Introduction
Preeclampsia is a serious pregnancy-related syndrome
that remains a leading cause of maternal and perinatal morbidity
and mortality. It is characterized by hypertension and proteinuria,
with symptoms usually arising after 20 weeks of gestation (1). Despite research into preeclampsia,
the molecular mechanisms underlying the onset and progression of
the syndrome remain to be completely elucidated (2–4).
Trophoblast cells are able to proliferate, migrate and invade the
pregnant uterus during normal placental development. However,
trophoblasts fail to invade the uterus in preeclamptic placentas
(5). Therefore, an improved
understanding of the molecular mechanisms underlying trophoblast
invasion is required.
Protein Jumonji (JARID2) is a member of the Jumonji
family of proteins (6). Previous
studies have demonstrated that JARID2 acts as an accessory
component of Polycomb repressive complex 2 (PRC2), which regulates
important gene expression patterns during fetal development
(7–9). JARID2 is associated with the
maintenance of pluripotency and differentiation of embryonic stem
cells (10). It has previously
been observed that JARID2 is involved in the development and
progression of a number of types of tumor such as lung and colon
cancer (11,12). Recently, Lei et al (13) reported that downregulation of
JARID2 inhibits hepatocellular carcinoma cell migration, invasion
and proliferation in vitro, and metastasis in vivo.
However, little is known about the role of JARID2 in the metastasis
of placenta trophoblast cells. In the present study, the effect and
underlying molecular mechanism of JARID2 in trophoblast cell
viability and invasion was examined. The expression of JARID2 in
placental tissues was analyzed by performing reverse
transcription-quantitative polymerase chain reaction and western
blotting. HTR8/SVneo cells were transfected with si-JARID2 or
scramble for 24 h, then cell viability, migration and invasion were
evaluated. The expression levels of matrix metallopeptidase 2
(MMP2), MMP9, phosphorylated phosphatidylinositol 3-kinase
(p-PI3K), PI3K, phosphorylated AKT serine/threonine kinase (p-Akt)
1 and Akt were also detected in HTR8/SVneo cells using western
blotting. It was demonstrated that the knockdown of JARID2
inhibited trophoblast cell viability and migration through
inactivation of the Rac-α Akt signaling pathway.
Materials and methods
Tissue specimens
Fresh placental tissues at 26–28 weeks of gestation
were obtained from 3 healthy pregnant women (age, 24.2±2.4 years)
and from 3 female patients with preeclampsia (age, 25.7±1.8 years)
who were recruited from the Department of Obstetrics and
Gynecology, Fujian Provincial Hospital (Fujian, China), between
July 2015 and December 2015. The specimens were immediately
snap-frozen, and stored in liquid nitrogen until use. The present
study was conducted with the approval of the Ethics Committee of
Fujian Provincial Hospital (Fuzhou, China), and informed consent
was obtained from all patients.
Cell culture
The human trophoblast cell line HTR8/SVneo was
obtained from the American Type Culture Collection (Manassas, VA,
USA). The cells were maintained in Dulbecco's modified Eagle's
medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% fetal bovine serum (FBS;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), 100 U/ml penicillin
and 100 mg/ml streptomycin (Sigma-Aldrich; Merck KGaA) at 37°C in a
humidified 5% CO2/95% air atmosphere.
RNA interference and transfection
The small interfering RNA against JARID2
(siRNA-JARID2; 5′-AGGAAGAGGAGGAGGACAA-3′) and control siRNA
(scramble; 5′-GAGUGGGUCUGGGUCUUCCCGUAGA-3′) were chemically
synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China).
