Introduction
Staphylococcus aureus (S. aureus) is the most
common causative organism in osteomyelitis (1,2),
which is characterized by severe inflammation and progressive bone
destruction (3). S. aureus
infection often causes excessive bone destruction and leads to the
formation of bone defect (4,5).
However, the precise mechanisms underlying the bone loss caused by
S. aureus infection is not well understood.
Bone is a dynamic organ that is constantly remodeled
throughout life, and this physiological process is tightly
regulated by osteoblasts (mediating bone formation) and osteoclasts
(mediating bone resorption) (6).
The balance between bone formation and bone resorption serves a
great role in the maintenance of bone shape and mineralization
(7). However, under the condition
of bone infection, the balance is destroyed, and because of this,
much research on the mechanism of bone defect infected by S.
aureus focuses on bone formation (8). It is clear that S. aureus
suppresses osteogenic differentiation of marrow mesenchymal stem
cells (9) and inhibits osteoblast
proliferation (10). In addition,
S. aureus can be internalized by osteoblast (11,12)
and subsequently induces osteoblast death (13). However, with respect to the bone
resorption, previous studies have demonstrated that the
surface-associated material (SAM) (14) and Surface-Associated Proteins
(15,16) of S. aureus stimulate
osteoclast formation and enhance bone resorption, but the active
moiety in the SAM is unknown.
Mature osteoclasts are multinucleated cells,
deriving from hematopoietic cells of the monocyte/macrophage family
(17). Current studies have
demonstrated that macrophage-colony stimulating factor (M-CSF) and
receptor activator of nuclear factor (NF)-κB ligand (RANKL) serves
an important role in the process of osteoclast differentiation.
M-CSF promotes the survival of osteoclast precursors and
osteoclasts (18,19) and induces RANK expression in
osteoclast precursors (20). While
RANKL is a key osteoclastogenic cytokine, the binding of RANKL to
its receptor RANK recruits tumor necrosis factor
receptor-associated factor 6, resulting in the activation of NF-κB,
phosphatidylinositol 3-kinase (PI-3K)/Akt, p38, c-Jun N-terminal
kinase (JNK) and extracellular signal-regulated kinase (ERK)
(21), which are involved in the
activation of c-Fos, activator protein 1 (AP-1), microphthalmia
transcription factor (MITF) and PU.1 (22). In the nucleus, the recruitment of
activated NF-κB and nuclear factor of activated T-cells (NFATc) 2
in the promoter of NFATc1 initiates the early activation of NFATc1,
which subsequently complexes with MITF, AP-1, PU.1 and cAMP
response element-binding protein to induce the expression of
osteoclast-specific genes (23),
such as acid-resistant acid phosphatase (TRAP), matrix
metalloproteinase-9 (MMP-9), cathepsin K, calcitonin receptors
(CTR), d2 isoform of the vacuolar ATPase Vo domain (Atp6v0d2) and
β3 integrin (23).
Staphylococcus aureus protein A (SpA) which
is expressed by the majority of S. aureus is an important
virulence factor anchored in the staphylococcal cell wall (24), which interacts with a large number
of human immunoglobulins and exists in a membrane-associated and
secreted form. It is reported that when SpA binds to osteoblasts it
induces cell apoptosis and death (13,25,26)
inhibiting bone formation and mineralization (10,27).
However, the direct effect of SpA on osteoclasts has not been
reported. In the present study, the effect of SpA on osteoclast
differentiation and bone resorption was investigated and the
underlying mechanisms was explored for the first time, to the best
of our knowledge. Results demonstrated that SpA induced osteoclast
differentiation and promoted bone resorption in the absence and
presence of RANKL, and that the NF-κB signaling pathway serves an
important role in this process.
Materials and methods
Materials
The SpA was purchased from Sino Biological (Beijing,
China); fetal bovine serum (FBS) was purchased from Gibco; Thermo
Fisher Scientific Inc. (Waltham, MA, USA); penicillin-streptomycin
solution and high-glucose Dulbecco's modified Eagle's medium (DMEM)
were purchased from HyClone; GE Healthcare (Chicago, IL, USA).
Soluble RANKL was obtained from R&D Systems Inc. (Minneapolis,
MN, USA); JSH-23 was from Selleck Chemicals (Houston, TX, USA);
acid phosphatase leukocyte kit (TRAP, 387A) and methyl thiazolyl
tetrazolium (MTT) were obtained from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). A GeneJET RNA purification kit was purchased
from Thermo Fisher Scientific Inc.; A PrimeScript™ RT reagent kit
with gDNA Eraser (Perfect Real Time) and SYBR Premix Ex Taq™ II
(Tli RNase H Plus) were purchased from Takara Biotechnology Co.,
Ltd. (Dalian, China). Oligonucleotide primer sets were synthesized
by Augct DNA-Syn Biotechnology Co., Ltd. (Beijing, China).
Antibodies against ERK1/2 (cat. no. 9926), p38 (cat. no. 9926), JNK
(cat. no. 9926), NF-κB p65 (cat. no. 8242), inhibitor of κB-α
(IκB-α; cat. no. 4812), protein kinase (Akt; cat. no. 4691), GAPDH
(cat. no. 8884) and NFATc1 (cat. no. 8032), or the phosphorylated
form of p38 (cat. no. 9910), ERK1/2 (cat. no. 9910), JNK (cat. no.
9910), Akt (cat. no. 4060), and NF-κB p65 (cat. no. 3033) were
purchased from Cell Signaling Technology, Inc. (Danvers, MA USA);
Immobilon Western Chemiluminescent horseradish peroxidase (HRP)
Substrate and polyvinylidene difluoride (PVDF) membranes were
purchased from Merck KGaA. A mouse tumor necrosis factor (TNF)-α
SimpleStep ELISA® kit (cat. no. ab208348), an
interleukin-1 (IL)-1α mouse in vitro SimpleStep ELISA™ kit
(cat. no. ab199076), and an IL-6 mouse ELISA kit (cat. no. ab46100)
were purchased from Abcam (Cambridge, UK).
Cell culture
The Raw264.7 mouse monocytes/macrophage cell line
(TIB-71; American Type Culture Collection, Manassas, VA, USA) was
used as osteoclast precursors, which can differentiate into
osteoclast-like cells in the presence of RANKL (28). The cells were grown in high-glucose
DMEM, supplemented with 10% FBS and 1% penicillin-streptomycin
solution, in a humidified atmosphere of 95% air and 5%
CO2 at 37°C; media were changed every 3 days.
Cell viability assay (MTT assay)
Raw264.7 cells were plated in 96-well plates at a
density of 1×104 cells/well with 10 replicates in each
group and cultured for 24 h. Cells were induced with SpA
(concentrations of 50, 100, 200, 400, 800 and 1,600 ng/ml) and
equal volume of phosphate buffered saline (PBS) alone, as control
for 1, 3 and 5 days; media and stimuli were changed every 3 days.
