Introduction
Endometriosis is a benign yet common gynecological
disorder observed in 10% of women of reproductive age and is
characterized by a growth of endometrial tissue in locations other
than the uterine cavity (1).
However, its etiology and pathogenesis remain obscure.
Endometriosis results from a complex trait, influenced by genetic
and environmental factors; however, few details are known regarding
the way that candidate genes function (2).
The genetic contribution into endometriosis has been
widely investigated thus far. Candidate gene studies, gene
association and genome wide association studies (GWAS) have been
conducted and already yielded over 30 candidate genes (3). However, the usefulness of these genes
for understanding the pathogenesis of endometriosis needs to be
proven by performing functional studies. At present, 19 independent
SNPs have been robustly associated with endometriosis upon GWAS,
explaining 5.19% of the disease variance (4). Of note, the number of these loci is
on the increase as the proportion of cases analyzed are limited to
more severe disease stages (stage III/IV endometriosis) rather than
stage I/II, thus indicating that moderate to severe endometriosis
cases have greater genetic burden relative to minimal or mild
disease (5). In the Greek
population, we previously reported that the combination of
CYP1A1 polymorphism and GSTM1 null deletion was
closely associated with penetration of the endometriosis phenotype
(2). Additionally, we investigated
the risk of familial endometriosis among first degree relatives
(6).
In the present study, we described a unique family
that was observed among 1,000 cases with endometriosis, where seven
members within a three-generation family were detected with
endometriosis. Furthermore, we investigated whether any
genotype-phenotype association for endometriosis exists in the
affected members of this family by analyzing five SNPs mapped to
WNT4, VEZT, FSHB and IL-16 gene loci,
which have been associated with endometriosis.
Materials and methods
Patient population and study
design
Over a 30-year period, 1,000 women with
endometriosis from two different geographic locations underwent
surgical treatment for endometriosis. The Ethics Committees of the
Human Research of Yale University School (New Haven, CT, USA) (HIC
no. 12590) and Venizeleio General Hospital of Heraklion (Heraklion,
Greece) (ECHR no. 46/6686) approved the overall study. The data
were collected by the clinicians and pathologists reported the
medical records, including surgical procedures and findings.
Staging of the disease was performed with the criteria proposed by
the American Society of Reproductive Medicine (Birmingham, AL, USA)
(7). Clinical characteristics of
the unique family, which was found in Crete, suffering from
endometriosis were examined. Venous blood samples were collected
from the family members as described below.
Genotyping
We selected a panel of SNP markers mapping to five
recently analyzed endometriosis susceptibility loci upon genotyping
of a Greek cohort of patients and healthy controls, including
WNT4, VEZT, FSHB and IL-16 genes.
Genomic DNA was isolated from peripheral blood leukocytes by using
the commercial kit (PureLink® Genomic DNA mini kit;
Invitrogen Life Technologies; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturers instructions.
Allelic discrimination of the WNT4, VEZT and
FSHB SNPs was carried out using pre-made TaqMan SNP
genotyping assays (from Applied Biosystems; Thermo Fisher
Scientific, Inc.) on an Applied Biosystems ViiA™7 Real-Time PCR
system. Allelic discrimination plots were all reviewed individually
for quality. Each assay was run with negative controls. Genotyping
of two common SNPs in the IL-16 gene (located on chromosome
15), rs4072111 (Pro434Ser) and rs11556218 (Asn446Lys) was performed
by following the restriction fragment length polymorphism approach,
by using BsmAI and NdeI, respectively, as described
elsewhere (8,9).