HTR8/SVneo cells were transfected for 24 h with siRNA-JARID2 or
scramble using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's
protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from frozen tissues or cells
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
cDNA was synthesized from the extracted RNA (5 µg) using the
EasyScript First-Strand cDNA Synthesis SuperMix kit (Invitrogen;
Thermo Fisher Scientific, Inc.). qPCR analysis was performed using
a 7500 Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.) with Fast Start Universal SYBR Green Master
(Roche Diagnostics, Basel, Switzerland). PCR amplification was
performed using the following primers: JARID2,
5′-GACACCAAACCCAATCACCAC-3′ (sense) and 5′-GTTCAACCTGCCACTGACCTT-3′
(antisense); and β-actin, 5′-TTAGTTGCGTTACACCCTTTC-3′ (sense) and
5′-ACCTTCACCGTTCCAGTTT-3′ (antisense). The thermocycling conditions
were as follows: 95°C for 4 min, followed by 40 cycles of 95°C for
25 sec, 55°C for 30 sec and 72°C for 20 sec with 2 sec for plate
reading, then melting curve analysis from 65 to 95°C. Data were
analyzed using the formula: R=2−[ΔCq sample-ΔCq control]
(14).
Western blotting
Total protein was extracted from frozen tissues or
cells using radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China). Protein content was
determined using a bicinchoninic acid protein assay (Pierce; Thermo
Fisher Scientific, Inc.). Proteins (30 µg/lane) were separated
using SDS-PAGE on a 12% gel and transferred onto a nitrocellulose
membrane (EMD Millipore, Billerica, MA, USA). Following blocking
with 5% fat-free milk in PBS containing 0.1% (v/v) Tween-20 at room
temperature for 1 h, membranes were incubated with one of the
following mouse primary antibodies: anti-JARID2 (1:3,000; cat. no.
SAB2105079; Sigma; Merck KGaA), anti-MMP2 (1:2,000; cat. no.
sc-13594), anti-MMP9 (1:3,000; cat. no. sc-21733), anti-PI3K
(1:2,000; cat. no. sc-365290), anti-p-PI3K (1:2,500; cat. no.
sc-293115), anti-Akt (1:3,000; cat. no. sc-5298), anti-p-Akt
(1:2,500; cat. no. sc-52940) or anti-GAPDH (1:2,000; cat. no.
sc-47724; all Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at
4°C overnight. Membranes were subsequently incubated with goat
anti-mouse horseradish peroxidase (HRP)-conjugated immunoglobulin
(Ig)G (1:2,500; cat. no. sc-2005; Santa Cruz Biotechnology, Inc.)
or goat anti-rabbit HRP-conjugated IgG (1:3,000; cat. no. sc-2004;
Santa Cruz Biotechnology, Inc.) for 1 h at room temperature. The
target protein was visualized using enhanced chemiluminescence
(Pierce; Thermo Fisher Scientific, Inc.) and densitometry was
performed using Gel-Pro Analyzer software version 4.0 (Media
Cybernetics, Inc., Rockville, MD, USA).
Cell viability assay
Cell viability was measured using the Cell Counting
Kit-8 assay (CCK-8; Dojindo Molecular Technologies, Inc. Kumamoto,
Japan). HTR8/SVneo cells were plated at a density of
1×104 cells/well in 96-well plates and transfected with
siRNA-JARID2 or scramble. Following incubation for 24 h, the CCK-8
reagents were added and incubated with the cells for 1 h.
Subsequently, the absorbance at 450 nm was measured using an ELISA
plate reader.
Cell invasion and migration
assays
Cell invasion assays were performed using Transwell™
chambers (Costar; Corning Incorporated, Corning, NY, USA).
Transfected HTR8/SVneo cells (1×104 cells/well) were
plated in the top chamber of the insert, which had been precoated
with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA; 8 µm pore
size), and 500 µl DMEM containing 10% FBS was added to the lower
chamber. Following 24 h of incubation, cells transferred to the
lower surface of the base membrane were stained with hematoxylin
and eosin (Sigma-Aldrich; Merck KGaA), and counted per 4 high power
fields under a light microscope (magnification, ×100). The
migration assay was performed by the same procedure, except that
the inserts were not pre-coated with Matrigel.
Statistical analysis
Data are expressed as the mean ± standard deviation.
All experiments were repeated at least three times. Statistical
significance was analyzed using one-way factorial analysis of
variance followed by a Dunnett's post hoc test or Student's
two-tailed t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
JARID2 is underexpressed in
preeclamptic placentas
In order to investigate the role of JARID2 in
preeclampsia, the expression levels of JARID2 in placental tissues
were examined by RT-qPCR analysis and western blotting. As
presented in Fig. 1A, JARID2 mRNA
levels were significantly decreased in placentas obtained from
preeclamptic patients compared with placentas from healthy
subjects. Similarly, the results of the western blot analysis
indicated that the protein expression of JARID2 was significantly
decreased in preeclamptic placentas compared with the control group
(Fig. 1B).
