Introduction
Mesenchymal stem cells (MSCs) are often considered
to be a good source for the development of regenerative medicine
due to the self-renewal and multipotent properties of these cells.
The MSC secretome is another area of research that will ultimately
contribute to the development of cell-free therapy (1,2).
From soluble factors to extracellular vesicles, MSCs produce a
variety of biologically active secreted proteins. As only a small
percentage of transplanted MSCs are successfully engrafted,
differentiated and functional in the target tissue (3,4), it
was suggested that endocrine and immunomodulatory effects of the
MSC secretome may have important roles in tissue regeneration
(5,6).
MSCs can be obtained from various tissues, including
the bone marrow, adipose tissue, placenta and umbilical cord. In
2008, human tonsils were described as a novel source of MSCs and
these MSCs are termed tonsil-derived MSCs (T-MSCs) (7). The characteristics and potential of
MSCs to be used for cell banking have been previously reported
(8). The therapeutic potential of
T-MSCs in the treatment of liver (9,10),
pancreas (11), parathyroid gland
(12), skeletal muscle (13), and bone (14) diseases, as well as the mechanisms
that underlie their immunomodulatory effects (15–18),
have been described previously.
Adiponectin, which is primarily produced and
secreted by adipocytes, evokes several beneficial metabolic
functions via insulin-sensitizing, anti-inflammatory and
cardiovascular protective effects (19–21).
Although adiponectin is secreted from adipocytes, the levels of
this protein in circulation are not always correlated with body
mass index (BMI). Instead, weight reduction paradoxically increases
adiponectin in circulation (22,23).
Adiponectin circulates in different multimer forms: Low molecular
weight form (LMW; trimer), medium molecular weight form (hexamer)
and high molecular weight form (HMW; octadecamer or higher). LMW
and HMW adiponectin promote glucose and lipid metabolism in
hepatocytes, while LMW primarily acts on myocytes (19,21).
Thus, promotion of adiponectin multimerization rather than an
increase and/or decrease of total adiponectin affects the function
of adiponectin.
In a previous study, we observed that T-MSC CM
injection reduces the body weight of senescence-accelerated mouse
prone 6 (SAMP6) mice (14). In
addition, a selective decrease in visceral adiposity was observed
in the previous study, and we also reported that T-MSC CM has the
potential to inhibit adipogenesis in vitro. Given that
weight reduction is closely associated with the improvement of
whole body metabolism due, in part, to increased levels of
adiponectin in circulation, the present study aimed to determine
whether T-MSC CM regulates adiponectin expression, secretion,
and/or multimerization in SAMP6 aging mice and 3T3-L1
adipocytes.
Materials and methods
T-MSC culture and T-MSC CM
preparation
Previously isolated and characterized human T-MSCs
(8,24) were cultured in Dulbecco's modified
Eagle's medium (DMEM) high-glucose medium (Welgene, Inc.,
Gyeongsan, Korea) supplemented with 10% fetal bovine serum (FBS;
Welgene, Inc.), 100 IU/ml penicillin and 100 µg/ml streptomycin
(Welgene, Inc.). Cells were maintained at 37°C in a humidified 5%
CO2 atmosphere. When cells reached 80% confluency, fresh
culture medium was added and the cells were incubated for an
additional 48 h. T-MSC CM was harvested and concentrated 10-fold
using 3-kDa cut-off Amicon Ultra centrifugal filter units (Merck
KGaA, Darmstadt, Germany) at a speed of 3,800 × g for 30 min at
room temperature.
Animals
A total of 12 male SAMP6 mice (age, 4 months;
weight, 47.04±2.87 g) were purchased from Japan SLC, Inc.
(Hamamatsu, Japan) and maintained at 21–23°C with 51–54% humidity
and a 12-h light/dark cycle under conventional conditions, with
food and water supplied ad libitum. At 7 months of age (weight,
52.64±4.66 g), mice were divided into two groups (n=6 mice/group)
and injected via tail vein with either culture medium alone
(control) or culture supernatant that was collected and
concentrated after a 48 h incubation of 0.8×106 T-MSCs
(T-MSC CM). Treatments were performed twice a week for 2 weeks
using freshly prepared CM. Mice were sacrificed at 9 months of age
by cervical dislocation, and whole blood and organs were harvested
for further analysis. Experiments and procedures were approved by
the Animal Ethics Committee at Ewha Womans University School of
Medicine (Seoul, Korea; no. ESM 14-0278), and all experiments were
performed in accordance with relevant guidelines and
regulations.
Hematoxylin and eosin (H&E)
staining
Mouse epididymal adipose tissue (eAT) was isolated
and fixed with a 4% paraformaldehyde solution overnight at 4°C.
Paraffin-embedded eAT was sectioned at 4 µm and subjected to
H&E staining. Briefly, cells were stained with 0.7% hematoxylin
for 2 min and then with 5% eosin for 1.5 min at room temperature.
Stained sections were observed using an Olympus BX50 light
microscope (Olympus Corporation, Tokyo, Japan) and images were
captured under ×100 magnification.
Adiponectin ELISA
Blood samples (~200 µl) were collected in a tube
containing heparin sodium (JW Pharmaceutical, Seoul, Korea) from
the facial vein of 7- and 9-month-old mice following 6 h of
fasting. Plasma proteins were separated by centrifugation at 1,000
× g for 10 min at room temperature. The adiponectin concentration
was measured using the Adiponectin Total, HMW ELISA kit (cat. no.
47-ADPMS-E01; Alpco Diagnostics, Salem, NH, USA), according to the
manufacturer's protocol.
Cell culture
The mouse preadipocyte cell line 3T3-L1 was
purchased from the Korean Cell Line Bank (Seoul, Korea) and
maintained in DMEM high-glucose medium supplemented with 10% FBS,
100 IU/ml penicillin, and 100 µg/ml streptomycin. After reaching
100% confluency, the medium was changed and cells were maintained
for an additional 3 days. Cells were subsequently induced to
differentiate to adipocytes (day 0). To induce adipocyte
differentiation, high-glucose DMEM containing 10% FBS, 100 IU/ml
penicillin, and 100 µg/ml streptomycin supplemented with 2 mg/ml
insulin, 0.25 µM dexamethasone and 0.5 mM
3-isobutyl-1-methylxanthine (all from Sigma-Aldrich; Merck KGaA)
was used. On day 3, the medium was replaced with medium containing
insulin only and cells were cultured for another 4 days at 37°C in
a humidified 5% CO2 atmosphere. Concentrated culture
medium or T-MSC CM was added between days 7 and 10. On day 10, the
cells were rinsed with PBS and incubated in serum-free medium
overnight. Culture supernatants and cells were harvested the next
day for further analyses. To induce oxidative stress in mature
adipocytes, cells were pretreated with 50 mU/ml glucose oxidase
(Sigma-Aldrich; Merck KGaA) for 2 h, followed by overnight
treatment with T-MSC CM at 37°C.
