Introduction
Osteoarthritis (OA) is the most common disorder of
joints, which is characterized by alterations in the structure and
organization of chondrocytes in the articular cartilage (1,2). The
disintegration of cartilage is caused by the disturbance of
equilibrium between the formation and degradation of matrix
components (3). For the treatment
of early stage OA, strategies for the provision of symptomatic
relief have been developed, however, the advanced stage can be
treated only via surgical intervention (4). The apoptosis of chondrocytes also
leads to changes in the biosynthesis of cartilage matrix, which is
the primary factor causing the development of the OA (5). Inflammatory processes are associated
with the degradation of cartilage and the subsequent development of
OA (6). The inflammatory reactions
and apoptosis of chondrocytes are initiated by the expression of
cytokines (7,8). The apoptosis of chondrocytes is
caused by the increased expression of interleukin (IL)-1β through
the involvement of matrix metalloproteinases (MMPs) (9,10).
The effects of MMPs are regulated by the secreted tissue inhibitors
of metalloproteinase (TIMPs). Disturbance of the equilibrium
between TIMPs and MMPs is one of the factors involved in the
development of OA (11).
The use of anti-inflammatory agents has been a
subject of interest for those investigating the treatment of
various types of cancer, including breast, colorectal, esophageal,
lung and stomach cancer (12,13).
Anti-inflammatory compounds are used in clinical practice for the
treatment of carcinoma either in the form of herbal medicines or as
isolated compounds (14,15). The extract of the roots of
Salvia miltiorrhiza Bunge (Danshen), has a long history of
traditional medicinal importance in China for the treatment of
cardiovascular disorders and hepatitis. Phytochemical
investigations of this plant have led to the isolation of certain
compounds, including tanshinone I, tanshinone IIA and
cryptotanshinone (16). Analysis
of these compounds has revealed antibacterial (16), antioxidative (17), anti-inflammatory (18,19)
and cytotoxic activities (20,21),
and inhibitory effects on platelet aggregation (22). The present study investigated the
role of tanshinone II-A in preventing the induction of apoptosis
and cartilage matrix degradation. Tanshinone II-A was found to
inhibit the chondrocyte apoptosis and cartilage matrix degradation
induced by anterior cruciate ligament transection (ACLT) and medial
meniscus resection (MMx).
Materials and methods
Animals
Healthy 8-week-old male Sprague-Dawley rats,
weighing ~180 g, were purchased from the Shanghai Laboratory Animal
Commission (Shanghai, China) under license number SCXK 2013–012.
The experimental procedures involving animals were performed
according to the guidelines for the Care and Use of Laboratory
Animals 2010 by the Ministry of Science and Technology of the
People's Republic of China. The present study was approved by the
ethics committee of Shandong Jimin No. 1 People's Hospital
(Shandong, China). Osteoarthritis (OA) was induced in the rats by
the methods described by Ying et al (23). Briefly, rats were anesthetized with
ether, the right knee was exposed and the patella was dislocated
laterally. Subsequently, the right knee was fully flexed, followed
by anterior crucial ligament transection and medial meniscus
resection using micro-scissors.
Treatment strategy
The rats in the treatments groups were administered
intragastrically with 0.25, 0.30, 0.35, 0.40, 0.45 and 0.50 mg/kg
doses of tanshinone IIA for 28 days. The rats in the normal control
and ACLT + MMx groups received normal saline for the same duration.
The animals were housed under a 12-h light/dark cycle in a
humidity-controlled (60–64%) and sterilized room at 25°C with
access to fresh water and standard laboratory food ad libitum.
Histological analysis
On day 29 following the completion of treatment, the
animals were sacrificed following anesthetization with halothane.
The bones (tibia and femur) were removed and then subjected to
decalcification using EDTA. The paraffin-embedded bone was cut into
thin sections of 2 µm, which were de-paraffinized in boiling
xylene. The sections were subjected to hematoxylin and eosin
staining, followed by histopathological examination using a Mankin
scale, in which 0 indicated normal cartilage and 12 indicated full
disintegration (24). Changes in
the synovial lining were determined using the Image-Pro Plus 6.0
image analysis system (Media Cybernetics, Inc., Rockville, MD,
USA).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end-labeling (TUNEL) assay for the
detection of apoptotic cells
For the analysis of apoptotic cells in the cartilage
sections, the sections were washed with 1% PBS/BSA and then fixed
in 4% paraformaldehyde for 20 min. The sections were permeabilized
using 0.1% Triton-X 100 for 15 min on ice. A TUNEL assay using
fluorescein isothiocyanate (FITC)-conjugated dUTP, and an Apoptosis
Detection System kit (Roche Diagnostics GmbH, Mannheim, Germany)
were used for the analysis of apoptosis, according to the
manufacturer's instructions.
Flow cytometric analysis
The chondrocytes of the rats belonging to the ACLT +
MMx and Tanshinone IIA treatment groups were examined for apoptosis
using Annexin V binding and propidium iodine (PI) staining,
followed by flow cytometry. The cells were washed with ice-cold PBS
and double-stained with FITC-conjugated Annexin V protein and PI
for 30 min. Subsequently, 488-nm laser flow cytometry coupled to a
cell sorter (FACSCalibur; BD Biosciences, San Jose, CA, USA) was
used for analysis of the stained cells.