Knockdown of JARID2 inhibits
HTR8/SVneo cell viability
In order to gain further insight into the effect of
JARID2 on cell viability, JARID2 was downregulated using siRNA
against JARID2 in HTR8/SVneo cells. As demonstrated in Fig. 2A and B, respectively, siRNA
knockdown of JARID2 significantly decreased the expression of
JARID2 at the mRNA and protein levels in HTR8/SVneo cells. In
addition, the effects of si-JARID2 on HTR8/SVneo cell viability
were examined. The results of the CCK-8 assay demonstrated that,
compared with the scramble group, knockdown of JARID2 significantly
inhibited the viability of HTR8/SVneo cells (Fig. 2C).
Knockdown of JARID2 inhibits the
migration and invasion of HTR8/SVneo cells
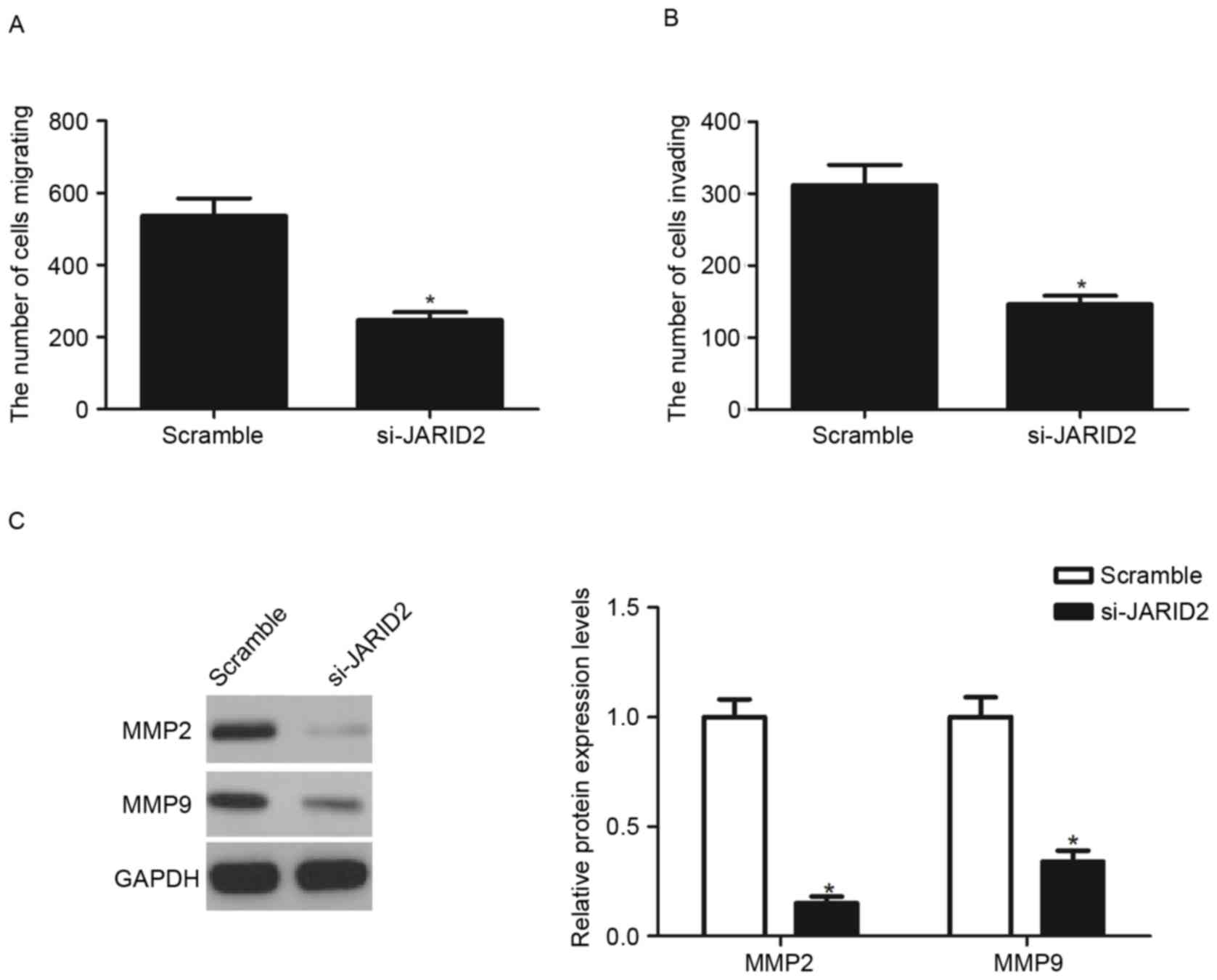
The present study investigated the effects of JARID2
on HTR8/SVneo cell migration and invasion. The Transwell migration
assay demonstrated that the knockdown of JARID2 significantly
inhibited the migratory capacity of HTR8/SVneo cells, compared with
the scramble group (Fig. 3A). The
Matrigel assay demonstrated that the number of cells that passed
through the Matrigel-coated membrane into the lower chamber was
significantly decreased in si-JARID2-transfected cells compared
with scramble-transfected cells (Fig.
3B). In addition, the present study analyzed the effect of
JARID2 on MMP expression in HTR8/SVneo cells. The results of the
western blot analysis demonstrated that knockdown of JARID2
significantly decreased the expression of MMP2 and MMP9 protein,
compared with the scramble group (Fig.
3C).
Knockdown of JARID2 inhibits the
activation of PI3K/Akt pathway in HTR8/SVneo cells
In order to gain insights into the downstream
signaling pathways modulated by si-JARID2 in preeclampsia
inhibition, the present study investigated the effect of si-JARID2
on the activation of the PI3K/Akt signaling pathway in HTR8/SVneo
cells. As presented in Fig. 4, the
knockdown of JARID2 significantly decreased the levels of
phosphorylated PI3K and Akt in HTR8/SVneo cells, compared with the
scramble group.
Discussion
Preeclampsia is a pregnancy-specific complication
and is associated with insufficient extravillous trophoblast
invasion. To the best of our knowledge, the present study is the
first to investigate the expression and role of JARID2 in
