Introduction
Temporomandibular joint dysfunction (TMD) is a
collective term describing a range of clinical symptoms that
involve the muscles of mastication and the temporomandibular joint
(TMJ), which is a bilateral synovial joint formed from the upper
temporal bone of the skull and lower jawbone (mandible). TMD is
characterized by restricted jaw motion and joint sound and pain and
often includes TMJ disc displacement, internal derangement, and
osteoarthritis (OA). In TMD, synovitis that often accompanies
intra-capsular pathologic conditions is characterized by chronic
inflammatory changes (1–3). The synovial membrane that covers the
inner wall of the TMJ capsule is populated by fibroblast-like
stromal cells and lining cells. Fibroblast-like cells have an
important role in the progression of inflammation in the TMJ due to
their ability to produce a number of pro-inflammatory mediators
(4–6). The process by which TMJ synovitis is
initiated and maintained remains to be elucidated; however, several
pro-inflammatory cytokines, such as tumour necrosis factor (TNF-α),
interleukin (IL)-1β, IL-6, IL-8 and interferon γ (IFNγ) have been
detected in either synovial fluid (7–13) or
in synovial tissue (5) obtained
from patients with internal derangement of the TMJ or OA of the
TMJ. Therefore, these cytokines may be involved in the
pathophysiology of TMJ internal derangement and OA.
Elastin is an extracellular matrix molecule
responsible for the mechanical resilience of tissues and was
initially believed to be restricted to this role (14). In keeping with its important
structural role, elastin has a very long half-life. However,
elastin turnover is dramatically accelerated in various disease
states including arthritis, atherosclerosis, emphysema and cancer
(15). Elastin degradation leads
to the production of bioactive peptides termed elastin-derived
peptides (EDPs) (16). The
hexapeptide VGVAPG, which may be detected in insoluble elastin and
EDPs, may responsible for bioactivity as it may bind to the elastin
receptor and exert numerous biological effects, including
atherosclerosis (17). Although
several other receptors have been suggested to bind EDPs
(αvβ3 and αvβ5
integrins, galectin-3), the principal EDPs receptor remains the
elastin receptor complex (18).
This heterotrimer is composed of a peripheral subunit, termed the
elastin binding protein (EBP), associated with the protective
protein/cathepsin A. The latter is bound to a membrane-associated
protein termed neuraminidase-1. Specific interactions between
inflammatory cells and EDPs previously established (19).
Anatomically, the TMJ disc is attached to the
capsule and its surrounding structures (20). In humans TMJ disc elastin is
abundant in both the anterior and posterior attachment structures,
particularly thick elastic fibres are located in the upper
bilaminar zone (21). It is of
note that, as a consequence of aging, there is a reduction in the
number of elastic fibres in the TMJ disc (22–24).
However, the absence of mastication movements in foetuses and
new-borns correlates with the presence of abundant elastic fibres
in all areas of the TMJ disc (24). In adults with teeth, as well as
edentulous older people, elastic fibre density has been identified
to be considerably reduced in the middle of the bilaminar
intermediate zone (25). It is
likely that mechanical factors, particularly pressure created
during mastication, may explain this phenomenon. Previous studies
on diseased human TMJ discs have revealed a significant reduction
in the number of elastic fibres in the bilaminar zone (23,26,27).
At present, there is minimal information regarding
the involvement of elastin degradation in the processes of
synovitis in human TMD. The present study aimed to investigate the
importance of EDPs in human TMD. To the best of our knowledge, the
present study was the first to reveal that EDPs in synovial fluid
of patients with TMD were correlated with the duration of TMJ
locking and VAS score, and that EDPs induce inflammatory responses
in human TMJ synovial cells. This important finding indicates that
elastin degradation is an event that may be involved in the
establishment of human TMD.
Materials and methods
Human samples
Experiments using human samples were approved by the
Ethics Committee of the Kanazawa University Graduate School of
Medical Science (IRB no. 2014-005, 351-2), and written informed
consent was obtained from patients providing the specimens.
Patients were diagnosed with closed lock disc disease in the
temporomandibular joint and were treated using pumping manipulation
at Kanazawa University Hospital (Kanazawa, Japan) between April
1997 and March 2000. Synovial fluid was obtained from the TMJ of 28
patients. The patients ranged between 16 and 66 years of age
(38.3±17.7 years old, mean ± SD), and 25 patients (89%) were
female. Samples were collected from the TMJ using a push and pull
technique, as previously described (28). Clinical examinations were performed
prior to synovial fluid collection. They included recordings of
maximum incisal opening, pain upon incisal opening, pain upon
palpation of muscles and the TMJ, subjective reports on pain and
function of the jaw, using a visual analogue scale (VAS), and the
duration of locking disc disease.
Cell culture
Human synovial fibroblast cells were prepared from
TMJ synovial tissues obtained at arthroplasty from 1 patient with
TMJ ankyloses (68 years old male) and 2 patients with condylar
fracture of the mandible (23-year-old female and 43-year-old male).
These 3 patients underwent surgery at Kanazawa University Hospital
between April 1997 and March 2000. Tissue samples were treated with
5% bacterial collagenase type I (Worthington Biochemical
Corporation, Freehold, NJ, USA) for 1 h at 37°C and 0.02% trypsin
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 30 min at
37°C. After the activities of these proteinases were blocked with
10% foetal bovine serum (FBS; GE Healthcare, Logan, UT, USA), the
1×105 cells were seeded in culture dishes and maintained
in Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) containing 10% FBS at 37°C in a
humidified 5% CO2 atmosphere. Human lung elastin
peptides were purchased from Elastin Products Company (Owensville,
MO, USA) (29). Cells below
passages 3–6 were used in the subsequent experiments. Prior to EDP
treatment, cells were pre-incubated at 37°C for 12 h in serum-free
DMEM medium and then incubated at 37°C for 24 h in DMEM containing
2% FBS and EDP (Elastin Products Company) at various concentrations
(0, 25, and 50 µg/ml). For the in vitro inhibition studies,
cells were pre-incubated for 12 h in serum-free DMEM medium with or
without the protein kinase A (PKA) inhibitor
N-[2-((p-bromocinnamyl) amino)ethyl]-5-isoquinolinesulfonamide
(H89; 2 µM; LKT Laboratories, Inc., St. Paul, MN, USA) or β-lactose
(20 mM; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) or vehicle
control (PBS only), and then incubated at 37°C for 24 h in DMEM
containing 2% FBS and with or without 50 µg/ml EDPs.