RNA extraction and reverse
transcription (RT)
To harvest adipocytes, the eAT was weighed and
finely minced. Minced tissue was transferred to a 4-times greater
volume of Hank's Balanced Salt Solution buffer (Welgene, Inc.)
containing 1 mg/ml collagenase I (Sigma-Aldrich; Merck KGaA) and
0.28 M fatty acid-free bovine serum albumin (BSA; Sigma-Aldrich;
Merck KGaA). Tissue digestion was performed by incubating the
tissue sample for 2 h at 37°C in a shaking incubator. Digested
tissue was filtered through a cell strainer and centrifuged at 100
× g for 15 min at room temperature. The floating adipocyte fraction
was transferred to a new tube for RNA extraction.
To extract RNA from mouse eAT or 3T3-L1 adipocytes
(9.5×105), TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was used. Tissue homogenization
was performed using a TissueRuptor (Qiagen GmbH, Hilden, Germany),
and following phase separation by centrifugation at 12,000 × g for
15 min at 4°C, RNA was isolated using NucleoSpin® RNA
Clean-up kit (Macherey-Nagel GmbH & Co. KG, Düren, Germany),
according to the manufacturer's protocol. After determination of
the concentration and purity of the RNA samples using BioPhotometer
D30 (Eppendorf, Hamburg, Germany), 1 µg RNA was used for cDNA
synthesis using a ReverTra Ace-α-kit (Toyobo Co., Ltd., Osaka,
Japan). Reverse transcription was performed by incubation at 30°C
for 10 min, at 42°C for 20 min and at 99°C for 5 min.
RT-quantitative polymerase chain
reaction (qPCR)
To determine the expression of target genes, a
primer pair (0.4 µM) and SYBR-Green Real-time PCR Maser Mix (Toyobo
Co., Ltd.) were mixed with the prepared cDNA. The primer sequences
are listed in Table I.
Amplification was performed in duplicate by 40 cycles of 15 sec
denaturation step at 95°C and a 1 min amplification and signal
acquisition step at 60°C using StepOnePlus Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Cycle
threshold (Cq) values were obtained, and the relative expression
level of a target gene was determined as 2 (cyclophilin
Cq-target gene Cq) (25).
 | Table I.Sequences of primers used for
quantitative polymerase chain reaction. |
Table I.
Sequences of primers used for
quantitative polymerase chain reaction.
|
|
| Primer sequence |
|---|
|
|
|
|
|---|
| Gene | GeneBank
accession | Forward | Reverse |
|---|
| Cyclophilin | NM_021130.4 |
5′-CGTTTTGGGTCCAGGAATGG-3′ |
5′-TACAGGACATTGCGAGCAGA-3′ |
| PPARγ | NM_011146.3 |
5′-GGAAGACCACTCGCATTCCTT-3′ |
5′-GTAATCAGCAACCATTGGGTCA-3′ |
| C/EBPα | NM_007678.3 |
5′-CAAGAACAGCAACGAGTACCG-3′ |
5′-GTCACTGGTCAACTCCAGCAC-3′ |
| Leptin | NM_008493.3 |
5′-GTGGCTTTGGTCCTATCTGTC-3′ |
5′-CGTGTGTGAAATGTCATTGATCC-3′ |
| Adiponectin | NM_009605.4 |
5′-GTTCCCAATGTACCCATTCGC-3′ |
5′-TGTTGCAGTAGAACTTGCCAG-3′ |
|
p40phox | NM_008677.2 |
5′-GCCGCTATCGCCAGTTCTAC-3′ |
5′-GCAGGCTCAGGAGGTTCTTC-3′ |
|
p47phox | NM_010876.4 |
5′-GATGTTCCCCATTGAGGCCG-3′ |
5′-GTTTCAGGTCATCAGGCCGC-3′ |
|
P67phox | NM_010877.5 |
5′-CTGGCTGAGGCCATCAGACT-3′ |
5′-AGGCCACTGCAGAGTGCTTG-3′ |
|
gp91phox | NM_007807.5 |
5′-TTGGGTCAGCACTGGCTCTG-3′ |
5′-TGGCGGTGTGCAGTGCTATC-3′ |
Western blot analysis
Whole protein lysates were isolated from mouse eAT
or 3T3-L1 adipocytes (9.5×105) using PRO-PREP Protein
Extraction Solution (Intron Biotechnology, Inc., Seongnam, Korea).
Protein concentrations were determined using the Bradford assay
with the Bio-Rad Protein assay solution (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Samples (4 µg for tissue lysates and 10
µg for cell lysates) were resolved by 10% SDS-PAGE and transferred
to Immobilon-P polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). Membranes were blocked with 5% skim milk in
TBS containing 0.1% Tween-20 (TBS-T) solution for 1 h at room
temperature and were subsequently incubated with primary antibodies
overnight at 4°C. The following primary antibodies were used:
Adiponectin (1:1,000, diluted in 5% skim milk containing TBST; cat.
no. ab25891; rabbit; Abcam, Cambridge, UK); and β-actin [1:2,000,
diluted in 2% BSA (Bovogen Biologicals, Pty, Ltd., East Keilor,
Victoria, Australia) containing TBST; cat. no. A1978; mouse;
Sigma-Aldrich; Merck KGaA]. The membranes were washed 3 times for
10 min in TBST and incubated with anti-rabbit (cat. no. BR170-6515;
Bio-Rad Laboratories, Inc.) or anti-mouse (cat. no. BR170-6516;
Bio-Rad Laboratories, Inc.) horseradish peroxidase-conjugated
secondary antibodies (1:3,000, diluted in TBST) for 1 h at room
temperature. Following incubation, membranes were washed 3 times
for 10 min in TBST and developed using SuperSignal West Femto
Maximum Sensitivity Substrate (Pierce; Thermo Fisher Scientific,
Inc.). Images were obtained using ImageQuant LAS 4000 (GE
Healthcare Life Sciences, Little Chalfont, UK).
