Introduction
Chronic heart failure (CHF) is the end stage of
various cardiovascular diseases and poses a serious threat to human
health, as it responds poorly to treatment (1). Previous clinical investigations
demonstrated that long-term vagus nerve stimulation (VNS) therapy
reduces ventricular remodeling and improves cardiac function, and
increases exercise tolerance and the quality of life following CHF
(2–4). Our previous study has demonstrated
that short-term VNS also improves left ventricular function in rats
with CHF (5). However, the
molecular mechanisms of short-term VNS treatment remain
unclear.
MicroRNAs (miRNAs/miRs) are highly conserved,
endogenous, single-stranded non-coding small RNA molecules. miRNAs
regulate gene expression by promoting degradation of target mRNA
and (or) inhibiting translation. miRNAs involve cellular
differentiation, proliferation, apoptosis and development processes
of a variety of biological tissues (6). Previous studies have identified that
specific miRNAs participate in the pathology of heart disease
development, particularly in the progression of heart failure (HF)
(7–10). Myocardial apoptosis is also
involved in the development of HF (11,12).
Reducing cardiac damage is a potential therapeutic target for HF
(13). miRNAs are also essential
for regulation of cardiomyocyte apoptosis (14,15).
Our earlier study hypothesized that miRNAs may be
cardioprotective of short-term VNS in rats with CHF. Therefore, the
differential expression of miRNAs was assessed using microarray
analysis (16). The present study
focused on miR-205, which was upregulated after the treatment with
sham stimulation (SS) and downregulated after the treatment with
short-term VNS following CHF in rats. In addition, the present
study selected baculoviral IAP repeat-containing protein 2 (Birc2),
an inhibitor of apoptosis protein, as a predicted target gene of
miR-205 through bioinformatics analysis. It was demonstrated that
downregulated miR-205 increased Birc2 expression and reduced
cardiac apoptosis in CHF rats.
Materials and methods
Rat model of CHF and VNS
The Animal Center of the Chinese Academy of Medical
Sciences (Beijing, China) provided 58 male Wistar rats (7–8 weeks
of age; weighing 250–300 g) to prepare the CHF model in the present
study. The use of all rats was in strict compliance with the
provisions of Animal Research Ethics Committee of Shengjing
Hospital of China Medical University (Shenyang, Liaoning, China).
The rats were kept in a stainless-steel net cage under standard
laboratory conditions (25°C; relative humidity 60%; 12-h light/dark
cycle) with free access to food and water. All animals were
randomly divided into three groups: i) treated with SS in sham
operated rats (SO-SS group, n=8), ii) treated with SS in CHF rats
(CHF-SS group, n=8), and iii) treated with VNS in CHF rats (CHF-VNS
group, n=8). As described previously, a thoracotomy was performed
under 1% pelltobarbitalum natricum (40 mg/kg) anesthesia
administered via an intraperitoneal injection. The left anterior
descending coronary artery was ligated firmly with prolene suture
(Ethicon, Inc., Cincinnati, OH, USA) to produce myocardial
infarction (17,18). The left anterior descending
coronary artery was tied loosely in the SO-SS group. At 3 weeks
after the myocardial infarction operation, echocardiography was
performed on the surviving rats. The rats with an infarction area
>40% of the left ventricular wall area were enrolled for
research (5). This method of
research was approved by the ethics committee of Shengjing Hospital
of China Medical University (Shenyang, Liaoning, China).
As described in our previous study (5), a pair of Teflon-coated stainless
steel wires (UL1330; Triumph Cable Co., Ltd., Dongguan, Guangdong,
China) was looped around the right vagus nerve in the neck for
electrical stimulation. The wires were connected to the output
terminals of the stimulator (BL-420S Data Acquisition &
Analysis System; Chengdu Tme Technology Co, Ltd., Chengdu, Sichuan,
China). Rectangular pulses were used at 1 Hz, 5 V and 40 msec
duration for 72 h to stimulate the vagus nerve.
Cardiac tissue preparation
The rats were sacrificed following 72 h of
treatment. For each rat, the whole heart tissue was rapidly excised
and washed with cold phosphate buffer. Heart tissues were frozen at
−80°C for reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) and western blot analysis.
Cell culture
The H9c2 rat myoblasts and 293T human renal
epithelial cells (Cell Bank of the Chinese Academy of Sciences,
Shanghai, China) were cultured in Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% (v/v) FBS (Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA) at 37°C in a mixed atmosphere of 5% CO2
and 95% air.
Cell transfection
Cell transfection was carried out using Attractene
Transfection reagent (Qiagen GmbH, Hilden, Germany) following the
manufacturer's protocol. miR-205 mimics (miR-205m),
miR-205 inhibitor (miR-205i) and matched negative
controls (NCm, negative control mimics; NCi,
negative control inhibitor) were synthesized by GE Healthcare
Dharmacon, Inc. (Lafayette, CO, USA). The level of miRNA expression
in the cells was assayed by RT-qPCR.
miRNA microarray analysis
The miRNA microarray analysis was performed by
Kangchen Biotech Co., Ltd. (Shanghai, China). Total RNA was
extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and an miRNeasy mini kit (Qiagen GmbH) according
to manufacturer's protocol. The RNA quantity was measured using the
NanoDrop 1000. The samples were labeled using the miRCURY™
Hy3™/Hy5™ Power labeling kit and hybridized on the miRCURY™ LNA
Array (v.18.0). Following the washing steps, the slides were
scanned using the Agilent Scanner G2505C. Scanned images were
imported into GenePix Pro 6.0 software (Molecular Devices, LLC,
Sunnyvale, CA, USA) for grid alignment and data extraction. In
screening for differentially expressed miRNAs, the threshold was
set at fold difference ≥1.5 or ≤0.67, and P<0.05. Hierarchical
clustering was performed to demonstrate distinguishable miRNA
expression profiling among samples.
