Introduction
Systemic inflammatory response syndrome (SIRS) is
caused by an inappropriately strong inflammatory reaction to
infectious or non-infectious stimuli, and results in the release of
humoral and cellular inflammatory mediators (1). While a certain degree of inflammation
is appropriate and protective, SIRS is a pathophysiological process
that may cause extensive tissue injury (2). SIRS has become a leading cause of
mortality in patients with infectious disease (3). SIRS occurs as an intermediate step in
a pathophysiological process that spans: i) Injury; ii) stress
responses; iii) SIRS with multiple organ dysfunction syndrome; and
eventually iv) multiple organ failure (MOF) (4). Notably, although SIRS is a severe
complication, there is the opportunity for a cure or reversal of
the pathophysiological process prior to MOF.
Infections are an important cause of SIRS, and
>80% of bacterial infections are caused by Gram-negative
bacteria that produce lipopolysaccharide (LPS), which is one of the
principal triggers of SIRS (5,6).
Inflammatory cells, including activated neutrophils and
granulocytes, may exert an important role in the pathophysiological
processes of SIRS (7,8). In addition, tumor necrosis factor-α
(TNF-α) is an important inflammatory mediator released from cells
stimulated by LPS (9). TNF-α is
additionally hypothesized to be an important contributor to SIRS,
as it appears early, peaks rapidly and exhibits numerous host
functions (10). TNF-α exists as a
transmembrane protein (mTNF-α; 26 kDa) that is cleaved by a
metalloproteinase to release the ectodomain, which becomes mature
secreted TNF-α (sTNF-α; 17 kDa) during the inflammatory response
(11). High levels of sTNF-α are
cytotoxic and a major contributor to inflammation-mediated tissue
injury (12). Therefore, a better
understanding of how mature TNF-α is produced, and its biological
function, may inform the development of novel therapeutic
strategies to effectively inhibit LPS-induced TNF-α overproduction,
thereby preventing SIRS and MOF.
mTNF-α is processed by either matrix
metalloproteinases or disintegrin and metalloproteinase
domain-containing proteins (ADAMs). ADAM17, also termed TNF-α
converting enzyme (TACE), is highly specific for mTNF-α and cleaves
it to produce sTNF-α by hydrolyzing mTNF-α between Ala76 and Vla77
(12–14). Therefore, a potential method for
controlling the toxicity of sTNF-α is to develop TACE-specific
inhibitors.
The present study aimed to develop a novel platform
for inhibiting the LPS-induced inflammatory reaction leading to
SIRS. Therefore, a recombinant lentiviral vector was developed to
deliver short hairpin (sh)RNA targeting ADAM17, to effectively
inhibit ADAM17 expression. The vector was tested in vitro
using U937 cells stimulated with LPS, and in vivo in a mouse
model of endotoxemia.
Materials and methods
Ethics statement
The present study was performed in strict accordance
with the recommendations in the Guide for the Care and Use of
Laboratory Animals (15) of the
National Institutes of Health (Bethesda, MD, USA). The protocol was
approved by the Committee on the Ethics of Animal Experiments of
Wuhan University (Wuhan, China; permit no. WDRY2015-K006). All
surgery was performed under 30 mg/kg sodium pentobarbital
anesthesia, and all efforts were made to minimize suffering.
Reagents
T4 DNA ligase and the Plasmid Maxi kit were
purchased from Qiagen China Co., Ltd. (Shanghai, China). Human cell
lines 293T and U937 were purchased from the Experimental Cell
Center of Wuhan University, and Escherichia coli DH5α were
maintained in the laboratory. Lentiviral vectors and primers were
synthesized by Shanghai GeneChem Co., Ltd. (Shanghai, China).
Kunming mice were purchased from the Laboratory Animal Center,
Tongji Medical College (Wuhan, China). Other reagents noted below
were analytical grade (Takara Biotechnology Co., Ltd., Dalian,
China). The U937 cell line was authenticated by short tandem repeat
profiling conducted by Shanghai Zhong Qiao Xin Zhou Biotechnology
Co., Ltd. (Shanghai, China; sample no. 20170303-20).
Construction of lentiviral vectors
expressing shRNA
The shRNA sequence used to target ADAM17 was the
same as that previously described (11). Synthetic oligonucleotide sequences
were constructed, and annealed to create double-stranded DNA using
the following sequences: forward, 5′-ccg gcc TGG TTA CAA CTC ATG
AAT Tct cga gAA TTC ATG AGT TGA TTG TAA CCA ggt ttt tg-3′ and
reverse, 5′-aat tca aaa acc TGG TTA CAT GAA TTc tcg agA ATT CAT GAG
TTG TAA CCA gg-3′. The sequence written in uppercase represents the
stem and lowercase sequences are the loop. The target gene was
inserted into the AgeI and EcoRI cleaved pGC-LV
vector by homologous recombination using the T4 DNA ligase enzyme.
Competent DH5α cells were prepared with calcium chloride and
subsequently transformed. Positive clones were selected by
polymerase chain reaction (PCR) analysis using Taq enzyme (cat. no.
DR010s; Takara Biotechnology Co., Ltd.). The following primer
sequences were used for PCR: Forward, 5′-ccatgattccttcatatttgc-3′
and reverse, 5′-cgcgtggataaccgtattac-3′. The thermocycling
conditions for PCR were as follows: 5 min at 94°C, followed by 30
cycles of 30 sec at 94°C, 30 sec at 60°C and 30 sec at 72°C, and a
final extension at 72°C for 6 min. The recombinant positive clones
were sent to Invitrogen (Thermo Fisher Scientific, Inc., Waltham,
MA, USA) for sequencing.
Production of the lentiviral
vector
293T cells in the logarithmic growth phase were
digested with trypsin 24 h prior to being transfected. The cells
were resuspended at 6.0×108 cells/l in Dulbecco's
modified Eagle's medium containing 10% fetal bovine serum (FBS) and
maintained at 37°C in an atmosphere containing 5% CO2
for 24 h until the cells were 50–60% confluent. The cell culture
medium was replaced with serum-free medium 2 h prior to
transfection. DNA was extracted using the Plasmid Maxi kit (Qiagen
China Co., Ltd.), according to the manufacturer's instructions. A
solution containing the prepared DNA (20 µg pGC-LV-shRNA vector, 15
µg pHelper vector 1.0, and 10 µg pHelper vector 2.0) was added to a
sterile centrifuge tube and the final volume was brought to 2.5 ml.
The solution was incubated at room temperature for 5 min, and 293T
cells were transfected using Lipofectamine™ 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instruction. The transfected cells were incubated in serum-free
medium for 8 h, the medium was subsequently replaced with medium
containing 10% serum, and the cells were cultured for an additional
48–72 h.
