Introduction
Photoaging refers to premature skin aging caused by
chronic exposure to ultraviolet (UV) irradiation, especially the
UVA (315–400 nm) and UVB (280–315 nm) components, which are
regarded as the main cause of skin damage (1,2).
Photoaged skin is characterized by coarse wrinkles, dryness,
laxity, dyspigmentation, telangiectasia, a leathery appearance and
histological changes, including variable epidermal thickness, solar
elastosis and disorganization of collagen fibers (1,3).
UV irradiation induces the expression of
transcription factor activator protein-1 (AP-1), which plays an
important role in the mechanism of photoaging (4). AP-1, mainly composed of c-Jun and
c-Fos proteins, increases matrix metalloproteinases (MMPs)
transcription and decreases procollagen synthesis (3). MMPs are a large family of
zinc-containing endopeptidases that are responsible for the
degradation of collagen and other extracellular matrix (ECM)
proteins (5). Among them, MMP-1
(interstitial collagenase) initiates the cleavage of types I and
III fibrillar collagen in human skin, while MMP-3 (stromelysin-1)
activates proMMP-1 and further degrades the collagen fragments
(1,6). Rodents lack the MMP-1 gene, which is
functionally replaced by the MMP-13 (collagenase-3) gene (5,7).
Therefore, MMP-3 and MMP-13 are the primary UV-induced
collagenolytic enzymes in mouse skin. Type I procollagen is
synthesized by dermal fibroblasts and subsequently converted into
type I collagen, which is the major structural protein in dermal
ECM. In the process of photoaging, UV irradiation decreases type I
procollagen synthesis, resulting in the loss of collagen content
(3). Based on the underlying
mechanism of photoaging, the regulation of MMPs and type I
procollagen may be an effective strategy for the prevention and
treatment of photoaging.
Retinoic acid (RA), the bioactive metabolite of
vitamin A, plays a key role in regulating proliferation and
differentiation of cutaneous cells (8). It has been widely used in the
treatment of dermatological disorders, such as acne, psoriasis,
skin carcinoma and photoaging (9–12).
In particular, all-trans retinoic acid (ATRA) is considered the
gold standard to treat photoaged skin (13). Topical ATRA could improve several
clinical and histological signs of photoaged skin, including
improved skin appearance, increased anchoring fibrils and increased
dermal collagen (8,12–14).
Classically, the actions of RA are mediated by retinoid nuclear
receptors, retinoic acid receptor (RAR) and retinoid X receptor
(RXR) (15). However, whether RA
exerts its therapeutic effects on photoaged skin through retinoid
nuclear receptors has not yet been elucidated. Therefore, the
present study aimed to explore whether the therapeutic effects of
RA on photoaged skin are mediated by RAR and/or RXR in mice and to
investigate the underlying mechanism by histological examination of
collagen fibers, determination of collagen content and detection of
MMP-3, MMP-13, type I procollagen, c-Jun and c-Fos protein
expression in mouse skin.
Materials and methods
Animals
Male ICR mice (8 weeks old) were obtained from the
Laboratory Animal Center of Xi'an Jiaotong University (Xi'an,
China). The animals were housed under controlled temperature
(23±2°C), humidity (55±5%) and light (12-h light/dark cycle) with
free access to standard diet and water. After acclimatization for 1
week, 72 mice were randomly allocated into two groups:
non-irradiated group (n=8) and UV-irradiated group (n=64). The
dorsal skin of mice (2×3 cm2) was shaved using an
electric razor, and this operation was repeated before UV
irradiation and postirradiation treatment. All animal experiments
were approved by the Laboratory Animal Administration Committee of
Xi'an Jiaotong University and performed according to the Guidelines
for Animal Experimentation of Xi'an Jiaotong University and the
Guide for the Care and Use of Laboratory Animals published by the
US National Institutes of Health (NIH publication no. 85–23,
revised 2011).
UV irradiation
UV irradiation of mice was performed using the UV
light source provided by 2 UVA lamps (315–400 nm, peak wavelength:
365 nm) and 4 UVB lamps (280–315 nm, peak wavelength: 312 nm) (both
from Beijing Lighting Research Institute, Beijing, China). The
distance from the lamps to the animals' backs was 30 cm. The
minimal erythema dose (MED) was preliminarily measured with a UV
meter (Lutron UV-340A, Taipei, Taiwan), and 1,200 mJ/cm2
of UVA and 180 mJ/cm2 of UVB were assembled 1 MED in
this study. Mice were irradiated 3 times a week (Monday, Wednesday
and Friday) for 12 weeks. The irradiation dose was increased weekly
by 1 MED from 1 MED up to 4 MED and then maintained at 4 MED for
the rest weeks. The non-irradiated group was treated identically
with the lamps power off.
Postirradiation treatment
After 12 weeks of UV irradiation, mice in the
UV-irradiated group were randomly reallocated into eight groups
with 8 mice per group: UV-irradiated plus vehicle (ethanol:
propylene glycol, 7:3 v/v)-treated group (UV control group),
UV-irradiated plus ATRA (Sigma-Aldrich, St. Louis, MO, USA)-treated
group (ATRA group), UV-irradiated plus ATRA and AGN193109 (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA)-treated group (ATRA
+ RAR antagonist group), UV-irradiated plus ATRA and HX531 (Tocris
Bioscience, Bristol, UK)-treated group (ATRA + RXR antagonist
group), UV-irradiated plus TTNPB (Sigma-Aldrich)-treated group (RAR
agonist group), UV-irradiated plus TTNPB and AGN193109-treated
group (RAR agonist + RAR antagonist group), UV-irradiated plus
SR11237 (Sigma-Aldrich)-treated group (RXR agonist group) and
UV-irradiated plus SR11237 and HX531-treated group (RXR agonist +
RXR antagonist group). ATRA and retinoid receptor-specific agonists
and antagonists were applied topically 5 times a week in 100 µl
vehicle per treatment for 8 weeks. According to previous studies
(16–19), these agonists and antagonists were
applied at the following concentrations with slight modification:
ATRA, 160 nM; TTNPB, 160 nM; AGN193109, 400 nM; SR11237, 160 nM;
HX531, 400 nM. Eight mice in the non-irradiated group were used as
the normal group and treated with vehicle alone.
Histological examination
Twenty-four hour after the final treatment, mice
were sacrificed by cervical dislocation under anesthesia, and
dorsal skin was quickly removed. The skin samples were fixed in 10%
neutral buffered formalin for 24 h, embedded in paraffin and
sectioned at 5 µm. Masson's trichrome staining was performed to
examine the skin collagen fibers. The stained sections were
captured under an Olympus BX51 light microscope equipped with a
DP70 digital camera (Olympus, Tokyo, Japan).
Determination of collagen content
Hydroxyproline (Hyp) can be converted to the
equivalent of collagen by multiplying the factor 7.46, considering
Hyp is the almost exclusive amino acid of collagen and accounts for
13.4±0.24% of mammalian collagen in previous studies (20,21).