To detect secreted adiponectin in cell culture
medium, conditioned medium was prepared by incubating
differentiated 3T3-L1 adipocytes (3.8×105) in serum-free
DMEM (500 µl) for 18 h. Conditioned medium was concentrated 10-fold
using 3-kDa cut-off Amicon Ultra centrifugal filter units (Merck
KGaA) and centrifugation at 14,000 × g for 15 min at room
temperature. Concentrated medium (50 µl) was prepared in sample
buffer (0.5 M Tris/HCl pH 6.8, 25% glycerol, 10% SDS, 500 mM DTT,
1% bromophenol blue; Sigma-Aldrich; Merck KGaA) and 12 µl samples
were separated on 10% SDS-PAGE for the detection of total secreted
adiponectin. To detect adiponectin in multimer forms, concentrated
medium was prepared in non-reducing and non-heat-denaturing
conditions using sample buffer lacking reducing agents, and 12 µl
samples were subsequently separated on 4–15% precast polyacrylamide
gradient gels (Bio-Rad Laboratories, Inc.). Blots were
semi-quantified by densitometric analysis using UN-SCAN-IT gel
analysis software version 6.1 (Silk Scientific, Inc., Orem, UT,
USA).
Reactive oxygen species (ROS) and
reactive nitrogen species (RNS) measurement
ROS and RNS production in total cell lysates or
culture medium was measured using OxiSelect™ In
Vitro ROS/RNS assay kit (Cell Biolabs, Inc., San Diego, CA,
USA), according to the manufacturer's protocol. Cellular ROS/RNS
levels were normalized to total protein measured by the BCA Protein
assay kit (Pierce; Thermo Fisher Scientific, Inc.).
Statistical analysis
Student's t-test or one-way analysis of variance
with Dunnett's multiple comparisons test was performed for
statistical analysis using GraphPad Prism 5.0 (GraphPad Software,
Inc., La Jolla, CA, USA). Data are presented as the mean ± standard
error of the mean from 3–4 independent experiments. P<0.05 was
considered to indicate a statistically significant difference.
Results
T-MSC CM injection reduces mouse body
weight and eAT tissue mass
In our previous study, we demonstrated that T-MSC CM
produces anti-adipogenic effects. In the present study, these in
vitro findings were further evaluated in a mouse model of
accelerated senescence (SPAM6 mice), as these mice have been
reported to exhibit an obese phenotype (26). Control culture medium or T-MSC CM
was injected into 7-month-old SAMP6 mice, and body weight changes
were examined 2 months later. A significant decrease in the weight
of mice was observed at 9 months in mice injected with T-MSC CM,
whereas the mice injected with the control medium did not exhibit
alterations in weight (Fig. 1A).
Isolated organs were weighed and normalized to the body weight.
T-MSC CM injection led to reduced eAT mass compared with control
mice, whereas the inguinal adipose tissue, liver and muscle mass
were not affected by injection of T-MSC CM (Fig. 1B). Histological analysis of eAT