Enzyme-linked immunosorbent assay
(ELISA)
Human TMJ synovial fluid and supernatants, following
5 min centrifugation at 12,500 × g and 4°C, obtained from cultured
primary TMJ synovial cells were analysed for the presence of EDPs,
IL-1β, IL-6, TNF-α and matrix metalloproteinase-12 (MMP-12) using
the appropriate ELISA kit (EDPs, cat. no. SK00806-01; Aviscera
Bioscience Inc., Santa Clara, CA, USA; IL-1β, cat. no. DLB50; IL-6,
cat. no. D6050; TNF-α, cat. no. DTA00C; all from R&D Systems,
Inc., Minneapolis, MN, USA; MMP-12, cat. no. EK0950; Boster
Biological Technology, Pleasanton, CA, USA) according to the
manufacturer's protocols. Total protein concentrations in the
synovial fluid were determined by the dye binding method using an
acidic solution of Coomassie Brilliant Blue G-250 dye, according to
the manufacturer's protocol (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). Values were calculated as pg/mg total protein or ng/mg
total protein. Data are presented as the mean ± standard error of
the mean.
Statistical analysis
For comparisons between samples, data was analysed
by with analysis of variance and Tukey's multiple comparison test
using SPSS version 23 (IBM Corporation, Armonk, NY, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
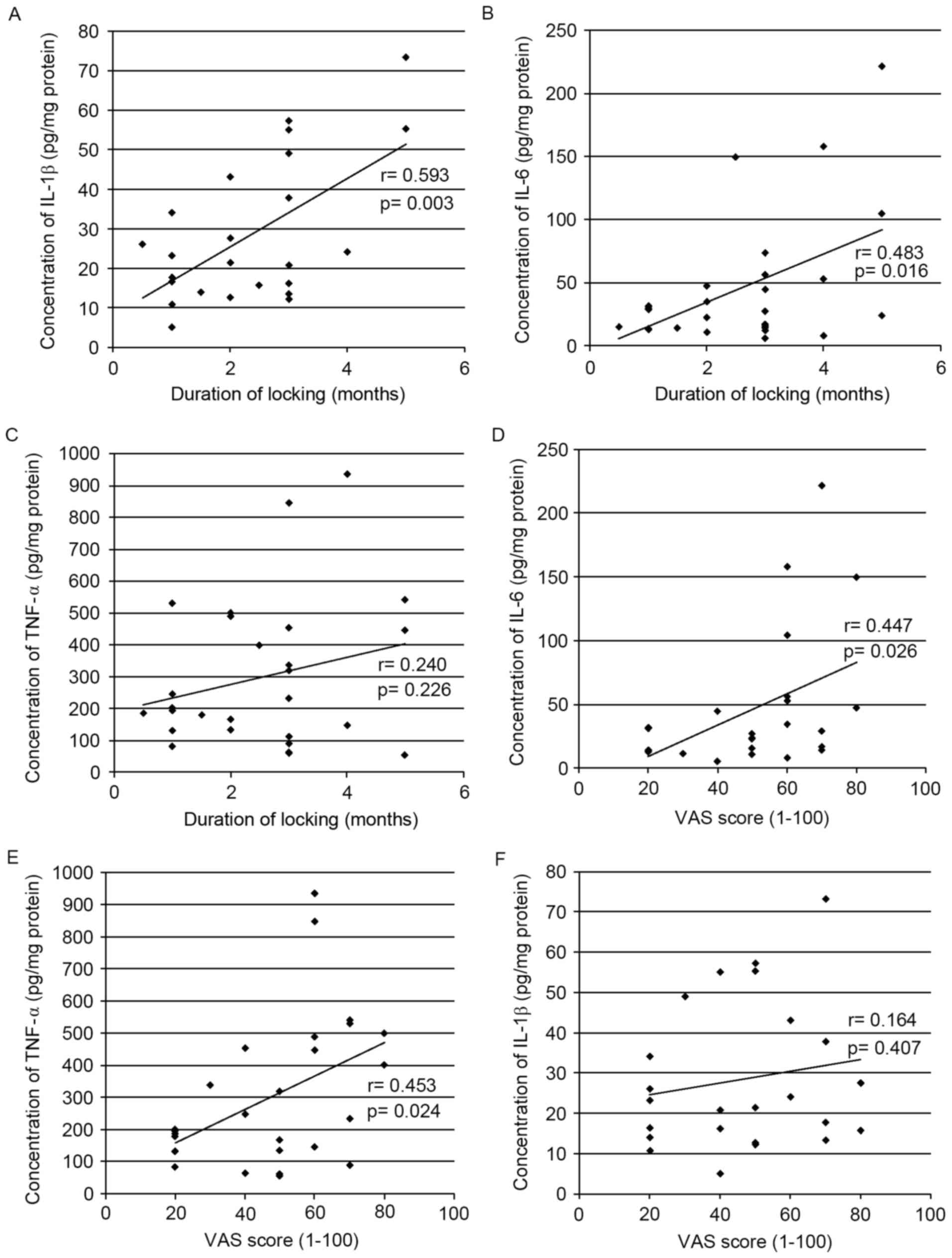
Concentrations of IL-1β, IL-6 and
TNF-α in the synovial fluid of patients with TMD are correlated
with the duration of TMJ disk locking or VAS score
The concentration of IL-1β, IL-6 and TNF-α in the
synovial fluid of patients with TMD was quantified using ELISA and
the correlation between the levels of these proteins and two
clinical parameters was determined. The duration of TMJ locking had
a positive correlation with IL-1β (r=0.593; P=0.003; Fig. 1A) and IL-6 levels (r=0.483;
P=0.016; Fig. 1B); however, no
correlation was identified with TNF-α levels (r=0.240; P=0.226;
Fig. 1C). In contrast, the VAS
score had a positive correlation with IL-6 (r=0.447; P=0.026;
Fig. 1D) and TNF-α (r=0.453;
P=0.024; Fig. 1E); however, no
correlation was identified with IL-1β levels (r=0.164; P=0.407;
Fig. 1F).