After culturing for the above indicated days, 10 µl MTT solution
was added to the cells and cells were incubated at 37°C for 4 h
away from light. Subsequently, media were removed, and 100 µl
dimethyl sulfoxide was added to the plates and cells were agitated
at room temperature for 10 min. Subsequently, the optical density
(OD) values of every well were detected with a Model 680 Microplate
Reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 570
nm.
In vitro osteoclastogenetic
assays
To differentiate into osteoclasts, Raw264.7 cells
were seeded in 96-well plates at a density of 1×104
cells/well with 5 duplicates in each group and cultured at 37°C for
24 h. Subsequently cells were stimulated with SpA (concentrations
of 50, 100, 200, 400 and 800 ng/ml), SpA + RANKL (50 ng/ml),
SpA+JSH-23 (20 µM), RANKL (100 ng/ml) or equal volumes of PBS
(control) at 37°C for 5 days; media and stimuli were replaced every
3 days. Following this, cells were fixed and stained using the TRAP
staining kit according to the manufacturer's protocol. All cells in
every well were counted, and TRAP-positive multi-nucleated (≥3
nuclei) cells were counted as osteoclast-like cells.
Bone resorption assay
Raw264.7 cells were seeded in 24-well
Corning® Osteo Assay Surface Multiple Well Plates
(Corning Inc., Corning, NY, USA) at a density of 5×104
cells/well with 4 duplicates in each group and cultured at 37°C for
24 h. Subsequently, cells were stimulated with SpA (concentrations
of 50, 100, 200, 400 and 800 ng/ml), SpA+ RANKL (50 ng/ml),
SpA+JSH-23 (20 µM), RANKL (100 ng/ml) and equal volumes of PBS
(control) for 5 days; media and stimuli were changed every 3 days.
After 5 days, media and cells were removed, and the areas of
resorption pits were imaged by an inverted microscope and analyzed
with Scion image software version 4.0.3.2 (Scion Corp., Frederick,
MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Raw264.7 cells were seeded in 6-well plates at a
density of 1×105 cells/well and cultured for 24 h.
Subsequently, cells were stimulated with SpA (concentrations of 50,
100, 200, 400 and 800 ng/ml), SpA+ RANKL (50 ng/ml), RANKL (100
ng/ml) or equal volumes of PBS (control) for 5 days; media and
stimuli were changed every 3 days. After 5 days, cells were
harvested and total RNA was isolated with a GeneJET RNA
purification kit according to the manufacturer's protocol, and the
concentration of total RNA was measured with a micro
spectrophotometer (Thermo Fisher Scientific Inc., NanoDrop 2000).
Total RNA of 500 ng was used to synthesize cDNA by reverse
transcription using PrimeScript™ RT reagent kit with gDNA Eraser.
PCR was performed in a CFX96 Real-time system (Bio-Rad
Laboratories, Inc.) with a SYBR® Premix Ex Taq™.
Reactions were initiated by incubation at 94°C for 5 min, and PCR
[94°C for 30 sec, 60°C (58°C for GAPDH) for 34 sec, 72°C for 30
sec] was performed for 40 cycles; all reactions were performed in
triplicate. Relative quantities of the tested genes were normalized
to GAPDH, and the normalized data were expressed using the
comparative 2−ΔΔCq method (29). Primers used in this study are
presented in Table I.
 | Table I.Primer sequences used in this
study. |
Table I.
Primer sequences used in this
study.
| Target gene | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| TRAP |
GCGACCATTGTTAGCCACATACG |
CGTTGATGTCGCACAGAGGGAT |
| MMP9 |
GCTGACTACGATAAGGACGGCA |
GCGGCCCTCAAAGATGAACGG |
| Cathepsin K |
AGCAGAACGGAGGCATTGACTC |
TTTAGCTGCCTTTGCCGTGGC |
| Calcitonin
receptor |
TGGTGCGGCGGGATCCTATAAGT |
AGCGTAGGCGTTGCTCGTCG |
| ATP6v0d2 |
ACGGTGATGTCACAGCAGACGT |
CCTCTGGATAGAGCCTGCCGCA |
| GAPDH |
CCCAGAAGACTGTGGATGG |
CAGATTGGGGGTAGGAACAC |
Western blot analysis
Raw264.7 cells were cultured in 25 cm2
cell culture flasks; when cells reached 80% confluency, the culture
medium was replaced, and cells were cultured at 37°C for another 1
h in the cell incubator. Following that, cells were stimulated with
SpA (400 ng/ml) for 0, 5, 10, 20, 40 and 60 min, and for NFATc1
measurement, Raw264.7 cells were treated with SpA (400 ng/ml) or
JSH-23+ SpA (400 ng/ml) for 0, 1, 2 and 3 days. At the indicated
time point, cells were harvested and lysed using high efficiency
tissue/cell lysis solution (Beijing Solarbio Science and Technology
Co, Ltd., Beijing, China), and were followed by centrifugation at
4°C and 13,800 × g for 15 min, proteins in the supernatant were
collected. Subsequently, protein concentrations were measured by
Bicinchoninic Acid protein assay kit (Beyotime Institute of
Biotechnology, Haimen, China), and protein samples were mixed with
the sample buffer and boiled at 95°C for 5 min. Following that,
protein samples (20 µg) were loaded onto 8–12% polyacrylamide gels,
transferred to PVDF membranes and subsequently blocked with 5%
skimmed milk or bovine serum albumin (Beijing Solarbio Science and
Technology Co, Ltd., Beijing, China)-TBS (0.05% Tween-20) for 1 h.
Following that, membranes were probed with specific antibodies
against total ERK1/2 (1:1,000), p38 (1:1,000), JNK (1:1,000), NF-κB
p65 (1:1,000), IκB-α (1:500), Akt (1:1,000), GAPDH (1:2,000) and
NFATc1 (1:1,000), or the phosphorylated form of p38 (1:1,000),
ERK1/2 (1:1,000), JNK (1:1,000), Akt (1:1,000) and NF-κB p65
(1:1,000), and were incubated at 4°C for 12 h. Detection was
carried out using a HRP-linked rabbit IgG antibody (cat. no. 7074;
1:1,000; Cell Signaling Technology, Inc. Danvers, MA USA) at room
temperature for 1 h, followed by an enhanced chemiluminescence
western blotting detection reagent at room temperature for 1 min.
Densitometry of the blots were performed using the Bio-Rad
Universal Hood 2 Electrophoresis Imaging Cabinet (Bio-Rad
Laboratories, Inc.), and were analyzed by Bio-Rad Image Lab
Software version 5.2.1 (Bio-Rad Laboratories, Inc.).
Statistical analysis
Each experiment was performed in triplicate and
similar results were obtained. Data are expressed as the mean ±
standard deviation. SPSS 19.0 software (IBM Corp., Armonk, NY, USA)
was used to perform the statistical analysis, and the statistical
significance was determined by one-way analysis of variance
followed by the Least Significant Difference/Student-Newman-Keuls
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of SpA on cell viability
The cytotoxic effect of SpA on the Raw264.7cells was
measured by MTT assay. As depicted in Fig. 1, SpA promoted cell proliferation on
the first day under the concentration of 100–400 ng/ml, but this
effect disappeared on days 3 and 5. Overall, SpA had no
cytotoxicity to Raw264.7 cells below the concentration of 800
ng/ml. However, when the concentration was raised to 1,600 ng/ml,
the cell proliferation activity was significantly inhibited. Since
SpA did not show any toxic effect on cell viability up to 800
ng/ml, concentrations of 50–800 ng/ml were used in the following
experiments.