RT-qPCR
All assays strictly followed the manufacturer's
protocol. Total RNA isolation was achieved using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). A PrimeScript RT kit
(Takara Biotechnology Co., Ltd., Dalian, Liaoning, China) was used
to create the cDNA library. RT-qPCR was performed using SYBR Premix
Ex Taq II (Takara Biotechnology, Co., Ltd.) on a LightCycler 480
Real-Time PCR system (Roche Diagnostics, Basel, Switzerland). For
qPCR, 1 µg total RNA from each sample was resuspended in 20 µl
final volume of reaction buffer. Relative quantification was based
on U6 and β-actin controls. All reactions were performed in
triplicate and three dishes were prepared in each group in H9c2
cells. The sequences of the primer pairs used were as follows:
miR-205 forward, 5′-GCGTCCTTCATTCCACCG-3′ and reverse,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACAGACTC-3′; U6
forward, 5′-CTCGCTTCGGCAGCACATATACT-3′ and reverse,
5′-AAAATATGGAAGGCTTCACAGATTTG-3′; Birc2 forward,
5′-CAGCTTTGTGCAGACTTTGCTTTC-3′ and reverse,
5′-CCTTGTTCCAGAGGTAGCGAGTG-3′; caspase-3 forward,
5′-AGATACCAGTGGAGGCCGAC-3′ and reverse,
5′-CACGAGTGAGGATGTGCATGA-3′; Bcl-2-associated X protein (Bax)
forward, 5′-CTGACATGTTTGCAGACGGC-3′ and reverse,
5′-AAGTCCAGTGTCCAGCCCAT-3′; B-cell lymphoma 2 (Bcl-2) forward,
5′-TGGTGGACAACATCGCTCTG-3′ and reverse,
5′-GAGAAATCAAACAGAGGTCGCA-3′; β-actin forward,
5′-ATAGCACAGCCTGGATAGCAACGTAC-3′ and reverse,
5′-CACCTTCTACAATGAGCTGCGTGTG-3′. The PCR was performed as follows:
95°C for 30 sec; 40 cycles of 95°C for 5 sec and 60°C for 30 sec;
95°C for 5 sec, 60°C for 60 sec; 50°C for 30 sec. Fold changes
relative to control samples were calculated using the
2−ΔΔCq method (19).
miR-205 target prediction
For predicting the potential target gene of miR-205,
a bioinformatics approach was implemented, using miRanda
(http://www.microrna.org/microrna/getDownloads.do),
TargetScan (version 6.2; www.targetscan.org/) and miRWalk algorithm (version
2.0; zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/genepub.html).
The common prediction results of three databases were used as
candidate genes. Birc2, an inhibitor of apoptosis protein, was
selected as a predicted target gene of miR-205.
Dual luciferase reporter gene
assay
The mutant 3′UTR and wild-type 3′UTR of Birc2 were
inserted downstream of firefly luciferase in pmiR-Glo to form a
luciferase reporter construct. 293T human renal epithelial cells
were co-transfected with miR-205 and mutant pmiR-Glo-Birc2-3′UTR or
wild-type pmiR-Glo-Birc2-3′UTR (Shanghai GenePharma Co., Ltd.,
Shanghai, China). Using Lipofectamine 2000, plasmid DNA and
miR-205m/NCm were co-transfected into 293T
cells. After 24 h, luciferase activity was measured using the
Dual-Luciferase Assay system (E1960, Promega Corporation, Madison,
WI, USA) and a Multilabel Plate Reader (PerkinElmer, Inc., Waltham,
MA, USA). Normalized luciferase activity was calculated as the
ratio of Renilla and firefly luciferase activities.
Western blotting
SDS lysis buffer was used to extract total protein
of rat tissues, and radioimmunoprecipitation assay lysis buffer was
used for lysis of H9c2 cells (Beyotime Institute of Biotechnology,
Shanghai, China). Proteins (40 µg) were separated by 12% SDS-PAGE
and transferred to polyvinylidene fluoride membranes (Roche
Diagnostics), blocked using 15% skimmed milk (Yili Group, Inner
Mongolia, China). Membranes were incubated overnight at 4°C with
primary antibodies against Bcl-2 (mouse monoclonal; 1:2,000
dilution; cat. no. sc-7382; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA), caspase-3 (rabbit polyclonal; 1:500 dilution; cat.
no. sc-7145; Santa Cruz Biotechnology, Inc.), Bax (mouse
monoclonal; 1:500 dilution; cat. no. sc-7480; Santa Cruz
Biotechnology, Inc.), Birc2 (rabbit polyclonal; 1:500 dilution;
cat. no. bs-4262R; BIOSS, Beijing, China) and β-actin (mouse
monoclonal; 1:1,000 dilution; cat. no. bsm-33036m; BIOSS). The
blots were then incubated at 37°C for 45 min with a horseradish
peroxidase-conjugated goat anti rabbit immunoglobulin G secondary
antibody (1:5,000; cat. no. A0208; Beyotime Institute of
Biotechnology). A UVP Bioimaging system (BioSpectrum 410; UVP Inc.,
Upland, CA, USA) and Gel-Pro-Analyzer software version 5.0 (Media
Cybernetics, Inc., Bethesda, MD, USA) were used for detection and
analysis. For H9c2 cells, three dishes were prepared in each group,
and experiments were repeated three times.
TdT-mediated dUTP nick end labeling
(TUNEL) assay
According to manufacturer's protocol, a TUNEL kit
was used to detect cell apoptosis (cat. no. 11684817910; Roche
Diagnostics). H9c2 cells were seeded into 12-well plates; 1 ml of
cell suspension was inoculated with 2×105 cells/well.
Cells were fixed with 4% paraformaldehyde at room temperature for
30–60 min, and then permeabilized with a 0.1% Triton X-100 solution
(50 µl; cat. no. ST795; Beyotime Institute of Biotechnology) in an
ice bath for 2–5 min. Cells were then quenched of endogenous
peroxidase activity with 3% H2O2 (cat. no.
10011218; Sinopharm Chemical Reagent Co., Ltd., Shanghai, China)
for 10 min at room temperature away from light, and incubated with
the TUNEL reaction mixture (Enzyme solution vs. Label Solution,
1:9) for 1 h at 37°C. Subsequently, cells were stained with 0.8%
hematoxylin for 3 min at room temperature. The apoptotic index was
evaluated in 5 random fields per section under a fluorescence
microscope (magnification, ×200) and counted for each group.