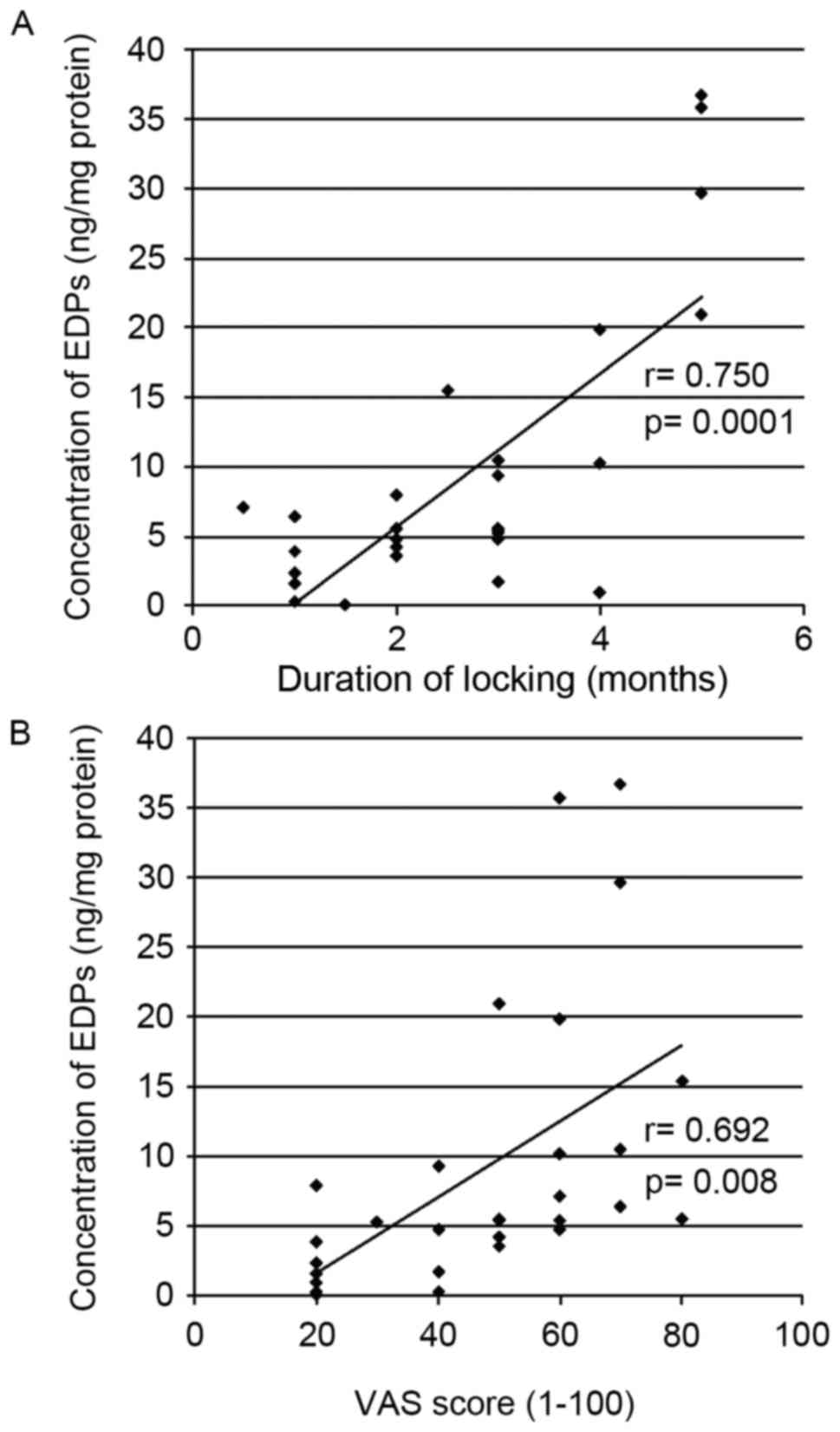
Concentration of EDPs in the synovial
fluid of patients with TMD and correlation with duration of TMJ
disk locking or the VAS score
The total concentration of EDPs in the synovial
fluid of patients with TMD was measured and the correlation between
the EDP levels and the clinical parameters was determined. The
locking duration of TMJ had a positive correlation with EDP levels
(r=0.750; P=0.0001; Fig. 2A).
Similarly, the VAS score also had a positive correlation with EDP
levels (r=0.692; P=0.008; Fig.
2B).