Enzyme-linked immunosorbent assay
(ELISA) analysis
For determination of inflammatory cytokines, blood
samples (4 ml) were collected from the aorta of the abdominal
region of the rats, and serum was separated by centrifugation at
3,000 × g for 10 min at 4°C. The levels of IL-1β (cat. no. EK0393),
tumor necrosis factor-α (TNF-α; cat no. EK0526) and inducible
nitric oxide synthase (iNOS; cat no. EK0472) were quantified using
commercially available ELISA kits (ScienCell Research Laboratories,
Carlsbad, CA, USA).
Western blot analysis
The cartilage of the rats was washed and placed into
lysis buffer (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
supplemented with phenylmethylsulfonyl fluoride and aprotinin for 4
h at 4°C. The content of protein was determined using a detergent
compatible protein assay kit (Bio-Rad Laboratories, Inc.). Equal
amounts of extracted protein samples (50 µg) was mixed with 2X SDS
buffer, and were separated on a 10% polyacrylamide gel by
electrophoresis. The proteins were then transferred onto a
nitrocellulose membrane (Bio-Rad Laboratories, Inc.) followed by
incubation in blocking buffer (PBS with 7.5% non-fat dry milk, 2%
BSA and 0.1% Tween-20) for 2.5 h at 4°C. The protein expression
levels of MMP-13 and TIMP-1 were determined following incubation at
4°C overnight with the following primary antibodies: Mouse
monoclonal anti-MMP-13 (cat. no. MA5-14247, 1:400 in blocking
buffer; Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
mouse monoclonal anti-TIMP-1 (cat. no. MA1-773, 1:500 in blocking
buffer; Pierce; Thermo Fisher Scientific, Inc.) and anti-β-actin
(cat. no. 2791, 1:800 in blocking buffer; OriGene Technologies,
Inc., Beijing, China). The membranes were washed with PBS and 0.1%
Tween-20, followed by incubation with secondary horseradish
peroxidase-conjugated goat polyclonal anti-rabbit antibodies (cat.
no. 31460, 1:10,000 dilution in blocking buffer; Invitrogen; Thermo
Fisher Scientific, Inc.) for 1 h at 4°C. The membranes were then
washed in PBS and developed using an enhanced chemiluminescence
detection system (GE Healthcare Life Sciences, Uppsala,
Sweden).
Statistical analysis
Data are expressed as the mean ± standard deviation.
The experiments were repeated at least three times, with the mean
of the results presented. The data were analyzed using one-way
analysis of variance followed by Student's t-test using SPSS
software version 21.0 (IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Tanshinone IIA prevents articular
cartilage disintegration in rats induced by ACLT + MMx
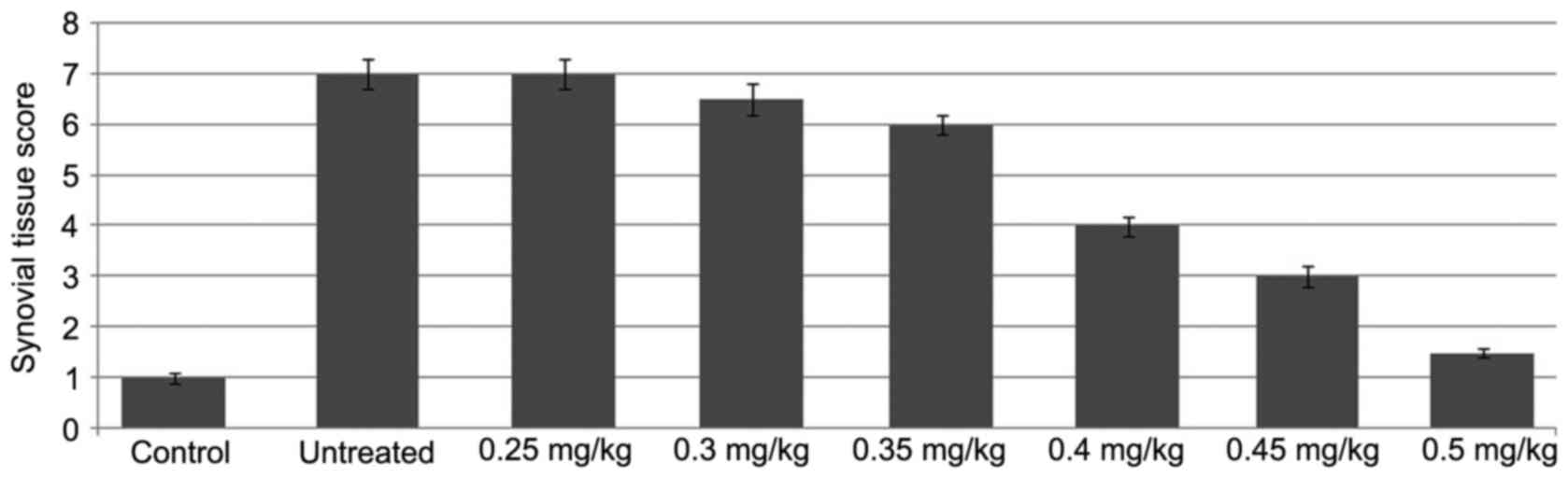
The knee joints of the rats in the ACLT + MMx group
showed marked alterations in articular cartilage histopathology,
which included roughness of the cartilage surface and random
distribution of chondrocytes (Fig.