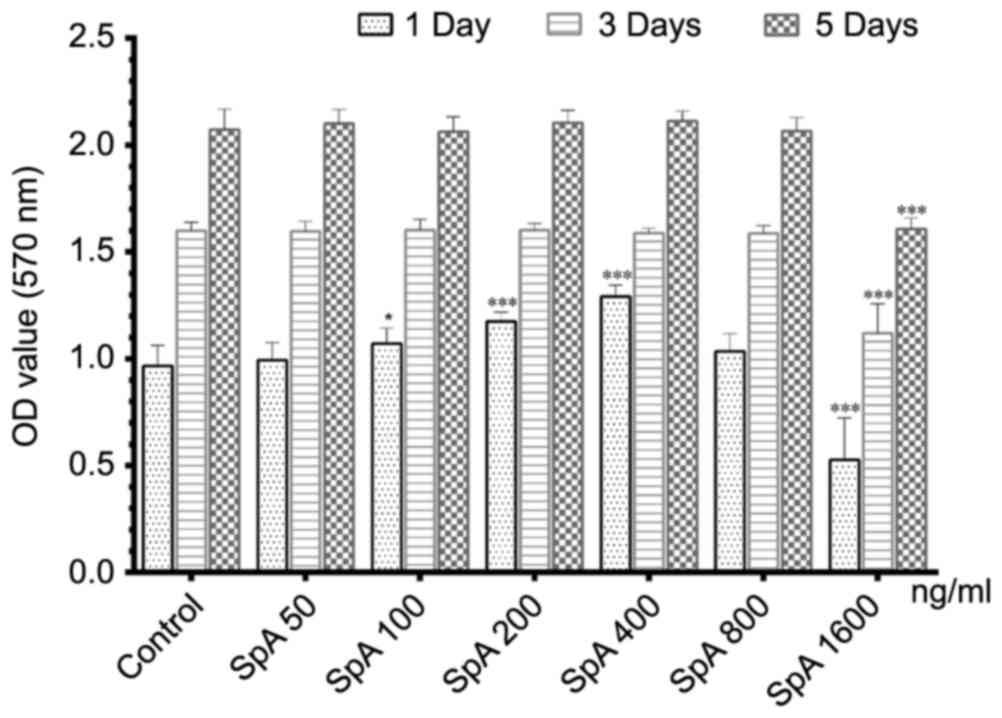 | Figure 1.Effect of SpA on the cell viability
of Raw264.7 cells treated with different concentrations of SpA (50,
100, 200, 400, 800 and 1600 ng/ml) and PBS alone (control), on days
1, 3 and 5. Data are presented as the mean ± standard deviation.
*P<0.05, ***P<0.001, vs. control. SpA, Staphylococcus aureus
protein A; OD, optical density. |
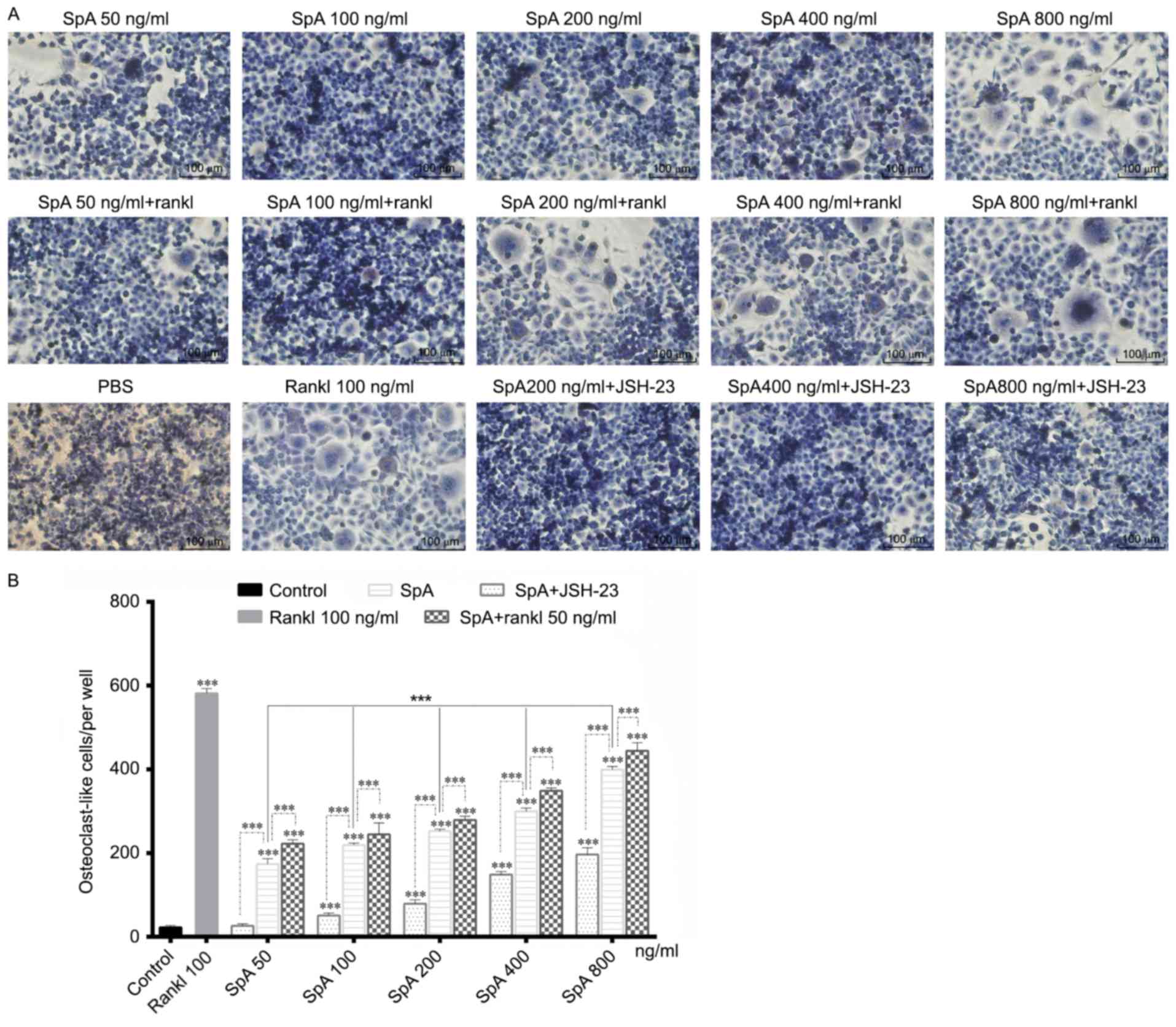
SpA induces the formation of
osteoclast-like cells from RAW264.7 cells in the absence or
presence of RANKL
SpA is an important virulence factor of S. aureus,
the effect of SpA on osteoclast differentiation was explored.