Results
Case description
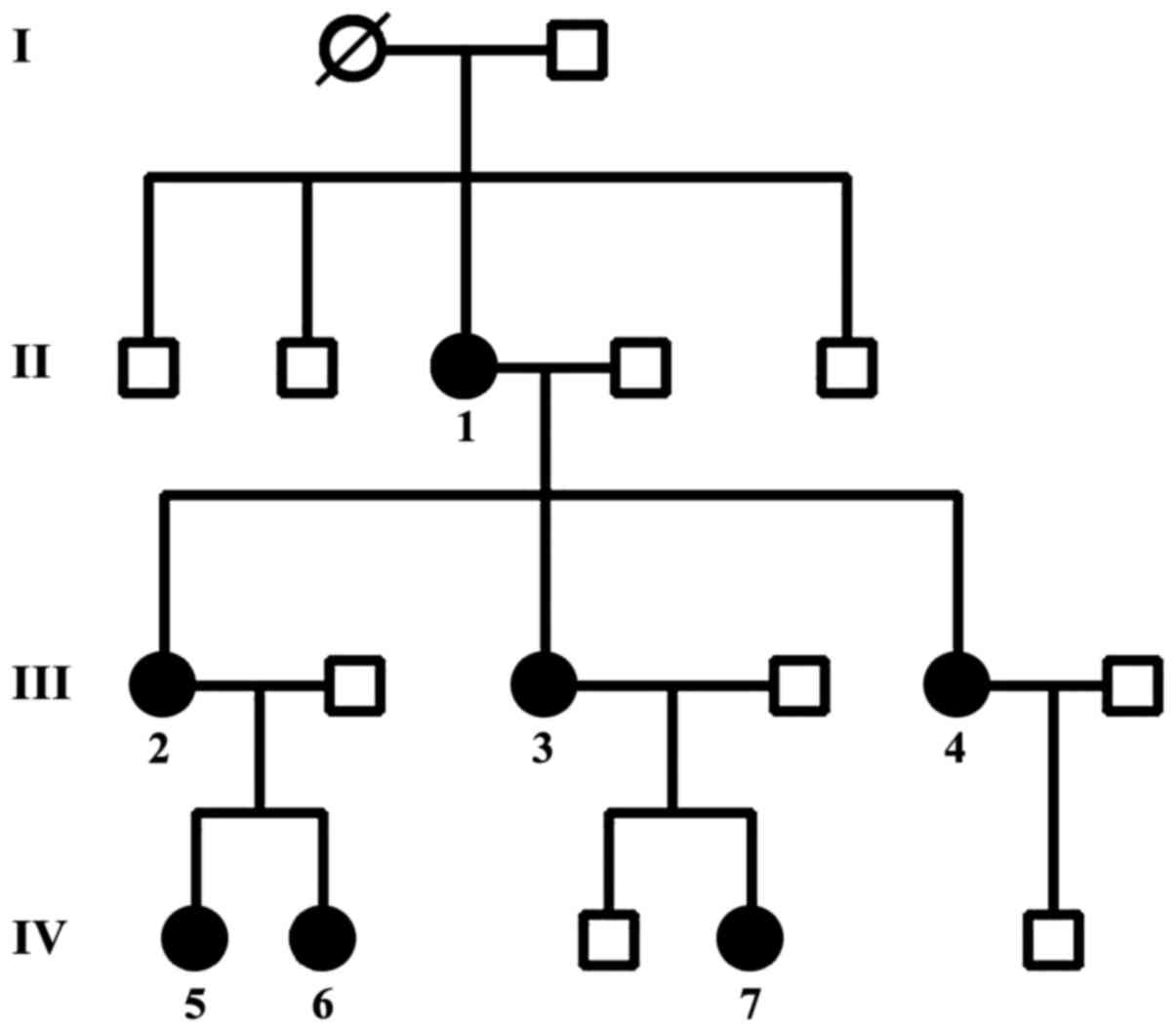
The pedigree of the family and the affected members
is shown in Fig. 1. Table I shows the characteristics of the
family members with endometriosis.
 | Table I.Characteristics of the family
members. |
Table I.
Characteristics of the family
members.
| Case no. | Age (years) | Symptoms | Stage | Pregnancy and
obstetric complications | Gynecologic
complications |
|---|
| 1 | 65 | Pelvic pain
dysmenorhea | IV | 3 | TAH for endometriosis
at age 32 |
| 2 | 49 | Pelvic pain
dysmenorhea | IV | 2 | TAH at age 33 |
| 3 | 46 | Pelvic pain | II | 1 child (IVF) | Endometrioma |
| 4 | 40 | Infertility
dysmenorhea | II | No children | Adenomyosis |
| 5 | 32 | Infertility | III | 2 children (IVF) | Endometrioma |
| 6 | 27 | Infertility pelvic
pain | II | No children | Endometrioma |
| 7 | 25 | Infertility | II | No children | Endometrioma |
The grandmother of the family had given birth to
four children who had no gynecologic problems. Her daughter (case
1), the 64-year-old mother, gave birth to three children from the
age of 16 to 24 years and underwent surgical hysterectomy when she
was 32 years of age due to stage IV bilateral ovarian
endometriosis. She underwent four surgeries for endometriosis. Her
first daughter (case 2) gave birth to two offspring (at the age of
14 and 18, respectively) and underwent laparotomy for bilateral
ovarian endometriosis at 31 years of age, which was followed by
total hysterectomy after two years of conservative treatment
because she had stage IV of the disease and severe clinical
symptoms. Her second daughter (case 3) gave birth to two children
between 17 and 22 years of age and underwent laparoscopy at 28
years of age, which confirmed stage II endometriosis. Her third
daughter (case 4) had severe dysmenorhea for four years and was
diagnosed with infertility at 25 years of age; stage II
endometriosis was found on laparoscopy. She underwent in
vitro fertilization (IVF) and gave birth to one child.