1). However, the knee joints of the rats in the normal group
exhibited smooth articular cartilage surface and ordered
arrangement of chondrocytes. The Mankin score was significantly
higher in the rats of the untreated group, compared with the normal
rats. Treatment of the rats with tanshinone IIA followed by
exposure to ACLT + MMx prevented degradation of the articular
cartilage. An increase in the dose of tanshinone IIA between 0.25
and 0.5 mg/kg had a significant inhibitory effect on the ACLT +
MMx-induced degradation of articular cartilage in the rats.
Tanshinone IIA treatment at a dose of 0.5 mg/kg significantly
reduced the Mankin score in the ACLT + MMx rats (P<0.002).
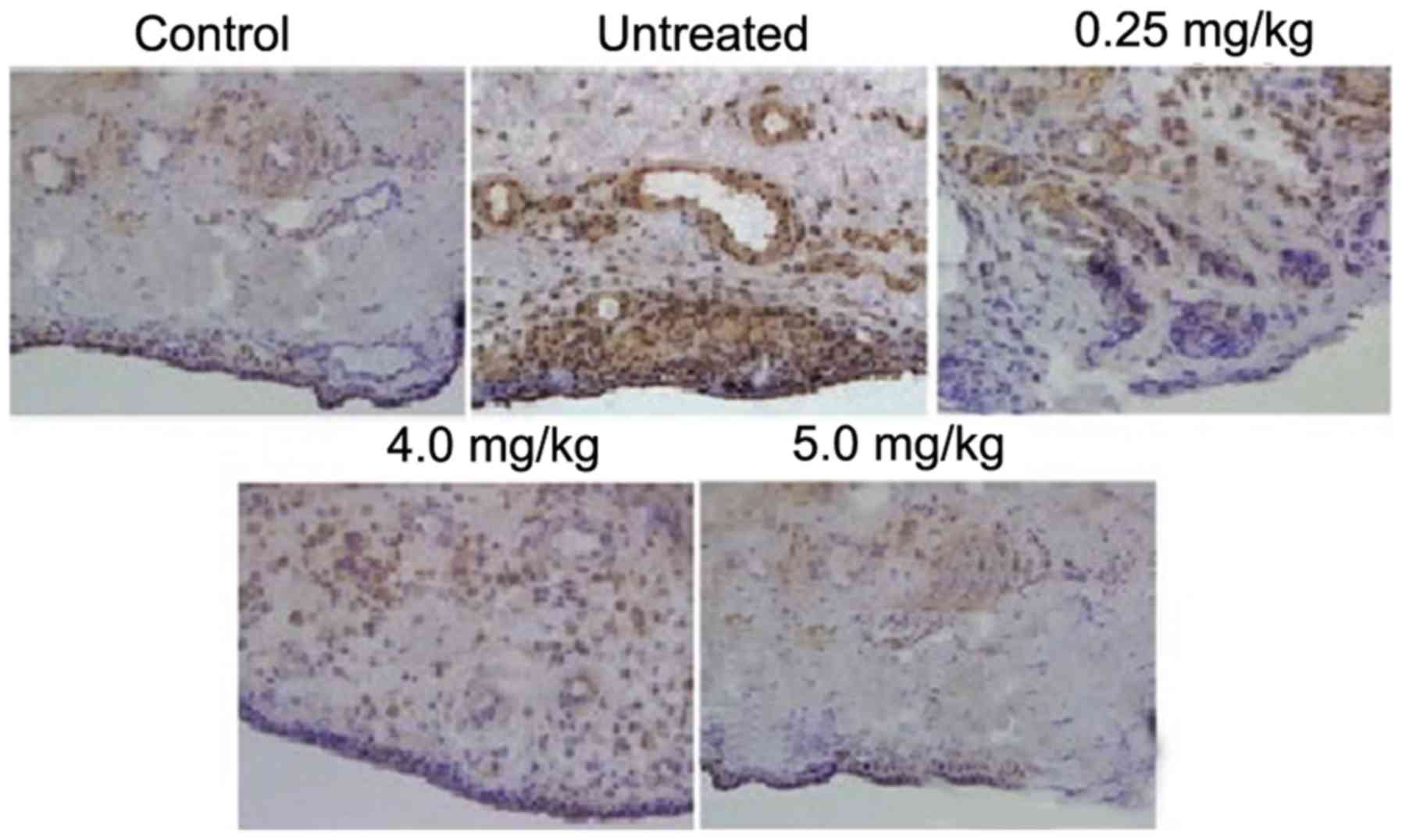
The rats in the ACLT + MMx group exhibited higher
expression levels of inflammatory cells in the tissues of the
synovium, compared with the rats in the normal group. The lining of
the synovium showed hyperplasia, which was absent in the normal
group of rats. Treatment of the rats with tanshinone IIA following
exposure to ACLT + MMx exhibited a concentration-dependent
inhibitory effect on the accumulation of inflammatory cells and
disintegration of the synovial lining (Fig. 2). Tanshinone IIA treatment at a
dose of 0.5 mg/kg completely inhibited the ACLT + MMx-induced
accumulation of inflammatory cells and disintegration of the
synovial lining in the rats.