Statistical analysis
All statistical analyses were performed using SPSS
16.0 software (SPSS, Inc., Chicago, IL, USA). All results are
expressed as the mean ± standard deviation. Comparisons among
various groups were determined using one-way analysis of variance
followed by the post hoc least significant difference test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Inhibition of miR-205 in the
myocardial tissue of CHF rats following short-term VNS
treatment
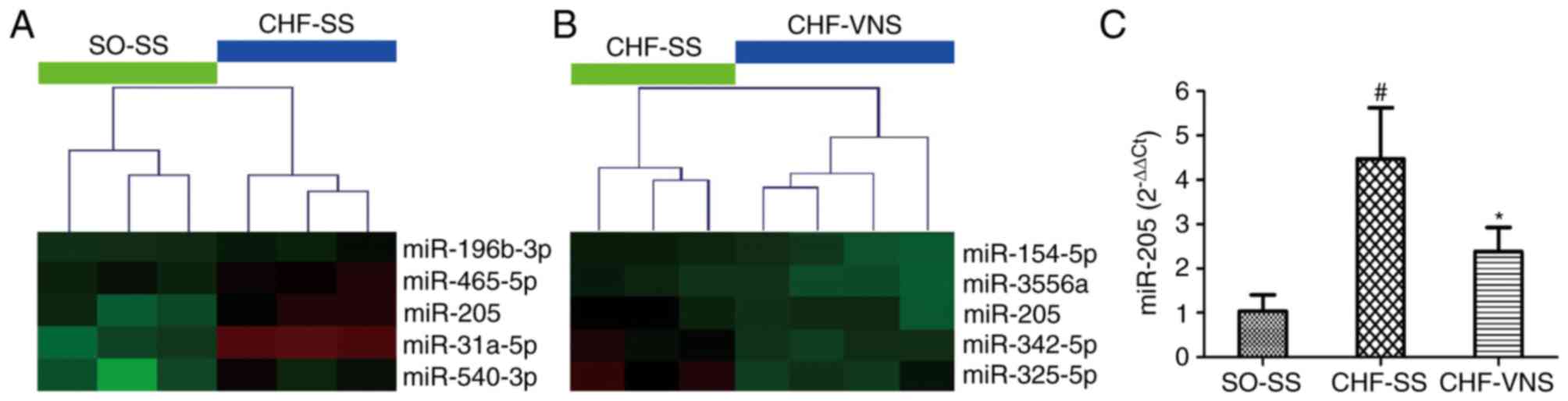
As presented in Fig. 1A
and B, miR-205 was screened by microarray analysis in our
previous study (16), which
revealed that the expression level of miR-205 was significantly
increased in the CHF-SS group (n=3; P=0.022 vs. SO-SS group) and
markedly decreased in the CHF-VNS group (n=4; P=0.026 vs. CHF-SS
group) (16). As presented in
Fig. 1C, these results were
verified in cardiac tissue by RT-qPCR. The expression level of
miR-205 in rats of the CHF-SS group was significantly activated
compared with the SO-SS group (4.48±1.15 vs. 1.04±0.37, P=0.002).
Following short-term VNS, the expression level of miR-205 was
gradually inhibited in CHF-VNS group compared with the CHF-SS group
(2.38±0.55 vs. 4.48±1.15, P=0.015).
Downregulation of miR-205 targeted to
increase the expression of Birc2 and further reduce cell
apoptosis
For predicting the potential target gene of miR-205,
the present study implemented a bioinformatic analysis using
miRanda, TargetScan and miRWalk algorithm. It was demonstrated that
Birc2 theoretically included an miR-205 binding site in the 3′UTR
(Fig. 2A). Birc2 is one of the
inhibitor of apoptosis proteins, and its activation results in cell
anti-apoptosis and cytoprotection. Studies have demonstrated that
miR-205 accelerates cell apoptosis (20–22);
the present study supported this by indicating that miR-205 may
regulate Birc2. To verify this hypothesis, the present study cloned
the 3′UTR of Birc2 into a pmiR-Glo-Birc2 vector (Fig. 2B) and co-transfected miR-205
mimics/inhibitor into the vector in 293T cells. Co-transfection of
miR-205 inhibited the luciferase activity of the reporter,
including wild-type pmiR-Glo-Birc2-3′UTR sequence (0.88±0.03 vs.
1.09±0.01, P<0.001, vs. negative control group; Fig. 2C), but failed to suppress that of
mutant pmiR-Glo-Birc2 by dual luciferase reporter gene assay
(P>0.05 vs. negative control group; Fig. 2C). These data suggested that
miR-205 could directly target the 3′UTR sequences of
pmiR-Glo-Birc2. Additionally, the protein expression level of Birc2
was significantly suppressed by miR-205 mimic transfection in H9c2
cells (0.62±0.13 vs. 1.02±0.08, P=0.003 vs. NCm group),
while the expression was markedly increased by the miR-205
inhibitor (1.28±0.11 vs. 0.99±0.19, P=0.02 vs. NCi
group; Fig. 2D). All these
findings demonstrated that miR-205 targets and regulates Birc2
protein expression. They also suggested that inhibition of miR-205
expression could serve an anti-apoptotic function by increasing
Birc2 expression.