Hence, in this study, total Hyp content in the skin was measured
using the Hyp assay kit (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China) according to the manufacturer's
instruction for the determination of collagen content.
Western blot analysis
Fresh skin samples were homogenized in ice-cold RIPA
lysis buffer (Heart Biological Technology, Co., Ltd., Xi'an,
China). Lysates were centrifuged at 14,000 × g for 10 min at 4°C,
and the supernatants were collected as the total proteins. Protein
concentration was measured using the BCA protein assay kit
(Beyotime Institute of Biotechnology, Shanghai, China). Each sample
was subsequently denatured by boiling in Laemmli loading buffer for
5 min. Equal amounts of protein were separated by 8–10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to PVDF membranes (Millipore, Billerica, MA, USA).
After blocking with 5% non-fat dried milk for 2 h at 37°C, the
membranes were incubated overnight at 4°C with the following
primary antibodies: rabbit anti-MMP-3 monoclonal antibody (Cat. no.
ab52915; 1:1,000 dilution), rabbit anti-MMP-13 polyclonal antibody
(Cat. no. ab39012; 1:1,000 dilution) (both from Abcam, Cambridge,
MA, USA), rabbit anti-c-Jun monoclonal antibody (Cat. no. 9165;
1:1,000 dilution), rabbit anti-c-Fos monoclonal antibody (Cat. no.
2250; 1:1,000 dilution) (both from Cell Signaling Technology, Inc.,
Danvers, MA, USA), goat anti-type I procollagen polyclonal antibody
(Cat. no. sc-8787; 1:500 dilution) and mouse anti-β-actin
monoclonal antibody (Cat. no. sc-47,778; 1:1,000 dilution) (both
from Santa Cruz Biotechnology, Inc.). After washing 3 times, the
membranes were incubated with the appropriate horseradish
peroxidase (HRP)-conjugated secondary antibodies for 1 h at 37°C,
followed by enhanced chemiluminescence (Millipore). The signals
were captured, and the intensity of the protein bands was
quantified using Image J software (NIH, Bethesda, MD, USA)
(22).
Statistical analysis
All data are presented as the means ± SD.
Statistical analysis was performed using SPSS 19.0 software (SPSS,
Inc., Chicago, IL, USA). Data were analyzed using one-way ANOVA or
a Student's t-test to perform comparisons between two groups. A
P-value of <0.05 was considered to indicate a statistically
significant difference.
Results
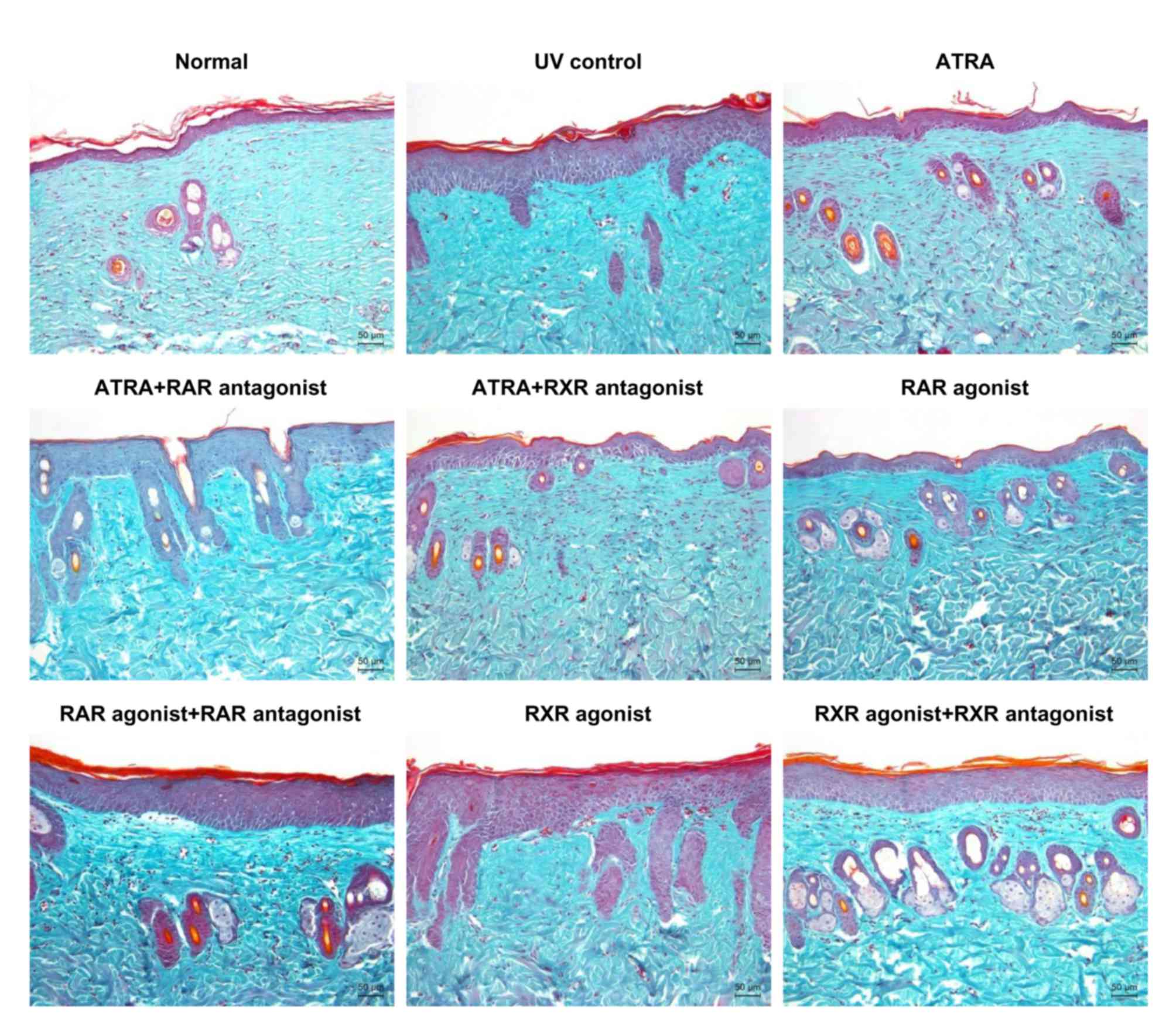
Histological analysis
Histological sections of the dorsal skin were
subjected to Masson's trichrome staining to visualize the changes
in collagen fibers in the dermis. As shown in Fig. 1, the normal group displayed
regularly arranged collagen fibers. Compared with the normal group,
UV irradiation caused large amounts of abnormal, fragmented and
disorganized collagen fibers in the UV control group. ATRA and RAR
agonist improved the UV-induced damage to collagen fibers, whereas
these effects were markedly inhibited by RAR antagonist. Similar
changes in collagen fibers were observed either between the ATRA
and ATRA + RXR antagonist groups or between the RXR agonist and UV
control groups. These results indicated that ATRA and RAR agonist
could ameliorate the UV-induced damage to skin collagen fibers
through RAR.