The culture supernatants were collected when the
cells expressed high levels of green fluorescence and cell fusion
was observed (48–72 h). The virus in the supernatant was
concentrated to the target volume by centrifugation (4°C, 4,000 ×
g, 10 min). The virus concentrate was removed and aliquoted into a
virus tube for long-term storage at −80°C. In order to determine
the viral titer, 100 µl viral stock was used to make 1 ml serial
dilutions (10−2-10−6), which were used to
infect 293T cells in a six-well plate (2×105 cells).
Following 48 h in culture, green fluorescent protein (GFP)
expression in each well was observed under a fluorescence
microscope (magnification, ×200). The number of cells expressing
GFP was counted and multiplied by the dilution ratio to determine
the viral titer [transducing units (TU)/ml].
Assessing the cytotoxicity of the
lentiviral vector
U937 cells were grown in RPMI-1640 medium
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) containing 10% FBS.
When the cells had reached 105-106 cells/ml,
they were diluted to 5×104 cells/ml with RMPI-1640,
inoculated into a 6-well plate and maintained in 5% CO2
at 37°C for 12 h. Recombinant lentivirus (0.5 ml, 100 ng/ml) was
added and the empty vector was used as a control. After 12 h, the
condition of the cells was observed. If there was no evidence of a
significant cytotoxic effect, the culture was continued for 24 h
prior to changing the culture medium. If cytotoxic effects were
observed, the medium was changed immediately. The GFP expression
was observed 3 days subsequent to infection, using a fluorescence
microscope.
Detection of protein expression by
western blotting
Total proteins from U937 cells infected with the
ADAM17 shRNA lentiviral vector or a negative control were extracted
using the radioimmunoprecipitation assay (RIPA; 1% Triton-100, 50
mmol/l TrisHCl, 150 mmol/l NaCl, 0.1 mmol/l phenylmethylsulfonyl
fluoride, 1 µmol/l pepstatin, 0.5 mg/ml leupeptin and 0.3 µmol/l
aprotinin) method, and protein concentrations were measured using a
bicinchoninic acid assay. LPS (Sigma-Aldrich; Merck KGaA)
stimulation with 2.5 mg/l LPS was performed prior to western
blotting for 6 h at room temperature. Total protein (50 µg) was
separated by 10% SDS-PAGE at 300 mA for 2 h, followed by transfer
to a nitrocellulose membrane. The membrane was blocked for 2 h in
TBS-Tween-20 (20% Tween-20; TBST) and 5% nonfat dry milk at room
temperature, and subsequently incubated with a primary antibody
against human ADAM17 (cat. no. 14-6202; 1:600; Abcam, Cambridge,
UK) at 4°C overnight. Subsequently, the membrane was washed three
times with TBST, and incubated with a horseradish
peroxidase-labeled secondary antibody (cat. no. sc-2004; 1:1,000;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at room
temperature for 1 h. The membrane was washed three times with TBST
and the bands were visualized using enhanced chemiluminescence
(PerkinElmer, Inc., Waltham, MA, USA). The film was scanned or
photographed, and analyzed using a gel imaging processing system
(Beijing Sage Creation Science Co., Ltd., Beijing, China; GSD800
system). The results were analyzed using Quantity One software
v4.62 (Bio-Rad Laboratories, Inc., Hercules, CA, USA). β-actin
(1:600; cat. no. sc-69879; Santa Cruz Biotechnology, Inc.) was used
as the internal control. The experiment was repeated three
times.
Measuring sTNF-α from U937 cells
following LPS stimulation using ELISA analysis
A total of three groups underwent the following
experiment: The shRNA + LPS group, the LPS + blank vector group,
and a blank vector without LPS stimulation as the control group. In
the shRNA + LPS group, the cells (1×105 /ml; in a
12-well plate at 1 ml/well) were infected with the ADAM17 shRNA
vector for 66 h and were subsequently stimulated with 2.5 mg/l LPS
(12) for an additional 6 h at
room temperature. The LPS group was stimulated with 2.5 mg/l LPS
for 6 h at room temperature. The supernatants from each well were
collected and the sTNF-α levels were measured using an ELISA
performed according to the manufacturer's protocol (cat. no.
550610; BD Biosciences, Franklin Lakes, NJ, USA). Using a
microplate reader, the absorbance of each well was detected at 495
nm.
Measuring mTNF-α on the surface of
U937 cells following LPS stimulation using western blotting
Western blotting was used to detect the expression
of mTNF-α on the surface of the U937 cells in the shRNA + LPS, LPS
and control groups. The RIPA method was used to extract the mTNF-α
proteins from U937 cells. Proteins were probed with the rabbit
anti-human mTNF-α primary antibody (cat. no. ab169616; 1:600;
Abcam). The detailed experimental procedure was the same as for the
aforementioned detection of ADAM17 protein.
Assessing the in vivo effects of
ADAM17 knockdown
Specific pathogen-free grade Kunming mice (male;
n=18; ~20 g; 8 weeks old) were randomly divided into three groups
(n=6/group). Rats were housed 2 or 3 to a cage under specific
pathogen-free conditions (controlled temperature of 24±3°C and
humidity of 55±15%) with a 12-h light/dark cycle and ad
libitum access to tap water and food. The three treatment
groups were: i) PBS control group; ii) endotoxemia group; iii)
endotoxemia + lentivirus group. Endotoxemia was established by
injecting 0.1 ml of a solution containing 10 mg D-ammonium
galactosamine and 2 µg LPS into the caudal vein (16). For the endotoxemia + lentivirus
group, 48 h prior to inducing endotoxemia, the mice were injected
with the shRNA ADAM17 lentivirus (4×108 TU/mouse)
through the caudal vein. The mice in the control group were
injected with the blank vector in the same manner as those in the
endotoxemia + lentivirus group. The reaction of each group of mice
was observed. The mice were sacrificed 6 h post-LPS injection using
cervical dislocation. Sterile saline (5 ml) was immediately
injected into the abdomen, which was gently massaged for 1 min.
Subsequently, 10 ml peritoneal fluid was extracted into a
centrifuge tube and centrifuged for 5 min at 1,000 × g at room
temperature. The cells were washed twice with PBS, resuspended in
RPMI-1640 containing 10% FBS and cultured at 37°C under 5%
CO2 for 1 h. Once the cells had adhered to the wall of
the tube, the non-adherent cells were removed and fresh medium was
added.
The livers, kidneys and lungs were also removed.
Some tissues were used immediately to prepare frozen sections (at
−4°C; 7 mm sections) for the detection of GFP expression under a
fluorescence microscope (BX53; Olympus Corporation, Tokyo, Japan).
