosis inducing ligand. World J Gastroenterol. 10:22–25.
2004.PubMed/NCBI
|
|
37
|
Chang MS, Lee HS, Jung EJ, Kim CW, Lee BL
and Kim WH: Cell-cycle regulators, bcl-2 and NF-kappaB in
Epstein-Barr virus-positive gastric carcinomas. Int J Oncol.
27:1265–1272. 2005.PubMed/NCBI
|
|
38
|
Dolcet X, Llobet D, Pallares J and
Matias-Guiu X: NF-kB in development and progression of human
cancer. Virchows Arch. 446:475–482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Buglio D, Palakurthi S, Byth K, Vega F,
Toader D, Saeh J, Neelapu SS and Younes A: Essential role of TAK1
in regulating mantle cell lymphoma survival. Blood. 120:347–355.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Park BB, Yoon Js, Kim Es, Choi J, Won Yw,
Choi Jh and Lee YY: Inhibitory effects of eupatilin on tumor
invasion of human gastric cancer MKN-1 cells. Tumour Biol.
34:875–885. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang QW, Liu L, Chen R, Wei YQ, Li P, Shi
HS and Zhao YW: Matrix metalloproteinase-9 as a prognostic factor
in gastric cancer: A meta-analysis. Asian Pac J Cancer Prev.
13:2903–2908. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hinz M, Krappmann D, Eichten A, Heder A,
Scheidereit C and Strauss M: NF-kappaB function in growth control:
Regulation of cyclin D1 expression and G0/G1-to-S-phase transition.
Mol Cell Biol. 19:2690–2698. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lam CR, Tan C, Teo Z, Tay CY, Phua T, Wu
YL, Cai PQ, Tan LP, Chen X, Zhu P and Tan NS: Loss of TAK1
increases cell traction force in a ROS-dependent manner to drive
epithelial-mesenchymal transition of cancer cells. Cell Death Dis.
4:e8482013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Omori E, Matsumoto K, Zhu S, Smart RC and
Ninomiya-Tsuji J: Ablation of TAK1 upregulates reactive oxygen
species and selectively kills tumor cells. Cancer Res.
70:8417–8425. 2010. View Article : Google Scholar : PubMed/NCBI
|