Introduction
Oxidative stress is an imbalance between oxidative
and antioxidative processes in vivo and is involved in the
pathogenesis of numerous diseases. Vascular disease is an
inflammatory process, and oxidative stress has been implicated in
vascular pathology (1,2). A previous study demonstrated that
treatment with fucoidan, which is an aggregate name for algal
fucose-enriched sulfated polysaccharides extracted from the
extracellular matrix of seaweeds, may increase oxidative stress
production by activating endothelial nitric oxide synthase and
promoting Akt phosphorylation in myocardial disease and stroke
(3). A recent study suggested that
oxidative stress may be involved in high-fat diet-induced aortic
remodeling, which may be exacerbated by Zn2+ deficiency,
as the antioxidative molecule resveratrol was demonstrated to
prevent high-fat diet-induced vascular inflammation, oxidative
stress and pathological remodeling (4). Therefore, investigating the
implications of oxidative stress in the pathogenesis of vascular
diseases and exploring the underlying molecular mechanisms are
imperative for the development of effective preventive and
therapeutic strategies aimed at patients with vascular
diseases.
Signaling pathways related to oxidative stress are
associated with endothelial cells that line the inner walls of
blood vessels, which are continuously exposed to various stresses
owing to the mechanical force exerted by blood flow and pressure,
and are sensitive to oxidative damage (5,6). A
recent study demonstrated that the natural antioxidant allicin
protected human umbilical vein endothelial cells (HUVECs) from
oxidized low-density lipoprotein-induced injury by preventing
apoptosis through the inhibition of caspase-3 and
nicotinamide-adenine dinucleotide phosphate oxidase-mediated
proapoptotic signaling (7).
Another recent study reported that resveratrol (polyphenolic
compounds) treatment ameliorated high glucose-induced HUVEC injury
through the activation of 5′ adenosine monophosphate-activated
protein kinase α, leading to an increase in reductive reactions and
a corresponding decrease in oxidative stress (8). These studies suggested that oxidative
stress may serve crucial roles during the development of vascular
endothelial cell injury. Therefore, the present study aimed to
investigate the effects of oxidative stress in HUVECs in
vitro.
MicroRNAs (miRNAs) are small (~22 nucleotides long)
non-coding RNA molecules, which modulate the stability and the
translational efficiency of target mRNA transcripts (9). miRNAs are involved in the
post-transcriptional regulation of gene expression and are
implicated in numerous biological processes, including
proliferation, differentiation, senescence and death (10). Previous studies have suggested that
miRNAs may be involved in the regulation of oxidative stress during
vascular disease pathogenesis, serving important roles in oxidative
stress-induced endothelial dysfunction and mitochondrial metabolism
dysregulation (1,11). Several miRNAs have been implicated
in endothelial development, including miRNA (miR)-20a, miR-23b-3p,
miR-150, miR-195 and miR-200b (11,12).
Previous studies have also revealed crucial roles for miRNAs in
endothelial physiology and pathology, thus suggesting that miRNAs
may have potential as novel therapeutic targets for the treatment
of patients with vascular diseases (13,14).
miR-4463 has garnered attention as a prognostic and
diagnostic biomarker in patients with polycystic ovary syndrome
(15). In addition, our previous
studies have demonstrated the aberrant expression of miR-4463 in
vascular diseases, including arteriosclerosis obliterans (16) and carotid artery stenosis (17), which suggested that miR-4463 may
also have potential as a biomarker for the early diagnosis of
vascular diseases. However, the roles of miR-4463 in the regulation
of oxidative stress in endothelial cells, and the molecular
mechanisms underlying its actions, have yet to be elucidated.
Therefore, the present study aimed to investigate the putative
relationship between miR-4463 and oxidative stress, and explore the
molecular mechanisms that may be involved in HUVECs.
Materials and methods
Cell culture and oxidative stress
model
The HUVEC cell line was purchased from ScienCell
Research Laboratories, Inc. (Carlsbad, CA, USA) and cultured in
low-glucose Dulbecco's modified Eagle's medium (DMEM; Hyclone, GE
Healthcare Life Sciences, Logan, UT, USA), supplemented with 10%
heat-inactivated fetal bovine serum (FBS; Gibco, Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and maintained in a humidified
5% CO2 atmosphere. Cells between passages 4 and 6, in
the logarithmic phase of growth, were used in the subsequent
experiments. The morphology of the cells was observed using a
phase-contrast microscope (cellSens standard software version 1.6,
DP72; Olympus Corporation, Tokyo, Japan).
Hydrogen peroxide (H2O2) is
commonly used in models of oxidative stress-induced apoptosis
(18). In the present study,
HUVECs were treated with various concentrations of
H2O2 (200, 500, 700 and 1,000 µmol/l;
Table I) using 0.3%
H2O2 stock solution, or 0 µl
H2O2 (low-gluocse DMEM alone containing 10%
FBS) for 16 h to induce oxidative stress, as previously described
(19–21).
 | Table I.Various concentrations of
H2O2. |
Table I.