The remaining tissues were collected and fixed with 4%
paraformaldehyde for 24 h at room temperature then dehydrated (75%
alcohol for 4 h, 85% alcohol for 2 h, 90% alcohol for 1.5 h, 95%
alcohol for 1 h, absolute ethanol I for 30 min and absolute ethanol
II for 30 min), cleared, dipped in wax (60°C for 1 h, three times),
paraffin-embedded and sliced into sections (4-mm). Following
dewaxing (xylene I for 10 min, xylene II for 10 min, absolute
ethanol I for 5 min, absolute ethanol for 5 min, 95% alcohol for 3
min, 85% alcohol for 2 min, 70% alcohol for 2 min and distilled
water for 2 min), the sections were stained with hematoxylin for 7
min at room temperature, and then counterstained with eosin for 2
min at room temperature. The stained tissues were observed under a
fluorescence microscope (BX53; Olympus Corporation) and images were
captured for analysis; a total of 10 fields of view were
observed.
Flow cytometric analysis of mTNF-α on
the surface of peritoneal macrophages
Following trypsinization and two washes with PBS
containing 5% bovine serum albumin (BSA; Sigma-Aldrich; Merck
KGaA), the murine peritoneal macrophages were resuspended at
106 cells/ml (1 ml). Following blocking with PBS
containing 5% BSA (Sigma-Aldrich; Merck KGaA) for 1 h at 4°C, the
cells were incubated with a primary goat anti-mouse TNF-α
polyclonal antibody (cat. no. ab8348; 1:100; Abcam) for 1 h at 4°C.
The cells were washed twice with PBS, followed by incubation with a
FITC-conjugated rabbit anti-goat secondary antibody (cat. no.
BA1101; 1:60; Boster Biological Technology, Pleasanton, CA, USA)
for 1 h at 4°C in the dark. Following centrifugation 400 × g for 2
min at 4°C) and two washes with PBS, the cells were resuspended in
PBS at 4°C and immediately subjected to flow cytometry (BD
FACSCalibur™ flow cytometer and analysis using BD CellQuest Pro
software version 5.2.1 (both BD Biosciences). The average
fluorescence of 5×104 cells was used to determine the
expression levels of mTNF-α.
Statistical analysis
Experiments were repeated three times and
statistical analyses were performed using SPSS version 11.0 (SPSS,
Inc., Chicago, IL, USA). All data are presented as the mean ±
standard deviation. Where there was homogeneity of variance, a
paired Student's t-test was used to evaluate differences between
two groups. Otherwise, differences among three groups were
determined by one-way analysis of variance with a
Student-Newman-Keuls post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Construction of the ADAM17 shRNA
lentiviral vector
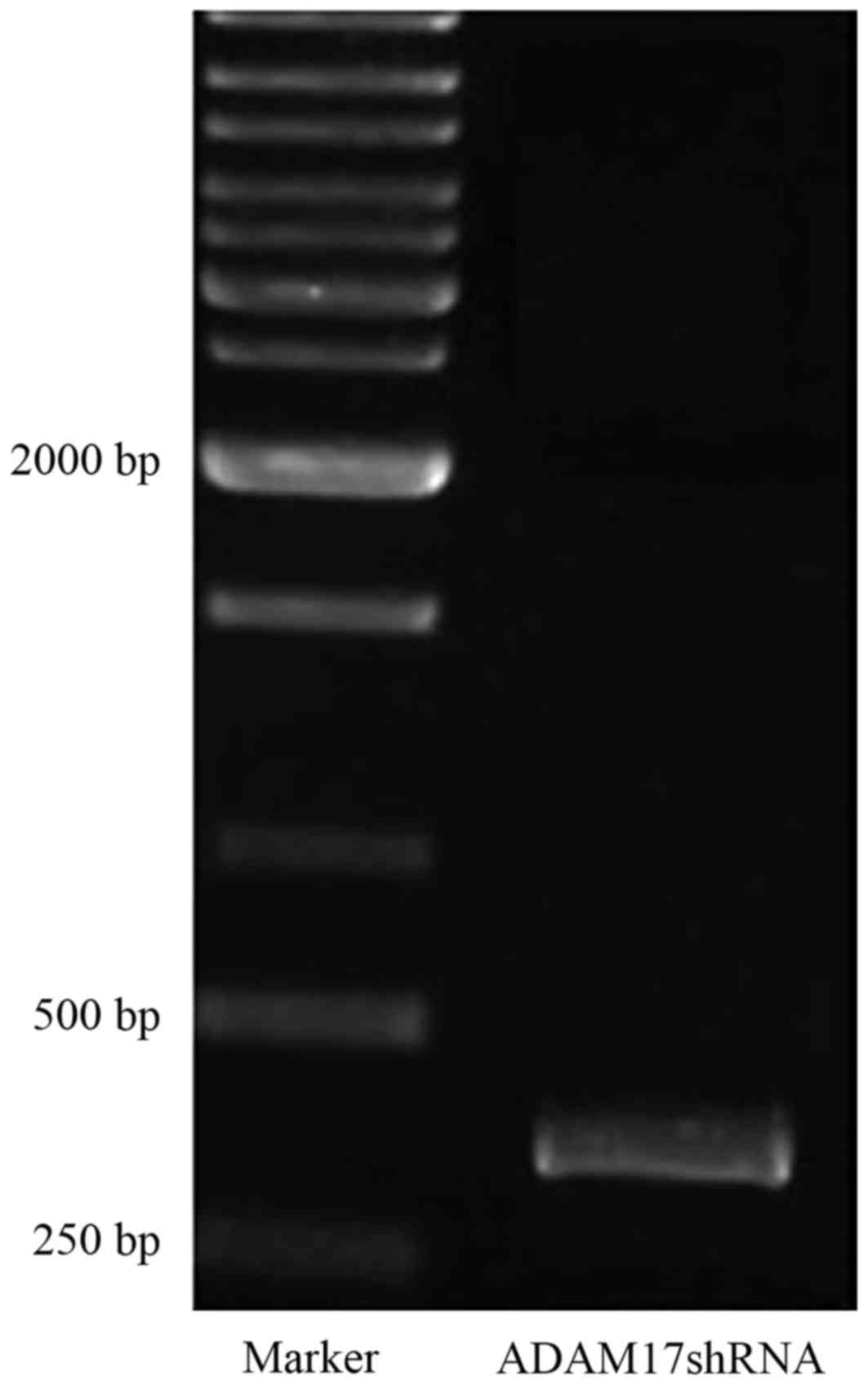
In order to construct the ADAM17 shRNA lentivirus
(pGC-LV-ADAM17), bacterial clones that had incorporated the target
shRNA sequence were identified using PCR analysis; the expected
product size was 341 bp (Fig. 1).