demonstrated a reduction in the size of adipocytes in the T-MSC
CM-injected mice compared with control mice (Fig. 1C). To determine the mRNA expression
of adipogenic markers in eAT adipocytes, collagen digestion was
performed, followed by floating adipocyte fraction separation.
Together with the reduction in eAT mass and adipocyte size, the
mRNA expression of the adipogenic markers peroxisome
proliferator-activated receptor γ (PPARγ), CCAAT/enhancer-binding
protein α (C/EBPα) and leptin were significantly decreased, while
adiponectin expression was increased in eAT adipocytes, compared
with control-treated mice (Fig.
1D).
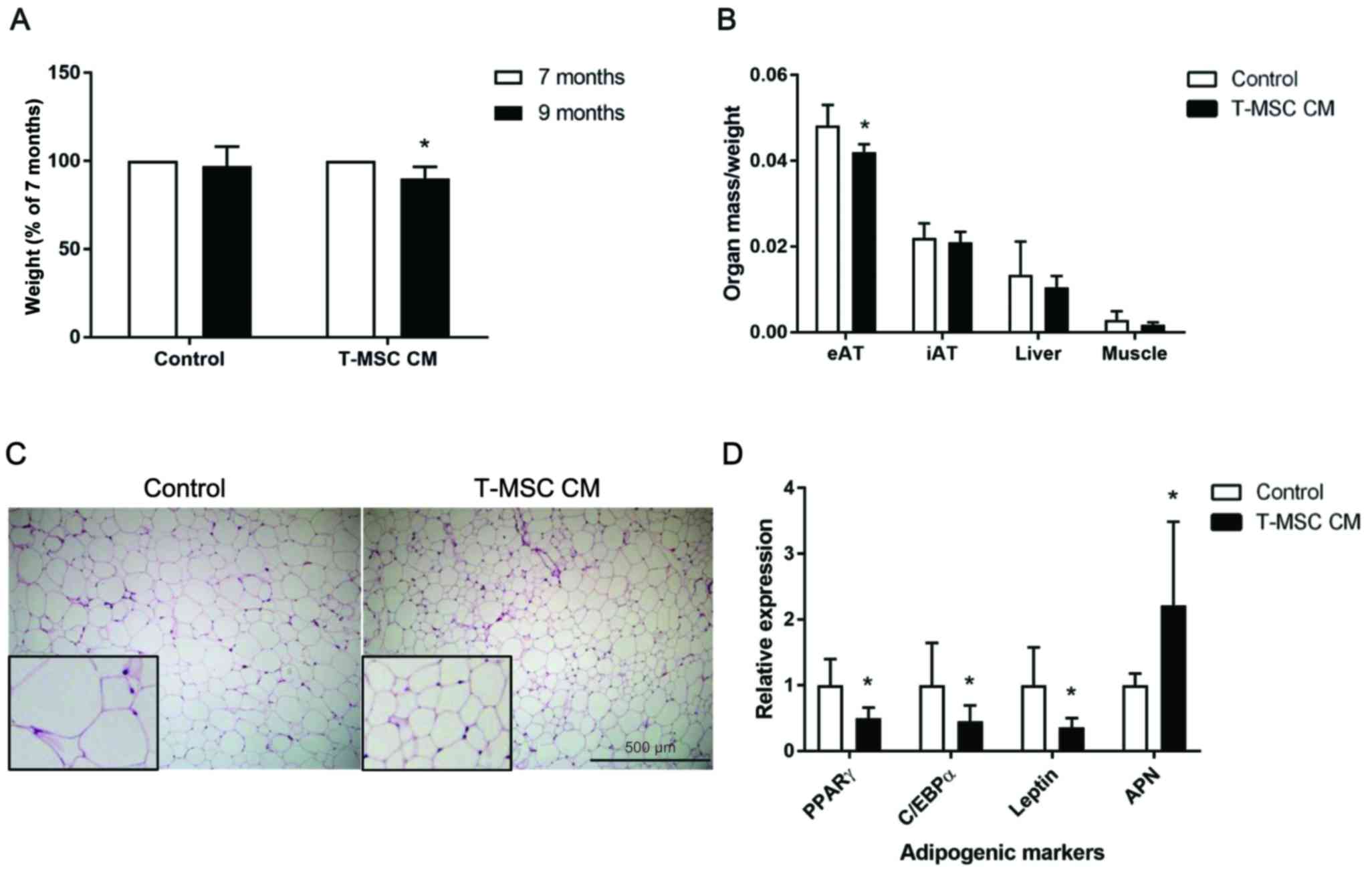 | Figure 1.T-MSC CM injection reduced body weight
and eAT accumulation in SAMP6 mice. (A) Mouse body weights were
measured prior to (7-month-old) and following (9-month-old mice)
treatment with control or T-MSC CM. (B) eAT, iAT, liver and
skeletal muscle were harvested and weighed upon sacrifice of
treated mice. Organ weights were normalized to the body weight of
each mouse. (C) Hematoxylin and eosin staining of mouse eAT was
performed. Figures are representative of images taken from 10–12
fields of each mouse eAT section. Magnification, ×100. Inserts,
magnification ×250. (D) mRNA expression of the adipogenic genes
PPARγ, C/EBPα, leptin and APN in fractionated eAT adipocytes were
assessed by reverse transcription-quantitative polymerase chain
reaction. Data are presented as the mean ± standard error of the
mean, n=6. *P<0.05 vs. control. T-MSC CM, tonsil-derived
mesenchymal stem cell conditioned medium; eAT, epididymal adipose
tissue; SAMP6, senescence-accelerated mouse prone 6; iAT, inguinal
AT; PPARγ, peroxisome proliferator-activated receptor γ; C/EBPα,
CCAAT/enhancer-binding protein α; APN, adiponectin. |
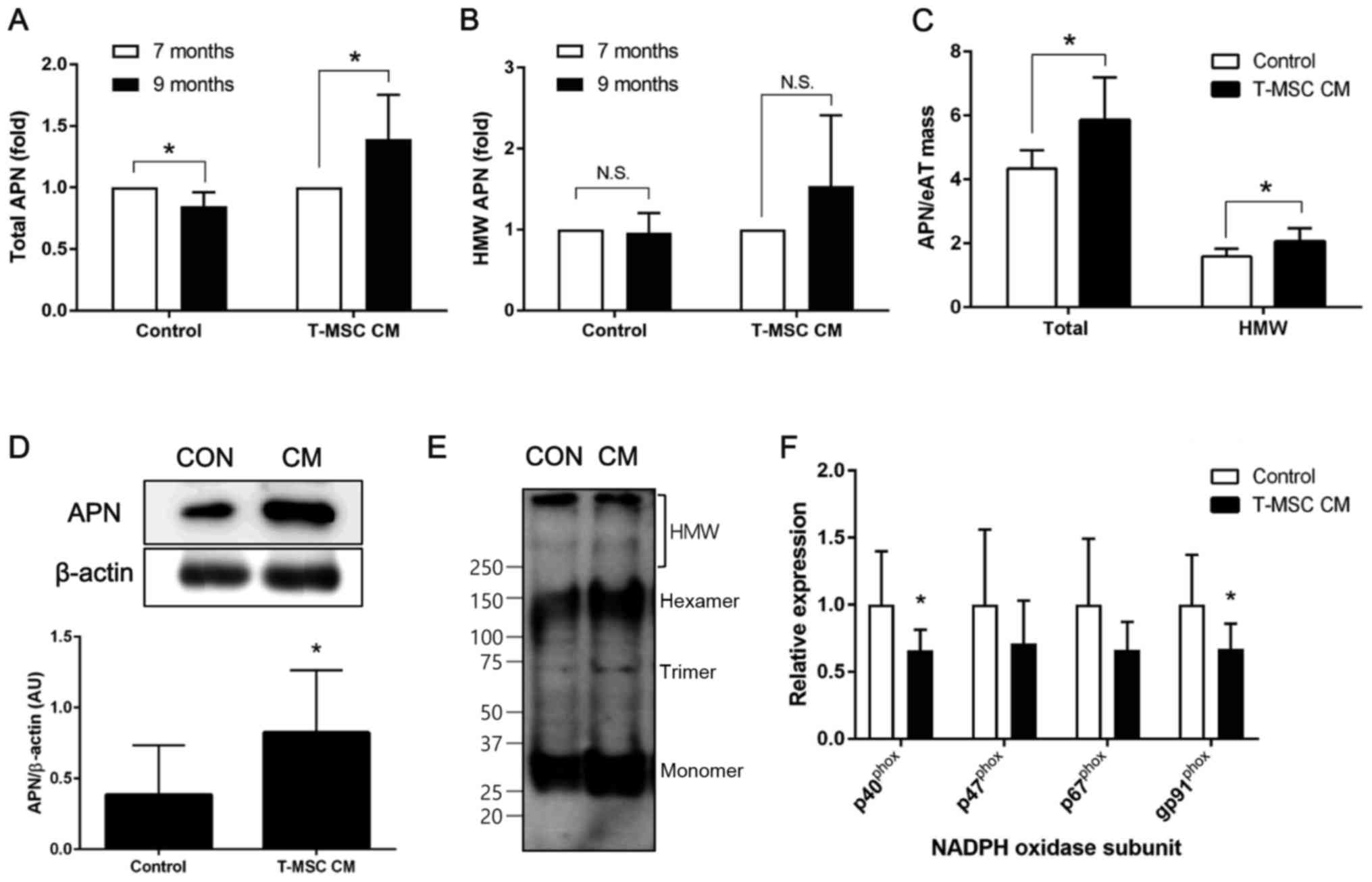
T-MSC CM injection increases
adiponectin in the circulation by upregulating expression in
eAT
As weight reduction is associated with an increase
in circulating adiponectin levels, the present study investigated
total and HMW adiponectin in mouse plasma prior to (7 months) and
following (9 months) treatment with T-MSC CM. A significant
increase in total adiponectin following T-MSC CM treatment was
observed, while levels of adiponectin were significantly decreased
in the mice injected with control medium (Fig. 2A). In addition, levels of HMW
adiponectin were increased following T-MSC CM injection, although
this was not statistically significant (Fig. 2B). Total and HMW adiponectin levels
in mouse plasma were normalized to the eAT mass and the results
demonstrated a significant increase in the levels of total and HMW
adiponectin following treatment with T-MSC CM, compared with
control-treated mice (Fig. 2C).