Various concentrations of
H2O2.
|
H2O2
concentration | 0.3%
H2O2 | Low-glucose DMEM
with 10% FBS |
|---|
| 0 µmol/l | 0.0 µl | 500.0 µl |
| 200 µmol/l | 11.4 µl | 488.6 µl |
| 500 µmol/l | 28.5 µl | 471.5 µl |
| 700 µmol/l | 39.9 µl | 460.1 µl |
| 1,000 µmol/l | 57.0 µl | 443.0 µl |
Transfection
The intracellular level of miR-4463 was upregulated
or downregulated by the transfection of miRNA mimics and inhibitor
respectively, according to the manufacturer's protocol (Guangzhou
RiboBio Co., Ltd., Guangzhou, China). The miRNA mimics synthesized
by the chemical method induces a high level of expression of mature
miRNA in the cell, and it enhances the regulation of endogenous
miRNA and gains certain functions in cells. miRNA inhibitors
inhibit the effects of mature miRNA, and weaken the role of
endogenous gene regulation and cell function. When the cell density
reached 50–70%, the transfection efficiency was optimal (Table II). Transfection was performed
using 100 µmol/l miR-4463 mimic or inhibitor for 15 min at room
temperature, and the efficiency of transfection was determined by
RT-qPCR. Finally, 100 nmol/l NC, miR-4463 mimics and miR-4463
inhibitor was chosen for the next step after 3 days transfection.
NC, miR-4463 mimics and miR-4463 inhibitor sequences are listed on
the Guangzhou RiboBio Co., Ltd. Website: http://ribobio.bioon.com.cn.
 | Table II.Transfection reagent components. |
Table II.
Transfection reagent components.
| Final
concentration | Final volume | Culture medium | 1x riboFECT™ CP
buffer | NC, miR-4463 mimics
or miR-4463 inhibitor | riboFECT™ CP
reagent |
|---|
| 100 nmol/l NC | 500 µl | 464.5 µl | 30 µl | 2.5 µl NC | 3 µl |
| 100 nmol/l miR-4463
mimic | 500 µl | 464.5 µl | 30 µl | 2.5 µl miR-4463
mimic | 3 µl |
| 100 nmol/l miR-4463
inhibitor | 500 µl | 464.5 µl | 30 µl | 2.5 µl miR-4463
inhibitor | 3 µl |
RNA exaction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Following treatment with H2O2,
total RNA was extracted from HUVECs (2–3×105 cells)
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
Total RNA (500 ng) was reverse transcribed into cDNA using an
miScript II RT kit (catalog no. 218160 and 21816; Qiagen GmbH,
Hilden, Germany), according to manufacturer's protocol. The
specific reaction components of reverse-transcription are mentioned
in Table III. The mix was
incubated for 60 min at 37°C, incubated for 5 min at 95°C and
stored at −20°C. Acquired cDNA was used for RT-qPCR according to
the manufacturer's protocol using the miScript SYBR-Green PCR kit
(catalog no. 208054; Qiagen GmbH). Mature has-miR-4463 primer
(catalog no. MIMAT0018987) and U6 primer (catalog no. MS00044996)
were purchased from Qiagen GmbH. The specific reaction components
of RT-qPCR are mentioned in Table
IV, and the cycling conditions are mentioned in Table V. qPCR was performed using the
StepOnePlus version 2.2.3 real-time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). Relative expression levels were
quantified using the 2−ΔΔCq method (22) and normalized to the expression of
U6; Ct values >35 were excluded. Experiments were performed in
triplicate.
 | Table III.Reverse-transcription reaction
components. |
Table III.
Reverse-transcription reaction
components.
| Component |
Volume/reaction |
|---|
| 5X miScript HiSpec
buffer | 4 µl |
| 10x nucleics
mix | 2 µl |
| RNase-free
water | Variable |
| miScript reverse
transcriptase mix | 2 µl |
| Template RNA | Variable (500
ng) |
| Total volume | 20 µl |
 | Table IV.Reaction mix for miScript SYBR Green
polymerase chain reaction. |
Table IV.
Reaction mix for miScript SYBR Green
polymerase chain reaction.
| Component | Volume/reaction
(96-well) |
|---|
| 2x QuantiTect
SYBR-Green | 12.5 µl |
| PCR Master Mix |
|
| 10x miScript
universal primer | 2.5 µl |
| 10x miScript primer
assay | 2.5 µl |
| RNase-free
water | 6.5 µl |
| Diluted cDNA | 1.0 µl |
| Total volume | 25.0 µl |
 | Table V.Cycling conditions of miScript SYBR
Green polymerase chain reaction. |
Table V.
Cycling conditions of miScript SYBR
Green polymerase chain reaction.
| Cycling
conditions |
|---|
|
|---|
| Step | Time | Temperature | Additional
comments |
|---|
| Initial activation
step 3-step cycling: | 15 min | 95°C |
HotStarTaq® DNA polymerase is
activated by this heating step. |
| Denaturation | 15 sec | 94°C |
|
| Annealing | 30 sec | 55°C |
|
| Extension | 30 sec | 70°C | Perform
fluorescence data collection. |
| Cycle number | 40 cycles |
| Cycle number
depends on the amount of template cDNA and abundance of the
target. |
Apoptosis assay
Apoptosis and necrosis were assessed using the
fluorescein isothiocyanate (FITC) Annexin V and propidium iodide
(PI) detection kit (BD Pharmingen; BD Biosciences, Franklin Lakes,
NJ, USA), according to the manufacturer's protocol. Following
treatment with H2O2, HUVECs (5×105
cells) were washed with 10X Annexin V binding buffer, and stained
for 10 min with 5 µl FITC Annexin V and 5 µl PI at room temperature
in the dark. Stained cells were analyzed by flow cytometry using a
BD FACSVerse and BD FACSuite™ software version 1.0.3 (BD
Biosciences), and the experiment was repeated three times.