The recombinant positive clones were sent to Invitrogen (Thermo
Fisher Scientific, Inc.) for sequencing, and the nucleotide
sequence of the synthetic ADAM17 shRNA was confirmed along with
correct insertion into the vector. Subsequently, pGC-LV-ADAM17 was
used to transfect 293T cells. The viral titer was determined based
on GFP expression observed under an inverted microscope. The stock
viral titer was 8×108 TU/ml, indicating efficient
transfection and successful virus packaging. The infection
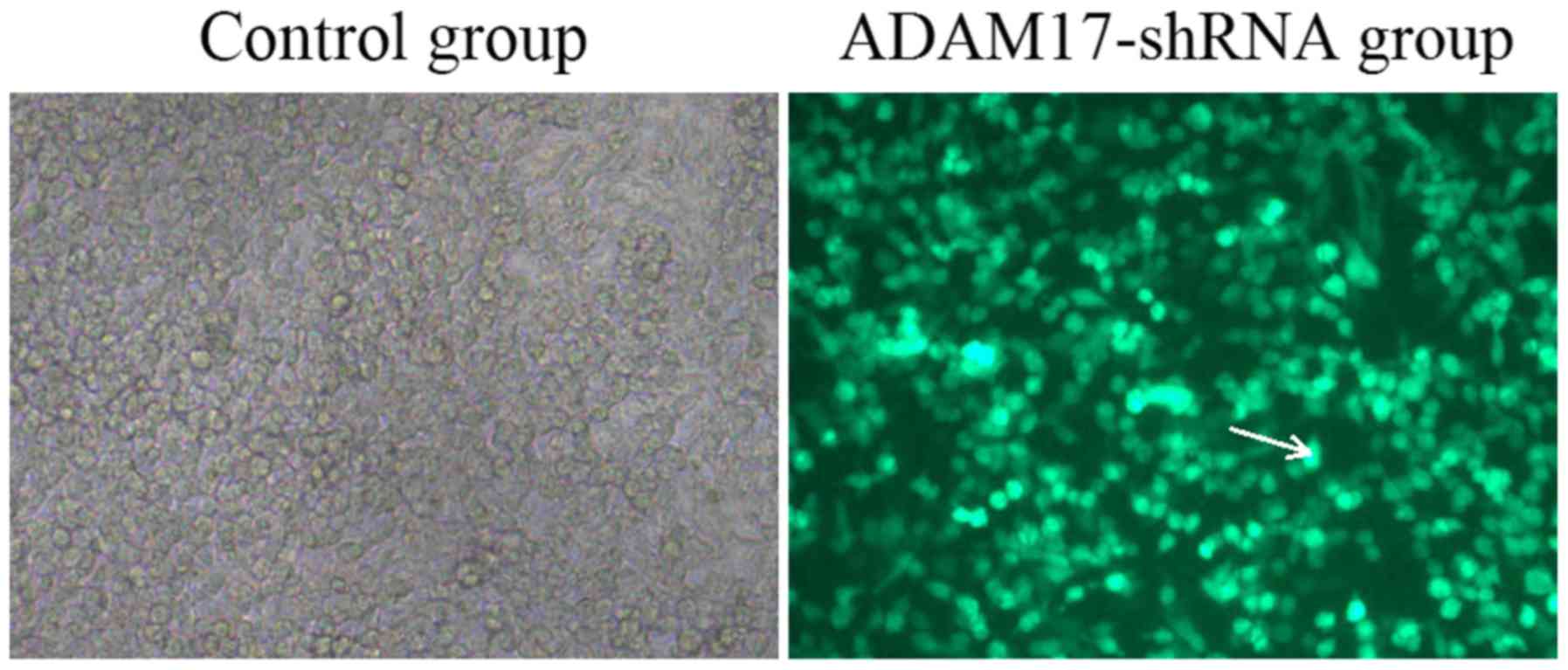
efficiency in U937 cells was subsequently assessed. A total of 72 h
post-infection, ~90% of the infected U937 cells expressed GFP,
indicating that the ADAM17-shRNA lentivirus exhibited a strong
tropism for U937 cells (Fig.
2).
Detection of ADAM17 protein using
western blotting
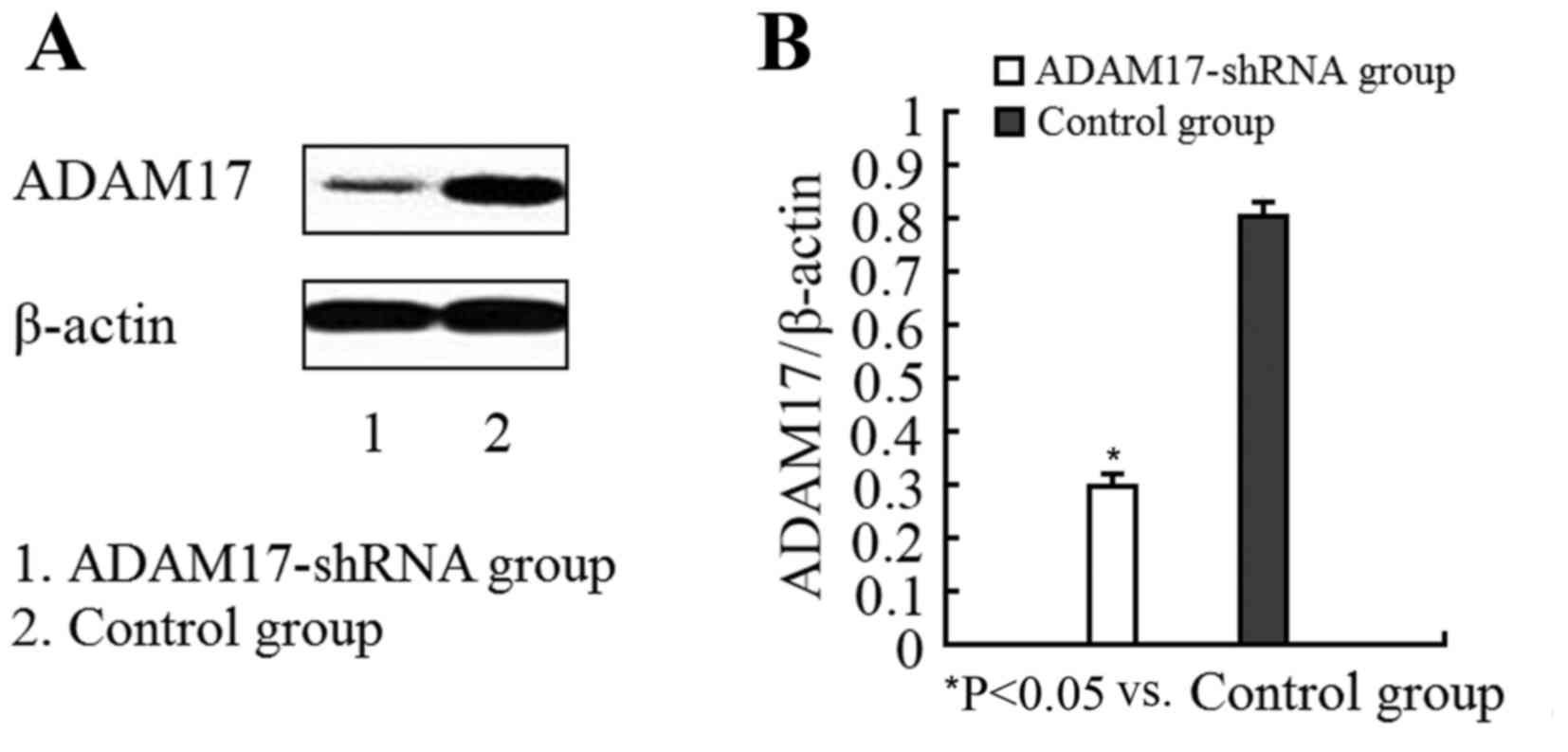
In order to determine whether the ADAM17-shRNA
lentivirus reduced ADAM17 protein expression, the levels of ADAM17
in infected and control cells were compared. Compared with the
control group, the ADAM17 protein levels in infected cells were
significantly decreased (Fig. 3)
demonstrating that the ADAM17-shRNA lentivirus effectively silenced
the ADAM17 gene and inhibited expression at the protein level.