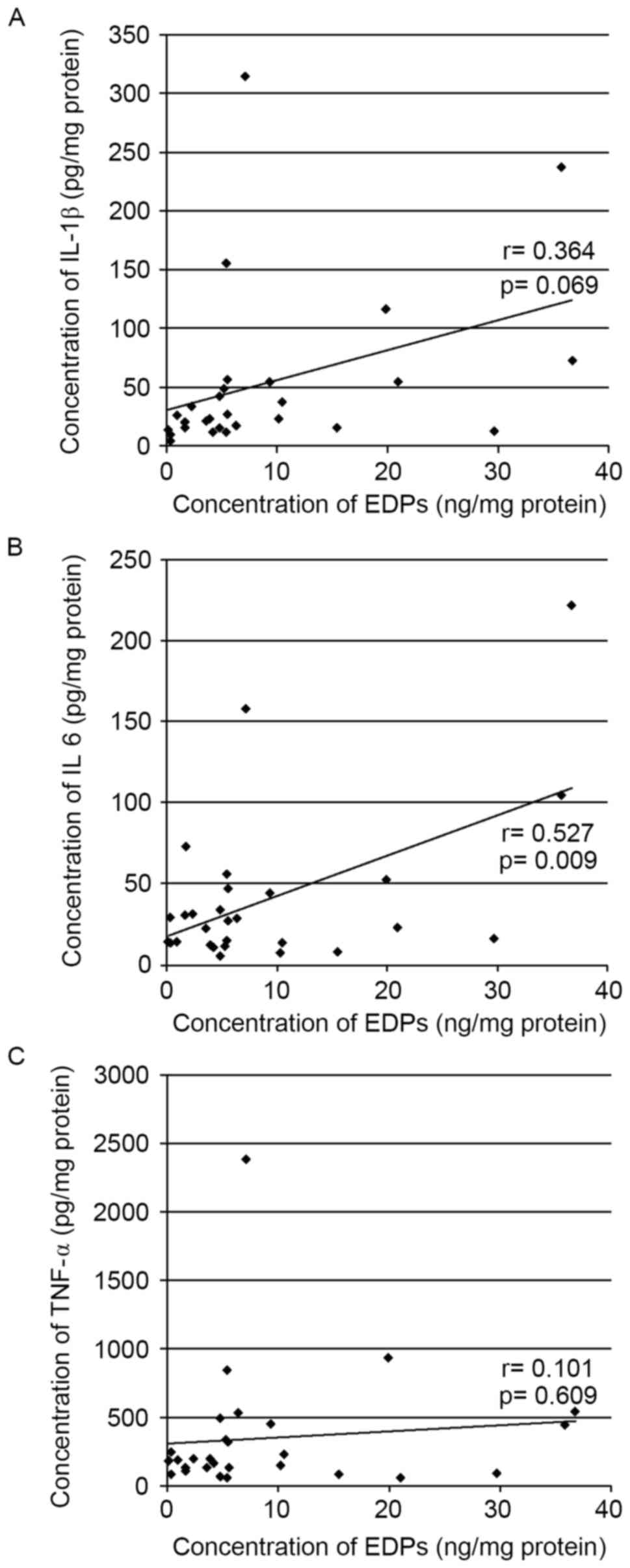
Correlation between EDP levels and IL-1β, IL-6 or
TNF-α levels in synovial fluid from patients with TMD. The
correlation between EDP levels and IL-1β, IL-6 or TNF-α levels in
synovial fluid from patients with TMD was determined (Fig. 3). EDP levels had a positive
correlation with IL-6 (r=0.527; P=0.009; Fig. 3B); however, no correlation was
identified with IL-1β (r=0.364; P=0.069; Fig. 3A) or TNF-α levels (r=0.101;
P=0.609; Fig. 3C).
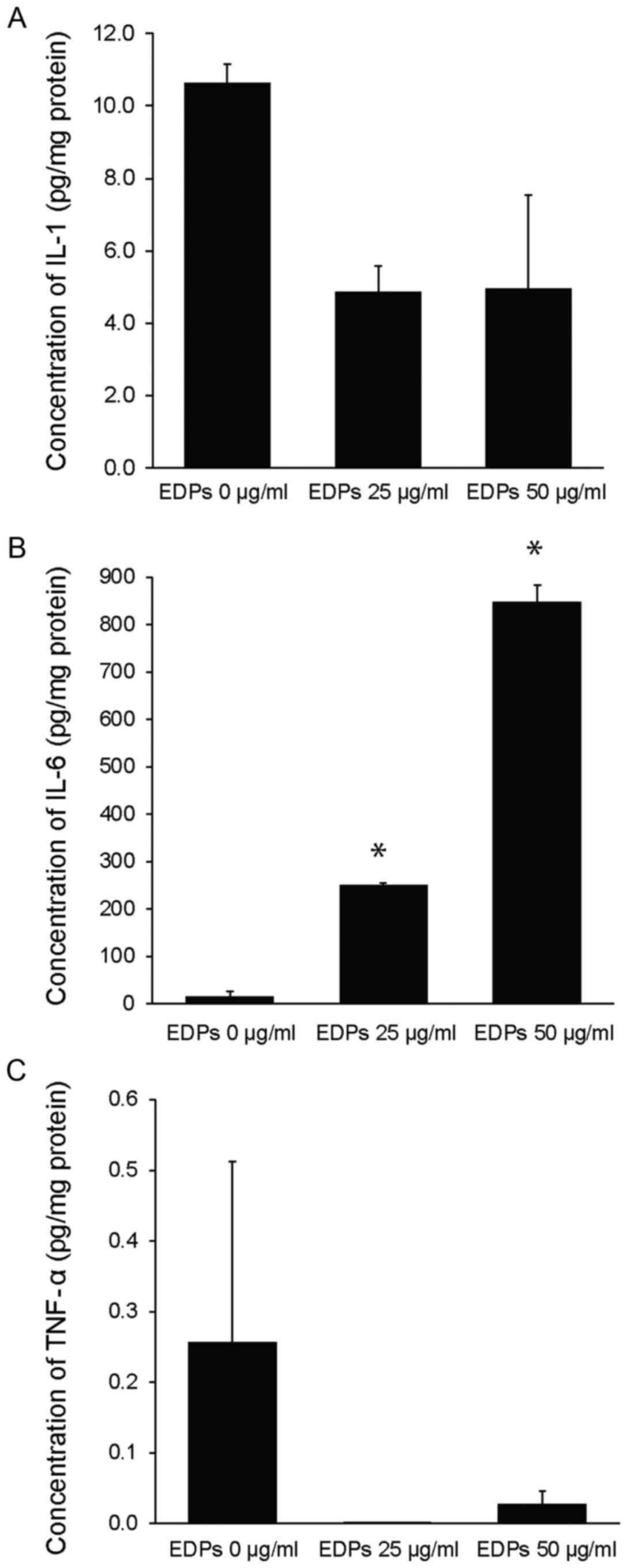
EDPs stimulate IL-6 protein production
in TMJ human synovial fibroblast cells
To determine the role of EDPs during the
inflammation process in TMD, cultured human TMJ synovial fibroblast
cells were treated with EDPs at a concentration of 0, 25 and 50 and
the levels of IL-1β, IL-6 and TNF-α were measured in the culture
media (Fig. 4). EDP treatment
significantly increased IL-6 protein levels compared with the
vehicle treated (EDPs 0 µg/ml) cells (250.01±5.09 pg/mg at 25
µg/ml; 848.44±34.29 pg/mg at 50 µg/ml compared to 15.33±9.27 pg/mg
for the vehicle control group, P<0.05; Fig. 4B). No significant effect was
observed on IL-1β and TNF-α protein levels (Fig. 4A and C).