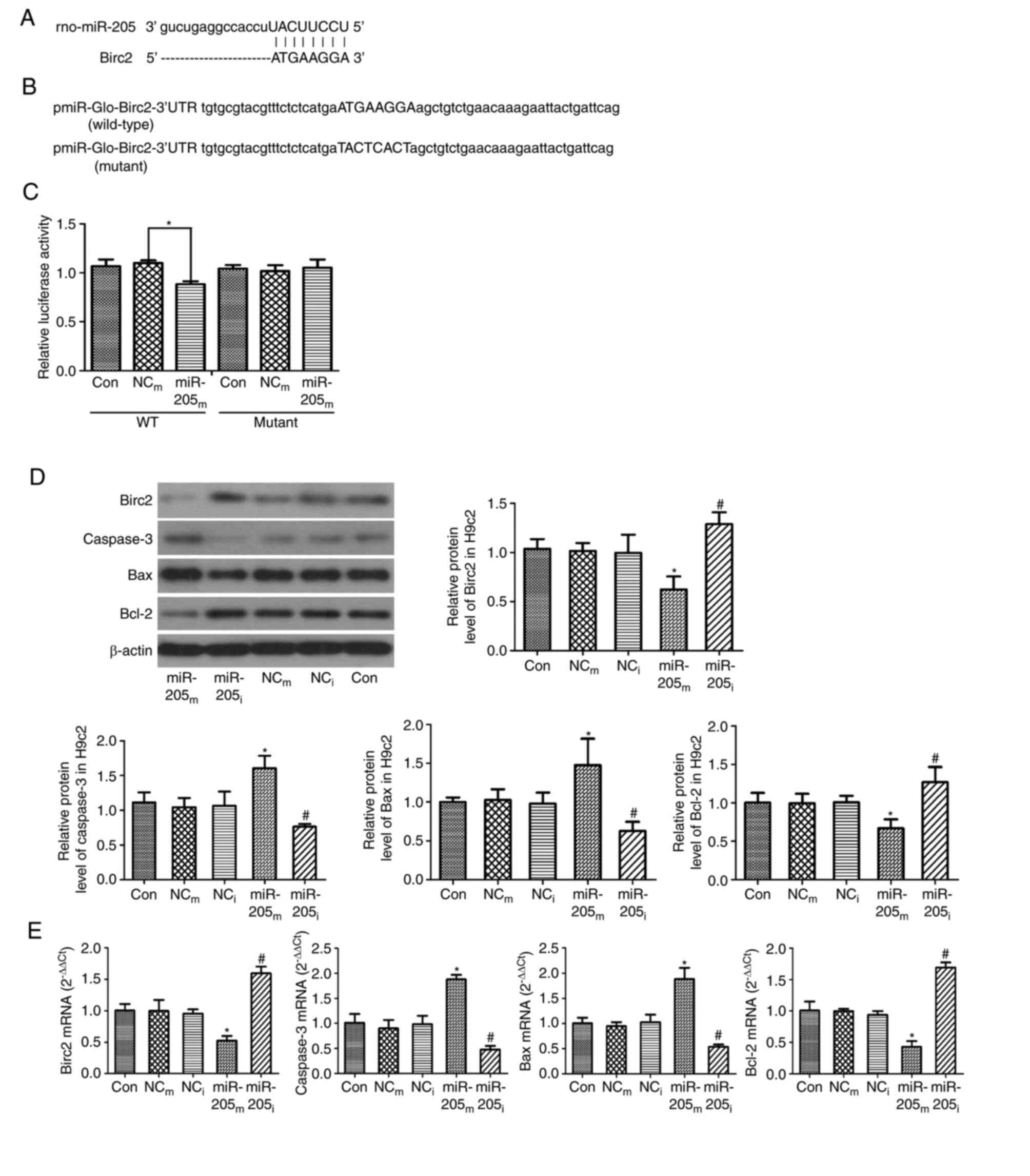 | Figure 2.Birc2 is a target of miR-205 and
downregulation of miR-205 reduces cell apoptosis in H9c2 cells. (A)
Sequence alignment of miR-205 and 3′UTR of Birc2. (B) 3′UTR of
Birc2 or a mutated sequence was cloned into pmiR-Glo vector. (C)
Luciferase reporter assay with co-transfection of wild-type or
mutant 3′UTR and miR-205m or NCm in 293T
cells. (D) Protein and (E) mRNA expression levels of Birc2,
caspase-3, Bax and Bcl-2 in H9c2 cells transfected with miR-205 as
an internal control. β-actin served as a loading control. Data are
expressed as the mean ± standard deviation. *P<0.05 vs.
NCm; #P<0.05 vs. NCi.
miR-205m, miR-205 mimics; miR-205i, miR-205
inhibitor; NCm, negative control mimics; NCi,
negative control inhibitor; Birc2, baculoviral IAP
repeat-containing protein 2; miR, microRNA; 3′UTR, 3-untranslated
region; Con, control; WT, wild-type; Bax, Bcl-2-associated X
protein; Bcl-2, B-cell lymphoma 2. |
Furthermore, for clarifying the mechanisms of
apoptosis, RT-qPCR and western blot analyses were performed to
measure the expression levels of Birc2, caspase-3, Bax and Bcl-2.
As presented in Fig. 2D, the
protein expression levels of caspase-3 and Bax were higher in the
miR-205m group compared with the NCm group
(1.61±0.18 vs. 1.05±0.13 and 1.48±0.34 vs. 1.03±0.14, P=0.001 and
P<0.014, respectively), and were markedly decreased in the
miR-205i group compared with the NCi group
(0.76±0.04 vs. 1.06±0.21 and 0.63±0.12 vs. 0.98±0.14, P=0.036 and
P=0.042, respectively). The protein expression level of Bcl-2 was
lower in the miR-205m group (0.67±0.12 vs. 0.99±0.12,
P=0.015 vs. NCm group), and was markedly higher in the
miR-205i group (1.27±0.2 vs. 1.01±0.09, P=0.037 vs.
NCi group).
As presented in Fig.
2E, consistent with the western blot results, the mRNA
expression levels of caspase-3 and Bax were significantly increased
in the miR-205m group compared with the NCm
group (1.88±0.09 vs. 0.90±0.17 and 1.88±0.22 vs. 0.95±0.08,
P<0.001, respectively); however, the levels of caspase-3 and Bax
were lower in the miR-205i group compared with the
NCi group (0.48±0.07 vs. 0.98±0.17 and 0.54±0.05 vs.
1.03±0.15, P=0.001, respectively). The mRNA expression levels of
Birc2 and Bcl-2 were markedly lower in the miR-205m
group compared with the NCm group (0.52±0.08 vs.
0.99±0.18 and 0.43±0.09 vs. 0.99±0.04, P<0.001 respectively).
However, the levels of Birc2 and Bcl-2 were increased in the
miR-205i group compared with the NCi group
(1.60±0.11 vs. 0.95±0.07 and 1.69±0.08 vs. 0.94±0.06, P=0.001
respectively). These data demonstrated that downregulation of
miR-205 reduced cell apoptosis.