Assessing the effects of the
ADAM17-siRNA lentivirus on LPS-induced TNF-α secretion in U937
cells
In order to identify the biological function of the
ADAM17-siRNA lentivirus, sTNF-α production in response to LPS
stimulation in U937 cells was measured using an ELISA (Table I). While LPS stimulation did elicit
a TNF-α response in cells infected with the ADAM17-shRNA
lentivirus, the concentration of sTNF-α in the ADAM17-shRNA + LPS
group was significantly decreased compared with in the LPS group.
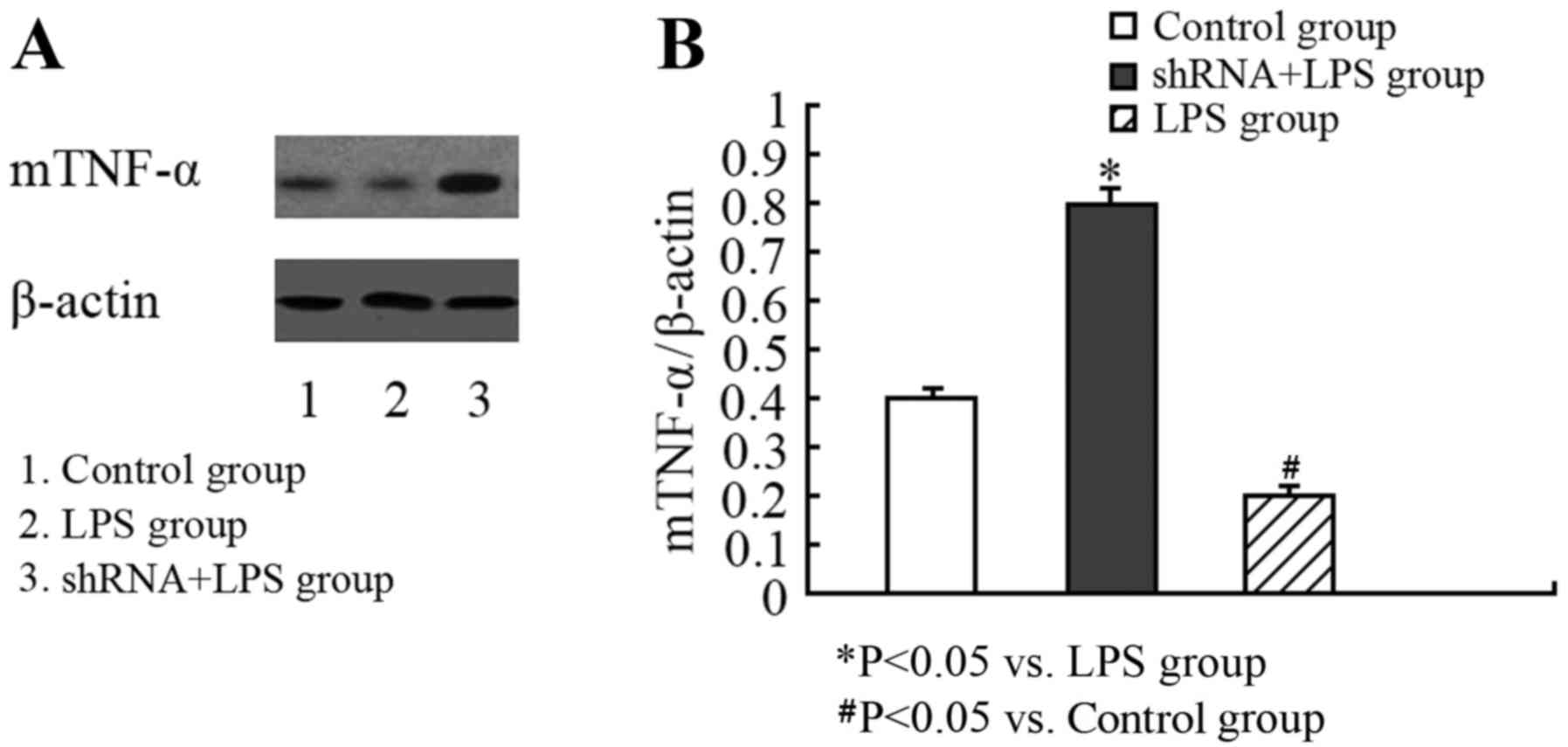
Since sTNF-α levels are associated with the levels of mTNF-α, the
present study additionally assessed whether there were alterations
in mTNF-α in LPS-stimulated U937 cells transfected with the
ADAM17-shRNA lentivirus. As mTNF-α is a transmembrane protein,
western blotting was performed, as described previously (11), to determine the expression of the
protein localized to the surface of U937 cells. Compared with the
LPS group, there was significantly increased expression of mTNF-α
on the surface of the U937 cells in the ADAM17-shRNA + LPS group
(Fig. 4), indicating inhibition of
TACE activity.
 | Table I.sTNF-α concentration in U937 culture
supernatants (mean ± standard deviation; n=3). |
Table I.
sTNF-α concentration in U937 culture
supernatants (mean ± standard deviation; n=3).