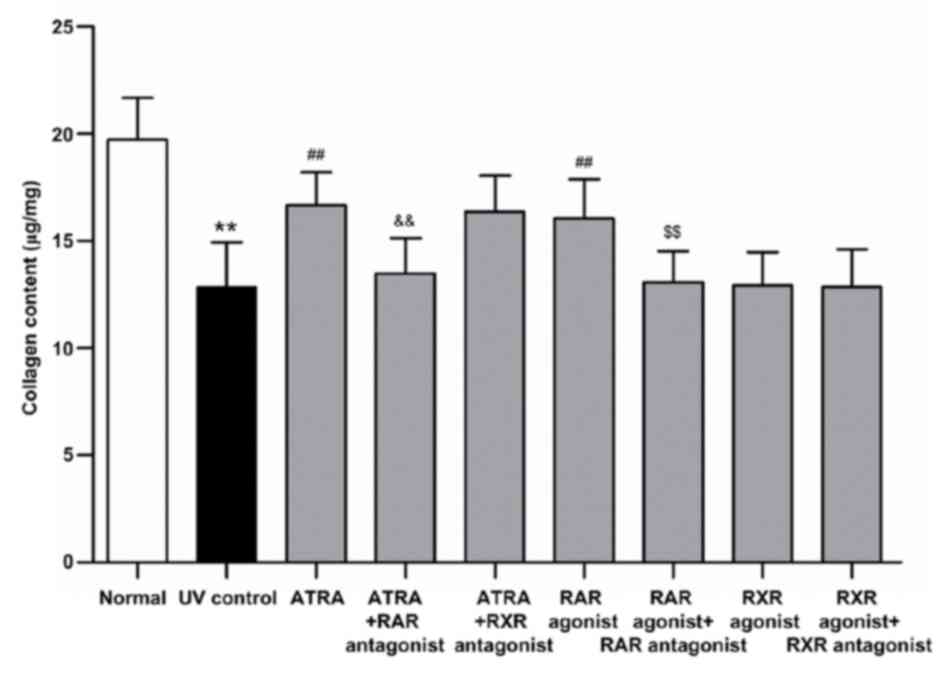
Estimation of collagen content
The collagen content was determined through
measuring the amount of Hyp. As shown in Fig. 2, UV irradiation induced a 34.86%
decrease in the collagen content (P<0.01, vs. normal group).
ATRA and RAR agonist significantly increased the collagen content
by 29.83 and 25.00%, respectively, as compared with the UV control
group (all P<0.01, vs. UV control group). However, these effects
were significantly suppressed by RAR antagonist (all P<0.01, vs.
ATRA and RAR agonist groups, respectively). There was no
significant difference in the collagen content either between the
ATRA and ATRA + RXR antagonist groups or between the RXR agonist
and UV control groups. These results were consistent with those of
Masson's trichrome staining.
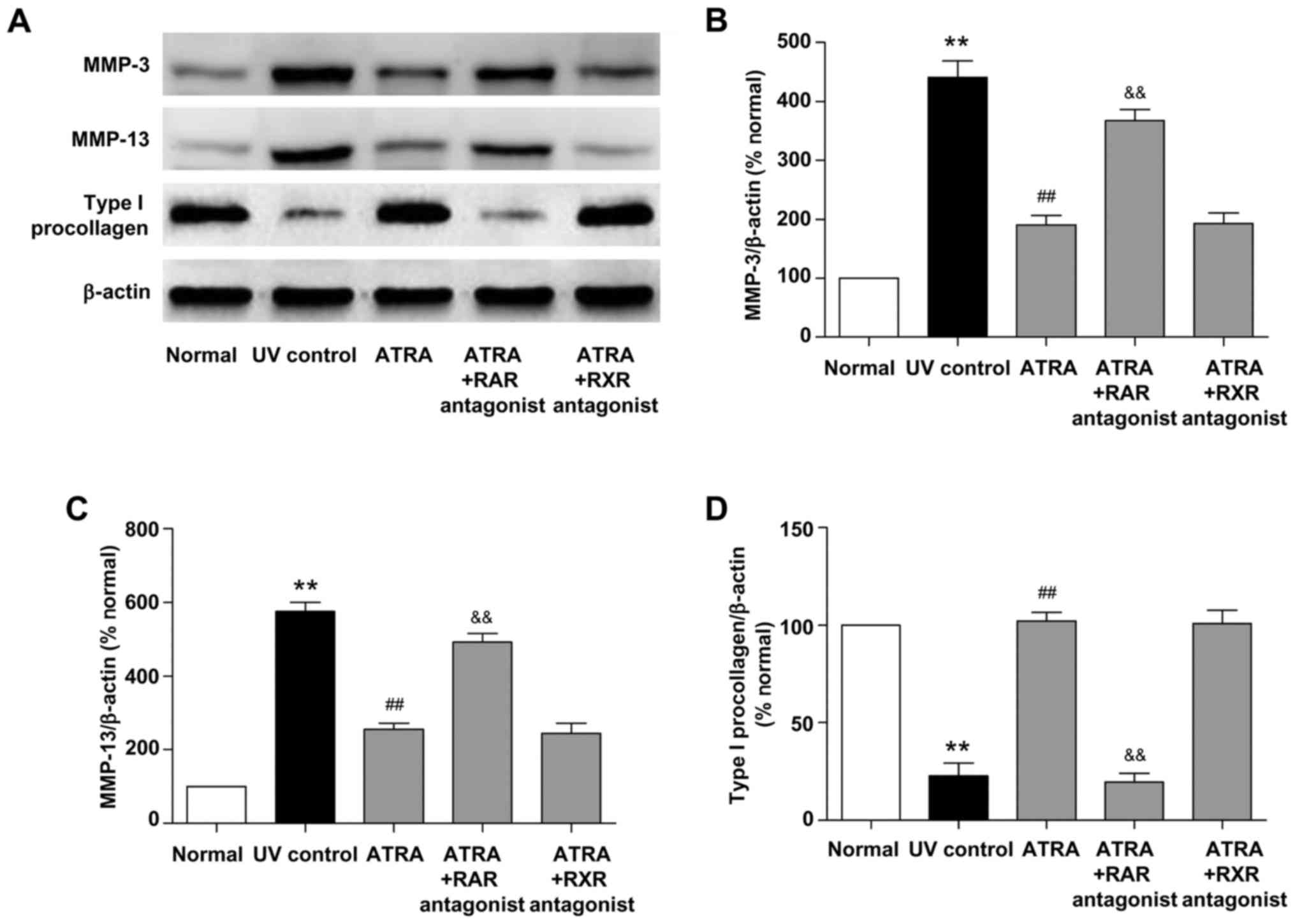
Effects of ATRA on the protein
expression of MMPs and type I procollagen in photoaged skin
UV irradiation stimulates collagen breakdown and
inhibits procollagen synthesis (1). MMP-3 and MMP-13 are the key
regulators of collagen degradation in photoaged mouse skin. To
investigate the mechanism underlying the beneficial histological
effects of ATRA on photoaged skin, the protein expression of MMP-3,
MMP-13 and type I procollagen was detected by western blotting. As
shown in Fig. 3, UV irradiation
significantly increased the protein levels of MMP-3 and MMP-13 and
decreased the protein level of type I procollagen (all P<0.01,
vs. normal group). ATRA significantly reduced MMP-3 and MMP-13
protein levels and increased type I procollagen protein level (all
P<0.01, vs. UV control group), while these effects were markedly
inhibited by RAR antagonist (all P<0.01, vs. ATRA group). There
was no significant difference in the protein levels of MMP-3,
MMP-13 and type I procollagen between the ATRA and ATRA + RXR
antagonist groups. These data indicated that ATRA could stimulate
the protein expression of type I procollagen and inhibit the
protein expression of MMP-3 and MMP-13 through RAR in photoaged
skin.