Raw264.7cells were grown in 96-well plates and stimulated with
various concentrations of SpA in the absence or presence of RANKL
for 5 days. SpA significantly promoted the formation of
osteoclast-like cells in a dose-dependent manner in the absence of
RANKL, but the number of osteoclast-like cells formed was lower
than that of RANKL -induced (Fig. 2A
and B). As reported previously, Raw264.7 cells stimulated by
RANKL alone could differentiate into mature osteoclasts (28), however, Staphylococcal lipoteichoic
acid, another virulence factor of S. aureus, inhibits osteoclast
differentiation in the presence of RANKL (30). Therefore, the induction effect of
SpA on osteoclast differentiation in the presence of RANKL was also
examined (Fig. 2A). However, SpA
did not inhibit the formation of osteoclast-like cells in the
presence of RANKL, and the number of osteoclast-like cells was
higher than that without RANKL under the same concentration
(Fig. 2B), which indicated that
RANKL enhanced the promotion effect of SpA on osteoclast
differentiation. In addition, when treated with JSH-23, an
inhibitor of NF-κB activation, the formation of osteoclast-like
cells induced by SpA was inhibited (Fig. 2).
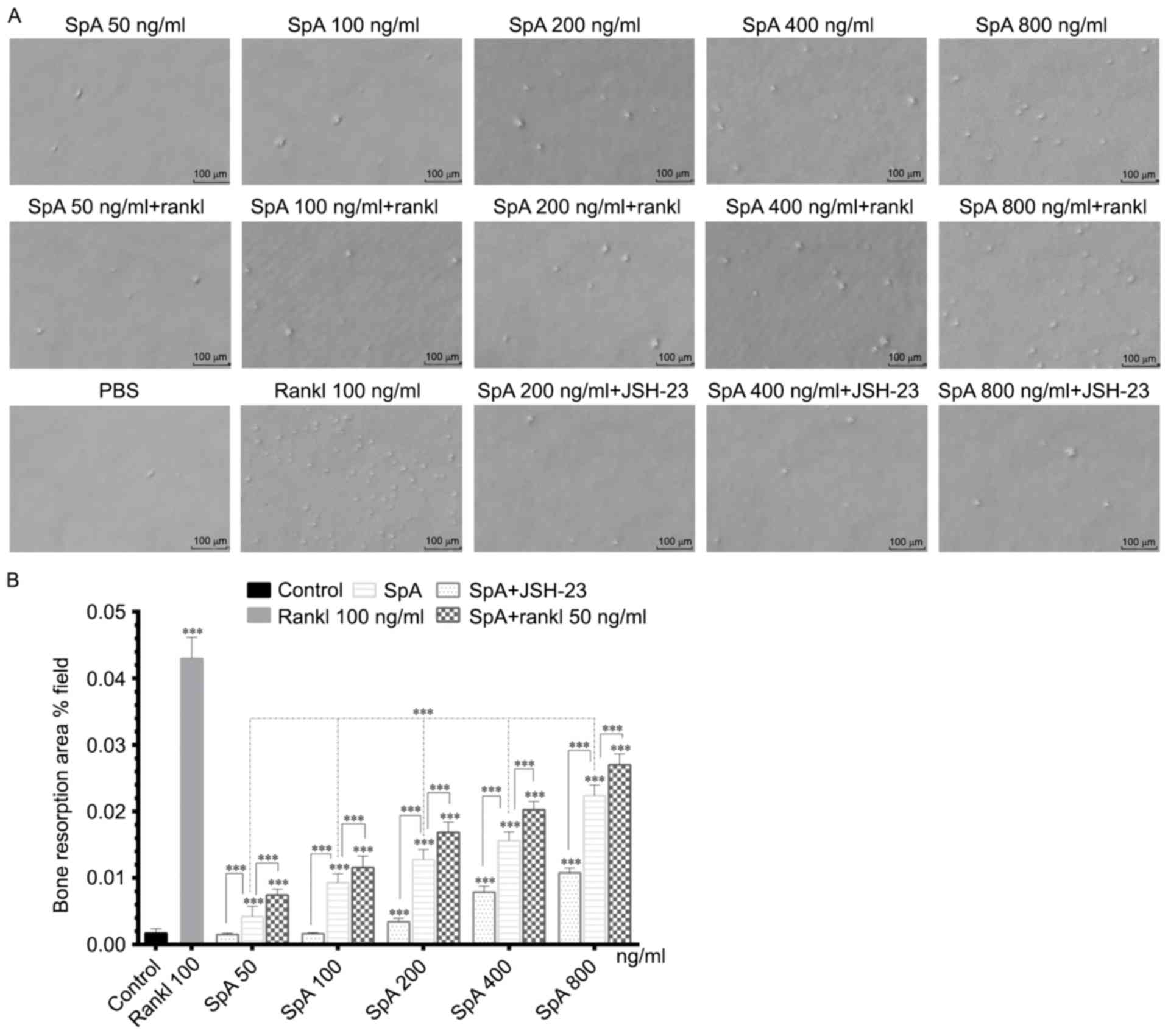
SpA promotes the formation of
resorption pits in the absence or presence of RANKL
Since SpA promoted the formation of osteoclast-like
cells derived from Raw264.7 cells, and bone resorption is the
feature that identifies mature osteoclasts (31), the bone resorption activity of
osteoclast-like cells induced by SpA from Raw264.7 cells was
evaluated next. In the bone resorption assay, Raw264.7 cells were
grown in COAS plates and stimulated with various concentrations of
SpA and RANKL for 5 days. As illustrated in Fig. 3, SpA induced the formation of
resorption pits in a dose-dependent manner in the absence or
presence of RANKL (Fig. 3A and B),
and the area of resorption pits with RANKL were higher than that
without RANKL under the same SpA concentration (200, 400 and 800
ng/ml; Fig. 3B). However, when
treated with JSH-23, the formation of resorption pits induced by
SpA was decreased (Fig. 3).
Collectively, these results suggested that SpA induced the
formation of osteoclasts with bone resorption activity, and this
effect was inhibitedby JSH-23.
SpA increases the mRNA expression
levels of osteoclast-specific genes in Raw264.7 cells
Osteoclasts originated from hematopoietic cells of
the monocyte/macrophage family (17), and Raw264.7 cells have been
demonstrated to differentiate into mature osteoclasts treated with
RANKL alone (17,32). mRNA expression levels of
osteoclast-specific genes, such as TRAP, MMP-9, cathepsin K, CTR
and Atp6v0d2 were measured. As demonstrated in Fig. 4, SpA increased the mRNA expression
levels of TRAP, MMP-9, cathepsin K, CTR and Atp6v0d2 in a
dose-dependent manner, and these expression levels were higher in
the presence of RANKL rather than when treated with SpA alone.