| Group | Concentration of
sTNF-α, µg/l |
|---|
| Control |
4.21±2.34 |
| LPS |
19.56±5.01a |
| LPS +
ADAM17-shRNA |
10.35±3.37b |
Effects of the ADAM17-shRNA lentivirus
on a murine model of endotoxemia
In order to determine whether the ability of the
ADAM17-shRNA lentivirus to inhibit the production of sTNF-α was
physiologically relevant, it was tested in a mouse model of
endotoxemia. The lentivirus was injected into the caudal vein of
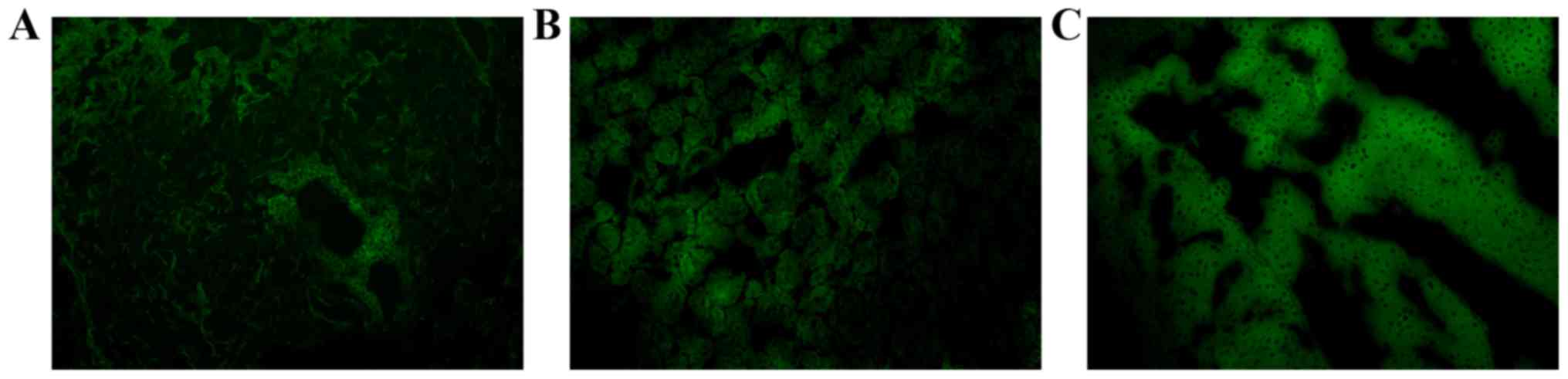
the mice and, upon necropsy, GFP was observed in the liver, lung
and kidney, indicating that the recombinant lentivirus was
successfully introduced into the mice (Fig. 5).
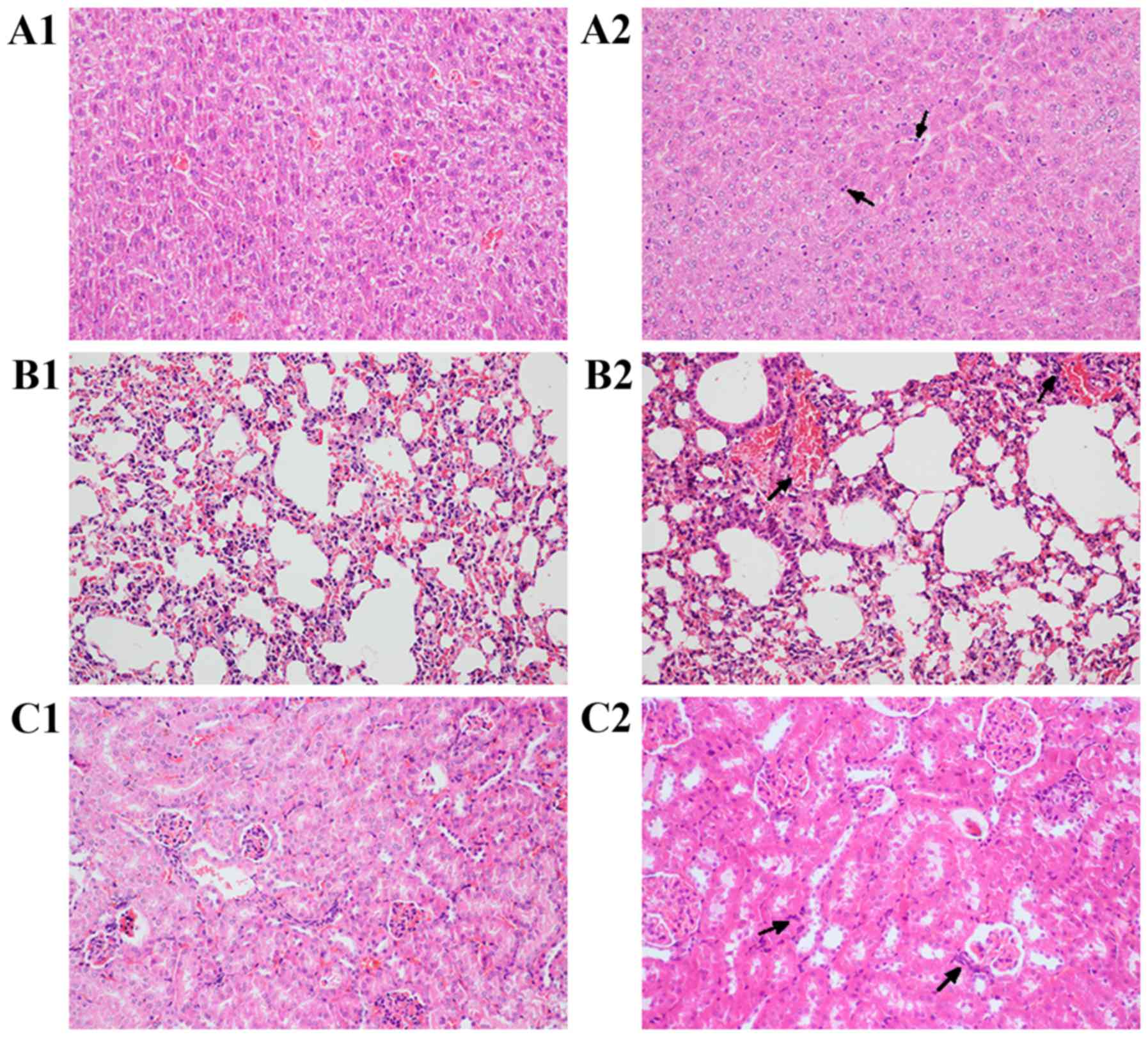
In response to an LPS challenge, the endotoxemia
group exhibited evidence of significant degeneration and necrosis
(Fig. 6); this was observed in the
liver, kidney and lung tissues 6 h post-challenge, in addition to a
large number of inflammatory cell infiltrates (Fig. 6A2, B2 and C2). Conversely, the mice
exposed to the ADAM17-shRNA lentivirus exhibited less LPS-mediated
inflammation in the liver, kidney and lung tissues, and fewer signs
of degeneration and necrosis. In the liver, hepatic lobule
structures were present and there was slight edema in the
hepatocytes, in addition to a small amount of inflammatory cell
infiltration (Fig. 6A1). In the
kidney, the glomerular structure was present and there was slight
edema in the proximal tubule epithelium, in addition to mild
luminal stenosis (Fig. 6C1). In
the lungs, there was a small amount of inflammatory cell
infiltration in the bronchi and alveolar septum, although no
significant inflammation was observed (Fig. 6B1).