In addition, we detected the granddaughter of case
1, who is 32 years of age (case 5) with endometriosis stage III and
her sister who is 27 years of age with endometriosis stage II (case
6).
Finally, the seventh case who is 25 years of age had
endometriosis stage II without offspring (Table I).
Genetic analysis of the family. The entire family
was genotyped for five SNPs, which were previously identified to be
associated with endometriosis. Genotypes of the family members for
the gene variants analyzed are presented in Table II. The family members were
homozygous for the wild-type allele C for the rs7521902 variant in
the WNT4 locus. Similarly, G/T heterozygotes for the
rs1156218 SNP of IL-16 locus were found for family members at
stages II, II or IV of endometriosis and therefore, the carriage of
the risk allele G cannot be associated with severity of
endometriosis. When a second SNP of the IL-16 gene,
rs4072111, was analyzed, the risk allele T was carried by patients
of both stages II and III, while it was absent from patients of
stage IV.
 | Table II.Genetic variants. |
Table II.
Genetic variants.
| Case no. | WNT4
(rs7521902) | VEZT
(rs10859871) | FSHB
(rs11031006) | IL-16
(rs11556218) | IL-16
(rs407211) |
|---|
| 1 | CC | AA | GG | GT | CC |
| 2 | CC | AA | GG | GT | CC |
| 3 | CC | AC | GG | GT | CC |
| 4 | CC | AC | GG | GT | CT |
| 5 | CC | AA | GG | GT | CT |
| 6 | CC | AA | GG | TT | CC |
| 7 | CC | AA | GG | TT | CC |
Homozygotes for the wild-type allele A of rs10859871
of the VEZT gene were identified for family members at
stages II, III or IV of endometriosis and, therefore, no
genotype-phenotype association was detected for this SNP
either.
Upon genotyping for rs11031006 SNP of FSHB
locus, all the members of the family analyzed were homozygous for
the risk allele G apart from the stage of endometriosis. Of note,
this genotype showed the highest frequency in women with
endometriosis in Greece (85.84%) (data not shown).
Discussion
Genome-wide association studies have identified many
genetic risk loci associated with endometriosis, which opened new
avenues to the better understanding of the molecular mechanisms
leading to the disease (4).
Nevertheless, the genetics of this disease are mainly unsolved and
much investigation remains to be conducted aiming to understand
better the precise role of the various biological pathways involved
in the pathogenesis of endometriosis.
Endometriosis is characterized by a non-clearly
defined phenotype, considering the various clinical manifestations
and obstetric complications of the disease (10). Apart from the progress succeeded in
the identification of new endometriosis risk genes, the literature
available concerning genetic associations related to the diseases
severity is limited. Notably, most of the currently confirmed SNPs
associated with endometriosis were more strongly associated with
stages III and IV, than stages I and II (5). It is possible that familial cases may
provide interesting information both from a phenotypic and
genotypic point of view. Thus, based on results from a replication
study in the Greek population that is currently in progress,
focusing on five SNPs previously reported to be associated with
endometriosis, we aimed to identify a genotype-phenotype
correlation. It has been suggested that although the
genotype-phenotype association is just at the beginning of
endometriosis research, it may be promiscuous regarding its
diagnostic and treatment applications (11).
All the family members were homozygous for the
wild-type allele C for the rs7521902 variant in the WNT4
locus. Notably, familial cases were also homozygous for allele C of
the same SNP in another study referring to an Italian family
(11). The frequency of this
genotype was high in both the Greek and Italian populations (63.29
and 70.5%, respectively) (10,data not shown).
In a former study investigating the probable effect
of IL-16 polymorphisms on disease progression, a significant
difference between allele frequencies of rs4072111 SNP regarding
mild/moderate and severe stages of endometriosis was detected
(9). In particular, the risk for a
severe phenotype among endometriosis patients that carried the T
allele of rs4072111 was reported to be higher, a finding that has
not been replicated in the present study.