Furthermore, adiponectin protein levels in eAT lysates were
significantly increased by T-MSC CM treatment compared with control
treatment (Fig. 2D). Non-reduced
and non-denatured tissue lysates were used to determine adiponectin
multimerization. These experiments indicated that T-MSC CM enhances
formation of adiponectin multimers (Fig. 2E). In addition, the present study
investigated whether oxidative stress in the adipose tissue, an
established negative regulator of adiponectin expression in aging,
is modulated in the presence or absence of T-MSC CM injection
(27). The mRNA expression of
subunits of NADPH oxidase was investigated by RT-qPCR, and the
results demonstrated a significant reduction of p40phox
and gp91phox in eAT from mice injected with T-MSC CM
compared with controls (Fig.
2F).
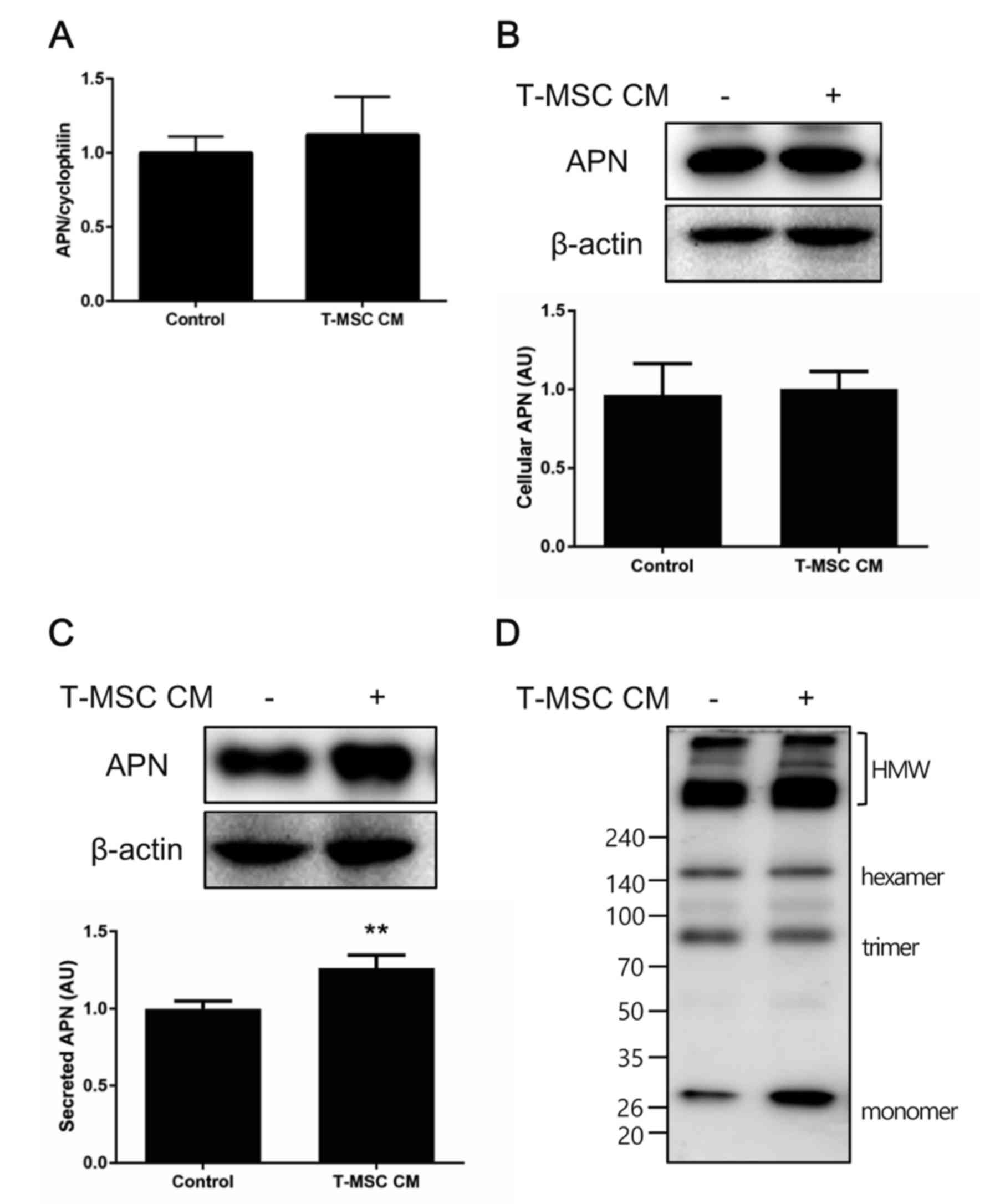
Adiponectin secretion and
multimerization are enhanced by T-MSC CM treatment
The current study confirmed these in vivo
findings in vitro by performing similar experiments with the
murine adipocyte cell line 3T3-L1. Preadipocytes were induced to
differentiate to adipocytes for 7 days and subsequently treated
with control medium or T-MSC CM for an additional 3 days. Total RNA
or whole cell lysates were collected, and adiponectin expression
was examined by RT-qPCR and western blot analysis. No significant
changes in the cellular levels of adiponectin mRNA or protein were
observed following treatment with T-MSC CM, compared with controls
(Fig. 3A and B), however,
adiponectin secretion into the culture medium was significantly
increased in cells treated with T-MSC CM compared with controls
(Fig. 3C). Adiponectin multimer
formation was also investigated under non-reduced and
non-heat-denatured conditions. T-MSC CM treatment slightly
increased the formation of HMW adiponectin by differentiated 3T3-L1
adipocytes (Fig. 3D).