Oxidative stress assays
Reactive oxygen species (ROS) production and lipid
peroxidation were measured in order to assess oxidative stress in
HUVECs, using ROS and malondialdehyde (MDA) detection kits
(Beyotime Institute of Biotechnology, Haimen, China). Experiments
were repeated >3 times. To detect ROS production,
H2O2-treated HUVECs (1×105 cells)
were incubated with the fluorescent probe
2′,7′-dichlorodihydrofluorescein diacetate (chloromethyl
derivative) at 37°C for 30 min, as previously described (23–25).
Subsequently, samples were digested by trypsin-EDTA solution
(Beyotime Institute of Biotechnology), centrifuged (1,000 × g for 5
min at room temperature) and transferred to black 96-well plates.
The fluorescence was measured using an excitation wavelength of 488
nm and an emission wavelength of 525 nm using a microplate reader.
MDA is a natural product of lipid oxidation in organisms. The level
of lipid oxidation may be measured by detecting the level of MDA,
and therefore the determination of MDA is widely used as an
indicator of lipid oxidation. To assess MDA contents in HUVECs
(1×105 cells), cells were lysed and then centrifuged
(1,600 × g for 10 min at 4°C). A total of 100 µl supernatant and
200 µl MDA detection working fluid (150 µl TBA dilute solution, 50
µl TBA storage solution and 3 µl antioxidant) were mixed, heated at
100°C for 15 min and cooled to room temperature. The reaction
mixture was centrifuged (1,000 × g for 10 min at room temperature),
and subsequently transferred to 96-well plates and the absorbance
was measured at 532 nm using a microplate reader, as previously
described (26,27).
Cell proliferation assay
Cellular proliferation was evaluated using a Cell
Counting Kit-8 (CCK-8; Beyotime Institute of Biotechnology), as
previously described (28,29). Following treatment with
H2O2 for 16 h, HUVECs (1×104
cells/well) were seeded in 96-well plates (5 wells/experimental
group) in 100 µl serum-free DMEM (Hyclone; GE Healthcare Life
Sciences) and 10 µl CCK-8 solution were added to each well and
cells were incubated at 37°C for 35 min. The absorbance of each
sample was measured at 450 nm using a microplate reader as
previously described (30). The
percentage of living cells was calculated as a ratio of the optical
density of treated cells over untreated control cells.
Western blot analysis
Following treatment with H2O2,
total protein was extracted from HUVECs (2×106 cells)
using Radioimmunoprecipitation Assay Lysis buffer (Beyotime
Institute of Biotechnology) as previously described (2,31).
Equal amounts (20 µg) of protein in each sample (extracted using a
bicinchoninic acid assay) were separated by 12% SDS-PAGE and
transferred onto polyvinylidene difluoride membranes. Then,
membranes were blocked for 2 h in 0.5% nonfat milk at room
temperature, and washed three times for 5 mins with PBS containing
0.1% Tween-20. Next, membranes were incubated with the following
primary antibodies at 4°C overnight: Anti-poly (adenosine
diphosphate-ribose) polymerase 1 (PARP1; 1:1,000; catalog no.
9532), anti-X-linked inhibitor of apoptosis protein (XIAP; 1:1,000;
catalog no. 14334), and anti-B cell lymphoma-2 (Bcl-2)-associated X
protein (Bax; 1:1,000; catalog no. 14796) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA); anti-active
(cleaved)-caspase-3 (C-caspase-3; 1:500; catalog no. ab32042) was
purchased from Abcam (Cambridge, UK); and anti-Bcl-2 (1:1,000;
catalog no. AB112-1) and anti-GAPDH (1:2,000; catalog no. AF0006)
were purchased from Beyotime Institute of Biotechnology. GAPDH
served as the loading control (18,19).
Subsequently, membranes were incubated with the following
horseradish peroxidase-conjugated secondary antibodies for 1 h at
37°C: Anti-rabbit immunoglobulin (Ig)G (1:2,000; catalog no. A0208)
and anti-mouse IgG (1:3,000; catalog no. A0216) purchased from
Beyotime Institute of Biotechnology. Protein bands were visualized
by enhanced chemiluminescence detection reagent (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), and blots were
semi-quantified by densitometric analysis using Quantity One
version 4.6.2 software (Bio-Rad Laboratories, Inc.). Experiments
were performed >3 times.
Statistical analysis
Statistical analysis was performed using SPSS
software version 19.0 (IBM Corp., Armonk, NY, USA). Data are
expressed as the mean ± standard deviation. The statistical
significance of the differences between groups was assessed using
one-way analysis of variance followed by a post hoc Dunnett's
two-tailed test for multiple comparisons. P<0.05 was considered
to indicate a statistically significant difference.