preeclampsia. The results of the present study demonstrated that
JARID2 is underexpressed in human preeclamptic placentas. The
knockdown of JARID2 significantly inhibited the viability and
migration/invasion of HTR8/SVneo cells. Additionally, the knockdown
of JARID2 reduced the activation of PI3K and Akt in HTR8/SVneo
cells.
JARID2 has been observed to regulate the
proliferation of a number of types of cells. Mejetta et al
(10) confirmed that silencing
JARID2 decreases the proliferation of rhabdomyosarcoma cells by
inducing myogenic differentiation. By contrast, it has been
reported that the knockdown of JARID2 promotes leukemia cell
proliferation via acceleration of the G1/S transition (15). These previous findings suggest that
JARID2 may serve promotive or inhibitory functions depending on the
cell type or context. In the present study, it was observed that
JARID2 is underexpressed in human preeclamptic placentas, and that
the knockdown of JARID2 inhibited the viability of HTR8/SVneo
cells. The present results suggested that JARID2 may act as a
suppressor in the development and progression of preeclampsia.
Previous studies have demonstrated that deficient
migration and shallow invasion of trophoblasts may lead to
preeclampsia (16,17). JARID2 has been suggested to
regulate tumor cell migration and invasion; a previous study
revealed that knockdown of JARID2 markedly inhibits hepatocellular
carcinoma cell migration and invasion (13). Consistent with the results of this
previous study, in the present study, it was observed that the
knockdown of JARID2 significantly inhibited the viability and
migration/invasion of HTR8/SVneo cells. Additionally, MMPs serve
important roles in cell migration and invasion by remodeling the
extracellular matrix (18,19). During early pregnancy, for embryo
implantation and placentation, the invasion of human trophoblast
cells depends on the secretion of MMPs, primarily MMP2 and MMP9
(20,21). In the present study, it was
observed that the knockdown of JARID2 significantly inhibited the
expression of MMP2 and MMP9 in HTR8/SVneo cells. The present study
provides evidence to suggest that the knockdown of JARID2 may lead
to decreased MMP2 and MMP9 expression, which may lead to
insufficient trophoblast cell migration and invasion, thereby
contributing to preeclampsia.