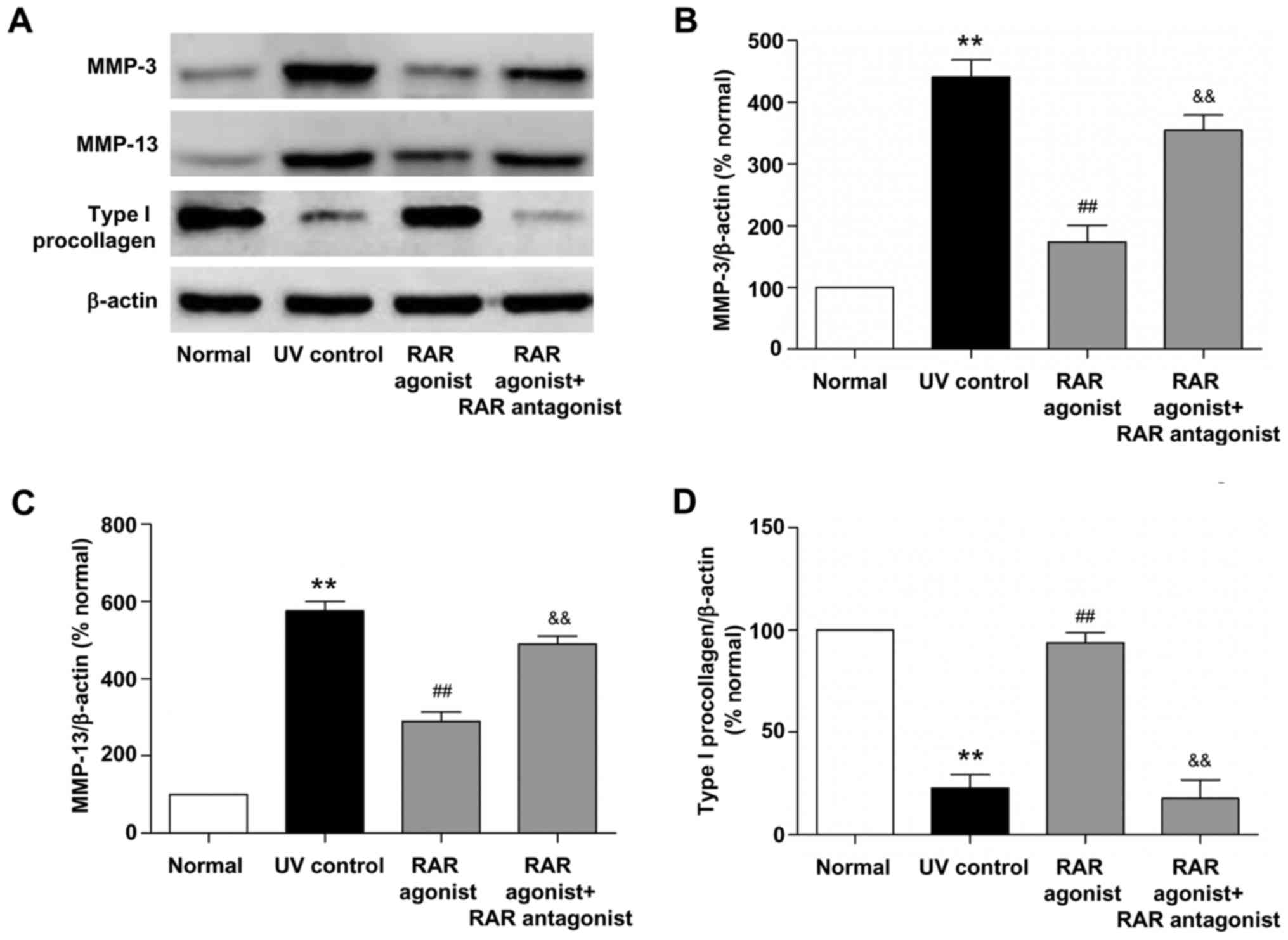
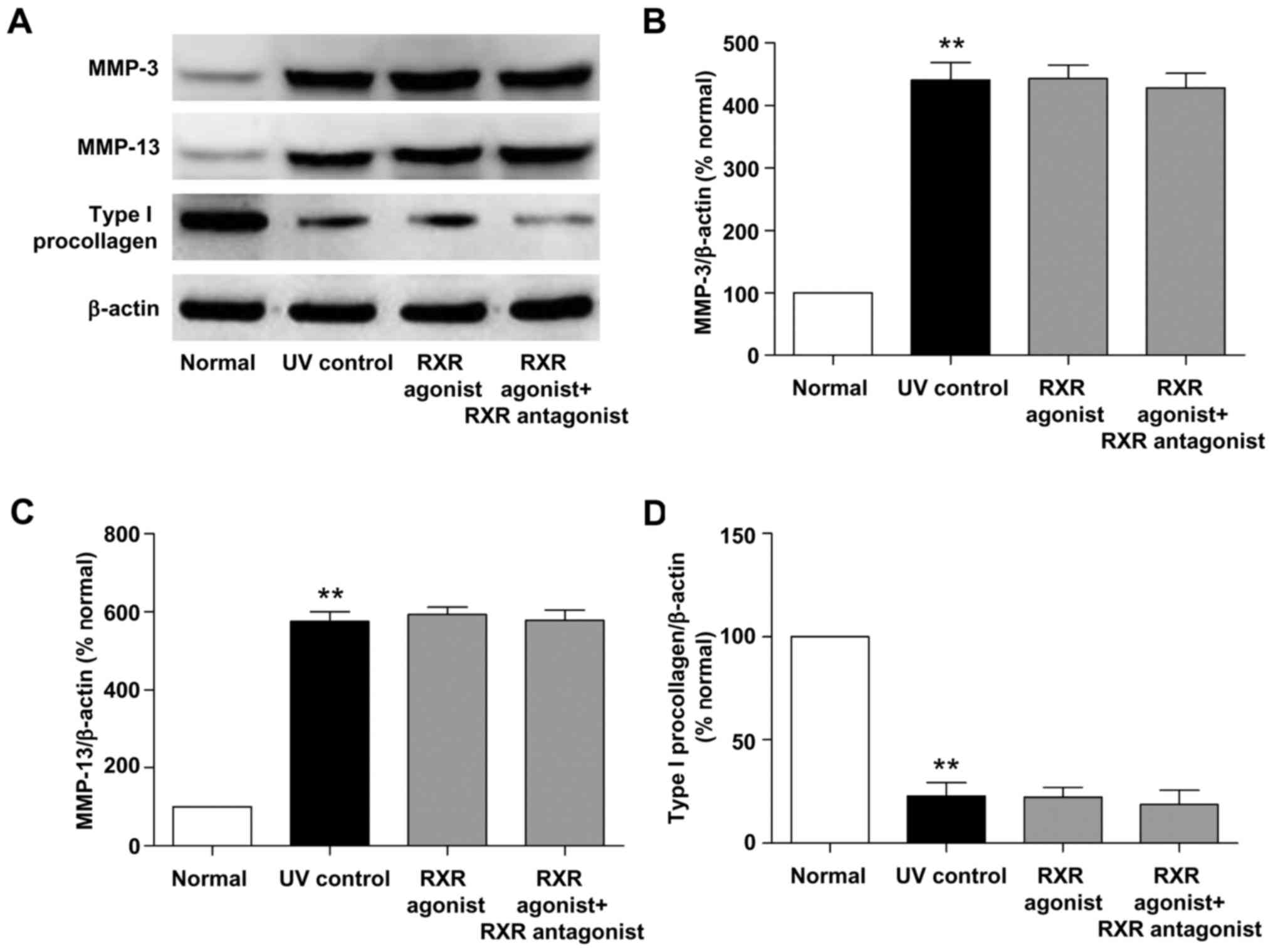
Effects of RAR and RXR agonists on the
protein expression of MMPs and type I procollagen in photoaged
skin
We also examined the effects of RAR and RXR agonists
on the protein expression of MMP-3, MMP-13 and type I procollagen
in photoaged skin. As shown in Figs.