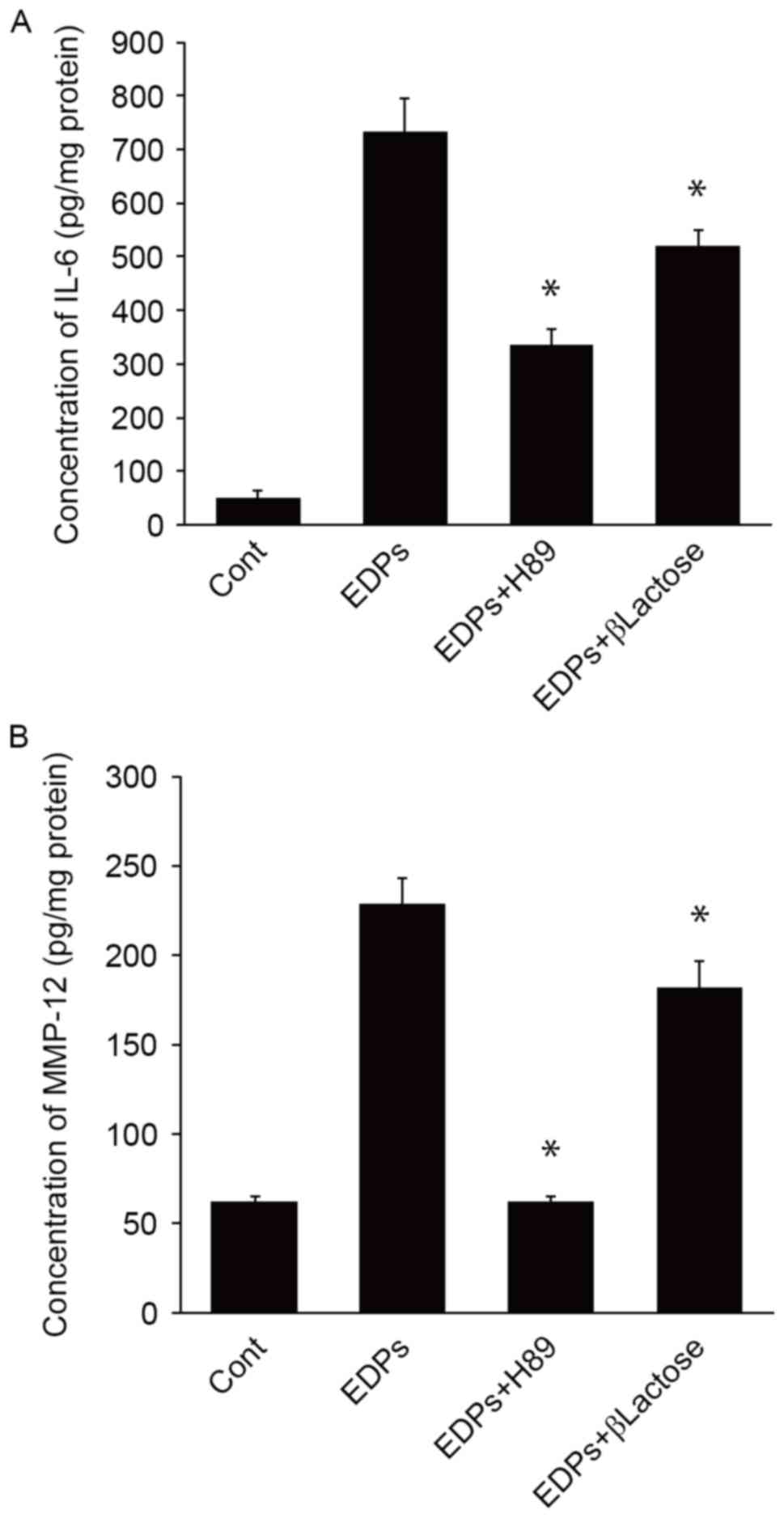
EDPs induce IL-6 and MMP-12 expression
through EBP
The correlation between EDP levels and IL-6
expression observed in clinical samples was validated using in
vitro inhibition. A previous study demonstrated that PKA was a
mediator of elastin-induced intracellular signalling through EBP
(30). Therefore, H89, a PKA
inhibitor, and β-lactose, another inhibitor of EBP mediated
signalling (30), was used to
determine the role of EBP in the stimulation of IL-6 release in
response to EDPs in TMJ human synovial fibroblast cells. IL-6
levels treated with EDP (777.40±61.99 pg/mg) were significantly
inhibited by the H89 (345.01±29.29 pg/mg; P<0.05) and β-lactose
treatments (539.51±30.71 pg/mg; P<0.05; Fig. 5A). EDPs also significantly
upregulated MMP-12 expression in the cultured synovial fibroblasts
(228.47±14.48 pg/mg; P<0.05) compared with cells treated with
the vehicle control (62.5±3.00 pg/mg; Fig. 5B). The upregulation of MMP-12 was
significantly inhibited following treatment with H89 (64.16±3.26
pg/mg; P<0.05) and β-lactose (182.22±14.63 pg/mg; P<0.05;
Fig. 5B).
Discussion
Previous studies have demonstrated that cytokines
including IL-1β (9,11,12,31,32),
IL-6 (9,11,12,31,33–37)
and TNF-α (8,11,31,32,34,35)
are associated with inflammation in synovial joints and loss of the
connective tissue in TMJ. Therefore, it is possible that increased
levels of these cytokines may be observed in the synovial fluid of
patients with TMD. In the present study, IL-6 expression
significantly correlated with the two clinical parameters
investigated, duration of the TMJ disc locking and VAS score. The
data of the present study was therefore consistent with a previous
study which stated that IL-6 has a major role in the development of
osteoarthritis in the TMJ (12).