Tanshinone IIA inhibits ACLT +
MMx-induced apoptosis in chondrocytes
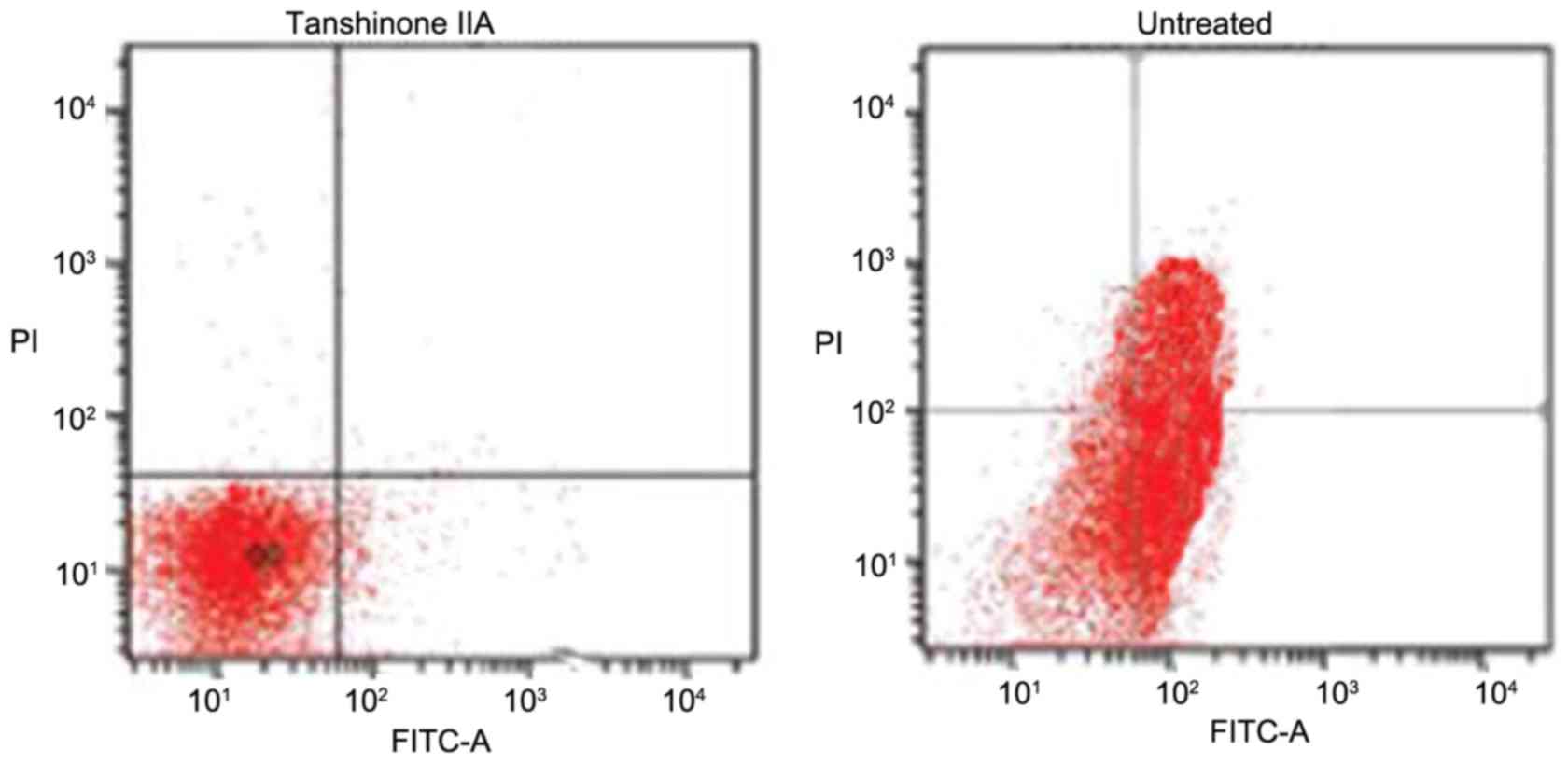
In the rats of the untreated group, the proportion
of apoptotic chondrocytes was significantly higher (Fig. 3). The proportion of apoptotic cells
increased to 52% following 28 days of ACLT + MMx. However,
tanshinone IIA treatment inhibited the induction of apoptosis in
chondrocytes in a concentration-dependent fashion. An increase in
the dose of tanshinone IIA between 0.25 and 0.5 mg/kg reduced the
proportion of apoptotic chrondrocytes from 41 to 2% on day 29.