Previous studies have reported that the PI3K/Akt
signaling pathway may serve important roles in regulating the
proliferation, migration and invasion of trophoblast cells
(22–24). Akt is a serine/threonine protein
kinase, and the phosphorylation of Akt has been observed to be
decreased in the preeclamptic placenta (25). In addition, activated Akt is able
to promote cell motility and invasion (26,27).
Therefore, the suppression of PI3K/Akt signaling may be a promising
approach for treating preeclampsia. A recent study reported that
JARID2 promotes the activation of Akt in hepatocellular carcinoma
(13). In the present study, it
was observed that the knockdown of JARID2 suppressed the levels of
phosphorylated PI3K and Akt in HTR8/SVneo cells. The results of the
present study suggested that the knockdown of JARID2 inhibited the
viability and invasion of trophoblast cells in preeclampsia by
suppressing the PI3K/Akt signaling pathway.
In conclusion, the results of the present study
demonstrated that JARID2 may serve an important role in the
progression of preeclampsia. The knockdown of JARID2 inhibited the
viability and invasion of trophoblast cells in preeclampsia by
suppressing the PI3K/Akt signaling pathway. Therefore, JARID2 may
serve as a novel potential target for treating preeclampsia.
References
|
1
|
Backes CH, Markham K, Moorehead P, Cordero
L, Nankervis CA and Giannone PJ: Maternal preeclampsia and neonatal
outcomes. J Pregnancy. 2011:2143652011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dieplinger H: Method for diagnosing
preeclampsia. Patent WO2013000992 A1. Filed June 28, 2012; issued
January 3. 2013.
|
|
3
|
Bernardi F, Guolo F, Bortolin T,
Petronilho F and Dal-Pizzol F: Oxidative stress and inflammatory
markers in normal pregnancy and preeclampsia. J Obstet Gynaecol
Res. 34:948–951. 2008.PubMed/NCBI
|
|
4
|
Berzan E, Doyle R and Brown CM: Treatment
of preeclampsia: Current approach and future perspectives. Curr
Hypertens Rep. 16:4732014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lyall F: Mechanisms regulating
cytotrophoblast invasion in normal pregnancy and pre-eclampsia.
Aust N Z J Obstet Gynaecol. 46:266–273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kooistra SM and Helin K: Molecular
mechanisms and potential functions of histone demethylases. Nat Rev
Mol Cell Biol. 13:297–311. 2012.PubMed/NCBI
|
|
7
|
Herz HM and Shilatifard A: The JARID2-PRC2
duality. Genes Dev. 24:857–861. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pasini D, Cloos PA, Walfridsson J, Olsson
L, Bukowski JP, Johansen JV, Bak M, Tommerup N, Rappsilber J and
Helin K: JARID2 regulates binding of the Polycomb repressive
complex 2 to target genes in ES cells. Nature. 464:306–310. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kinkel SA, Galeev R, Flensburg C, Keniry
A, Breslin K, Gilan O, Lee S, Liu J, Chen K, Gearing LJ, et al:
Jarid2 regulates hematopoietic stem cell function by acting with
polycomb repressive complex 2. Blood. 125:1890–1900. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mejetta S, Morey L, Pascual G, Kuebler B,
Mysliwiec MR, Lee Y, Shiekhattar R, Di Croce L and Benitah SA:
Jarid2 regulates mouse epidermal stem cell activation and
differentiation. EMBO J. 30:3635–3646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tange S, Oktyabri D, Terashima M, Ishimura
A and Suzuki T: JARID2 is involved in transforming growth
factor-beta-induced epithelial-mesenchymal transition of lung and
colon cancer cell lines. PLoS One. 9:e1156842014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Manceau G, Letouzé E, Guichard C, Didelot
A, Cazes A, Corté H, Fabre E, Pallier K, Imbeaud S, Le
Pimpec-Barthes F, et al: Recurrent inactivating mutations of ARID2