However, when JSH-23 was added, the mRNA expression levels of
osteoclast-specific genes induced by SpA were inhibited (Fig. 4).
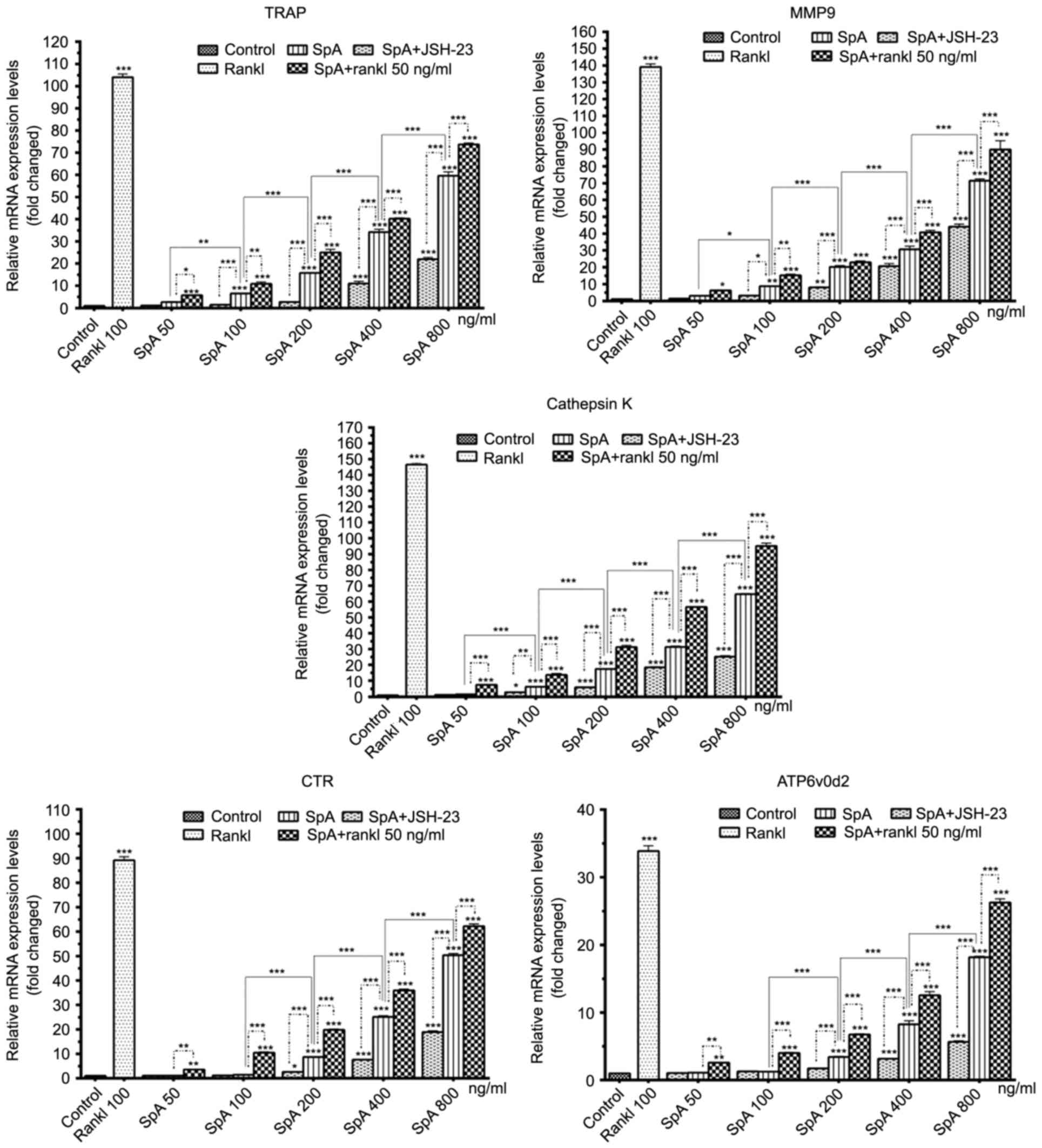 | Figure 4.Effect of SpA (50–800 ng/ml), RANKL
and JSH-23 on the mRNA expression levels of osteoclast-specific
genes (TRAP, MMP-9, Cathepsin K, CTR and ATP6v0d2). Data are
presented as the mean ± standard deviation, *P<0.05,
**P<0.01, ***P<0.001 vs. control. SpA, Staphylococcus aureus
protein A; RANKL, receptor activator of nuclear factor κB-ligand;
TRAP, acid-resistant acid phosphatase; MMP, matrix
metalloproteinase; CTR, calcitonin receptor; ATP6v0d2, d2 isoform
of the vacuolar ATPase Vo domain. |
NF-κB signaling pathway holds a role
in SpA-induced osteoclast differentiation
It was reported that, three mitogen-activated
protein kinase (MAPK; including p38, ERK and JNK) (33), NF-κB (21,34)
and PI-3K/Akt (23,35) signaling pathways have been involved
in osteoclast differentiation. To elucidate the signaling pathways
by which SpA promoted osteoclast differentiation, the protein
expression of NFATc1 and the above-mentioned signaling pathways was
measured in Raw264.7 cells stimulated by SpA. As illustrated in
Fig. 5, the degradation of IκBα
occurred 10 min after SpA treatment (Fig. 5A and B), while the phosphorylation
of NF-κB increased markedly at 5 and 10 min (Fig. 5A and C). Furthermore, proteins in
the signaling pathways of Akt and MAPKs showed no obvious change
when treated with SpA (Fig. 5A).
Because of this, the culture medium was supplemented with JSH-23,
which is an inhibitor of NF-κB (36,37).
Subsequently, Raw264.7 cells were treated with SpA and the
formation of osteoclast-like cells and resorption pits was
decreased; the expression levels of osteoclast-specific genes were
inhibited too, which indicated that NF-κB signaling pathway was
involved in the SpA-induced osteoclast differentiation (Fig. 5A and C).