However, in another previous study, levels of IL-6 were determined
to be comparable in the synovial fluids from patients with TMJ and
OA, and healthy patients with TMJ (32). Previous studies (32,36–38)
have revealed a negative correlation between frequently isolated
cytokines, IL-6 (32), TNF-α
(37,38) and IL-1β (36,38)
and TMD. Kaneyama et al (38) suggested that no correlation was
observed between the IL-1β or TNF-α levels and condyle degenerative
changes due to the rapid turnover of cytokines within the joint
cavity (38). However, it is
difficult to elucidate the reasons behind these controversial
findings, and standardization of the sampling methods, assay
methods and patient cohort will be performed in future studies. The
present findings may be affected by numerous clinical variables,
including patient age, gender, duration and intensity of TMJ
disease. Therefore, in the future, carefully designed clinical
trials will need to be conducted with the aim of using the levels
of pro-inflammatory cytokines to predict the extent and severity of
TMJ pathology.
The pathophysiology of TMD has been previously
investigated and several mechanistic studies have been reported.
For example, loss of disc elastic fibres was observed following
induction of anterior disc displacement or disc perforation
(39,40). It has also been reported that the
abundance of elastic fibres in the bilaminar zone was reduced in
patients with internal disc derangement (23,26,27).
A previous study demonstrated that the presence and distribution of
newly formed elastic fibres, was associated with the degree of disc
tissue damage to, or the complete absence of collagen bundles
(41).
Previous studies (7–13)
have focused on examining pro-inflammatory cytokines, to the best
of our knowledge the present study was the first to determine the
quantity of EDPs present in synovial fluid. In current study
determined the concentration of EDPs in the synovial fluid of
patients with TMD and revealed that the levels of EDPs were
significantly correlated with VAS score or duration of TMJ disc
locking as well as IL-6 expression in the synovial fluid of
patients with TMD. The biological role of elastin, as the
fundamental unit of an elastic fibre, was originally believed to be
restricted only to mechanical maintenance of tissue architecture.
This simple view of elastin has now evolved as EDPs have been
revealed to be biologically active in a range of normal and
transformed cells (42,43). However, despite these new insights,
the role for EDPs in the pathophysiology of TMD, to the best of our
knowledge, has not been previously investigated.
In elastin-rich tissues such as arteries, lungs and
skin, inflammation is concomitant with elastolysis, leading to the
generation of EDPs (42,44,45).
In these cases, a direct association between inflammation and EDP
levels has been established. The present study determined that EDPs
may act selectively on human TMJ synovial cells to stimulate IL-6
production, with no significant effect on IL-1β or TNF-α
production. IL-6 is produced at the site of inflammation and has a
key role in the acute phase response (46). Following the onset of the
inflammatory response, IL-6 secreted by synovial cells in the TMJ
may act as a chemoattractant for other cell types important to
tissue degradation. In a previous study, stimulation of cartilage
explants with IL-6 potentiated proteoglycan (aggrecan) catabolism
in articular cartilage. This catabolism was associated with
aggrecanase activity (47). In
this regard, upregulation of IL-6 is critical for the progression
of arthritic diseases. Under inflammatory conditions, changes in
the levels of polarized Th1 and Th2 cells may also to be important
(48,49). Therefore, the expression levels of
a Th1 cytokine (TNF-α) and a Th2 cytokine (IL-6) were examined in
EDP-treated TMJ synovial cells. The expression of IL-6; however,
not TNF-α, was increased by EDPs suggesting that EDPs stimulate a
Th2 cellular response. Characterization of Th1 and Th2 response has
been previously performed using synovial tissue samples obtained
from a patient with erosive rheumatoid arthritis (50). The synovial tissue sample used in
the present study had an unusual Th2 dominant pattern. A previous
study has demonstrated that IL-6 deficient mice had reduced Th2
responses and increased arthritis (51). These findings demonstrated that a
predominantly Th2 response may be associated with arthritis.
EDPs may act via EBP, which is located on the
membrane of fibroblasts, granulocytes, lymphocytes, monocytes and
cancer cells (52). H89 inhibition
of PKA was used to determine the role of PKA as a key intracellular
signalling mediator downstream of EBP (30). EBP is inhibited by β-lactose
(30); however, α-lactose is only
a partial inhibitor due to its partial conversion to β-lactose by
anomerization (53). The present
study revealed that H89 and β-lactose inhibited EDP stimulated
expression of IL-6 in TMJ synovial cells. These findings suggest
that the induction of IL-6 expression by EDPs involved the EBP and
a PKA signalling cascades. MMP-12 is a member of a group of enzymes
that are able to degrade elastin. The present study revealed that
MMP-12 is secreted from EDP-treated TMJ synovial cells and that,
similarly to IL-6, this process was also inhibited by H89 and
β-lactose. Therefore, from these findings a model for the
initiation of the inflammatory process in the TMJ may be proposed.
Harmful mechanical stimuli, and in particular pressure, may lead to
tissue damage in the TMJ. EDPs are then generated, as endogenous
danger signals and induce a pro-inflammatory cascade by activating
EBP signalling. Subsequently, pro-inflammatory mediators, such as
IL-6 and MMP-12 are upregulated and trigger further tissue damage
leading to increased EDP levels. Therefore, a positive feedback
loop is established and may lead to chronic inflammation of the
TMJ. Therefore, significantly higher levels of a combination of
EDPs and IL-6 in the synovial fluid of patients with TMJ may be
indicators of the pathological condition of the joint.
Acknowledgements
The authors would like to thank the members of the
Department of Oral and Maxillofacial Surgery of Kanazawa University
for their helpful suggestions and assistance and Elsevier Language
Editing Services for assistance with language editing. The present
study was supported by grants-in-aid for Scientific Research from
the Ministry of Education, Science, Sports and Culture, Japan
(grant no. 15H05042 to Dr Shuichi Kawashiri and grant no. 25462882
to Dr Hiroyuki Nakamura).