T-MSC CM treatment ameliorates
oxidative stress and restores adiponectin multimerization
In order to extend the in vivo findings of
the reduction in NADPH oxidase subunit transcription, we induced
oxidative stress in mature 3T3-L1 adipocytes using glucose oxidase
(28). Cells were differentiated
for 10 days, challenged with glucose oxidase for 2 h and treated
with control medium or T-MSC CM for the following 24 h. Cell
culture medium and cells were collected and ROS/RNS production was
measured. The results demonstrated that enhanced ROS/RNS generation
in glucose oxidase-treated cells was significantly ameliorated in
cells incubated with T-MSC CM, in culture medium and cells
(Fig. 4A and B). Adiponectin
expression was also determined, and T-MSC CM treatment was not able
to reverse reduced mRNA expression induced by glucose oxidase
(Fig. 4C), however, HMW
adiponectin formation was restored to a certain extent in cells
treated with T-MSC CM (Fig.
4D).
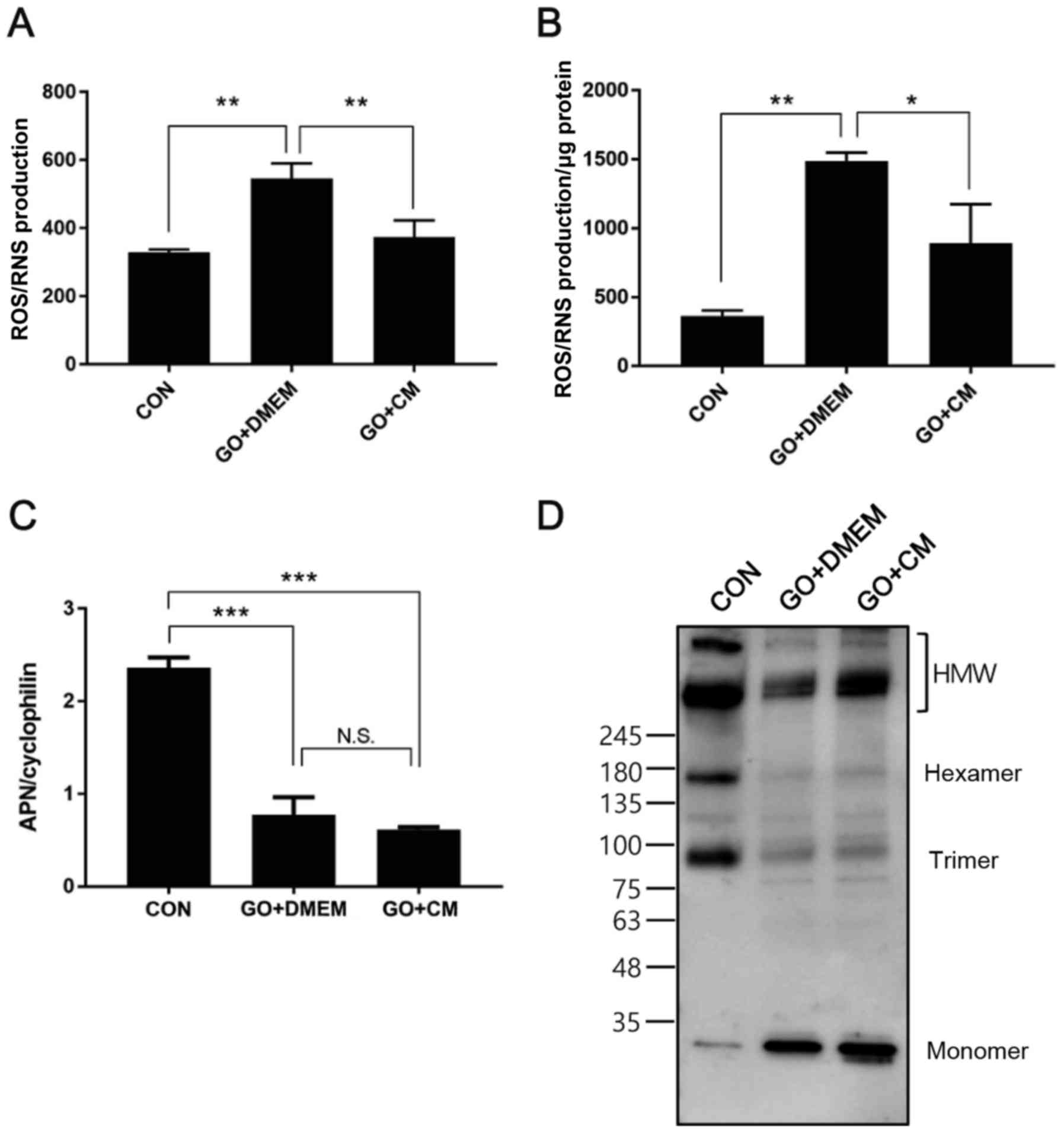 | Figure 4.T-MSC CM treatment reduces oxidative
stress and restores HMW APN formation in 3T3-L1 adipocytes. 3T3-L1
adipocytes were differentiated for 10 days and challenged with GO
for 2 h, followed by incubation in serum-free medium overnight in
the presence or absence of T-MSC CM. ROS/RNS production in (A)
culture supernatants and (B) cell lysates was measured. (C) mRNA
expression of APN was measured using reverse
transcription-quantitative polymerase chain reaction. (D) APN
multimer composition in culture supernatants was analyzed by
SDS-PAGE on gradient gels under non-reducing and
non-heat-denaturing conditions. Data are presented as the mean ±
standard error of the mean, n=3. *P<0.05, **P<0.01 and
***P<0.001, as indicated. T-MSC CM, tonsil-derived mesenchymal
stem cell conditioned medium; HMW, high molecular weight; APN,
adiponectin; GO, glucose oxidase; ROS, reactive oxygen species;
RNS, reactive nitrogen species; CM, T-MSC CM; DMEM, Dulbecco's
modified Eagle's medium; CON, control; N.S., not significant. |
Discussion
The present study has demonstrated the effects of
T-MSC CM treatment on weight reduction and the promotion of
adiponectin secretion from adipocytes using SAMP6 aging mice and
3T3-L1 adipocytes. Obesity increases inflammation and insulin
resistance in organs. This pathophysiology is associated with a
reduction in the anti-inflammatory adipokine adiponectin the
formation of HMW adiponectin in adipocytes. The results of the
current study indicated that T-MSC CM infusion in mice reduces eAT
accumulation and increases adiponectin synthesis, secretion and
multimerization.
T-MSC CM injection resulted in weight loss in mice
via a reduction in visceral adiposity and adipocyte size.
Investigation of the expression of adipogenic markers in eAT
adipocytes revealed downregulation of the transcription factors
PPARγ and C/EBPα following T-MSC CM injection, and these results
are consistent with our previous findings that T-MSC CM treatment
inhibits adipocyte differentiation in vitro (14). The reduced expression of adipogenic
regulators in T-MSC CM-injected mice confirmed the anti-adipogenic
effects of T-MSC CM in vivo.