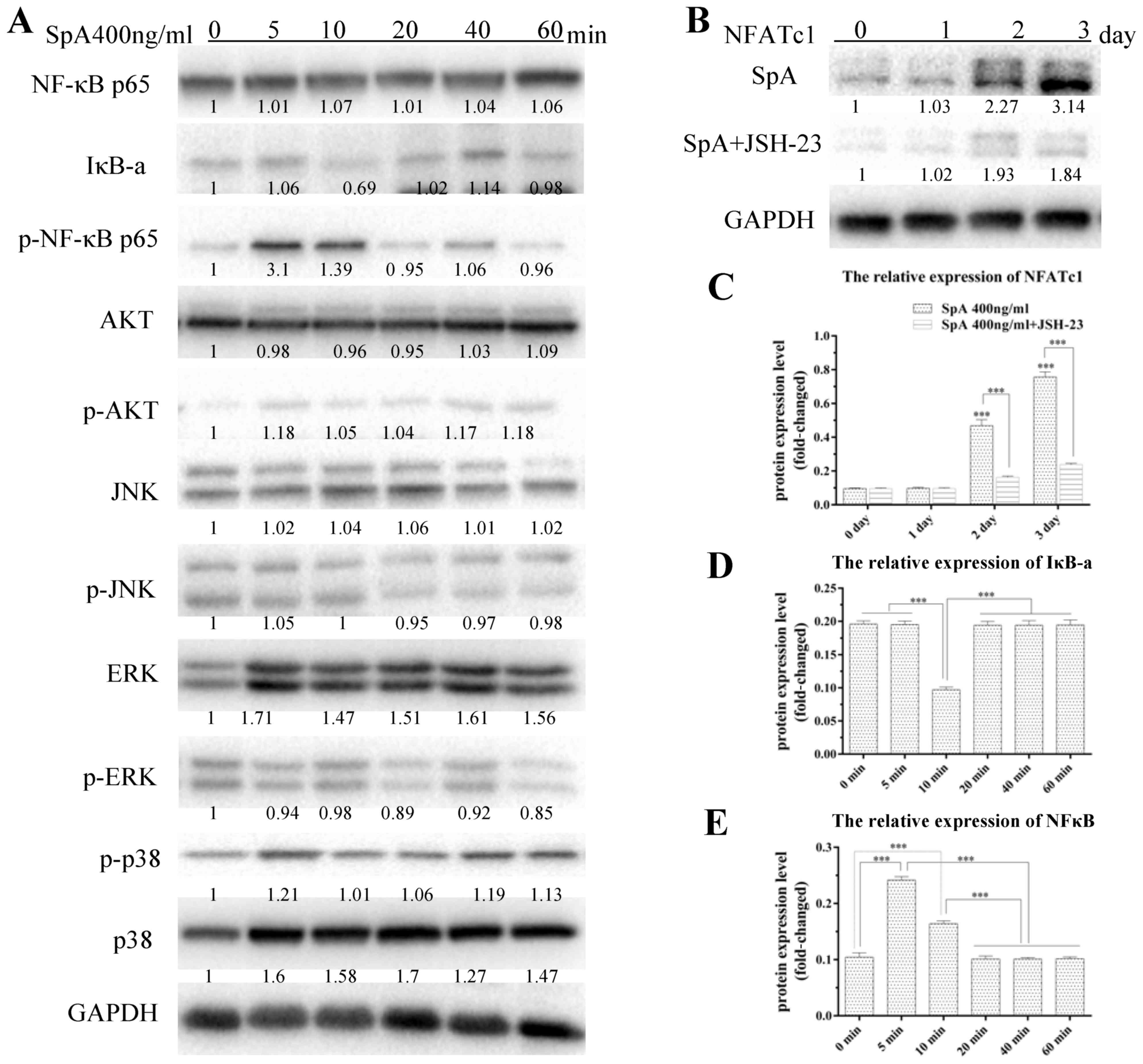 | Figure 5.Effects of SpA on the NF-κB, MAPK and
AKT signaling pathways and NFATc1 protein expression levels.
Raw264.7 cells were treated with SpA (400 ng/ml) and SpA+JSH-23 for
the indicated times (0, 5, 10, 20, 40, 60 min and 1, 2, 3 days).
(A) Representative western blot images of NF-κB, MAPK and AKT
signaling pathway-associated protein expression levels. (B)
Representative western images and (C) quantification of NFATc1
protein expression levels. Quantification of (D) IκB-α, and (E)
NF-κB protein expression levels. GAPDH served as an internal
control. All experiments were performed at least three times. Data
are presented as the mean ± standard deviation, *P<0.05,
**P<0.01, ***P<0.001 vs. control. SpA, Staphylococcus aureus
protein A; RANKL, receptor activator of nuclear factor κB-ligand;
ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminus
kinase; NF-κB, nuclear factor-κB; IκB-α, inhibitor of IκB; Akt,
protein kinase B; NFATc1, nuclear factor of activated T-cells; p,
phosphorylated. |
NFATc1 is an important transcription factor in
osteoclast differentiation; the induction of NFATc1 is a hallmark
in the cell terminal determination of osteoclasts (38,39);
and its presence in osteoclast precursors could promote their
differentiation into mature osteoclasts in the absence of RANKL
(39). Thus, the expression of
NFATc1 in Raw264.7 cells treated with SpA for 1, 2 and 3 days was
examined, and the results demonstrated that SpA significantly
promoted the expression of NFATc1 on days 2 and 3 (Fig. 5D and E), but JSH-23 inhibited the
expression of NFATc1 induced by SpA. These findings suggested that
SpA may induce osteoclast differentiation through NF-κB activation,
which subsequently activates NFATc1 determining osteoclast
differentiation.
Discussion
In the present study, the effect of SpA on
osteoclastogenesis and the potential underlying mechanisms were
investigated. It was identified that SpA induced osteoclast
differentiation, promoted bone resorption and increased the
expression of osteoclast-specific genes, such as TRAP, MMP-9,
cathepsin K, CTR and ATP6v0d2, in a concentration-dependent manner.
In addition, the effect of SpA-induced osteoclastogenesis was
enhanced in the presence of RANKL. Moreover, it was demonstrated
that the NF-κB signaling pathway may serve a role in SpA-induced
osteoclastogenesis.
Raw264.7 cells express RANK and can differentiate
into mature osteoclasts induced by RANKL (32), and therefore, serve an important
role in studies of osteoclast formation and function in
vitro (40). This is why
Raw264.7 cells were chosen in this study as osteoclast precursors.
However, Mendoza Bertelli et al (41) obtained osteoclast precursors by
using M-CSF to induce bone marrow cells from BALB/c, C57BL/6 or
tnfr1−/−mice, which is another method of obtaining
osteoclast precursors. They obtained a similar result, where SpA
induced osteoclast differentiation and promoted bone resorption.
However, Staphylococcal lipoteichoic acid, another toxic component
of the cell wall of S. aureus, inhibited osteoclast
differentiation in the presence of RANKL (30). This phenomenon may be associated
with the different receptors and structures of these two virulence
factors. Lipoteichoic acid is recognized by toll-like receptor
(TLR) 2 (42), and stimulation by
TLRs inhibits osteoclast differentiation (43), but SpA can bind to a variety of
ligands, such as the TNF receptor-1 (TNFR-1) (44), the epidermal growth factor receptor
(EGFR) (45) and Fc region of IgG
(46), and different ligands may
trigger different intracellular signaling pathways. Furthermore,
Trouillet-Assant et al (47) have demonstrated that live S.
aureus inhibits osteoclastogenesis. In their studies, murine
bone marrow cells were infected with live S. aureus only for
2 h, then the cells were induced with M-CSF and RANK-L and
differentiated into activated macrophages instead of osteoclasts.