Glossary
Abbreviations
Abbreviations:
|
TMJ
|
temporomandibular joint
|
|
EDPs
|
elastin-derived peptides
|
|
TMD
|
temporomandibular dysfunction
|
|
EBP
|
elastin binding protein
|
|
VAS
|
visual analog scale
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
OA
|
osteoarthritis
|
|
ADD
|
anterior disc displacement
|
References
|
1
|
Carls FR, von Hochstetter A, Makek M and
Engelke W: Diagnostic accuracy of TMJ arthroscopy in correlation to
histological findings. J Craniomaxillofac Surg. 23:75–80. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dijkgraaf LC, Liem RS and de Bont LG:
Synovial membrane involvement in osteoarthritic temporomandibular
joints: A light microscopic study. Oral Surg Oral Med Oral Pathol
Oral Radiol Endod. 83:373–386. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gynther GW, Holmlund AB, Reinholt FP and
Lindblad S: Temporomandibular joint involvement in generalized
osteoarthritis and rheumatoid arthritis: A clinical, arthroscopic,
histologic, and immunohistochemical study. Int J Oral Maxillofac
Surg. 26:10–16. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gynther GW, Dijkgraaf LC, Reinholt FP,
Holmlund AB, Liem RS and de Bont LG: Synovial inflammation in
arthroscopically obtained biopsy specimens from the
temporomandibular joint: A review of the literature and a proposed
histologic grading system. J Oral Maxillofac Surg. 56:1281–1287.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kardel R, Ulfgren AK, Reinholt FP and
Holmlund A: Inflammatory cell and cytokine patterns in patients
with painful clicking and osteoarthritis in the temporomandibular
joint. Int J Oral Maxillofac Surg. 32:390–396. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Satoh K, Ogura N, Akutsu M, Kuboyama N,
Kuyama K, Yamamoto H and Kondoh T: Expression of cyclooxygenase-1
and −2 in IL-1beta-induced synovitis of the temporomandibular
joint. J Oral Pathol Med. 38:584–590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shafer DM, Assael L, White LB and
Rossomando EF: Tumor necrosis factor-alpha as a biochemical marker
of pain and outcome in temporomandibular joints with internal
derangements. J Oral Maxillofac Surg. 52:786–792. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fu K, Ma X, Zhang Z and Chen W: Tumor
necrosis factor in synovial fluid of patients with
temporomandibular disorders. J Oral Maxillofac Surg. 53:424–426.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kubota E, Kubota T, Matsumoto J, Shibata T
and Murakami KI: Synovial fluid cytokines and proteinases as
markers of temporomandibular joint disease. J Oral Maxillofac Surg.
56:192–198. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sandler NA, Buckley MJ, Cillo JE and Braun
TW: Correlation of inflammatory cytokines with arthroscopic
findings in patients with temporomandibular joint internal
derangements. J Oral Maxillofac Surg. 56:534–544. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takahashi T, Kondoh T, Fukuda M, Yamazaki
Y, Toyosaki T and Suzuki R: Proinflammatory cytokines detectable in
synovial fluids from patients with temporomandibular disorders.
Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 85:135–141. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaneyama K, Segami N, Nishimura M, Suzuki
T and Sato J: Importance of proinflammatory cytokines in synovial
fluid from 121 joints with temporomandibular disorders. Br J Oral
Maxillofac Surg. 40:418–423. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nishimura M, Segami N, Kaneyama K, Sato J
and Fujimura K: Comparison of cytokine level in synovial fluid
between successful and unsuccessful cases in arthrocentesis of the
temporomandibular joint. J Oral Maxillofac Surg. 62:284–288. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Muiznieks LD, Weiss AS and Keeley FW:
Structural disorder and dynamics of elastin. Biochem Cell Biol.
88:239–250. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hornebeck W and Robert L: Elastase-like
enzymes in aortas and human breast carcinomas: Quantitative
variations with age and pathology. Adv Exp Med Biol. 79:145–164.
1977. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hornebeck W, Emonard H, Monboisse JC and
Bellon G: Matrix-directed regulation of pericellular proteolysis
and tumor progression. Semin Cancer Biol. 12:231–241. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gayral S, Garnotel R, Castaing-Berthou A,
Blaise S, Fougerat A, Berge E, Montheil A, Malet N, Wymann MP,
Maurice P, et al: Elastin-derived peptides potentiate
atherosclerosis through the immune Neu1-PI3Kγ pathway. Cardiovasc
Res. 102:118–127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scandolera A, Odoul L, Salesse S, Guillot
A, Blaise S, Kawecki C, Maurice P, El Btaouri H, Romier-Crouzet B,
Martiny L, et al: The elastin receptor complex: A unique
matricellular receptor with high anti-tumoral potential. Front
Pharmacol. 7:322016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Duca L, Floquet N, Alix AJ, Haye B and
Debelle L: Elastin as a matrikine. Crit Rev Oncol Hematol.
49:235–244. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bravetti P, Membre H, El Haddioui A,
Gérard H, Fyard JP, Mahler P and Gaudy JF: Histological study of
the human temporo-mandibular joint and its surrounding muscles.
Surg Radiol Anat. 26:371–378. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scapino RP: Histopathology associated with
malposition of the human temporomandibular joint disc. Oral Surg
Oral Med Oral Pathol. 55:382–397. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Benigno MI, Azeredo RA, Lemos JL, Júnior B
König and Liberti EA: The structure of the bilaminar zone in the
human temporomandibular joint: A light and scanning electron
microscopy study in young and elderly subjects. J Oral Rehabil.
28:113–119. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hall MB, Brown RW and Baughman RA:
Histologic appearance of the bilaminar zone in internal derangement
of the temporomandibular joint. Oral Surg Oral Med Oral Pathol.
58:375–381. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Minarelli AM and Liberti EA: A microscopic
survey of the human temporomandibular joint disc. J Oral Rehabil.
24:835–840. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thilander B, Carlsson GE and Ingervall B:
Postnatal development of the human temporomandibular joint I. A
histological study. Acta Odontol Scand. 34:117–126. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Toller PA: Ultrastructure of the condylar
articular surface in severe mandibular pain-dysfunction syndrome.
Int J Oral Surg. 6:297–312. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pereira FJ, Lundh H, Eriksson L and
Westesson PL: Microscopic changes in the retrodiscal tissues of
painful temporomandibular joints. J Oral Maxillofac Surg.
54:461–469. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alstergren P, Kopp S and Theodorsson E:
Synovial fluid sampling from the temporomandibular joint: Sample
quality criteria and levels of interleukin-1 beta and serotonin.