Tanshinone IIA exhibits inhibitory
effects on the ACLT + MMx-induced increased expression of MMP-13
and reduced expression of TIMP-1
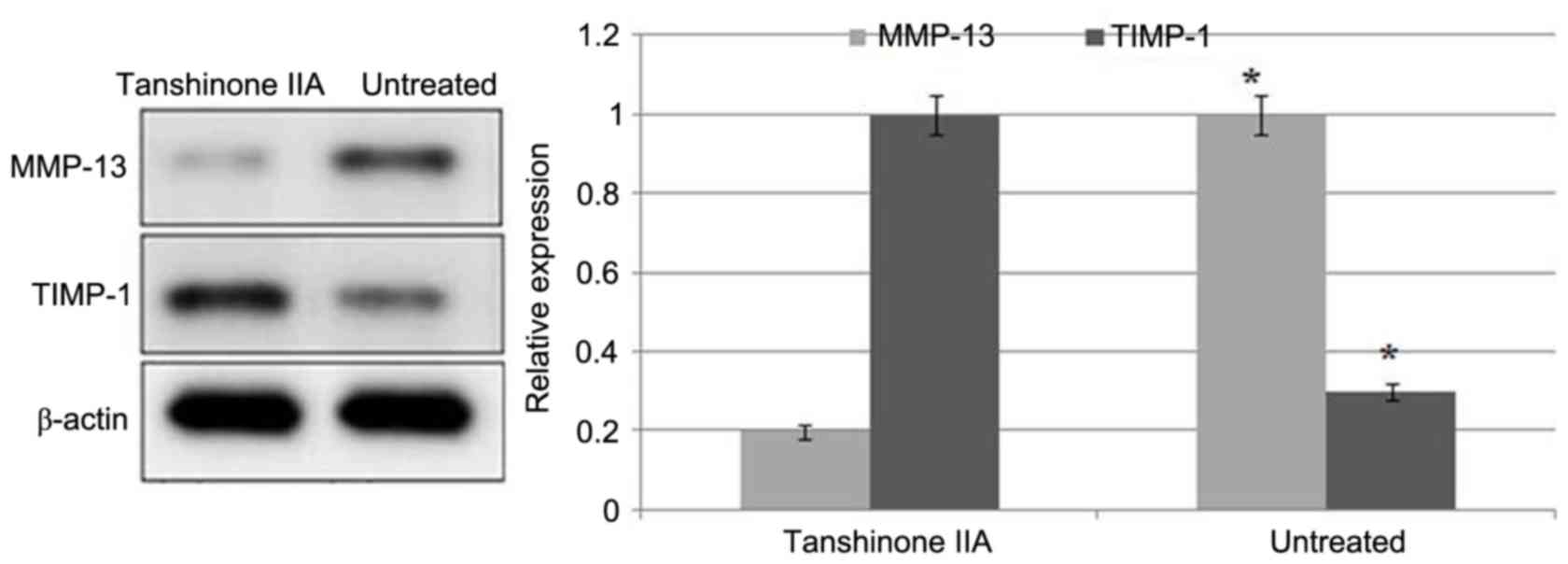
ACLT + MMx caused a significant increase in the
expression of MMP-13 and reduction in the expression of TIMP-1 in
the articular cartilage of the rats (Fig. 4). Tanshinone IIA treatment
inhibited the ACLT + MMx-induced increased expression of MMP-13 and
decreased expression of TIMP-1 in a dose-dependent manner.
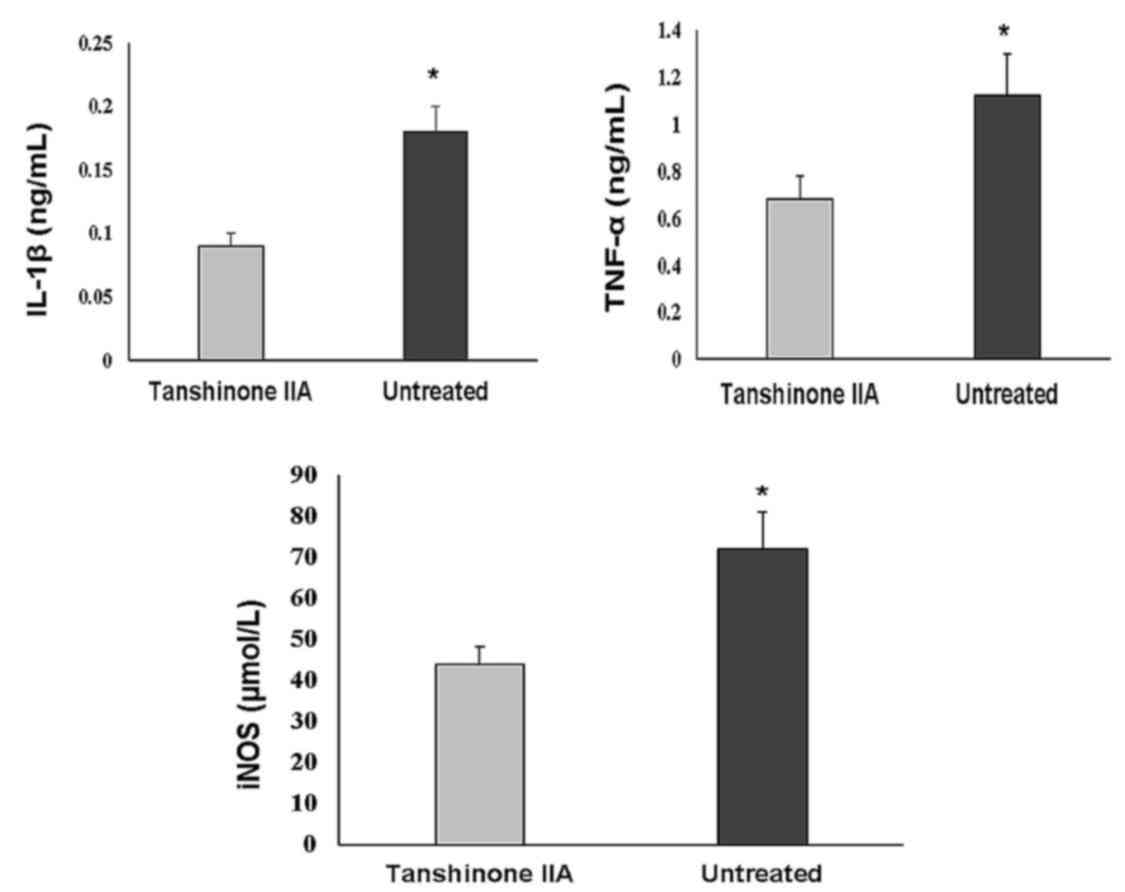
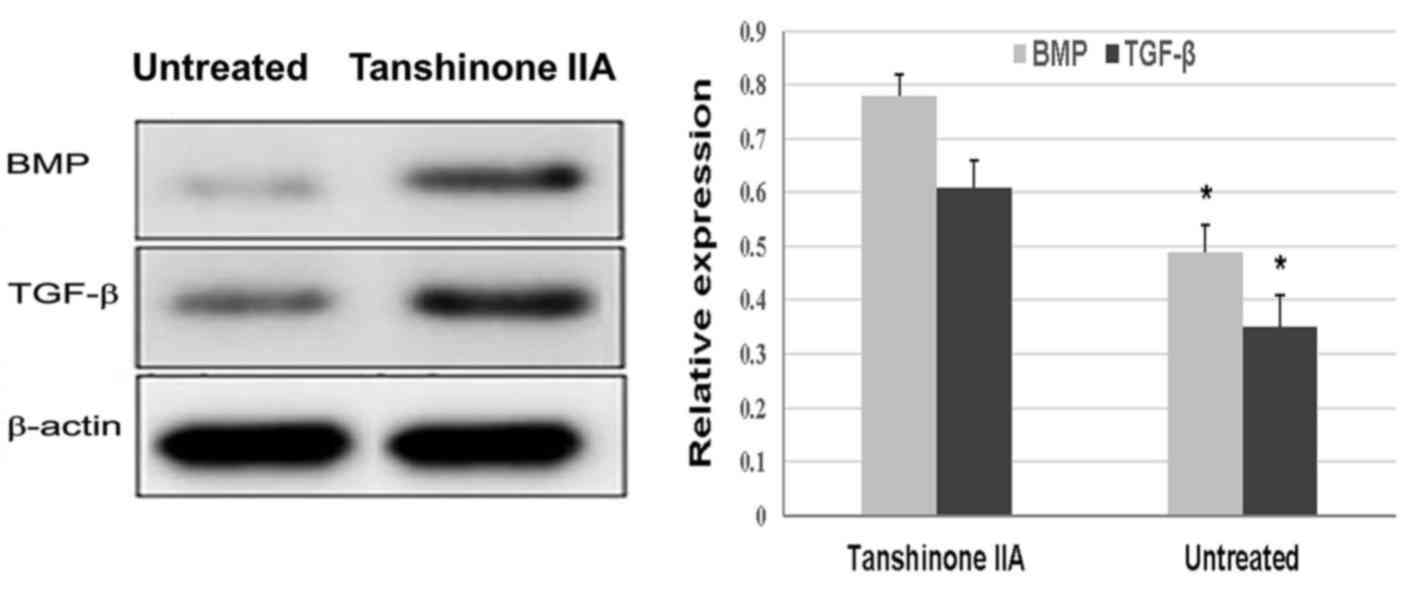
Tanshinone IIA inhibits the production
of IL-1β, TNF-α, and iNOS, and promotes the protein expression of
BMP and TGF-β
The serum levels of cytokines involved in
inflammatory processes, including TNF-α, IL-1β and iNOS, were
significantly increased in the ACLT + MMx group. Treatment with
tanshinone IIA at a dose of 0.5 mg/kg significantly reduced the
serum levels of these inflammatory cytokines (Fig. 5). ACLT + MMx caused a marked
decrease in the protein expression of BMP and TGF-β in the rat
chondrocytes. However, in the rats treated with tanshinone IIA, the
protein expression of BMP and TGF-β was significantly increased
compared with in the untreated group (Fig. 6).
Discussion
In the development of OA, the apoptosis of
chondrocytes and onset of inflammatory processes are vital.