This result may have been as a result of a too short infection time
and with the extension of infection time, live S. aureus may
promote osteoclast formation. In addition, although mature
osteoclasts are derived from hematopoietic cells, they can be
directly differentiated from the monocyte/macrophage family. This
means that if they had treated bone marrow cells with M-CSF to
obtain monocytes and infected them with live S. aureus after
that, different conclusions may have been obtained. Furthermore, in
the present study, although SpA induced the formation of osteoclast
with bone resorption activity, compared with RANKL, the induction
effect was weaker, because the formed number of osteoclast-like
cells and the area of resorption pits stimulated with SpA were
lower than those treated with RANKL, which emphasizes that, RANKL,
may be a necessary and sufficient cytokine, serving a great role in
osteoclast differentiation.
In the present study, it was demonstrated that the
degradation of IκB-α and phosphorylation of NF-κB in Raw264.7 cells
occurred under the stimulation of SpA, but proteins in MAPKs and
PI-3 K signaling pathways presented no obvious change, which
indicated that the NF-κB signaling pathway might be involved in
SpA-induced osteoclast differentiation. Additionally, JSH-23 was
used to inhibit NF-κB activation, and it was identified that the
formation of osteoclast-like cells and resorption pits induced by
SpA was obviously decreased, and the expression of
osteoclast-specific genes (TRAP, MMP-9, cathepsin K, CTR and
ATP6v0d2) were inhibited too, which confirmed that the NF-κB
signaling pathway was involved in the SpA-induced osteoclast
differentiation. As reported previously, NFATc1 serves a decisive
role in the terminal differentiation of osteoclasts (39), and NF-κB induces the initial
activation of NFATc1 (39), which
evokes the auto-amplification of NFATc1 and determines its
sufficient role in osteoclast differentiation (48). Therefore, the expression of NFATc1
was measured and it was demonstrated that its high expression
occurred 2 and 3 days after SpA stimulation. However, when JSH-23
was added, the expression of NFATc1 was decreased, which suggested
that SpA promotes osteoclast differentiation by activating the
NF-κB signaling pathway, which subsequently activates NFATc1 and
induces osteoclast differentiation. As Mendoza Bertelli et
al (41) mentioned, SpA
induces osteoclastogenesis via TNFR1 and EGFR, whereas Claro et
al (26) demonstrated that SpA
binding to TNFR-1 activates NF-κB and induces the release of IL-6,
and Yi et al (49)
demonstrated that EGFR cross-talks with RANK to induce osteoclast
differentiation. Based on the above studies, whether SpA activates
NF-κB by TNFR-1 and EGFR, and then activates NFATc1, which
subsequently promotes osteoclast differentiation, requires further
experimentation.
In conclusion, the present study indicated that SpA
induces osteoclast differentiation and promotes bone resorption
in vitro, that the NF-κB signaling pathway serves a role in
this process, and that these findings provide a molecular basis in
order to understand the pathogenesis of S. aureus-mediated
osteomyelitis.
Acknowledgements
The present study was funded by the National Natural
Sciences Foundation of China (grant no. 81472096).
References
|
1
|
Jorge LS, Chueire AG and Rossit AR:
Osteomyelitis: A current challenge. Braz J Infect Dis. 14:310–315.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lew DP and Waldvogel FA: Osteomyelitis.
Lancet. 364:369–379. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Klosterhalfen B, Peters KM, Tons C,
Hauptmann S, Klein CL and Kirkpatrick CJ: Local and systemic
inflammatory mediator release in patients with acute and chronic
posttraumatic osteomyelitis. J Trauma. 40:372–378. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parsons B and Strauss E: Surgical
management of chronic osteomyelitis. Am J Surg. 188 1A
Suppl:S57–S66. 2004. View Article : Google Scholar
|
|
5
|
Giannoudis PV and Atkins R: Management of
long-bone non-unions. Injury. 38 Suppl 2:S1–S2. 2007. View Article : Google Scholar
|
|
6
|
Feng X and McDonald JM: Disorders of bone
remodeling. Annu Rev Pathol. 6:121–145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taichman RS: Blood and bone: Two tissues
whose fates are intertwined to create the hematopoietic stem-cell
niche. Blood. 105:2631–2639. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Josse J, Velard F and Gangloff SC:
Staphylococcus aureus vs. Osteoblast: Relationship and consequences
in osteomyelitis. Front Cell Infect Microbiol. 5:852015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fiedler T, Salamon A, Adam S, Herzmann N,
Taubenheim J and Peters K: Impact of bacteria and bacterial
components on osteogenic and adipogenic differentiation of
adipose-derived mesenchymal stem cells. Exp Cell Res.
319:2883–2892. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin T, Zhu YL, Li J, Shi J, He XQ, Ding J
and Xu YQ: Staphylococcal protein A, Panton-Valentine leukocidin
and coagulase aggravate the bone loss and bone destruction in
osteomyelitis. Cell Physiol Biochem. 32:322–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ellington JK, Reilly SS, Ramp WK, Smeltzer
MS, Kellam JF and Hudson MC: Mechanisms of Staphylococcus aureus
invasion of cultured osteoblasts. Microb Pathog. 26:317–323. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hudson MC, Ramp WK, Nicholson NC, Williams
AS and Nousiainen MT: Internalization of Staphylococcus aureus by
cultured osteoblasts. Microb Pathog. 19:409–419. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rasigade JP, Trouillet-Assant S, Ferry T,
Diep BA, Sapin A, Lhoste Y, Ranfaing J, Badiou C, Benito Y, Bes M,
et al: PSMs of hypervirulent Staphylococcus aureus act as
intracellular toxins that kill infected osteoblasts. PLoS One.