Effects of the ADAM17-siRNA lentivirus
on the surface expression of mTNF-α in peritoneal macrophages from
mice with endotoxemia
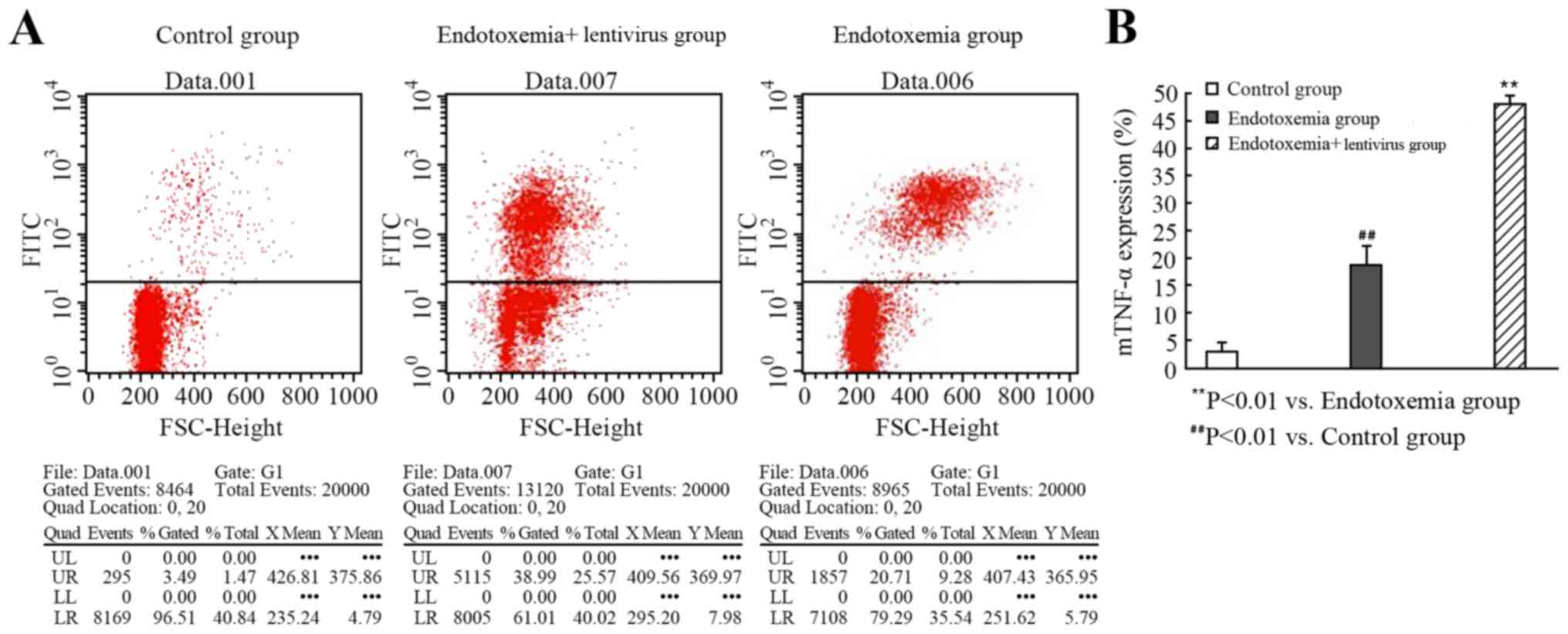
Flow cytometry was used to determine whether
exposure to the ADAM17-shRNA lentivirus altered the expression of
mTNF-α on peritoneal macrophages following an LPS challenge in
mice. The fluorescence intensity of mTNF-α staining in the
peritoneal macrophages from the mice exposed to the ADAM17-shRNA
lentivirus was increased, compared with the endotoxemia group. This
result indicated that the activity of ADAM17 was inhibited, thereby
reducing ADAM17-mediated sTNF-α production and increasing the
expression of mTNF-α on the cell surface (P<0.01; Fig. 7; Table II).
 | Table II.mTNF-α expression on macrophages, as
detected by flow cytometry (mean ± standard deviation; n=3). |
Table II.
mTNF-α expression on macrophages, as
detected by flow cytometry (mean ± standard deviation; n=3).
| Group | mTNF-α expression
(%) |
|---|
| Control |
2.947±1.119 |
| Endotoxemia |
18.743±3.40a |
| Endotoxemia +
lentivirus |
48.15±1.438b |
Discussion
The present study aimed to determine whether the
pathophysiological effects of TNF-α, produced in response to an LPS
challenge, may be attenuated by regulating the ADAM17-mediated
hydrolysis of mTNF-α. At present, the only known natural inhibitor
of TACE in vivo is metalloproteinase inhibitor 3 (17). The majority of strategies used to
reduce sTNF-α have utilized methods to prevent the production of
sTNF-α or to elicit enhanced clearance of sTNF-α through
vaccinations or antibodies. However, these approaches have led to
notable side effects, including inflammation (18). The present study utilized RNA
interference (RNAi), which is a relatively novel molecular biology
technique. Utilizing lentiviral vectors as a delivery system has
several advantages, including: i) Low immunogenicity; ii) the
ability to infect cells that are dividing and non-dividing; and
iii) the ability to integrate into the host cell genome (19,20).
This approach is fundamentally different from the current
approaches that utilize anti-sTNF-α antibodies or vaccines. The
ADAM17-mediated processing pathway for mTNF-α was selected in the
present study as a novel target for inhibiting the overproduction
of sTNF-α.
In the present study, a lentiviral vector expressing
shRNA targeting ADAM17 was constructed, and it was demonstrated
in vitro that the lentivirus effectively decreased the
protein expression of ADAM17 in U937 cells. The transduction rate
exceeded 90%, demonstrating that the lentiviral vector effectively
inserted the foreign gene into the host cells. Concurrent with the
reduction of ADAM17, levels of sTNF-α in response to an LPS
challenge were decreased in cells exposed to the ADAM17-shRNA
lentivirus, and protein expression levels of mTNF-α were
significantly increased, thus suggesting that the ADAM17-shRNA
lentivirus efficiently inhibited the biological activity of
ADAM17.
LPS has a strong pyrogenic effect in vivo,
and is an important cause of inflammation and various pathological
reactions, including SIRS (21).
Since the cDNA structure of ADAM17 in mice shares 85% homology with
that in humans, and 91.9% amino acid similarity, the ADAM17-shRNA
lentivirus was tested in a mouse model of endotoxemia. It has
previously been observed that lentivirus-mediated RNAi technology
is able to achieve high infection efficiency and stable silencing
effects in experimental animals (22). Consistent with these findings, the
present study observed high levels of GFP expression in the liver,
lung and kidney of mice exposed to the ADAM17-shRNA lentivirus. In
addition, the mice treated with the ADAM17-shRNA lentivirus had
fewer signs of inflammation and less tissue damage in the liver,
lungs and kidneys, compared with the control mice, following an LPS
challenge. Flow cytometric analysis confirmed that the ADAM17-shRNA
lentivirus inhibited the enzymatic cleavage of mTNF-α into sTNF-α,
resulting in a marked increase in mTNF-α expression in pertioneal
macrophages. The results of the present study demonstrated that the
ADAM17-shRNA lentivirus may inhibit ADAM-17 mTNF-α cleavage,
thereby preventing sTNF-α secretion in response to an LPS
challenge.