In the three-generation family under investigation
in the present study, a woman with endometriosis gave birth to
three daughters with endometriosis (case nos. 2, 3 and 4) of a
varying severity. All the sisters became pregnant and gave birth to
daughters with endometriosis. However, the first sister (no. 2)
with severe endometriosis (stage IV) gave birth to two daughters of
milder stages of endometriosis. By contrast, the second sister (no.
3) gave birth to a daughter with endometriosis of the same stage
with her (II). Thus, it appears that there is no pattern of
familial inheritance dealing with the stage of disease.
In the present study, despite the peculiar clinical
manifestations exhibited by the familial cases from the disease
severity point of view, no correlation between genotype and
phenotype could be determined. However, analysis of the expression
profile from patients of stages I/II vs. III/IV may contribute to
the identification of molecularly defined subtypes and the further
dissection of endometriosis from a molecular viewpoint. A first
attempt in this direction has been previously performed by
Tamaresis et al (12).
To conclude, additional studies involving more SNPs
associated with endometriosis in the members of this particular
family may result in the identification of gene polymorphisms that
may confer new knowledge concerning the genotype-phenotype
correlation. Analogous studies in families from other ethnic
populations may be valuable in this direction but requires large
national and international collaborations and sharing of datasets.
The new findings can be used for the detection of new potential
therapeutic targets and the development of new targeted therapies
depending on the stage of endometriosis, thus leading to improved
care and beneficial management of women with endometriosis.
Acknowledgements
We would like to thank all the practitioners for
providing the data and pathology reports used in this study.
References
|
1
|
Olive DL and Schwartz LB: Endometriosis. N
Engl J Med. 328:1759–1769. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arvanitis DA, Goumenou AG, Matalliotakis
IM, Koumantakis EE and Spandidos DA: Low-penetrance genes are
associated with increased susceptibility to endometriosis. Fertil
Steril. 76:1202–1206. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zondervan KT, Rahmioglu N, Morris AP,
Nyholt DR, Montgomery GW, Becker CM and Missmer SA: Beyond
endometriosis genome-wide association study: from genomics to
phenomics to the patient. Semin Reprod Med. 34:242–254. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sapkota Y, Steinthorsdottir V, Morris AP,
Fassbender A, Rahmioglu N, De Vivo I, Buring JE, Zhang F, Edwards
TL, et al: Meta-analysis identifies five novel loci associated with
endometriosis highlighting key genes involved in hormone
metabolism. Nat Commun. 8:155392017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rahmioglu N, Nyholt DR, Morris AP, Missmer
SA, Montgomery GW and Zondervan KT: Genetic variants underlying
risk of endometriosis: Insights from meta-analysis of eight
genome-wide association and replication datasets. Hum Reprod
Update. 20:702–716. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matalliotakis M, Goulielmos GN, Zervou MI,
Matalliotaki CH, Koumantakis G and Matalliotakis I: Familial
predisposition of endometriosis in Greece. J Endometr Pelvic Pain
Disord. Jun 7–2017.(Epub ahead of print). doi:
https://doi.org/10.5301/jeppd.5000290.
|
|
7
|
No authors listed: American Society for
reproductive: Revised American Society for reproductive medicine
classification of endometriosis: 1996. Fertil Steril. 67:817–821.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao LB, Rao L, Wang YY, Liang WB, Li C,
Xue H, Zhou B, Sun H, Li Y, Lv ML, et al: The association of
interleukin-16 polymorphisms with IL-16 serum levels and risk of
colorectal and gastric cancer. Carcinogenesis. 30:295–299. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Azimzadeh P, Khorshid HR Khorram, Akhondi
MM and Shirazi A: Association of interleukin-16 polymorphisms with
disease progression and susceptibility in endometriosis. Int J
Immunogenet. 43:297–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Viganò P, Somigliana E, Panina P,
Rabellotti E, Vercellini P and Candiani M: Principles of phenomics
in endometriosis. Hum Reprod Update. 18:248–259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Buggio L, Pagliardini L, Gentilini D, De
Braud L, Viganò P and Vercellini P: A rare familial case of
endometriosis with very severe gynecological and obstetric
complications: Novel genetic variants at a glance. Gynecol Obstet
Invest. 77:201–204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tamaresis JS, Irwin JC, Goldfien GA,
Rabban JT, Burney RO, Nezhat C, DePaolo LV and Giudice LC:
Molecular classification of endometriosis and disease stage using
high-dimensional genomic data. Endocrinology. 155:4986–4999. 2014.
View Article : Google Scholar : PubMed/NCBI
|