Results
Alterations in HUVEC morphology and
miR-4463 expression following treatment with
H2O2
When examined under an inverted phase-contrast
microscope, HUVECs in the untreated control group exhibited
physiological morphology, characterized by spindle- or round-shaped
cell architecture, cells that were adherent and formed paving
stone-like cultures (32,33). Conversely, the number of HUVECs in
culture appeared to be markedly reduced following
H2O2 treatment, with undefined boundaries and
observable necrotic and disintegrative phenomena among the cells,
which concurred with previously published data (34–36)
(Fig. 1).
The expression levels of miR-4463 were assessed in
HUVECs treated with various concentrations of
H2O2. The results demonstrated that miR-4463
expression levels were significantly upregulated in
H2O2-treated HUVECs in a dose-dependent
manner compared with in untreated control cells (Fig. 2). miR-4463 expression appeared to
be the highest following treatment with H2O2
at a concentration of 700 µmol/l. The high concentration of
H2O2 (1,000 µmol/l) may promote cell death
and inhibit the expression of the cells under oxidative stress.
H2O2 induces
apoptosis in HUVECs
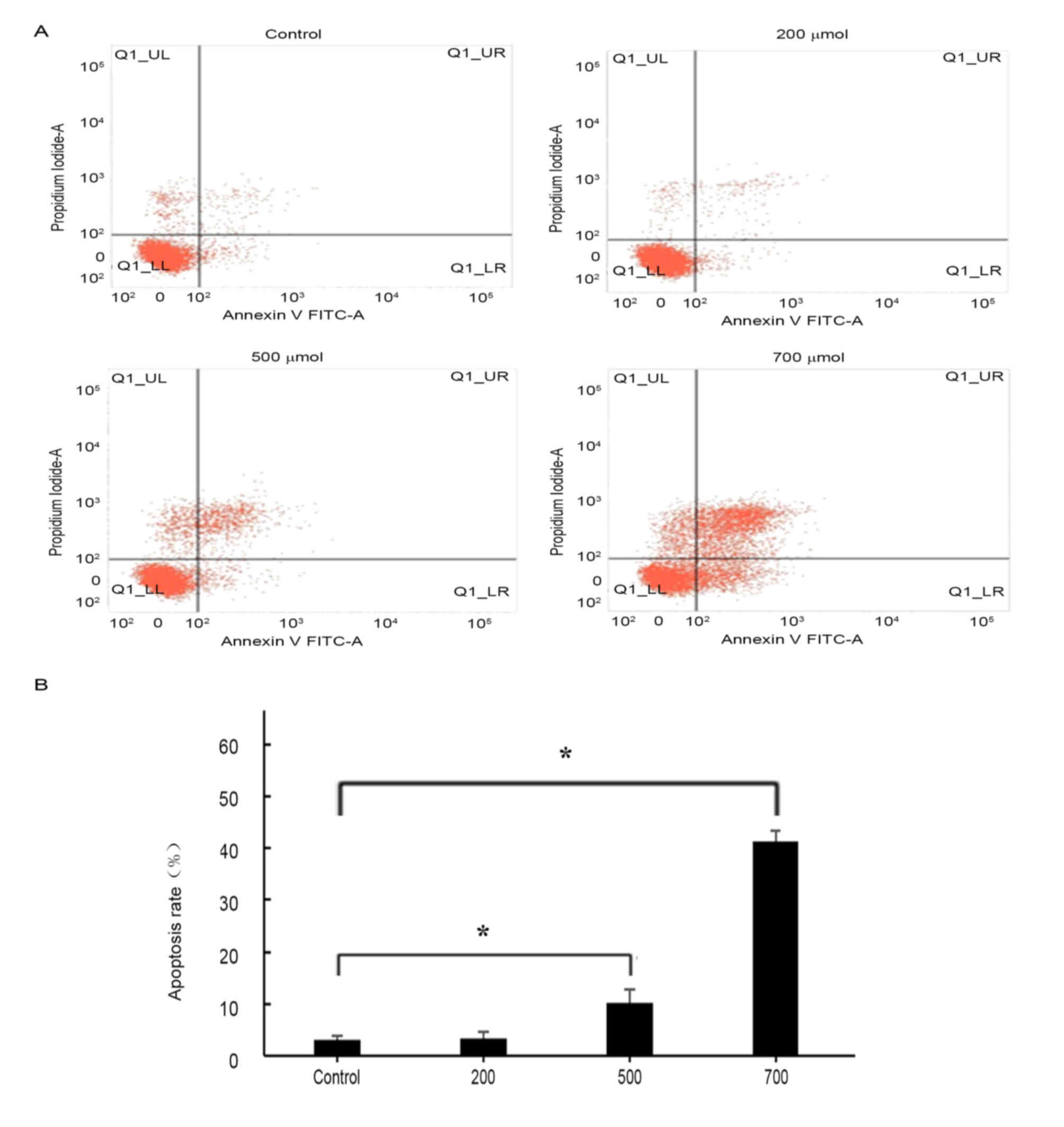
Flow cytometric analysis revealed that treatment
with H2O2 was able to induced apoptosis in
HUVECs (Fig. 3). Annexin
V-positive cells and cellular uptake of PI were evaluated in order
to distinguish between living, apoptotic and necrotic cells, as
previously described (37). The
proapoptotic effects of H2O2 treatment
appeared to be concentration-dependent (Fig. 3B): No significant difference in the
apoptotic rate was detected following treatment with 200 µmol/l
H2O2; however, HUVEC apoptosis was
significantly increased following treatment with 500 and 700 µmol/l
H2O2 compared with control untreated cells
(P<0.05). As H2O2 at a concentration of
700 µmol/l appeared to produce the greatest increase in apoptotic
rate (43%) and necrotic rate (8.25%), this concentration was
selected for subsequent experiments.