In conclusion, the present study successfully
constructed a shRNA lentiviral vector targeting the ADAM17 gene,
which had obvious in vitro and in vivo effects on
TNF-α processing in response to an LPS challenge. The results of
the present study may aid the design and improvement of drugs
designed to inhibit the function of ADAM17, and suggested a novel
means of controlling inflammation and its associated processes.
Acknowledgements
The present study was supported by the Nature
Science Foundation of Hubei Province (grant no. 2012FB04418) and
the National Natural Science Foundation of China (grant no.
8100094).
Glossary
Abbreviations
Abbreviations:
|
SIRS
|
systemic inflammatory response
syndrome
|
|
TNF-α
|
tumor necrosis factor-α
|
|
LPS
|
lipopolysaccharide
|
|
MOF
|
multiple organ failure
|
|
mTNF-α
|
transmembrane TNF-α
|
|
sTNF-α
|
secreted TNF-α
|
|
ADAM
|
disintegrin and metalloproteinase
domain-containing protein
|
|
TACE
|
TNF-α converting enzyme
|
References
|
1
|
Zheng Z, Jiany L, Ye L, Gao Y, Tang L and
Zhang M: The accuracy of presepsin for the diagnosis of sepsis from
SIRS: A systematic review and meta-analysis. Ann Intensive Care.
5:482015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ratzinger F, Schuardt M, Eichbichler K,
Tsirkinidou I, Bauer M, Haslacher H, Mitteregger D, Binder M and
Burgmann H: Utility of sepsis biomarkers and the infection
probability score to discriminate sepsis and systemic inflammatory
response syndrome in standard care patients. PLoS One.
8:e829462013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim JH, Seo JW, Mok JH, Kim MH, Cho WH,
Kim KU, Jeon D, Park HK, Kim YS, Kim HH and Lee MK: Usefulness of
plasma procalcitonin to predict severity in elderly patients with
community-acquired pneumonia. Tuberc Respir Dis (Seoul).
74:207–214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moss ML, Jin Sl, Milla ME, Bickett DM,
Burkhart W, Carter HL, Chen WJ, Clay WC, Didsbury JR, Hassler D, et
al: Cloning of a disintegrin metalloproteinase that processes
precursor tumour-necrosis factor-alpha. Nature. 385:733–736. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kamisoglu K, Haimovich B, Calvano SE,
Coyle SM, Corbett SA, Langley RJ, Kingsmore SF and Androulakis IP:
Human metabolic response to systemic inflammation: Assessment of
the concordance between experimental endotoxemia and clinical cases
of sepsis/SIRS. Crit Care. 19:712015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rampanelli E, Dessing MC, Claessen N,
Teske GJ, Joosten SP, Pals ST, Leemans JC and Florquin S:
CD44-deficiency attenuates the immunologic responses to LPS and
delays the onset of endotoxic shock-induced renal inflammation and
dysfunction. PLoS One. 8:e844792013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu CW, Juan LI, Hsu SC, Chen CK, Wu CW,
Lee CC and Wu JY: Role of procalcitonin in the diagnosis of
infective endocarditis: A meta-analysis. Am J Emerg Med.
31:935–941. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Azevedo JR, Torres OJ, Czeczko NG, Tuon
FF, Nassif PA and Souza GD: Procalcitonin as a prognostic biomarker
of severe sepsis and septic shock. Rev Col Bras Cir. 39:456–461.
2012.(In English, Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shu J, He X, Zhang L, Li H, Wang P and
Huang X: Human amnion messnchrmal cells inhibit
lipopolysaceharide-induced TNF-α and IL-1β production in THP-1
cells. Bio Res. 48:692015. View Article : Google Scholar
|
|
10
|
Olofsson PS, Rosas-Ballina M, Levine YA
and Tracey KJ: Rethinkinginflammation: Neural circuit s in the
regulation of immunity. Immunol Rev. 248:188–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Quang P, Braggio E, Badalian-Very
G, Flores L, Zhang Y, Sacco A, Maiso P, Azab AK, Azab F, et al:
Novel tumor suppressor function of glucocorticoid-induced TNF
receptor GITR in multiple myeloma. PLoS One. 8:e669822013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Er A and Dik B: The effects of florfenicol
on the values of serum tumor necrosis factor-α and other
biochemical markers in lipopolysaccharide-induced endotoxemia in
brown trout. Mediators Inflamm. 2014:4643732014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Locksley RM, Killeen N and Lenardo MJ: The
TNF and TNF receptor superfamilies: Integrating mammalian biology.
Cell. 104:487–501. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee YJ, Park CH, Yun JW and Lee YS:
Predictive comparisons of procalcitonin (PCT) level, arterial
ketone body ratio (AKBR), APACHE III score and multiple organ
dysfunction score (MODS) in systemic inflammatory response syndrome
(SIRS). Yonsei Med J. 45:29–37. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press; Washington (DC): pp. 11–35.
2011
|
|
16
|
Fan J, Shek PN, Suntres ZE, Li YH,
Oreopoulos GD and Rotstein OD: Liposomal antioxidants provide
prolonged protection against acute respiratory distress syndrome.
Surgery. 128:332–338. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Salem ES, Grobe N and Elased KM: Insulin
treatment attenuates renal ADAM17 and ACE2 shedding in diabetic
Akita mice. Am J Physiol Renal Pyhsiol. 306:F629–F639. 2014.
View Article : Google Scholar
|
|
18
|
Nicoll JA, Wilkinson D, Holmes C, Steart
P, Markham H and Weller RO: Neuropathology of human Alzheimer
disease after immunization with amyloid-beta peptide: A case
report. Nat Med. 9:448–452. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Katzourakis A, Tristem M, Pybus OG and
Gifford RJ: Discovery and analysis of the first endogenous
lentivirus. Proc Natl Acad Sci USA. 104:6261–6265. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chai N, Chang HE, Nicolas E, Gudima S,
Chang J and Taylor J: Assembly of hepatitis B virus envelope
proteins onto a lentivirus pseudotype that infect sprimary human
hepatocytes. J Virol. 81:10897–10904. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huynh T, Uaesoontrachoon K, Quinn JL,
Tatem KS, Heier CR, Van Der Meulen JH, Yu Q, Harris M, Nolan CJ,
Haegeman G, et al: Selective modulation through the glucocorticoid
receptor ameliorates muscle pathology in mdx mice. J Pathol.
231:223–235. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hope TJ: VIROLOGY. Visualizing
trans-infection. Scinence. 350:511–512. 2015. View Article : Google Scholar
|