4 and 5, UV irradiation
resulted in a significant increase in the protein levels of MMP-3
and MMP-13 and a significant decrease in the protein level of type
I procollagen (all P<0.01, vs. normal group). RAR agonist
prominently reduced MMP-3 and MMP-13 protein levels and increased
type I procollagen protein level (all P<0.01, vs. UV control
group), whereas these effects were significantly suppressed by RAR
antagonist (all P<0.01, vs. RAR agonist group). There was no
significant difference in the protein levels of MMP-3, MMP-13 and
type I procollagen between the RXR agonist and UV control groups.
These data demonstrated that RAR agonist rather than RXR agonist
could stimulate the protein expression of type I procollagen and
inhibit the protein expression of MMP-3 and MMP-13 through RAR in
photoaged skin.
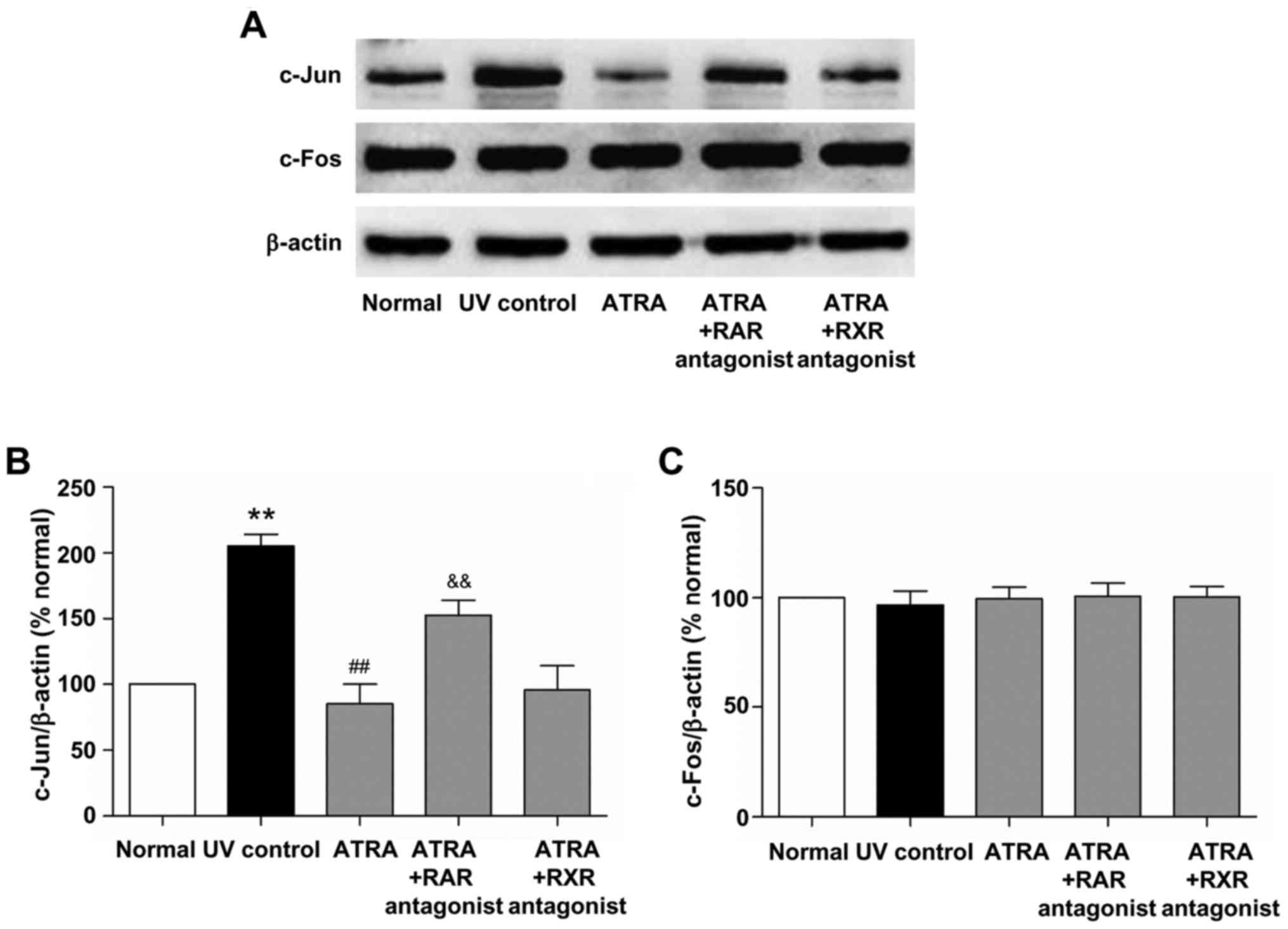
Effects of ATRA on the protein
expression of c-Jun and c-Fos in photoaged skin
Transcription factor AP-1 is mainly composed of
c-Jun and c-Fos proteins and plays an important role in the
mechanism of photoaging (3). To
investigate the effect of ATRA on AP-1 expression in photoaged
skin, the protein expression of c-Jun and c-Fos was detected by
western blotting. As shown in Fig.