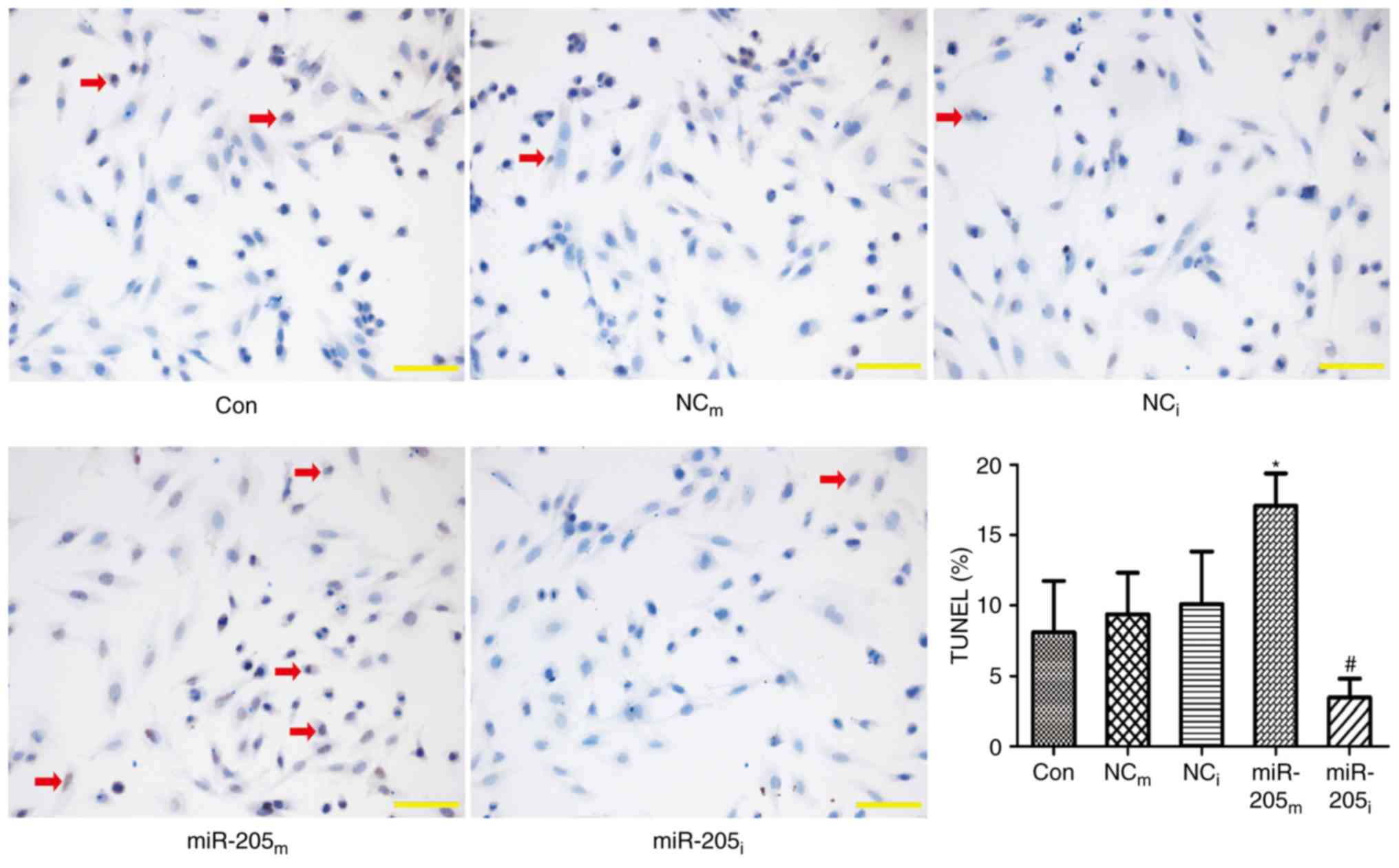
TUNEL assay was also performed to evaluate apoptosis
of H9c2 cells transfected with the miR-205 vector. As presented in
Fig. 3, the apoptosis of H9c2
cells was markedly increased in the miR-205m group
(13.61±1.44 vs. 8.21±1.56, P<0.001 vs. NCm group),
while the apoptosis was markedly reduced in the miR-205i group
(4.96±0.70 vs. 8.27±0.94, P<0.001 vs. NCi group).
Anti-apoptosis effect in the
myocardial tissue of rats with CHF following short-term VNS
treatment
The above studies demonstrated that miR-205 mediates
Birc2 to reduce myocardial apoptosis. To further determine the
anti-apoptosis effect of short-term VNS treatment in the myocardial
tissue of CHF rats, the present measured the expression levels of
Birc2, caspase-3, Bax and Bcl-2 using RT-qPCR and western blot
analyses in the three experimental groups of rats. As presented in
Fig. 4A, the mRNA expression
levels of caspase-3 and Bax were significantly increased in the
CHF-SS group compared with the SO-SS group (2.07±0.13 vs. 1.05±0.4
and 2.63±0.48 vs. 1.01±0.19, P=0.004 and P=0.001, respectively) and
were reduced in the CHF-VNS group compared with the CHF-SS group
(1.39±0.23 vs. 2.07±0.13 and 1.65±0.15 vs. 2.63±0.48, P=0.023 and
P=0.008, respectively). The mRNA expression levels of Birc2 and
Bcl-2 were markedly lower in the CHF-SS group compared with the
SO-SS group (0.37±0.14 vs. 1.01±0.21 and 0.44±0.05 vs. 1.02±0.22,
P=0.002 and P=0.004, respectively) and were increased in the
CHF-VNS group compared with the CHF-SS group (0.78±0.1 vs.
0.37±0.14 and 0.87±0.14 vs. 0.44±0.05, P=0.018 and P=0.016,
respectively).