An increase in adiponectin expression was observed
in T-MSC CM-treated mice, while PPARγ expression was reduced, which
is a master regulator of adipogenesis. Regarding promotion or
inhibition of adipogenesis, conflicting results currently exist.
The promotion of adipogenesis is required for the generation of
small and insulin-sensitive adipocytes. This restrains expansion of
adipose tissue via adipocyte hypertrophy, which may lead to insulin
resistance and ectopic lipid accumulation (29). On the other hand, it has also been
demonstrated that blocking adipocyte differentiation using a PPARγ
antagonist protected mice from high fat diet-induced adipocyte
hypertrophy and insulin resistance (30). The results of the present study
demonstrate that anti-adipogenic effect of T-MSC CM involves
reduction of adipocyte size and increased adiponectin synthesis.
These effects may yield medical benefits and further studies are
required to elucidate whether T-MSC CM may also be able to improve
inflammation and insulin sensitivity.
The SAMP6 mouse strain is useful for the study of
obesity in aging as reduced physical performance and energy
metabolism lead to the development of obesity in this mouse strain.
Furthermore, a previous report demonstrated that SAMP6 mice develop
characteristics of obesity (26).
A significantly higher BMI and the increase of obesity parameters
in the plasma, including glucose, triglyceride, insulin and leptin,
were reported in SAMP6 mice compared with age-matched AKR/J or
SAMR1 mice. In addition, adiponectin in plasma was reported to be
significantly lower in SAMP6 mice compared with control mice, and
low adiponectin is an established characteristic of obesity
(31,32). However, the observations in the
present study should be confirmed using a diet-induced obesity
mouse model. More detailed analyses on the consequences of
adiponectin induction on body metabolism are required. The action
of T-MSC CM on other metabolic organs, such as the liver or
skeletal muscle, needs to be determined. Identification of
adiponectin receptors that are involved in T-MSC CM-induced
adiponectin function and changes in the signal transduction may
explain the association between T-MSC CM and adiponectin. In
addition, the association between increases in adiponectin and
improvement in metabolic parameters requires further investigation.
Measurement of blood glucose, triglyceride and inflammatory
markers, as well as performing glucose tolerance and insulin
tolerance tests in the presence or absence of T-MSC CM may increase
our understanding of the advantages of using T-MSC CM in the
regulation of metabolic diseases.
Oxidative stress is one of the key contributors of
aging pathologies. In obesity, systemic oxidative stress is closely
associated with the incidence of metabolic syndrome. One potential
mechanism of action is that high levels of ROS production may lead
to suppressed adiponectin secretion (27,33).
In addition, a recent report demonstrated a positive correlation
between plasma adiponectin levels and antioxidant capacity in the
elderly (34). The present study
elucidated effects of T-MSC CM on the promotion of adiponectin
secretion in the setting of both obesity and aging. Furthermore,
the results demonstrated that T-MSC CM led to an increase in the
HMW adiponectin formation with the amelioration of oxidative
stress. Further studies are required to identify potential
antioxidant molecules secreted by T-MSCs and their mechanisms of
actions.
In conclusion, we demonstrated that T-MSC CM
injection reduces mouse body weight and eAT mass. Reduction in body
weight increased adiponectin in circulation by upregulating
adiponectin expression and multimerization. Amelioration of
oxidative stress may be the mechanism by which T-MSC CM leads to
increased adiponectin secretion. Furthermore, these results, which,
to the best of our knowledge, provide the first description of the
role of T-MSC CM on adiponectin synthesis and secretion, may
provide the framework for future development of cell therapy to
combat obesity or obesity-associated metabolic diseases.
Acknowledgements
This study was supported by the Bio & Medical
Technology Development Program of the National Research Foundation
of Korea funded by the Ministry of Science, ICT & Future
Planning (grant no. 2012M3A9C6049823), Ministry of Education (grant
no. 2016R1A6A3A11933360) and Intramural Research Promotion Grants
from Ewha Womans University School of Medicine (Seoul, Korea).
References
|
1
|
Lavoie JR and Rosu-Myles M: Uncovering the
secretes of mesenchymal stem cells. Biochimie. 95:2212–2221. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Konala VB, Mamidi MK, Bhonde R, Das AK,
Pochampally R and Pal R: The current landscape of the mesenchymal
stromal cell secretome: A new paradigm for cell-free regeneration.
Cytotherapy. 18:13–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li TS, Takahashi M, Ohshima M, Qin SL,
Kubo M, Muramatsu K and Hamano K: Myocardial repair achieved by the
intramyocardial implantation of adult cardiomyocytes in combination
with bone marrow cells. Cell Transplant. 17:695–703. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel G, Krause P, Wöhrle S, Nowak P,
Ayturan M, Kluba T, Brehm BR, Neumeister B, Köhler D, Rosenberger
P, et al: Bone marrow-derived human mesenchymal stem cells express
cardiomyogenic proteins but do not exhibit functional
cardiomyogenic differentiation potential. Stem Cells Dev.
21:2457–2470. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bi B, Schmitt R, Israilova M, Nishio H and
Cantley LG: Stromal cells protect against acute tubular injury via
an endocrine effect. J Am Soc Nephrol. 18:2486–2496. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cantinieaux D, Quertainmont R, Blacher S,
Rossi L, Wanet T, Noël A, Brook G, Schoenen J and Franzen R:
Conditioned medium from bone marrow-derived mesenchymal stem cells
improves recovery after spinal cord injury in rats: An original
strategy to avoid cell transplantation. PLoS One. 8:e695152013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Janjanin S, Djouad F, Shanti RM, Baksh D,
Gollapudi K, Prgomet D, Rackwitz L, Joshi AS and Tuan RS: Human
palatine tonsil: A new potential tissue source of multipotent
mesenchymal progenitor cells. Arthritis Res Ther. 10:R832008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ryu KH, Cho KA, Park HS, Kim JY, Woo SY,
Jo I, Choi YH, Park YM, Jung SC, Chung SM, et al: Tonsil-derived
mesenchymal stromal cells: Evaluation of biologic, immunologic and
genetic factors for successful banking. Cytotherapy. 14:1193–1202.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ryu KH, Kim SY, Kim YR, Woo SY, Sung SH,
Kim HS, Jung SC, Jo I and Park JW: Tonsil-derived mesenchymal stem
cells alleviate concanavalin A-induced acute liver injury. Exp Cell
Res. 326:143–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park M, Kim YH, Woo SY, Lee HJ, Yu Y, Kim
HS, Park YS, Jo I, Park JW, Jung SC, et al: Tonsil-derived
mesenchymal stem cells ameliorate CCl4-induced liver fibrosis in
mice via autophagy activation. Sci Rep. 5:86162015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim SY, Kim YR, Park WJ, Kim HS, Jung SC,
Woo SY, Jo I, Ryu KH and Park JW: Characterisation of
insulin-producing cells differentiated from tonsil derived
mesenchymal stem cells. Differentiation. 90:27–39. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park YS, Kim HS, Jin YM, Yu Y, Kim HY,
Park HS, Jung SC, Han KH, Park YJ, Ryu KH and Jo I: Differentiated
tonsil-derived mesenchymal stem cells embedded in Matrigel restore
parathyroid cell functions in rats with parathyroidectomy.