H2O2 induces
oxidative stress in HUVECs
To confirm the induction of oxidative stress
following H2O2 treatment in HUVECs, oxidative
stress products were detected and cellular viability was evaluated,
using commercially available kits. MDA is an end product of lipid
oxidation that is involved in the oxidative damage of the cellular
membrane; therefore, MDA contents may be measured as an indication
of the degree of lipid peroxidation and the extent of membrane
damage (38,39). In HUVECs treated with 700 µmol/l
H2O2, MDA levels were increased by 1.5-fold
compared with the untreated control cells (P<0.05; Fig. 4A); whereas no significant
differences were indicated in HUVECs treated with 200 or 500 µmol/l
H2O2. In addition, intracellular ROS
production was significantly enhanced in
H2O2-treated cells, as indicated by the
3.75-fold increase in ROS levels following treatment with 700
µmol/l H2O2 compared with the control.
Treatment with 500 µmol/l H2O2 increased ROS
levels by ~2-fold compared with the control, and there was no
significant increase after treatment with 200 µmol/l
H2O2 (Fig.
4B). Notably, no significant difference in cellular
proliferation was detected between 200 µmol/l
H2O2-treated HUVECs and control cells
(Fig. 4C). However, cell
proliferation and viability was significantly reduced following
treatment with 500 or 700 µmol/l H2O2
(Fig. 4C). These findings
suggested that treatment with H2O2 may induce
oxidative stress in HUVECs and suppress their viability in a
concentration-dependent manner.
miR-4463 promotes
H2O2-induced increases in oxidative
stress
The apoptotic rate of HUVECs pretreated with
miR-4463 mimics prior to H2O2 exposure was
significantly increased compared with negative control-treated
H2O2-exposed cells (Fig. 5), thus suggesting that miR-4463 may
serve a role in promoting apoptosis following
H2O2 treatment. Detecting NC-treated
H2O2 as the control group eliminates any
background effects of the transfection reagent. The roles of
miR-4463 in H2O2-induced oxidative stress
were also investigated, and the results revealed that intracellular
ROS and MDA levels were significantly increased in
H2O2-treated HUVECs following transfection
with miR-4463 mimics; however, the miR-4463 inhibitor did not
induce a significant difference. This suggested that miR-4463
overexpression enhanced H2O2-induced
oxidative stress (Fig. 6A and B).
However, the miR-4463 mimics did not have an exact effect on cell
proliferation (Fig. 6C). These
findings suggested that miR-4463 may enhance oxidative stress on
HUVECs but have no apparent effect on the proliferation of
HUVECs.
miR-4463 promotes proapoptotic signaling in
H2O2-treated HUVECs. The protein expression
levels of pro- and antiapoptotic markers, including C-caspase-3,
Bax, Bcl-2, XIAP and PARP1, were detected in
H2O2-treated HUVECs by western blot analysis
(Fig. 7). The results demonstrated
that following H2O2 treatment, the expression
levels of the antiapoptotic proteins Bcl-2 and XIAP were
downregulated, whereas the expression of the proapoptotic
C-caspase3, PARP1 and Bax was enhanced. Notably, HUVECs pretreated
with miR-4463 mimics prior to H2O2 exposure
exhibited significantly increased C-caspase3, cleaved PARP and Bax
protein expression levels, whereas the expression of Bcl-2 and XIAP
was significantly suppressed. The present findings suggested that
miR-4463 overexpression may increase the proapoptotic effects of
H2O2 on HUVECs.
 | Figure 7.miR-4463 promotes proapoptotic
signaling in H2O2-treated HUVECs. (A) HUVECs
were pretreated with miR-4463 mimics, a miR-4463 inhibitor or a NC
miRNA, and then exposed to 700 µmol/l H2O2
for 16 h; Control cells did not receive treatment. (B) Western blot
analysis was used to assess the protein expression levels of the
proapoptotic factors PARP1, Bax and C-caspase3, and the
antiapoptotic factors XIAP and Bcl-2 (n=3). *P<0.05 vs. 700+NC.
Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma-2; C,
cleaved; H2O2, hydrogen peroxide; HUVEC,
human umbilical vein endothelial cell; miR, microRNA; NC, negative
control; PARP1, poly (adenosine diphosphate-ribose) polymerase 1;
XIAP, X-linked inhibitor of apoptosis protein. |
Discussion
miRNAs are able to combine with complementary
sequences in the 3′-untranslated region of target mRNA transcripts,
and thus regulate the transcription of target genes and ultimately
inhibit the translation of specific proteins (9). miRNAs have been demonstrated
previously to be involved in the regulation of HUVEC proliferation,
migration and apoptosis (12). In
addition, miRNAs have been associated with processes involved in
the development of oxidative stress (24). However, the roles of miRNAs in the
pathophysiology of vascular diseases, as well as their clinical
value, have yet to be elucidated. Our previous study reported that
miR-4463 has a greater change in the plasma levels of vascular
patients (16). Therefore, the
present study hypothesized that miR-4463 may have an effect on the
pathophysiological processes associated with vascular diseases.