Acta Odontol Scand. 57:16–22. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Skeie JM, Hernandez J, Hinek A and Mullins
RF: Molecular responses of choroidal endothelial cells to elastin
derived peptides through the elastin-binding protein (GLB1). Matrix
Biol. 31:113–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Almine JF, Wise SG, Hiob M, Singh NK,
Tiwari KK, Vali S, Abbasi T and Weiss AS: Elastin sequences trigger
transient proinflammatory responses by human dermal fibroblasts.
FASEB J. 27:3455–3465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kaneyama K, Segami N, Sun W, Sato J and
Fujimura K: Analysis of tumor necrosis factor-alpha, interleukin-6,
interleukin-1beta, soluble tumor necrosis factor receptors I and
II, interleukin-6 soluble receptor, interleukin-1 soluble receptor
type II, interleukin-1 receptor antagonist, and protein in the
synovial fluid of patients with temporomandibular joint disorders.
Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 99:276–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vernal R, Velásquez E, Gamonal J,
Garcia-Sanz JA, Silva A and Sanz M: Expression of proinflammatory
cytokines in osteoarthritis of the temporomandibular joint. Arch
Oral Biol. 53:910–915. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fu K, Ma X, Zhang Z, Pang X and Chen W:
Interleukin-6 in synovial fluid and HLA-DR expression in synovium
from patients with temporomandibular disorders. J Orofac Pain.
9:131–137. 1995.PubMed/NCBI
|
|
34
|
Kaneyama K, Segami N, Sato J, Nishimura M
and Yoshimura H: Interleukin-6 family of cytokines as biochemical
markers of osseous changes in the temporomandibular joint
disorders. Br J Oral Maxillofac Surg. 42:246–250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee JK, Cho YS and Song SI: Relationship
of synovial tumor necrosis factor alpha and interleukin 6 to
temporomandibular disorder. J Oral Maxillofac Surg. 68:1064–1068.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shinoda C and Takaku S: Interleukin-1
beta, interleukin-6, and tissue inhibitor of metalloproteinase-1 in
the synovial fluid of the temporomandibular joint with respect to
cartilage destruction. Oral Dis. 6:383–390. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wake M, Hamada Y, Kumagai K, Tanaka N,
Ikeda Y, Nakatani Y, Suzuki R and Fukui N: Up-regulation of
interleukin-6 and vascular endothelial growth factor-A in the
synovial fluid of temporomandibular joints affected by synovial
chondromatosis. Br J Oral Maxillofac Surg. 51:164–169. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kaneyama K, Segami N, Nishimura M, Sato J,
Suzuki T and Fujimura K: Osteoclastogenesis inhibitory
factor/osteoprotegerin in synovial fluid from patients with
temporomandibular disorders. Int J Oral Maxillofac Surg.
32:404–407. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sato S, Goto S, Kamakura S and Motegi K:
Morphologic changes in the elastic fibers of the temporomandibular
joint after experimental disc perforation in the rabbit. J Oral
Maxillofac Surg. 56:753–759. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ali AM, Sharawy M, O'Dell NL and al-Behery
G: Morphological alterations in the elastic fibers of the rabbit
craniomandibular joint following experimentally induced anterior
disk displacement. Acta Anat (Basel). 147:159–167. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Leonardi R, Villari L, Bernasconi G and
Caltabiano M: Histochemical study of the elastic fibers in
pathologic human temporomandibular joint discs. J Oral Maxillofac
Surg. 59:1186–1192. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Duca L, Lambert E, Debret R, Rothhut B,
Blanchevoye C, Delacoux F, Hornebeck W, Martiny L and Debelle L:
Elastin peptides activate extracellular signal-regulated kinase 1/2
via a Ras-independent mechanism requiring both p110gamma/Raf-1 and
protein kinase A/B-Raf signaling in human skin fibroblasts. Mol
Pharmacol. 67:1315–1324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Simionescu A, Philips K and Vyavahare N:
Elastin-derived peptides and TGF-beta1 induce osteogenic responses
in smooth muscle cells. Biochem Biophys Res Commun. 334:524–532.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Houghton AM, Quintero PA, Perkins DL,
Kobayashi DK, Kelley DG, Marconcini LA, Mecham RP, Senior RM and
Shapiro SD: Elastin fragments drive disease progression in a murine
model of emphysema. J Clin Invest. 116:753–759. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Robinet A, Fahem A, Cauchard JH, Huet E,
Vincent L, Lorimier S, Antonicelli F, Soria C, Crepin M, Hornebeck
W and Bellon G: Elastin-derived peptides enhance angiogenesis by
promoting endothelial cell migration and tubulogenesis through
upregulation of MT1-MMP. J Cell Sci. 118:343–356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gabay C: Interleukin-6 and chronic
inflammation. Arthritis Res Ther. 8:(Suppl 2). S32006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Flannery CR, Little CB, Hughes CE, Curtis
CL, Caterson B and Jones SA: IL-6 and its soluble receptor augment
aggrecanase-mediated proteoglycan catabolism in articular
cartilage. Matrix Biol. 19:549–553. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Romagnani S: Lymphokine production by
human T cells in disease states. Annu Rev Immunol. 12:227–257.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Abbas AK, Murphy KM and Sher A: Functional
diversity of helper T lymphocytes. Nature. 383:787–793. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Aarvak T, Chabaud M, Thoen J, Miossec P
and Natvig JB: Changes in the Th1 or Th2 cytokine dominance in the
synovium of rheumatoid arthritis (RA): A kinetic study of the Th
subsets in one unusual RA patient. Rheumatology (Oxford).
39:513–522. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Anguita J, Rincón M, Samanta S, Barthold
SW, Flavell RA and Fikrig E: Borrelia burgdorferi-infected,
interleukin-6-deficient mice have decreased Th2 responses and
increased lyme arthritis. J Infect Dis. 178:1512–1515. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Helbig G and Krzemień S: Clinical
significance of elastin turnover-focus on diseases affecting
elastic fibres. Wiad Lek. 57:360–363. 2004.PubMed/NCBI
|
|
53
|
Jawad R, Elleman C, Vermeer L, Drake AF,
Woodhead B, Martin GP and Royall PG: The measurement of the β/α
anomer composition within amorphous lactose prepared by spray and
freeze drying using a simple (1)H-NMR method. Pharm Res.
29:511–524. 2012. View Article : Google Scholar : PubMed/NCBI
|