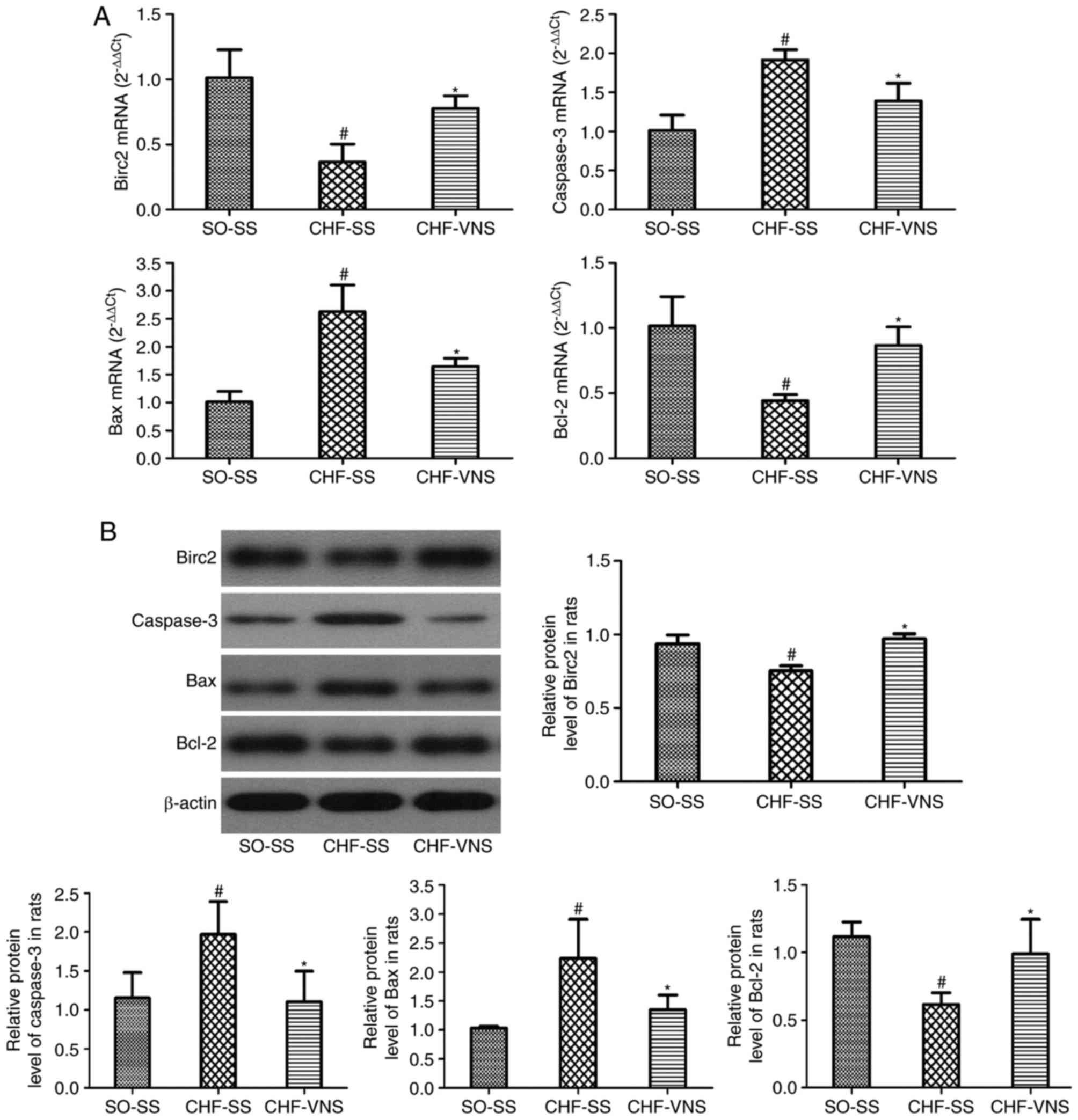 | Figure 4.mRNA and protein expression levels of
apoptosis-associated factors in the SO-SS, CHF-SS and CHF-VNS
groups (n=8/group). (A) mRNA and (B) protein expression levels of
Birc2, caspase-3, Bax and Bcl-2. β-actin served as an internal
control. Data are expressed as the mean ± standard deviation.
#P<0.05 vs. SO-SS; *P<0.05 vs. CHF-SS. miR,
microRNA; SO, sham-operated; CHF, chronic heart failure; SS, sham
stimulation; VNS, vagus nerve stimulation; Bax, Bcl-2-associated X
protein; Bcl-2, B-cell lymphoma 2; Birc2, baculoviral IAP
repeat-containing protein 2. |
As presented in Fig.
4B, consistent with the RT-qPCR results, the protein expression
levels of caspase-3 and Bax were significantly higher in the CHF-SS
group compared with the SO-SS group (1.97±0.42 vs. 1.15±0.33 and
2.23±0.68 vs. 1.03±0.03, P=0.041 and P=0.012, respectively).
Following short-term VNS, the levels of caspase-3 and Bax were
markedly lower in the CHF-VNS group compared with the CHF-SS group
(1.10±0.39 vs. 1.97±0.42 and 1.35±0.25 vs. 2.23±0.68, P=0.033 and
P=0.042, respectively). The protein expression levels of Birc2 and
Bcl-2 in the CHF-SS group were significantly reduced compared with
the SO-SS group (0.75±0.04 vs. 0.94±0.06 and 0.62±0.09 vs.
1.12±0.11, P=0.002 and P=0.011, respectively). Following short-term
VNS, the level of Birc2 and Bcl-2 were markedly higher in the
CHF-VNS group compared with the CHF-SS group (0.97±0.03 vs.
0.75±0.04 and 0.99±0.26 vs. 0.62±0.09, P=0.001 and P=0.034,
respectively). All these findings suggested that short-term VNS
treatment serves an anti-apoptosis effect in the myocardial tissue
of rats following CHF.
Discussion
Heart failure is the dysfunction of contraction
and/or diastole, leading to insufficiency of blood circulation in
the heart, and is a major cause of mortality from cardiovascular
disease (1,23,24).
The morbidity of HF is increased with the aging population
(25). The clinical treatments of
HF have made more progress; however, traditional therapies are
insufficient for many patients with HF, who eventually progress to
end-stage disease (1). Animal
experiments and clinical investigations have reported that VNS
treatment improves the cardiac function and prognosis of CHF, and
reduces the fatality rate (26–28).
Our previous study demonstrated that short-term VNS has a
significant beneficial effect on attenuation of LV remodeling,
alters the components of cardiac excitation-contraction coupling
associated protein, and improves the cardiac function in rats with
CHF (5).
miRNAs participate in regulating gene expression in
different diseases. Previous research has demonstrated that
regulating the expression of miRNAs serves a protective role in HF
(29–32). The present study demonstrated that
short-term VNS reduces miR-205 expression in CHF-VNS rats. miR-205
serves a different role in diverse range of tumor cell types
(33–36). miR-205 induces inflammation and
atherosclerosis in vascular endothelial cells and targets to
regulate tissue inhibitor of metalloproteinase 3, which serves a
protective role in vascular endothelial cells through interfering
the expression of miR-205 (37,38).
miR-205 was also demonstrated to regulate the expression of
phosphatase and tensin homolog directly in endometrial cancer
cells, as well as inhibit apoptosis (39). Myocardial apoptosis serves an
important role in the development of HF. A variety of myocardial
cell injuries, such as myocardial infarction, ischemia/reperfusion,
and genetic and metabolic cardiomyopathy, can induce myocardial
apoptosis and eventually develop into HF; thus, it is a potential
therapeutic target to reduce myocardial damage. Apoptosis is
tightly regulated by a variety of active and programmed death
processes under certain physiological or pathological conditions,
and it is an important homeostatic mechanisms (40). Birc2 is a member of the
anti-apoptotic gene family, which mainly serves its function by
inhibiting the activation of caspase-3, 7, 9 and other enzymes
(41). Endoplasmic reticulum
stress induces the Birc2 expression of inhibition apoptosis through
the phosphoinositide 3-kinase/protein kinase B signaling pathway
and serves an important role in the adaptation of cellular stress
(42,43). Caspase-3 is a common downstream
effector of the apoptosis signaling pathway. Bcl-2 and Bax are
involved in cell survival and apoptosis via regulating the
mitochondria. Bcl-2 is a direct effect of the substrate caspase-3,
and the N-terminal variable loop region can be cut by caspase-3 at
Asp34, and cleave fragments of similar Bax, overall promoting
apoptosis (44). Birc2, caspase-3,
Bcl-2 and Bax can interact and influence each other, so as to
participate in the regulation of apoptosis.