H2O2 is one of the main ROS,
which has been reported to decrease cellular proliferation and
induce apoptosis, in a process involving several miRNAs (28). Exposure of endothelial cells to
H2O2 has been reported to cause cell
dysfunction and promote apoptosis, thus inducing endothelial
inflammation. For example, Yu et al (40) previously demonstrated that miR-200c
expression was upregulated following 100 µmol/l
H2O2 treatment in BV-2 mouse microglial cells
in vitro, whereas Zhang et al (38) reported that miR-92a overexpression
in HUVECs enhanced capillary tube formation under conditions of
oxidative stress. The present study investigated the expression of
miR-4463 in H2O2-treated HUVECs, and RT-qPCR
analysis revealed that miR-4463 expression was upregulated
following treatment with H2O2 in a
concentration-dependent manner. Notably, in HUVECs treated with
1,000 µmol/l H2O2, miR-4463 expression was
comparable to baseline levels. Yu et al (40) also reported similar changes in
miR-200c expression levels following spinal cord injury in murine
BV-2 cells; however, the reason remains unclear and further studies
are required to investigate whether high H2O2
concentration (1,000 µmol/l) may increase cell necrosis. Regardless
of this discrepancy, the present results suggested that miR-4463
may serve a role in oxidative processes in HUVECs, and its
expression may be regulated by H2O2 in a
concentration-dependent manner.
Oxidative stress has been implicated in cellular
apoptosis. In the present study, HUVEC apoptosis was investigated
using flow cytometry following cell exposure to
H2O2. The present results demonstrated that
H2O2 increased the apoptotic rate in HUVECs
in a concentration-dependent manner. Once generated, ROS readily
react with unsaturated fatty acids and cholesterol molecules
present in cell membranes, thus leading to cellular apoptosis
through mitochondrial pathways involving nuclear factor-κB, p53 and
stress-activated protein kinase (41). In the present study, oxidative
stress was investigated in HUVECs by assessing ROS production and
MDA concentration; the results of which revealed that ROS and MDA
levels were significantly increased following
H2O2 treatment, whereas cell viability was
significantly reduced, thus indicating that
H2O2 was able to induced apoptosis in HUVECs,
which is in accordance with previous studies (27,37).
To investigate the roles of miR-4463 in oxidative
pathways and cellular apoptosis, HUVECs were transfected with
miR-4463 mimics or inhibitors prior to H2O2
exposure. The present results demonstrated that the apoptotic rate
and the levels of oxidation products were significantly increased
in HUVECs overexpressing miR-4463. However, miR-4463 inhibition did
not produce a significant effect. These results suggested that
miR-4463 upregulation may enhance
H2O2-induced HUVEC apoptosis; however,
further studies are required to confirm the exact roles of miR-4463
in HUVECs under oxidative stress and to determine the possible
underlying molecular mechanisms that are involved.
Oxidative stress is a harmful mechanism for
self-protection, therefore, excessive activation may enhance this
effect. The results of the present study revealed that
H2O2 treatment altered miR-4463 expression in
HUVECs, and miR-4463 overexpression enhanced
H2O2-induced oxidative stress. Therefore, the
molecular mechanisms underlying the involvement of miR-4463 in
apoptotic pathways were investigated in HUVECs, by assessing the
expression levels of apoptosis-associated marker proteins.
Following miR-4463 overexpression in
H2O2-treated HUVECs, the proapoptotic
proteins C-caspase3, PARP1 and Bax were significantly upregulated,
whereas the protein expression of the anti-apoptotic effectors
Bcl-2 and XIAP was significantly reduced. These findings suggested
that miR-4463 may be implicated in the regulation of
apoptosis-related protein expression in HUVECs treated with
H2O2. However, further studies are required
to fully elucidate the roles of miR-4463 and its target genes in
apoptotic pathways.
In conclusion, the present results suggested that
miR-4463 may be implicated in the pathogenesis of cardiovascular
disease through the regulation of oxidative processes. Future
studies will address the roles of specific target genes during the
regulation of HUVEC oxidative stress and apoptosis, and the effects
of miRNAs in the pathophysiological mechanisms involved in
oxidative processes will be explored. The results of the present
study suggested that miR-4463 may have potential for the
development of novel strategies for the diagnosis, prognosis and
treatment of cardiovascular diseases.
References
|
1
|
Ono K: MicroRNA-133a in the development of
arteriosclerosis obliterans. J Atheroscler Thromb. 22:342–343.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rajput C, Tauseef M, Farazuddin M, Yazbeck
P, Amin MR, Br V Avin, Sharma T and Mehta D: MicroRNA-150
suppression of angiopoetin-2 generation and signaling is crucial
for resolving vascular injury. Arterioscler Thromb Vasc Biol.