6, UV irradiation significantly increased the protein level of
c-Jun (P<0.01, vs. normal group). ATRA significantly reduced
c-Jun protein level (P<0.01, vs. UV control group), while this
effect was markedly inhibited by RAR antagonist (P<0.01, vs.
ATRA group). There was no significant difference in the protein
level of c-Jun between the ATRA and ATRA + RXR antagonist groups.
Moreover, no significant difference in the c-Fos protein level was
observed between all groups. These results indicated that ATRA
could down-regulate the protein expression of c-Jun through RAR in
photoaged skin.
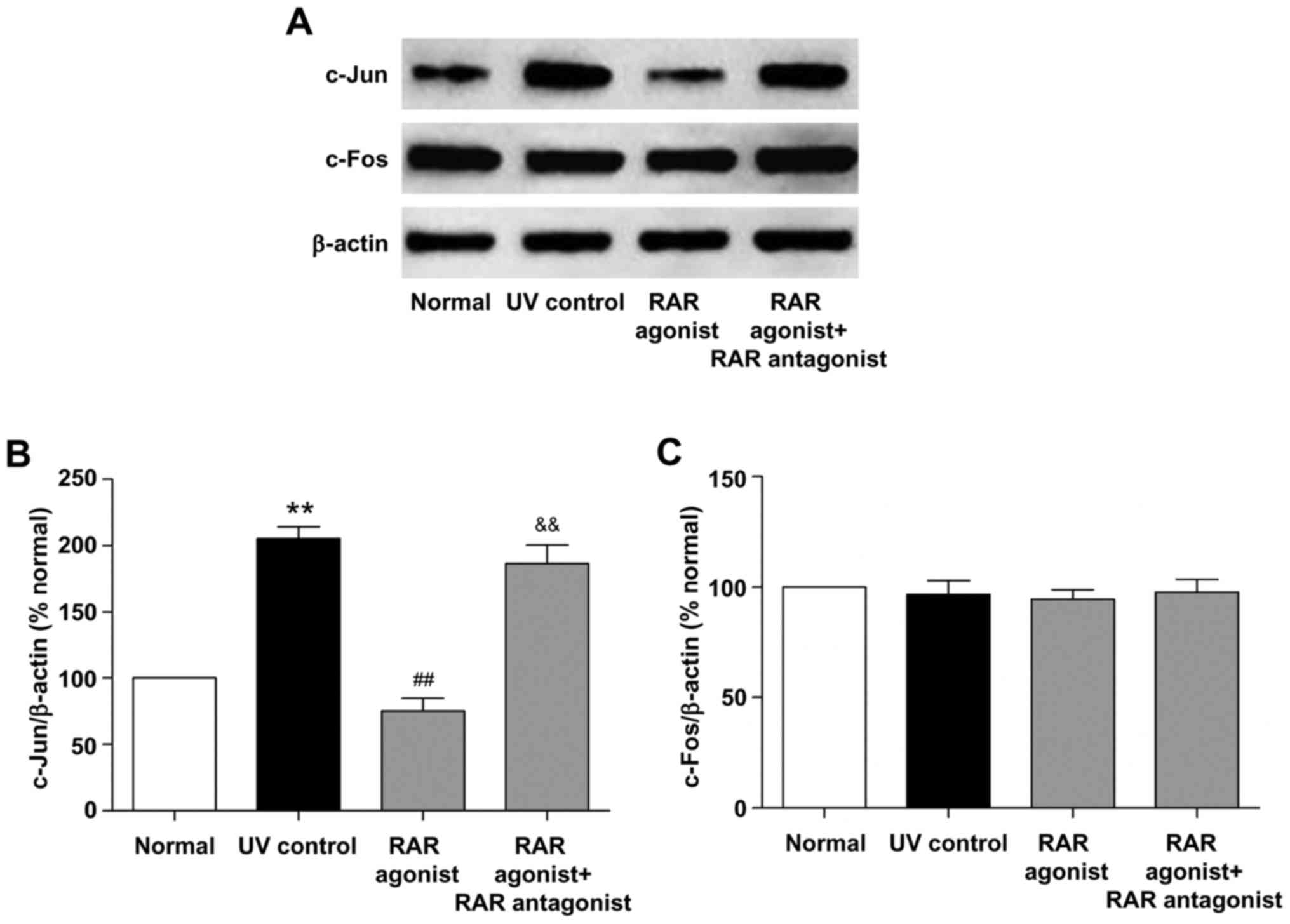
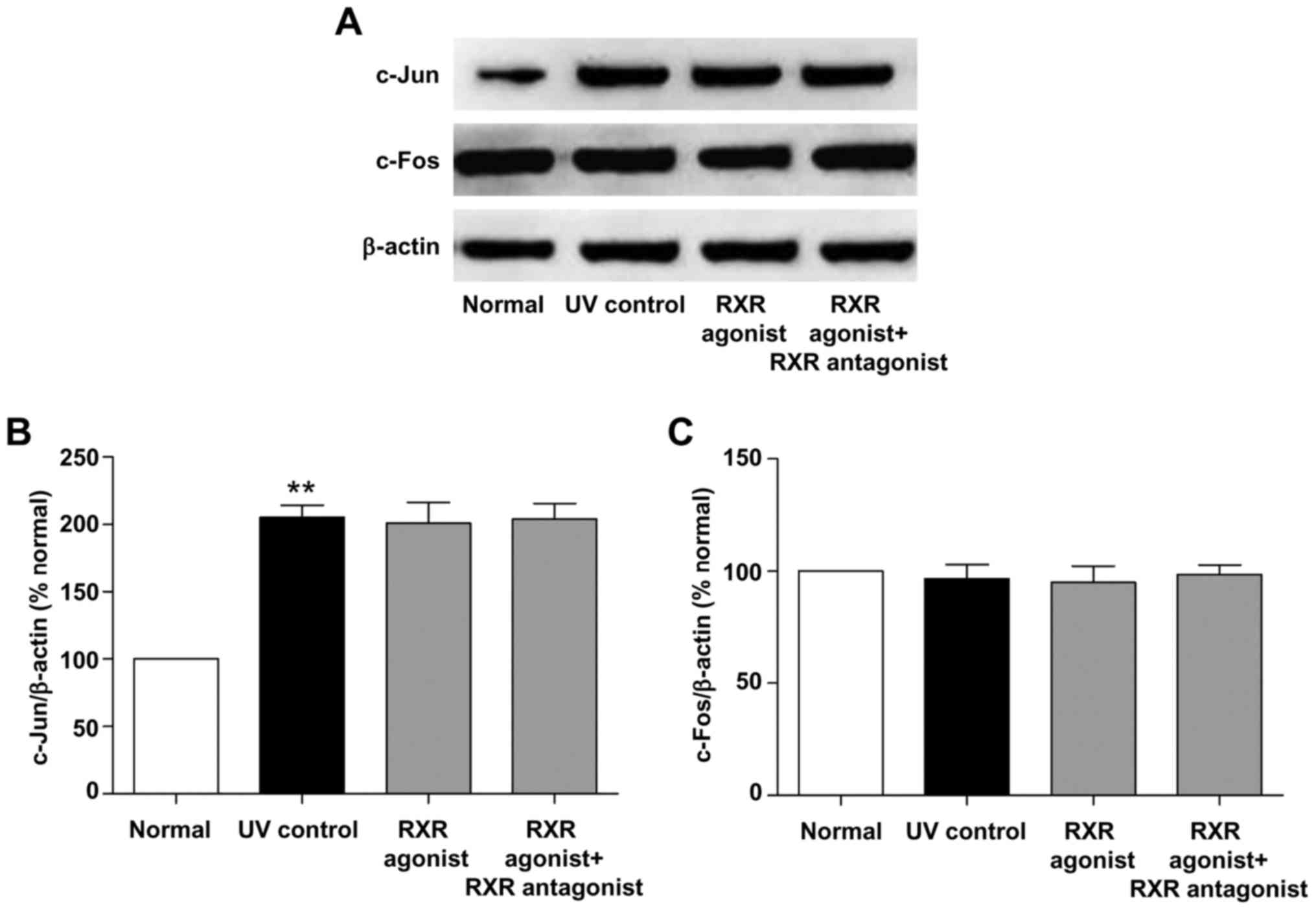
Effects of RAR and RXR agonists on the
protein expression of c-Jun and c-Fos in photoaged skin
We also examined the effects of RAR and RXR agonists
on the protein expression of c-Jun and c-Fos in photoaged skin. As
shown in Figs. 7 and 8, UV irradiation resulted in a
significant increase in the protein level of c-Jun (P<0.01, vs.