8:e631762013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lau YS, Wang W, Sabokbar A, Simpson H,
Nair S, Henderson B, Berendt A and Athanasou NA: Staphylococcus
aureus capsular material promotes osteoclast formation. Injury. 37
Suppl 2:S41–S48. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meghji S, Crean SJ, Hill PA, Sheikh M,
Nair SP, Heron K, Henderson B, Mawer EB and Harris M:
Surface-associated protein from staphylococcus aureus stimulates
osteoclastogenesis: Possible role in S. Aureus-induced bone
pathology. Br J Rheumatol. 37:1095–1101. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nair S, Song Y, Meghji S, Reddi K, Harris
M, Ross A, Poole S, Wilson M and Henderson B: Surface-associated
proteins from Staphylococcus aureus demonstrate potent bone
resorbing activity. J Bone Miner Res. 10:726–734. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Woo KM, Kim HM and Ko JS: Macrophage
colony-stimulating factor promotes the survival of osteoclast
precursors by up-regulating Bcl-X(L). Exp Mol Med. 34:340–346.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fuller K, Owens JM, Jagger CJ, Wilson A,
Moss R and Chambers TJ: Macrophage colony-stimulating factor
stimulates survival and chemotactic behavior in isolated
osteoclasts. J Exp Med. 178:1733–1744. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arai F, Miyamoto T, Ohneda O, Inada T,
Sudo T, Brasel K, Miyata T, Anderson DM and Suda T: Commitment and
differentiation of osteoclast precursor cells by the sequential
expression of c-Fms and receptor activator of nuclear factor kappaB
(RANK) receptors. J Exp Med. 190:1741–1754. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Soysa NS, Alles N, Aoki K and Ohya K:
Osteoclast formation and differentiation: An overview. J Med Dent
Sci. 59:65–74. 2012.PubMed/NCBI
|
|
22
|
Nakashima T and Takayanagi H: New
regulation mechanisms of osteoclast differentiation. Ann N Y Acad
Sci. 1240:E13–E18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shinohara M and Takayanagi H: Novel
osteoclast signaling mechanisms. Curr Osteoporos Rep. 5:67–72.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peacock SJ, Moore CE, Justice A, Kantzanou
M, Story L, Mackie K, O'Neill G and Day NP: Virulent combinations
of adhesin and toxin genes in natural populations of Staphylococcus
aureus. Infect Immun. 70:4987–4996. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tuchscherr L, Heitmann V, Hussain M,
Viemann D, Roth J, von Eiff C, Peters G, Becker K and Löffler B:
Staphylococcus aureus small-colony variants are adapted phenotypes
for intracellular persistence. J Infect Dis. 202:1031–1040. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Claro T, Widaa A, McDonnell C, Foster TJ,
O'Brien FJ and Kerrigan SW: Staphylococcus aureus protein A binding
to osteoblast tumour necrosis factor receptor 1 results in
activation of nuclear factor kappa B and release of interleukin-6
in bone infection. Microbiology. 159:147–154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Claro T, Widaa A, O'Seaghdha M, Miajlovic
H, Foster TJ, O'Brien FJ and Kerrigan SW: Staphylococcus aureus
protein A binds to osteoblasts and triggers signals that weaken
bone in osteomyelitis. PLoS One. 6:e187482011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hsu H, Lacey DL, Dunstan CR, Solovyev I,
Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, et
al: Tumor necrosis factor receptor family member RANK mediates
osteoclast differentiation and activation induced by
osteoprotegerin ligand. Proc Natl Acad Sci USA. 96:3540–3545. 1999;
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang J, Ryu YH, Yun CH and Han SH:
Impaired osteoclastogenesis by staphylococcal lipoteichoic acid
through Toll-like receptor 2 with partial involvement of MyD88. J
Leukoc Biol. 86:823–831. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chambers TJ, Revell PA, Fuller K and
Athanasou NA: Resorption of bone by isolated rabbit osteoclasts. J
Cell Sci. 66:383–399. 1984.PubMed/NCBI
|
|
32
|
Suda T, Takahashi N, Udagawa N, Jimi E,
Gillespie MT and Martin TJ: Modulation of osteoclast
differentiation and function by the new members of the tumor
necrosis factor receptor and ligand families. Endocr Rev.
20:345–357. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matsuzaki K, Udagawa N, Takahashi N,
Yamaguchi K, Yasuda H, Shima N, Morinaga T, Toyama Y, Yabe Y,
Higashio K and Suda T: Osteoclast differentiation factor (ODF)
induces osteoclast-like cell formation in human peripheral blood
mononuclear cell cultures. Biochem Biophys Res Commun. 246:199–204.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jimi E, Akiyama S, Tsurukai T, Okahashi N,
Kobayashi K, Udagawa N, Nishihara T, Takahashi N and Suda T:
Osteoclast differentiation factor acts as a multifunctional
regulator in murine osteoclast differentiation and function. J
Immunol. 163:434–442. 1999.PubMed/NCBI
|
|
35
|
Moon JB, Kim JH, Kim K, Youn BU, Ko A, Lee
SY and Kim N: Akt induces osteoclast differentiation through
regulating the GSK3β/NFATc1 signaling cascade. J Immunol.
188:163–169. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He Q, Zhang C, Wang L, Zhang P, Ma D, Lv J
and Liu F: Inflammatory signaling regulates hematopoietic stem and
progenitor cell emergence in vertebrates. Blood. 125:1098–1106.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He L, Sun F, Wang Y, Zhu J, Fang J, Zhang
S, Yu Q, Gong Q, Ren B, Xiang X, et al: HMGB1 exacerbates
bronchiolitis obliterans syndrome via RAGE/NF-κB/HPSE signaling to
enhance latent TGF-β release from ECM. Am J Transl Res.
8:1971–1984. 2016.PubMed/NCBI
|
|
38
|
Asagiri M, Sato K, Usami T, Ochi S,
Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E and
Takayanagi H: Autoamplification of NFATc1 expression determines its
essential role in bone homeostasis. J Exp Med. 202:1261–1269. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takayanagi H, Kim S, Koga T, Nishina H,
Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, et al:
Induction and activation of the transcription factor NFATc1 (NFAT2)
integrate RANKL signaling in terminal differentiation of
osteoclasts. Dev Cell. 3:889–901. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Collin-Osdoby P and Osdoby P:
RANKL-mediated osteoclast formation from murine RAW 264.7 cells.
Methods Mol Biol. 816:187–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bertelli A Mendoza, Delpino MV, Lattar S,
Giai C, Llana MN, Sanjuan N, Cassat JE, Sordelli D and Gómez MI:
Staphylococcus aureus protein A enhances osteoclastogenesis via
TNFR1 and EGFR signaling. Biochim Biophys Acta. 1862:1975–1983.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ellingsen E, Morath S, Flo T, Schromm A,
Hartung T, Thiemermann C, Espevik T, Golenbock D, Foster D, Solberg
R, et al: Induction of cytokine production in human T cells and
monocytes by highly purified lipoteichoic acid: Involvement of
Toll-like receptors and CD14. Med Sci Monit. 8:BR149–BR156.
2002.PubMed/NCBI
|
|
43
|
Takami M, Kim N, Rho J and Choi Y:
Stimulation by toll-like receptors inhibits osteoclast
differentiation. J Immunol. 169:1516–1523. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gómez MI, O'Seaghdha M, Magargee M, Foster
TJ and Prince AS: Staphylococcus aureus protein A activates TNFR1
signaling through conserved IgG binding domains. J Biol Chem.
281:20190–20196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gómez MI, Seaghdha MO and Prince AS:
Staphylococcus aureus protein A activates TACE through
EGFR-dependent signaling. EMBO J. 26:701–709. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cedergren L, Andersson R, Jansson B, Uhlén
M and Nilsson B: Mutational analysis of the interaction between
staphylococcal protein A and human IgG1. Protein Eng. 6:441–448.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Trouillet-Assant S, Gallet M, Nauroy P,
Rasigade JP, Flammier S, Parroche P, Marvel J, Ferry T, Vandenesch
F, Jurdic P and Laurent F: Dual impact of live Staphylococcus
aureus on the osteoclast lineage, leading to increased bone
resorption. J Infect Dis. 211:571–581. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhao Q, Wang X, Liu Y, He A and Jia R:
NFATc1: Functions in osteoclasts. Int J Biochem Cell Biol.
42:576–579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yi T, Lee HL, Cha JH, Ko SI, Kim HJ, Shin
HI, Woo KM, Ryoo HM, Kim GS and Baek JH: Epidermal growth factor
receptor regulates osteoclast differentiation and survival through
cross-talking with RANK signaling. J Cell Physiol. 217:409–422.
2008. View Article : Google Scholar : PubMed/NCBI
|