Therefore, understanding the mechanism of chondrocyte apoptosis and
inflammatory processes, and development of a strategy for their
inhibition, offer potential efficient treatment for OA. The present
study demonstrated the role of tanshinone IIA in the prevention of
OA in the ACLT + MMx rat model. The results revealed that
tanshinone IIA efficiently prevented ACLT + MMx-induced degradation
of articular cartilage through the inhibition of chondrocyte
apoptosis and secretion of inflammatory cytokines.
The apoptosis of chondrocytes degrades articular
cartilage, which is the primary factor contributing to the
development of the OA. The results obtained in the present study
showed that ACLT + MMx induced the apoptosis of chondrocytes in the
articular cartilage of the rats, compared with that in normal rats.
Treatment of the rats with tanshinone IIA following exposure to
ACLT + MMx significantly inhibited the induced apoptosis of
chondrocytes. Therefore, tanshinone IIA treatment prevented the
damage to the articular cartilage induced by ACLT + MMx in rats.
Another important factor responsible for the integrity of the
cartilage is collagen, and its breakdown has been observed in
patients with OA (25). The
commonly distributed collagen in cartilage is the type II collagen,
and its degradation by MMP-13 is prevented by the intervention of
TIMP-1 (26–28). Disturbance of the equilibrium
between MMP-13 and TIMPs due to various factors leads to the
development of OA (11). The
results of the present study revealed that ACLT + MMx increased the
expression of MMP-13 in the articular cartilage tissues of the
rats. Treatment of the rats with tanshinone IIA following exposure
to ACLT + MMx inhibited the increased expression of MMP-13, and
maintained the equilibrium between MMP-13 and TIMPs.
Inflammatory cytokines initiate inflammatory
reactions, which indicate the beginning of OA. Previous studies
have reported that the levels of proinflammatory cytokines,
including IL-1β, TNF-α and iNOS, were markedly increased in OA
patients compared with in healthy controls (29). Furthermore, NO radicals, generated
by the activity of iNOS, have been identified as responsible for
the induction of cell apoptosis and the increased secretion of
MMP-13 in tissues (30). The
results of the present study demonstrated that rats of the ACLT +
MMx group exhibited increased serum levels of iNOS, IL-1β and
TNF-α. Conversely, the levels of iNOS IL-1β and TNF-α were
significantly decreased following treatment with tanshinone IIA.
The biosynthesis of collagen in the cartilage matrix is regulated
by the activity of various factors, including TGF-β and BMP-2
(31). In the present study,
tanshinone IIA treatment in the ACLT + MMx rats increased the
expression levels of TGF-β and BMP-2.
In conclusion, the present study demonstrated that
tanshinone IIA effectively inhibited chondrocyte apoptosis and the
degradation of articular cartilage in the ACLT + MMx rat model
through inhibiting the expression of inflammatory cytokines.
Therefore, tanshinone IIA may be used for the treatment of OA.
References
|
1
|
Gabriel SE, Crowson CS, Campion ME and
O'Fallon WM: Direct medical costs unique to people with arthritis.
J Rheumatol. 24:719–725. 1997.PubMed/NCBI
|
|
2
|
March LM and Bachmeier CJ: Economics of
osteoarthritis: A global perspective. Baillieres Clin Rheumatol.
11:817–834. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martel-Pelletier J, Lajeunesse D and
Pelletier JP: Etiopathogenesis of osteoarthritisArthritis and
Allied conditions: A Textbook of Rheumatology. Koopman WJ and
Moreland LW: 2. 15th. Lippincott Williams & Wilkins;
Philadelphia: pp. 2199–2226. 2005
|
|
4
|
Buckwalter JA and Mankin HJ: Articular
cartilage: Degeneration and osteoarthritis, repair, regeneration
and transplantation. Instr Course Lect. 47:487–504. 1998.PubMed/NCBI
|
|
5
|
Aigner T and Kim HA: Apoptosis and
cellular vitality: Issues in osteoarthritic cartilage degeneration.
Arthritis Rheum. 46:1986–1996. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kapoor M, Martel-Pelletier J, Lajeunesse
D, Pelletier JP and Fahmi H: Role of proinflammatory cytokines in
the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 7:33–42.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aizawa T, Kon T, Einhorn TA and
Gerstenfeld LC: Induction of apoptosis in chondrocytes by tumor
necrosis factor-alpha. J Orthop Res. 19:785–796. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fernandes JC, Martel-Pelletier J and
Pelletier JP: The role of cytokines in osteoarthritis
pathophysiology. Biorheology. 39:237–246. 2002.PubMed/NCBI
|
|
9
|
Attur M, Al-Mussawir HE, Patel J, Kitay A,
Dave M, Palmer G, Pillinger MH and Abramson SB: Prostaglandin E2
exerts catabolic effects in osteoarthritis cartilage: Evidence for
signaling via the EP4 receptor. J Immunol. 181:5082–5088. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abramson SB: Osteoarthritis and nitric
oxide. Osteoarthritis Cartilage. 16 Suppl 2:S15–S20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Burger D, Rezzonico R, Li JM, Modoux C,
Pierce RA, Welgus HG and Dayer JM: Imbalance between interstitial
collagenase and tissue inhibitor of metalloproteinases 1 in
synoviocytes and fibroblasts upon direct contact with stimulated T
lymphocytes: Involvement of membrane-associated cytokines.