normal group). RAR agonist prominently reduced c-Jun protein level
(P<0.01, vs. UV control group), whereas this effect was
significantly suppressed by RAR antagonist (P<0.01, vs. RAR
agonist group). There was no significant difference in the protein
level of c-Jun between the RXR agonist and UV control groups.
Furthermore, RAR and RXR agonists had no significant effect on the
c-Fos protein level in photoaged skin. These results demonstrated
that RAR agonist rather than RXR agonist could down-regulate the
protein expression of c-Jun through RAR in photoaged skin.
Discussion
Chronic exposure to UV irradiation disrupts the
normal architecture of skin connective tissue, impairs skin
function and ultimately causes photoaging (1). RA, which is the bioactive metabolite
of vitamin A, has demonstrated efficacy in the treatment of
photoaged skin (12–14). However, the mechanism of its action
in the treatment of photoaged skin remains unclear. Thus, in the
present study, we applied ATRA, RAR and RXR agonists, as well as
RAR and RXR antagonists to the photoaged mouse skin, explored
whether the therapeutic effects of RA on photoaged skin are
mediated by RAR and/or RXR in mice and investigated the underlying
mechanism.
Disorganization of collagen fibers and reduction of
collagen content are the prominent features of photoaged skin
(1,3). In this study, Masson's trichrome
staining and Hyp assay were used to evaluate the effects of ATRA
and RAR and RXR agonists on the collagen fibers and collagen
content in photoaged skin. Our results showed that UV irradiation
caused the fragmented and disorganized collagen fibers and the
decreased collagen content, which was consistent with previous
studies (20,23,24).
ATRA and RAR agonist not only improved the UV-induced damage to
collagen fibers but also increased the collagen content in
photoaged skin. However, these beneficial effects were markedly
inhibited by RAR antagonist. RXR agonist had no significant effect
on the collagen fibers and collagen content in photoaged skin
(Figs. 1 and 2). Therefore, ATRA and RAR agonist could
ameliorate the UV-induced damage to skin collagen fibers and
increase the collagen content in photoaged skin through RAR.
UV irradiation causes alterations of dermal collagen
through stimulating collagen breakdown and inhibiting procollagen
synthesis (1). MMP-3 and MMP-13
are primarily responsible for the degradation of collagen in
photoaged mouse skin (5–7). Type I procollagen can be converted
into the major structural protein in dermal ECM. Thus, the
regulation of MMP-3, MMP-13 and type I procollagen may be an
effective strategy for the treatment of photoaged skin in mice. In
the present study, UV irradiation significantly increased the
protein levels of MMP-3 and MMP-13 and decreased the protein level
of type I procollagen. ATRA and RAR agonist significantly reduced
MMP-3 and MMP-13 protein levels and increased type I procollagen
protein level compared with the UV control group, whereas these
effects were markedly inhibited by RAR antagonist (Figs. 3 and 4). RXR agonist had no significant effect
on the protein levels of MMP-3, MMP-13 and type I procollagen in
photoaged skin (Fig. 5).
Therefore, ATRA and RAR agonist could stimulate the protein
expression of type I procollagen and inhibit the protein expression
of MMP-3 and MMP-13 through RAR in photoaged skin.
UV-induced transcription factor AP-1, which is
composed of elevated c-Jun and constitutively expressed c-Fos,
increases MMPs transcription and decreases procollagen synthesis in
the process of photoaging (3,25,26).
RA can antagonize UV activation of AP-1 by inhibiting the induction
of c-Jun protein in human skin (26). In this study, we further
investigated whether RA stimulated type I procollagen protein
expression and inhibited MMP-3 and MMP-13 protein expression
through AP-1 pathway in photoaged skin. Our results demonstrated
that ATRA and RAR agonist significantly reduced the protein level
of c-Jun in photoaged skin, while these effects were markedly
inhibited by RAR antagonist (Figs.
6 and 7). There was no
significant difference in the protein level of c-Jun between the
RXR agonist and UV control groups (Fig. 8). In addition, ATRA and RAR and RXR
agonists had no significant effect on the c-Fos protein level in
photoaged skin (Figs. 6–8). These findings indicated that ATRA and
RAR agonist could stimulate type I procollagen protein expression
and inhibit MMP-3 and MMP-13 protein expression by down-regulating
c-Jun protein expression in photoaged skin, which was mediated by
RAR.
In conclusion, our study indicates for the first
time that ATRA and RAR agonist could ameliorate the UV-induced
damage to skin collagen fibers and increase the collagen content in
photoaged skin through RAR. In addition, ATRA and RAR agonist could
stimulate type I procollagen protein expression and inhibit MMP-3
and MMP-13 protein expression by down-regulating c-Jun protein
expression in photoaged skin, which was mediated by RAR. Taken
together, these findings suggest that RA may ameliorate photoaged
skin through RAR-mediated pathway in mice, providing a theoretical
basis for clinical treatment of photoaged skin.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (no. 81271741).