36:380–388. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li X, Li J, Li Z, Sang Y, Niu Y, Zhang Q,
Ding H and Yin S: Fucoidan from Undaria pinnatifida prevents
vascular dysfunction through PI3K/Akt/eNOS-dependent mechanisms in
the l-NAME-induced hypertensive rat model. Food Funct. 7:2398–2408.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo R, Li W, Liu B, Li S, Zhang B and Xu
Y: Resveratrol protects vascular smooth muscle cells against high
glucose-induced oxidative stress and cell proliferation in vitro.
Med Sci Monit Basic Res. 20:82–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Csordas A, Kreutmayer S, Ploner C, Braun
PR, Karlas A, Backovic A, Wick G and Bernhard D: Cigarette smoke
extract induces prolonged endoplasmic reticulum stress and
autophagic cell death in human umbilical vein endothelial cells.
Cardiovasc Res. 92:141–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jeon BK, Kwon K, Kang JL and Choi YH:
Csk-induced phosphorylation of Src at tyrosine 530 is essential for
H2O2-mediated suppression of ERK1/2 in human
umbilical vein endothelial cells. Sci Rep. 5:127252015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen X, Pang S, Lin J, Xia J and Wang Y:
Allicin prevents oxidized low-density lipoprotein-induced
endothelial cell injury by inhibiting apoptosis and oxidative
stress pathway. BMC Complement Altern Med. 16:1332016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou DY, Su Y, Gao P, Yang QH, Wang Z and
Xu Q: Resveratrol ameliorates high glucose-induced oxidative stress
injury in human umbilical vein endothelial cells by activating
AMPK. Life Sci. 136:94–99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng D, Yu Y, Li M, Wang G, Chen R, Fan
GC, Martin C, Xiong S and Peng T: Inhibition of microRNA 195
prevents apoptosis and multiple-organ injury in mouse models of
sepsis. J Infect Dis. 213:1661–1670. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hecker M, Thamilarasan M, Koczan D,
Schröder I, Flechtner K, Freiesleben S, Füllen G, Thiesen HJ and
Zettl UK: MicroRNA expression changes during interferon-beta
treatment in the peripheral blood of multiple sclerosis patients.
Int J Mol Sci. 14:16087–16110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao S, Li T, Li J, Lu Q, Han C, Wang N,
Qiu Q, Cao H, Xu X, Chen H and Zheng Z: miR-23b-3p induces the
cellular metabolic memory of high glucose in diabetic retinopathy
through a SIRT1-dependent signalling pathway. Diabetologia.
59:644–654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li X, Yao N, Zhang J and Liu Z:
MicroRNA-125b is involved in atherosclerosis obliterans in vitro by
targeting podocalyxin. Mol Med Rep. 12:561–568. 2015.PubMed/NCBI
|
|
13
|
Correia AC, Moonen JR, Brinker MG and
Krenning G: FGF2 inhibits endothelial-mesenchymal transition
through microRNA-20a-mediated repression of canonical TGF-b
signaling. J Cell Sci. 129:569–579. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Savoia C, Sada L, Zezza L, Pucci L, Lauri
FM, Befani A, Alonzo A and Volpe M: Vascular inflammation and
endothelial dysfunction in experimental hypertension. Int J
Hypertens. 2011:2812402011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ding CF, Chen WQ, Zhu YT, Bo YL, Hu HM and
Zheng RH: Circulating microRNAs in patients with polycystic ovary
syndrome. Hum Fertil (Camb). 18:22–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He XM, Zheng YQ, Liu SZ, Liu Y, He YZ and
Zhou XY: Altered plasma microRNAs as novel biomarkers for
arteriosclerosis obliterans. J Atheroscler Thromb. 23:196–206.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu G, Wong MS, Xiong MZQ, Leung CK, Su XW,
Zhou JY, Poon WS, Zheng VZY, Chan WY and Wong GKC: Circulating
MicroRNAs in delayed cerebral infarction after aneurysmal
subarachnoid hemorrhage. J Am Heart Assoc. 6:pii: e005363. 2017.
View Article : Google Scholar
|
|
18
|
Liu X and Sun J: Endothelial cells
dysfunction induced by silica nanoparticles through oxidative
stress via JNK/P53 and NF-kappaB pathways. Biomaterials.
31:8198–8209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lesniewska MA, Dereziński P, Klupczyńska
A, Kokot ZJ, Ostrowski T, Zeidler J and Muszalska I: HPLC and
HPLC/MS/MS studies on stress, accelerated and intermediate
degradation tests of antivirally active tricyclic analog of
acyclovir. J AOAC Int. 98:1240–1247. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lennicke C, Rahn J, Lichtenfels R,
Wessjohann LA and Seliger B: Hydrogen peroxide - production, fate
and role in redox signaling of tumor cells. Cell Commun Signal.
13:392015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang H, Sheng C, Yin Y, Wen S, Yang G,
Cheng Z and Zhu Q: PABPC1 interacts with AGO2 and is responsible
for the microRNA mediated gene silencing in high grade
hepatocellular carcinoma. Cancer Lett. 367:49–57. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nagababu P, Barui AK, Thulasiram B, Devi
CS, Satyanarayana S, Patra CR and Sreedhar B: Antiangiogenic
activity of mononuclear copper(II) polypyridyl complexes for the
treatment of cancers. J Med Chem. 58:5226–5241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma Y and Li W, Yin Y and Li W: AST IV
inhibits H2O2-induced human umbilical vein
endothelial cell apoptosis by suppressing Nox4 expression through
the TGF-β1/Smad2 pathway. Int J Mol Med. 35:1667–1674.