in non-small cell lung carcinoma. Int J Cancer. 132:2217–2221.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lei X, Xu JF, Chang RM, Fang F, Zuo CH and
Yang LY: JARID2 promotes invasion and metastasis of hepatocellular
carcinoma by facilitating epithelial-mesenchymal transition through
PTEN/AKT signaling. Oncotarget. 7:40266–40284. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-tie quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Su CL, Deng TR, Shang Z and Xiao Y: JARID2
inhibits leukemia cell proliferation by regulating CCND1
expression. Int J Hematol. 102:76–85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suga N, Sugimura M, Koshiishi T, Yorifuji
T, Makino S and Takeda S: Heparin/heparan sulfate/CD44-v3 enhances
cell migration in term placenta-derived immortalized human
trophoblasts cells. Biol Reprod. 86:1341–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zou Y, Jiang Z, Yu X, Sun M, Zhang Y, Zuo
Q, Zhou J, Yang N, Han P, Ge Z, et al: Upregulation of long
noncoding RNA SPRY4-IT1 modulates proliferaiton, migraiton,
apoptosis, and netword formation in trophoblast cell HTR-8SV/neo.
PLoS One. 8:e795982013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim YH, Kwon HJ and Kim DS: Matrix
metalloproteinase 9 (MMP-9)-dependent processing of βig-h3 protein
regulates cell migration, invasion, and adhesion. J Biol Chem.
287:38957–38969. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Q, Jin M, Yang F, Zhu J, Xiao Q and
Zhang L: Matrix Metalloproteinases: Inflammatory regulators of cell
behaviors in vascular formation and remodeling. Mediators Inflamm.
2013:9283152013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Staun-Ram E, Goldman S, Gabarin D and
Shalev E: Expression and importance of matrix metalloproteinase 2
and 9 (MMP-2 and −9) in human trophoblast invasion. Reprod Biol
Endocrinol. 2:592004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seval Y, Akkoyunlu G, Demir R and Asar M:
Distribution patterns of metalloproteinase (MMP)-2 and-9 and their
inhibitors (TIMP-1 and TIMP-2) in the human decidua during early
pregnancy. Acta Histochem. 106:353–362. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng Q, Dai K, Cui X, Yu M, Yang X, Yan
B, Liu S and Yan Q: Leukemia inhibitory factor promote trophoblast
invasion via urokinase-type plasminogen activator receptor in
preeclampsia. Biomed Pharmacother. 80:102–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jia RZ, Ding GC, Gu CM, Huang T, Rui C,
Wang YX and Lu Q: CDX2 enhances HTR-8/SVneo trophoblast cell
invasion by altering the expression of matrix metalloproteinases.
Cell Physiol Biochem. 34:628–636. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu X, Mu H, Luo X, Xiao X, Ding Y, Yin N,
Deng Q and Qi H: Expression of Gadd45α in human early placenta and
its role in trophoblast invasion. Placenta. 35:370–377. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cudmore MJ, Ahmad S, Sissaoui S, Ramma W,
Ma B, Fujisawa T, Al-Ani B, Wang K, Cai M, Crispi F, et al: Loss of
Akt activity increases circulating soluble endoglin release in
preeclampsia: Identification of inter-dependency between Akt-1 and
heme oxygenase-1. Eur Heart J. 33:1150–1158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bulj Z, Duchi S, Bevilacqua A, Gherardi A,
Dozza B, Piccinini F, Mariani G Adalgisa, Lucarelli E, Giannini S,
Donati D and Marmiroli S: Protein kinase B/AKT isoform 2 drives
migration of human mesenchymal stem cells. Int J Oncol. 42:118–126.
2013.PubMed/NCBI
|
|
27
|
Yoeli-Lerner M and Toker A: Akt/PKB
signaling in cancer: A function in cell motility and invasion. Cell
Cycle. 5:603–605. 2006. View Article : Google Scholar : PubMed/NCBI
|