Biomaterials. 65:140–152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park S, Choi Y, Jung N, Yu Y, Ryu KH, Kim
HS, Jo I, Choi BO and Jung SC: Myogenic differentiation potential
of human tonsil-derived mesenchymal stem cells and their potential
for use to promote skeletal muscle regeneration. Int J Mol Med.
37:1209–1220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim YH, Park M, Cho KA, Kim BK, Ryu JH,
Woo SY and Ryu KH: Tonsil-derived mesenchymal stem cells promote
bone mineralization and reduce marrow and visceral adiposity in a
mouse model of senile osteoporosis. Stem Cells Dev. 25:1161–1171.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ryu JH, Park M, Kim BK, Ryu KH and Woo SY:
Tonsil-derived mesenchymal stromal cells produce CXCR2-binding
chemokines and acquire follicular dendritic cell-like phenotypes
under TLR3 stimulation. Cytokine. 73:225–235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park M, Kim YH, Ryu JH, Woo SY and Ryu KH:
Immune suppressive effects of tonsil-derived mesenchymal stem cells
on mouse bone-marrow-derived dendritic cells. Stem Cells Int.
2015:1065402015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cho KA, Lee JK, Kim YH, Park M, Woo SY and
Ryu KH: Mesenchymal stem cells ameliorate B-cell-mediated immune
responses and increase IL-10-expressing regulatory B cells in an
EBI3-dependent manner. Cell Mol Immunol. Jan 2–2017.(Epub ahead of
print). View Article : Google Scholar :
|
|
18
|
Kim JY, Park M, Kim YH, Ryu KH, Lee KH,
Cho KA and Woo SY: Tonsil-derived mesenchymal stem cells (T-MSCs)
prevent Th17-mediated autoimmune response via regulation of the
programmed death-1/programmed death ligand-1 (PD-1/PD-L1) pathway.
J Tissue Eng Regen Med. Jan 20–2017.(Epub ahead of print).
View Article : Google Scholar
|
|
19
|
Whitehead JP, Richards AA, Hickman IJ,
Macdonald GA and Prins JB: Adiponectin-a key adipokine in the
metabolic syndrome. Diabetes Obes Metab. 8:264–280. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu M and Liu F: Regulation of adiponectin
multimerization, signaling and function. Best Pract Res Clin
Endocrinol Metab. 28:25–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang ZV and Scherer PE: Adiponectin, the
past two decades. J Mol Cell Biol. 8:93–100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cnop M, Havel PJ, Utzschneider KM, Carr
DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD and Kahn
SE: Relationship of adiponectin to body fat distribution, insulin
sensitivity and plasma lipoproteins: Evidence for independent roles
of age and sex. Diabetologia. 46:459–469. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Furler SM, Gan SK, Poynten AM, Chisholm
DJ, Campbell LV and Kriketos AD: Relationship of adiponectin with
insulin sensitivity in humans, independent of lipid availability.
Obesity (Silver Spring). 14:228–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu Y, Park YS, Kim HS, Kim HY, Jin YM,
Jung SC, Ryu KH and Jo I: Characterization of long-term in vitro
culture-related alterations of human tonsil-derived mesenchymal
stem cells: Role for CCN1 in replicative senescence-associated
increase in osteogenic differentiation. J Anat. 225:510–518. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Niimi K, Takahashi E and Itakura C:
Adiposity-related biochemical phenotype in senescence-accelerated
mouse prone 6 (SAMP6). Comp Med. 59:431–436. 2009.PubMed/NCBI
|
|
27
|
Furukawa S, Fujita T, Shimabukuro M, Iwaki
M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M and
Shimomura I: Increased oxidative stress in obesity and its impact
on metabolic syndrome. J Clin Invest. 114:1752–1761. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo H, Ling W, Wang Q, Liu C, Hu Y and Xia
M: Cyanidin 3-glucoside protects 3T3-L1 adipocytes against H2O2- or
TNF-alpha-induced insulin resistance by inhibiting c-Jun
NH2-terminal kinase activation. Biochem Pharmacol. 75:1393–1401.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gustafson B, Hammarstedt A, Hedjazifar S
and Smith U: Restricted adipogenesis in hypertrophic obesity: The
role of WISP2, WNT, and BMP4. Diabetes. 62:2997–3004. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choi SS, Kim ES, Jung JE, Marciano DP, Jo
A, Koo JY, Choi SY, Yang YR, Jang HJ, Kim EK, et al: PPARγ
antagonist Gleevec improves insulin sensitivity and promotes the
browning of white adipose tissue. Diabetes. 65:829–839. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Matsubara M, Maruoka S and Katayose S:
Inverse relationship between plasma adiponectin and leptin
concentrations in normal-weight and obese women. Eur J Endocrinol.
147:173–180. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Asayama K, Hayashibe H, Dobashi K, Uchida
N, Nakane T, Kodera K, Shirahata A and Taniyama M: Decrease in
serum adiponectin level due to obesity and visceral fat
accumulation in children. Obes Res. 11:1072–1079. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fujita K, Nishizawa H, Funahashi T,
Shimomura I and Shimabukuro M: Systemic oxidative stress is
associated with visceral fat accumulation and the metabolic
syndrome. Circ J. 70:1437–1442. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gradinaru D, Margina D, Borsa C, Ionescu
C, Ilie M, Costache M, Dinischiotu A and Prada GI: Adiponectin:
Possible link between metabolic stress and oxidative stress in the
elderly. Aging Clin Exp Res. Sep 29–2016.(Epub ahead of print).
PubMed/NCBI
|