Glossary
Abbreviations
Abbreviations:
|
AP-1
|
activator protein-1
|
|
ATRA
|
all-trans retinoic acid
|
|
Hyp
|
hydroxyproline
|
|
MED
|
minimal erythema dose
|
|
MMP
|
matrix metalloproteinase
|
|
RA
|
retinoic acid
|
|
RAR
|
retinoic acid receptor
|
|
RXR
|
retinoid X receptor
|
|
UV
|
ultraviolet
|
References
|
1
|
Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ
and Fisher GJ: Matrix-degrading metalloproteinases in photoaging. J
Investig Dermatol Symp Proc. 14:20–24. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Svobodová A and Vostálová J: Solar
radiation induced skin damage: Review of protective and preventive
options. Int J Radiat Biol. 86:999–1030. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yaar M and Gilchrest BA: Photoageing:
Mechanism, prevention and therapy. Br J Dermatol. 157:874–887.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Quan T, He T, Voorhees JJ and Fisher GJ:
Ultraviolet irradiation induces Smad7 via induction of
transcription factor AP-1 in human skin fibroblasts. J Biol Chem.
280:8079–8085. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nelson AR, Fingleton B, Rothenberg ML and
Matrisian LM: Matrix metalloproteinases: Biologic activity and
clinical implications. J Clin Oncol. 18:1135–1149. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feng XX, Yu XT, Li WJ, Kong SZ, Liu YH,
Zhang X, Xian YF, Zhang XJ, Su ZR and Lin ZX: Effects of topical
application of patchouli alcohol on the UV-induced skin photoaging
in mice. Eur J Pharm Sci. 63:113–123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schorpp M, Mattei MG, Herr I, Gack S,
Schaper J and Angel P: Structural organization and chromosomal
localization of the mouse collagenase type I gene. Biochem J.
308:211–217. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ascenso A, Ribeiro H, Marques HC, Oliveira
H, Santos C and Simões S: Is tretinoin still a key agent for
photoaging management? Mini Rev Med Chem. 14:629–641. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Torok HM and Pillai R: Safety and efficacy
of micronized tretinoin gel (0.05%) in treating adolescent acne. J
Drugs Dermatol. 10:647–652. 2011.PubMed/NCBI
|
|
10
|
Weinstein GD, Koo JY, Krueger GG, Lebwohl
MG, Lowe NJ, Menter MA, Lew-Kaya DA, Sefton J, Gibson JR and Walker
PS; Tazarotene Cream Clinical Study Group, : Tazarotene cream in
the treatment of psoriasis: Two multicenter, double-blind,
randomized, vehicle-controlled studies of the safety and efficacy
of tazarotene creams 0.05% and 0.1% applied once daily for 12
weeks. J Am Acad Dermatol. 48:760–767. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lippman SM, Parkinson DR, Itri LM, Weber
RS, Schantz SP, Ota DM, Schusterman MA, Krakoff IH, Gutterman JU
and Hong WK: 13-cis-retinoic acid and interferon alpha-2a:
Effective combination therapy for advanced squamous cell carcinoma
of the skin. J Natl Cancer Inst. 84:235–241. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ho ET, Trookman NS, Sperber BR, Rizer RL,
Spindler R, Sonti S, Gotz V and Mehta R: A randomized,
double-blind, controlled comparative trial of the anti-aging
properties of non-prescription tri-retinol 1.1% vs. prescription
tretinoin 0.025%. J Drugs Dermatol. 11:64–69. 2012.PubMed/NCBI
|
|
13
|
Bouloc A, Vergnanini AL and Issa MC: A
double-blind randomized study comparing the association of Retinol
and LR2412 with tretinoin 0.025% in photoaged skin. J Cosmet
Dermatol. 14:40–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bagatin E, Guadanhim LR, Enokihara MM,
Sanudo A, Talarico S, Miot HA and Gibson L: Low-dose oral
isotretinoin versus topical retinoic acid for photoaging: A
randomized, comparative study. Int J Dermatol. 53:114–122. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Konta T, Xu Q, Furusu A, Nakayama K and
Kitamura M: Selective roles of retinoic acid receptor and retinoid
X receptor in the suppression of apoptosis by all-trans-retinoic
acid. J Biol Chem. 276:12697–12701. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mihály J, Gericke J, Lucas R, de Lera AR,
Alvarez S, Törőcsik D and Rühl R: TSLP expression in the skin is
mediated via RARγ-RXR pathways. Immunobiology. 221:161–165. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gericke J, Ittensohn J, Mihály J, Alvarez
S, Alvarez R, Töröcsik D, de Lera AR and Rühl R: Regulation of
retinoid-mediated signaling involved in skin homeostasis by RAR and
RXR agonists/antagonists in mouse skin. PLoS One. 8:e626432013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mukherjee S, Date A, Patravale V, Korting
HC, Roeder A and Weindl G: Retinoids in the treatment of skin
aging: An overview of clinical efficacy and safety. Clin Interv
Aging. 1:327–348. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kang S, Bergfeld W, Gottlieb AB, Hickman
J, Humeniuk J, Kempers S, Lebwohl M, Lowe N, McMichael A, Milbauer
J, et al: Long-term efficacy and safety of tretinoin emollient
cream 0.05% in the treatment of photodamaged facial skin: A
two-year, randomized, placebo-controlled trial. Am J Clin Dermatol.
6:245–253. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang XF, Huang YF, Wang L, Xu LQ, Yu XT,
Liu YH, Li CL, Zhan JY, Su ZR, Chen JN and Zeng HF:
Photo-protective activity of pogostone against UV-induced skin
premature aging in mice. Exp Gerontol. 77:76–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Neuman RE and Logan MA: The determination
of collagen and elastin in tissues. J Biol Chem. 186:549–556.
1950.PubMed/NCBI
|
|
22
|
Huang X, Zhu B, Wang X, Xiao R and Wang C:
Three-dimensional co-culture of mesenchymal stromal cells and
differentiated osteoblasts on human bio-derived bone scaffolds
supports active multi-lineage hematopoiesis in vitro: Functional
implication of the biomimetic HSC niche. Int J Mol Med.
38:1141–1151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kong SZ, Chen HM, Yu XT, Zhang X, Feng XX,
Kang XH, Li WJ, Huang N, Luo H and Su ZR: The protective effect of
18β-Glycyrrhetinic acid against UV irradiation induced photoaging
in mice. Exp Gerontol. 61:147–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hwang E, Park SY, Lee HJ, Lee TY, Sun ZW
and Yi TH: Gallic acid regulates skin photoaging in UVB-exposed
fibroblast and hairless mice. Phytother Res. 28:1778–1788. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hwang KA, Yi BR and Choi KC: Molecular
mechanisms and in vivo mouse models of skin aging associated with
dermal matrix alterations. Lab Anim Res. 27:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fisher GJ, Talwar HS, Lin J, Lin P,
McPhillips F, Wang Z, Li X, Wan Y, Kang S and Voorhees JJ: Retinoic
acid inhibits induction of c-Jun protein by ultraviolet radiation
that occurs subsequent to activation of mitogen-activated protein
kinase pathways in human skin in vivo. J Clin Invest.
101:1432–1440. 1998. View
Article : Google Scholar : PubMed/NCBI
|