2015.PubMed/NCBI
|
|
25
|
Yan S, Zhang X, Zheng H, Hu D, Zhang Y,
Guan Q, Liu L, Ding Q and Li Y: Clematichinenoside inhibits VCAM-1
and ICAM-1 expression in TNF-α-treated endothelial cells via NADPH
oxidase-dependent IκB kinase/NF-κB pathway. Free Radic Biol Med.
78:190–201. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu S, Luo R, Xiang Q, Xu X, Qiu L and
Pang J: Design and synthesis of novel xyloketal derivatives and
their protective activities against
H2O2-induced HUVEC injury. Mar Drugs.
13:948–973. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hou X, Tong Q, Wang W, Xiong W, Shi C and
Fang J: Dihydromyricetin protects endothelial cells from hydrogen
peroxide-induced oxidative stress damage by regulating
mitochondrial pathways. Life Sci. 130:38–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu W, Zhang D, Pan D, Zuo G, Ren X and
Chen S: Downregulation of vascular endothelial growth factor
receptor-2 under oxidative stress conditions is mediated by
b-transduction repeat-containing protein via glycogen synthase
kinase-3b signaling. Int J Mol Med. 37:911–920. 2016.PubMed/NCBI
|
|
29
|
Chen AY, Lü JM, Yao Q and Chen C:
Entacapone is an antioxidant more potent than vitamin C and vitamin
E for scavenging of hypochlorous acid and peroxynitrite and the
inhibition of oxidative stress-induced cell death. Med Sci Monit.
22:687–696. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang HB, Wen JK, Zhang J, Miao SB, Ma GY,
Wang YY, Zheng B and Han M: Flavonoids from Inula britannica
reduces oxidative stress through inhibiting expression and
phosphorylation of p47(phox) in VSMCs. Pharm Biol. 49:815–820.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fetahu IS, Tennakoon S, Lines KE, Gröschel
C, Aggarwal A, Mesteri I, Baumgartner-Parzer S, Mader RM, Thakker
RV and Kállay E: miR-135b- and miR-146b-dependent silencing of
calcium-sensing receptor expression in colorectal tumors. Int J
Cancer. 138:137–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu W, Wang M, Yin H, Yao C, He Q, Yin L,
Zhang C, Li W, Chang G and Wang S: MicroRNA-1298 is regulated by
DNA methylation and affects vascular smooth muscle cell function by
targeting connexin 43. Cardiovasc Res. 107:534–545. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu J, Li W and Wang S, Wu Y, Li Z, Wang
W, Liu R, Ou J, Zhang C and Wang S: miR-142-3p attenuates the
migration of CD4+ T cells through regulating actin
cytoskeleton via RAC1 and ROCK2 in arteriosclerosis obliterans.
PLoS One. 9:e955142014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mostafavi N, Haghjooy-Javanmard S,
Presidend N, Manssori NS and Kelishadi R: Persistence of
endothelial cell damage late after Kawasaki disease in patients
without coronary artery complications. Adv Biomed Res. 4:252015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Du P, Suhaeri M, Subbiah R, Van SY, Park
J, Kim SH, Park K and Lee K: Elasticity modulation of
fibroblast-derived matrix for endothelial cell vascular
morphogenesis and mesenchymal stem cell differentiation. Tissue Eng
Part A. 22:415–426. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song Z, Liu Y, Hao B, Yu S, Zhang H, Liu
D, Zhou B, Wu L, Wang M, Xiong Z, et al: Ginsenoside Rb1 prevents
H2O2-induced HUVEC senescence by stimulating
sirtuin-1 pathway. PLoS One. 9:e1126992014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yin YL, Li P, Yang J, Liu DW, Sun RL, Pan
GP, Wan GM and Wan GR: Protective effect of ultrafiltered XinMaiJia
extract against H2O2-induced injury in human
umbilical vein endothelial cells through NHE1 downregulation. Genet
Mol Res. 13:8436–8449. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang L, Zhou M, Qin G, Weintraub NL and
Tang Y: miR-92a regulates viability and angiogenesis of endothelial
cells under oxidative stress. Biochem Biophys Res Commun.
446:952–958. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kojima H, Otani A, Oishi A, Makiyama Y,
Nakagawa S and Yoshimura N: Granulocyte colony-stimulating factor
attenuates oxidative stress-induced apoptosis in vascular
endothelial cells and exhibits functional and morphologic
protective effect in oxygen-induced retinopathy. Blood.
117:1091–1100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu DS, Lv G, Mei XF, Cao Y, Wang YF, Wang
YS and Bi YL: miR-200c regulates ROS-induced apoptosis in murine
BV-2 cells by targeting FAP-1. Spinal cord. Dec 2–2014.(Epub ahead
of print).
|
|
41
|
Kong BS, Cho YH and Lee EJ: G
protein-coupled estrogen receptor-1 is involved in the protective
effect of protocatechuic aldehyde against endothelial dysfunction.
PLoS One. 9:e1132422014. View Article : Google Scholar : PubMed/NCBI
|