The present study demonstrated that miR-205 is
involved in VNS treatment by regulating myocardial apoptosis in
CHF, and downregulating miR-205 increased the expression of Birc2
in vitro. The present study also investigated the mRNA and
protein expression levels of caspase-3, Bax and Bcl-2. Prior to
short-term VNS treatment, the expression levels of caspase-3 and
Bax were higher and that of Bcl-2 was significantly lower in the
CHF-SS group compared with the SO-SS group. After short-term VNS
therapy, the expression levels of Bax and caspase-3 were lower and
Bcl-2 was significantly higher in the CHF-VNS group compared with
the CHF-SS group. To confirm these results in vitro, the
present study demonstrated that the upregulation of miR-205
increased the mRNA and protein expression levels of caspase-3 and
Bax, and decreased Birc2 and Bcl-2; however, the downregulation of
miR-205 served the opposite effect. TUNEL assay revealed consistent
results; myocardial apoptosis was increased by upregulation of
miR-205, but decreased by downregulation of miR-205. These results
indicated that the downregulation of miR-205 was one significant
reason for which short-term VNS treatment improved heart function
in CHF.
In conclusion, the present study demonstrated that
short-term VNS therapy decreases apoptosis and serves a protective
role in cardiomyocytes by downregulating miR-205, and improved
cardiac function in rats with CHF. Therefore, the results of the
present study provide basic evidence for the application of
short-term VNS in clinical treatments for CHF.
Acknowledgements
The present study was supported by Liaoning
Provincial Science and Technology Department (grant no.
2011404013-7).
References
|
1
|
Akat KM, Moore-McGriff D, Morozov P, Brown
M, Gogakos T, Da Rosa J Correa, Mihailovic A, Sauer M, Ji R,
Ramarathnam A, et al: Comparative RNA-sequencing analysis of
myocardial and circulating small RNAs in human heart failure and
their utility as biomarkers. Proc Natl Acad Sci USA.
111:11151–11156. 2014; View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schwartz PJ, De Ferrari GM, Sanzo A,
Landolina M, Rordorf R, Raineri C, Campana C, Revera M,
Ajmone-Marsan N, Tavazzi L and Odero A: Long term vagal stimulation
in patients with advanced heart failure: First experience in man.
Eur J Heart Fail. 10:884–891. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sabbah HN, Ilsar I, Zaretsky A, Rastogi S,
Wang M and Gupta RC: Vagus nerve stimulation in experimental heart
failure. Heart Fail Rev. 16:171–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zannad F, De Ferrari GM, Tuinenburg AE,
Wright D, Brugada J, Butter C, Klein H, Stolen C, Meyer S, Stein
KM, et al: Chronic vagal stimulation for the treatment of low
ejection fraction heart failure: Results of the neural cardiac
therapy for heart failure (NECTAR-HF) randomized controlled trial.
Eur Heart J. 36:425–433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Y, Xuan YH, Liu SS, Dong J, Luo JY and
Sun ZJ: Short-term vagal nerve stimulation improves left
ventricular function following chronic heart failure in rats. Mol
Med Rep. 12:1709–1716. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barringhaus KG and Zamore PD: MicroRNAs:
Regulating a change of heart. Circulation. 119:2217–2224. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cordes KR and Srivastava D: MicroRNA
regulation of cardiovascular development. Circ Res. 104:724–732.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eulalio A, Mano M, Dal Ferro M, Zentilin
L, Sinagra G, Zacchigna S and Giacca M: Functional screening
identifies miRNAs inducing cardiac regeneration. Nature.
492:376–381. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Latronico MV and Condorelli G: MicroRNAs
and cardiac pathology. Nat Rev Cardiol. 6:419–429. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Olivetti G, Abbi R, Quaini F, Kajstura J,
Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski
S, et al: Apoptosis in the failing human heart. N Engl J Med.
336:1131–1141. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wencker D, Chandra M, Nguyen K, Miao W,
Garantziotis S, Factor SM, Shirani J, Armstrong RC and Kitsis RN: A
mechanistic role for cardiac myocyte apoptosis in heart failure. J
Clin Invest. 111:1497–1504. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Katz MG, Fargnoli AS, Williams RD, Kendle
AP, Steuerwald NM and Bridges CR: MiRNAs as potential molecular
targets in heart failure. Future Cardiol. 10:789–800. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu C, Lu Y, Pan Z, Chu W, Luo X, Lin H,
Xiao J, Shan H, Wang Z and Yang B: The muscle-specific microRNAs
miR-1 and miR-133 produce opposing effects on apoptosis by
targeting HSP60, HSP70 and caspase-9 in cardiomyocytes. J Cell Sci.
120:3045–3052. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang Y, Zheng J, Sun Y, Wu Z, Liu Z and
Huang G: MicroRNA-1 regulates cardiomyocyte apoptosis by targeting
Bcl-2. Int Heart J. 50:377–387. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu SS, Xuan YH, Li Y, Dong J, Luo JY and
Sun ZJ: Short-term vagal nerve stimulation improves chronic heart
failure via miR-133a-3p upregulation in a rat model. Int J Clin Exp
Pathol. 10:50–60. 2017.
|
|
17
|
Cao JM, Fishbein MC, Han JB, Lai WW, Lai
AC, Wu TJ, Czer L, Wolf PL, Denton TA, Shintaku IP, et al:
Relationship between regional cardiac hyperinnervation and
ventricular arrhythmia. Circulation. 101:1960–1969. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gajarsa JJ and Kloner RA: Left ventricular
remodeling in the post-infarction heart: A review of cellular,
molecular mechanisms, and therapeutic modalities. Heart Fail Rev.
16:13–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Salajegheh A, Vosgha H, Md Rahman A, Amin
M, Smith RA and Lam AK: Modulatory role of miR-205 in angiogenesis
and progression of thyroid cancer. J Mol Endocrinol. 55:183–196.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guan B, Li Q, Li XH and Zhou XJ:
MicroRNA-205 targeted Kruppel-like factor 12 and regulated
MDA-MB-468 cells apoptosis in basal-like breast carcinoma. Zhonghua
Yi Xue Za Zhi. 96:2070–2075. 2016.(In Chinese). PubMed/NCBI
|
|
22
|
Tian L, Zhang J, Ge J, Xiao H, Lu J, Fu S,
Liu M and Sun Y: MicroRNA-205 suppresses proliferation and promotes
apoptosis in laryngeal squamous cell carcinoma. Med Oncol.