Arthritis Rheum. 41:1748–1759. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harris RE, Namboodiri KK and Farrar WB:
Nonsteroidal antiinflammatory drugs and breast cancer.
Epidemiology. 7:203–205. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baron JA and Sandler RS: Nonsteroidal
anti-inflammatory drugs and cancer prevention. Annu Rev Med.
51:511–523. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cohen I, Tagliaferri M and Tripathy D:
Traditional Chinese medicine in the treatment of breast cancer.
Semin Oncol. 29:563–574. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kumar NB, Allen K and Bell H:
Perioperative herbal supplement use in cancer patients: Potential
implications and recommendations for presurgical screening. Cancer
Control. 12:149–157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee DS, Lee SH, Noh JG and Hong SD:
Antibacterial activities of cryptotanshinone and dihydrotanshinone
I from a medicinal herb, Salvia miltiorrhiza Bunge. Biosci
Biotechnol Biochem. 63:2236–2239. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao EH, Liu XQ, Wang JJ and Xu NF: Effect
of natural antioxidant tanshinone II-A on DNA damage by lipid
peroxidation in liver cells. Free Radic Biol Med. 20:801–806. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kang BY, Chung SW, Kim SH, Ryu SY and Kim
TS: Inhibition of interleukin-12 and interferon-gamma production in
immune cells by tanshinones from Salvia miltiorrhiza.
Immunopharmacology. 49:355–361. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim SY, Moon TC, Chang HW, Son KH, Kang SS
and Kim HP: Effects of tanshinone I isolated from Salvia
miltiorrhiza Bunge on arachidonic acid metabolism and in vivo
inflammatory responses. Phytother Res. 16:616–620. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sung HJ, Choi SM, Yoon Y and An KS:
Tanshinone IIA, an ingredient of Salvia miltiorrhiza Bunge, induces
apoptosis in human leukemia cell lines through the activation of
caspase-3. Exp Mol Med. 31:174–178. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu WL, Chang WL and Chen CF: Cytotoxic
activities of tanshinones against human carcinoma cell lines. Am J
Chin Med. 19:207–216. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang N, Luo HW, Niwa M and Ji J: A new
platelet aggregation inhibitor from Salvia miltiorrhiza. Planta
Med. 55:390–391. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu Y, Dai GJ, Liu Q, Liu ZL, Song ZQ, Li
L, Chen WH and Lin N: Sanmiao formula inhibits chondrocyte
apoptosis and cartilage matrix degradation in a rat model of
osteoarthritis. Exp Ther Med. 8:1065–1074. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mankin HJ, Dorfman H, Lippiello L and
Zarins A: Biochemical and metabolic abnormalities in articular
cartilage from osteo-arthritic human hips. II Correlation of
morphology with biochemical and metabolic data. J Bone Joint Surg
Am. 53:523–537. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jubb RW and Fell HB: The breakdown of
collagen by chondrocytes. J Pathol. 130:159–167. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Naito K, Watari T, Muta T, Furuhata A,
Iwase H, Igarashi M, Kurosawa H, Nagaoka I and Kaneko K:
Low-intensity pulsed ultrasound (LIPUS) increases the articular
cartilage type II collagen in a rat osteoarthritis model. J Orthop
Res. 28:361–369. 2010.PubMed/NCBI
|
|
27
|
Goldring MB, Otero M, Plumb DA, Dragomir
C, Favero M, El Hachem K, Hashimoto K, Roach HI, Olivotto E, Borzì
RM and Marcu KB: Roles of inflammatory and anabolic cytokines in
cartilage metabolism: signals and multiple effectors converge upon
MMP-13 regulation in osteoarthritis. Eur Cell Mater. 21:202–220.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wetzel M, Li L, Harms KM, Roitbak T,
Ventura PB, Rosenberg GA, Khokha R and Cunningham LA: Tissue
inhibitor of metalloproteinases-3 facilitates Fas-mediated neuronal
cell death following mild ischemia. Cell Death Differ. 15:143–151.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hashimoto S, Nishiyama T, Hayashi S,
Fujishiro T, Takebe K, Kanzaki N, Kuroda R and Kurosaka M: Role of
p53 in human chondrocyte apoptosis in response to shear strain.
Arthritis Rheum. 60:2340–2349. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Del Carlo M Jr and Loeser RF: Nitric
oxide-mediated chondrocyte cell death requires the generation of
additional reactive oxygen species. Arthritis Rheum. 46:394–403.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shuler FD, Georgescu HI, Niyibizi C,
Studer RK, Mi Z, Johnstone B, Robbins RD and Evans CH: Increased
matrix synthesis following adenoviral transfer of a transforming
growth factor beta1 gene into articular chondrocytes. J Orthop Res.
18:585–592. 2000. View Article : Google Scholar : PubMed/NCBI
|