31:7852014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gaddam KK, Verma A, Thompson M, Amin R and
Ventura H: Hypertension and cardiac failure in its various forms.
Med Clin North Am. 93:665–680. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schannwell CM, Hennersdorf MG and Strauer
BE: Hypertension and cardiac failure. Internist (Berl). 48:909–920.
2007.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McMurray JJ, Adamopoulos S, Anker SD,
Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C,
Gomez-Sanchez MA, et al: ESC Guidelines for the diagnosis and
treatment of acute and chronic heart failure 2012: The task force
for the diagnosis and treatment of acute and chronic heart failure
2012 of the European Society of Cardiology. Developed in
collaboration with the Heart Failure Association (HFA) of the ESC.
Eur Heart J. 33:1787–1847. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hamann JJ, Ruble SB, Stolen C, Wang M,
Gupta RC, Rastogi S and Sabbah HN: Vagus nerve stimulation improves
left ventricular function in a canine model of chronic heart
failure. Eur J Heart Fail. 15:1319–1326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
De Ferrari GM, Crijns HJ, Borggrefe M,
Milasinovic G, Smid J, Zabel M, Gavazzi A, Sanzo A, Dennert R,
Kuschyk J, et al: Chronic vagus nerve stimulation: A new and
promising therapeutic approach for chronic heart failure. Eur Heart
J. 32:847–855. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Premchand RK, Sharma K, Mittal S, Monteiro
R, Dixit S, Libbus I, DiCarlo LA, Ardell JL, Rector TS, Amurthur B,
et al: Autonomic regulation therapy via left or right cervical
vagus nerve stimulation in patients with chronic heart failure:
Results of the ANTHEM-HF trial. J Card Fail. 20:808–816. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li X, Zhang X, Wang T, Sun C, Jin T, Yan
H, Zhang J, Li X, Geng T, Chen C, et al: Regulation by bisoprolol
for cardiac microRNA expression in a rat volume-overload heart
failure model. J Nanosci Nanotechnol. 13:5267–5275. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tolonen AM, Magga J, Szabó Z, Viitala P,
Gao E, Moilanen AM, Ohukainen P, Vainio L, Koch WJ, Kerkelä R, et
al: Inhibition of Let-7 microRNA attenuates myocardial remodeling
and improves cardiac function postinfarction in mice. Pharmacol Res
Perspect. 2:e000562014. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wong LL, Armugam A, Sepramaniam S,
Karolina DS, Lim KY, Lim JY, Chong JP, Ng JY, Chen YT, Chan MM, et
al: Circulating microRNAs in heart failure with reduced and
preserved left ventricular ejection fraction. Eur J Heart Fail.
17:393–404. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kuosmanen SM, Hartikainen J, Hippeläinen
M, Kokki H, Levonen AL and Tavi P: MicroRNA profiling of
pericardial fluid samples from patients with heart failure. PLoS
One. 10:e01196462015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wiklund ED, Bramsen JB, Hulf T, Dyrskjøt
L, Ramanathan R, Hansen TB, Villadsen SB, Gao S, Ostenfeld MS,
Borre M, et al: Coordinated epigenetic repression of the miR-200
family and miR-205 in invasive bladder cancer. Int J Cancer.
128:1327–1334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schaefer A, Jung M, Mollenkopf HJ, Wagner
I, Stephan C, Jentzmik F, Miller K, Lein M, Kristiansen G and Jung
K: Diagnostic and prognostic implications of microRNA profiling in
prostate carcinoma. Int J Cancer. 126:1166–1176. 2010.PubMed/NCBI
|
|
35
|
Karaayvaz M, Zhang C, Liang S, Shroyer KR
and Ju J: Prognostic significance of miR-205 in endometrial cancer.
PLoS One. 7:e351582012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Matsushima K, Isomoto H, Yamaguchi N,
Inoue N, Machida H, Nakayama T, Hayashi T, Kunizaki M, Hidaka S,
Nagayasu T, et al: MiRNA-205 modulates cellular invasion and
migration via regulating zinc finger E-box binding homeobox 2
expression in esophageal squamous cell carcinoma cells. J Transl
Med. 9:302011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Son DJ, Kumar S, Takabe W, Kim CW, Ni CW,
Alberts-Grill N, Jang IH, Kim S, Kim W, Won Kang S, et al: The
atypical mechanosensitive microRNA-712 derived from pre-ribosomal
RNA induces endothelial inflammation and atherosclerosis. Nat
Commun. 4:30002013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim CW, Kumar S, Son DJ, Jang IH,
Griendling KK and Jo H: Prevention of abdominal aortic aneurysm by
anti-microRNA-712 or anti-microRNA-205 in angiotensin II-infused
mice. Arterioscler Thromb Vasc Biol. 34:1412–1421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang G, Hou X, Li Y and Zhao M: MiR-205
inhibits cell apoptosis by targeting phosphatase and tensin homolog
deleted on chromosome ten in endometrial cancer Ishikawa cells. BMC
Cancer. 14:4402014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Meier P, Finch A and Evan G: Apoptosis in
development. Nature. 407:796–801. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
LaCasse EC, Baird S, Korneluk RG and
MacKenzie AE: The inhibitors of apoptosis (IAPs) and their emerging
role in cancer. Oncogene. 17:3247–3259. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Warnakulasuriyarachchi D, Cerquozzi S,
Cheung HH and Holcik M: Translational induction of the inhibitor of
apoptosis protein HIAP2 during endoplasmic reticulum stress
attenuates cell death and is mediated via an inducible internal
ribosome entry site element. J Biol Chem. 279:17148–17157. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hamanaka RB, Bobrovnikova-Marjon E, Ji X,
Liebhaber SA and Diehl JA: PERK-dependent regulation of IAP
translation during ER stress. Oncogene. 28:910–920. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kirsch DG, Doseff A, Chau BN, Lim DS, de
Souza-Pinto NC, Hansford R, Kastan MB, Lazebnik YA and Hardwick JM:
Caspase-3-dependent cleavage of Bcl-2 promotes release of
cytochrome c. J Biol Chem. 274:21155–21161. 1999. View Article : Google Scholar : PubMed/NCBI
|