Introduction
Systemic lupus erythematosus (SLE) is a chronic,
systemic autoimmune inflammatory disease characterized by the
production of numerous autoantibodies, particularly antinuclear
antibodies (ANA), increased immune complex deposition, and multiple
organ damage (1). To
quantitatively assess SLE disease activity, the SLE disease
activity index (SLEDAI) was developed by weighting organ
involvement; this index includes a list of 24 items, of which 16
pertain to clinical manifestations associated with various organ
systems and eight refer to laboratory test results (2). In addition, other laboratory findings
specific for SLE may facilitate the diagnosis of SLE, including
elevations in double-stranded DNA (dsDNA) antibodies, erythrocyte
sedimentation rate (ESR) and C-reactive protein (CRP), and
reductions in complement C3 and C4 levels, and lymphocyte count
(3).
Genetic and environmental factors may contribute to
the development of SLE; however, the precise etiology of SLE is not
fully understood. As a central player in SLE development, the
immune system, including T and B cells, contributes to the
pathogenesis of human SLE (4).
Historically, B cells are considered to be positive regulators of
humoral immune responses, due to their ability to terminally
differentiate into plasma cells and produce antigen-specific
antibodies (5). However, specific
B cell subsets negatively regulate immune responses and have been
termed regulatory B cells (Bregs) (6–8).
Bregs are associated with the cluster of differentiation (CD)
19+CD24highCD38high phenotype
(9), and are characterized by
production of the immunoregulatory cytokines interleukin (IL)-10
and transforming growth factor (TGF)-β (10–13).
Through the secretion of these immunosuppressive cytokines, Bregs
suppress other immune cells and have been studied extensively for
their potential role in the treatment of various autoimmune
diseases (13).
Previous studies have indicated that Bregs are
functionally impaired in patients with SLE, thus suggesting their
potential involvement in the pathogenesis of lupus (9,14).
However, to the best of our knowledge, no studies have
systematically characterized the status of Bregs, the serum levels
of IL-10 and TGF-β, or IL-10 signaling in SLE. Furthermore, their
associations with the SLEDAI and other laboratory parameters in
patients with SLE have yet to be examined. Therefore, the present
study aimed to address these issues, in order to significantly
improve understanding regarding the pathogenesis of SLE, and to
justify targeting Bregs as a therapeutic approach for the treatment
of SLE and potentially other autoimmune diseases.
Materials and methods
Patients and controls
The present study was approved by the Ethics
Committee of Bengbu Medical College (Bengbu, China), and written
informed consent was obtained from each participant. A total of 56
patients (female, n=53; male, n=3; age, 35.38±12.22) with SLE and
35 healthy individuals (controls; female, n=33; male, n=2; age,
32.77±7.36) were enrolled into the present study. The patients and
control participants were recruited between November 2014 and June
2015, and were matched for general demographic characteristics,
including age, sex, lifestyle and geographical lineage. The
diagnosis of SLE was established according to the SLE
Classification Criteria proposed by the American College of
Rheumatology in 1997 (15). Lupus
disease activities were assessed using the SLEDAI, as described
previously (2). According to the
presence of lupus nephritis (LN), the patients with SLE were
divided into two groups: Those with lupus nephritis (LN group;
n=24) and those without (SLE group; n=32). The diagnosis of LN was
based on persistence of proteinuria (>0.5 g/24 h), the presence
of cellular casts and persistent hematuria, or biopsy evidence of
LN. Whole blood was collected from all participants; blood samples
were split into three portions: One portion for serum isolation via
centrifugation (1,000 × g for 5 min at 4°C), which was stored at
−70°C until further use, one portion for flow cytometric analysis,
and one portion for the performance of laboratory tests.
Performance of laboratory tests
Routine laboratory tests were performed on one
portion of the blood samples. These tests included the
following:
Erythrocyte sedimentation rate (ESR)
determination
ESR was measured using the modified Westergren
method and the InteRRliner ESR Analyzer automated system (Sysmex
America, Inc., Lincolnshire, IL, USA).
Peripheral blood parameter
analysis
The peripheral complete blood cell parameters, red
blood cell (RBC) count and lymphocyte count, were determined using
the LH780 hematology auto-analyzer and the associated software
version 2D3 (Beckman Coulter, Inc., Brea, CA, USA) according to the
manufacturer's instructions.
Serum immunological index
detection
The concentrations of serum CRP, immunoglobulin (Ig)
G, IgM, IgA, and complement C3 and C4 were measured by rate
turbidimetry and nephelometry. The 6 reagent kits (IgG, cat. no.
OSR61172; IgA, cat. no. OSR61171; IgM, cat. no. OSR61173; CRP, cat.
no. 447280; C3, cat. no. 988462; C4, cat. no. 988471) were
purchased from the Beckman Coulter, Inc. and measurements were
taken using the IMMAGE 800 Immunochemistry System (Beckman Coulter,
Inc.), according to the manufacturer's instructions. An indirect
immunofluorescence assay was performed for the ANA titer (cat. no.
FA1510) and the anti-dsDNA antibody titer (cat. no. FA1572)
according to the manufacturer's instructions (both kits were
purchased from the EUROIMMUN, Lübeck, Germany).
Liver function tests for serum alanine
aminotransferase (ALT), aspartate aminotransferase (AST) and
alkaline phosphatase (ALP) determination
Liver function tests were performed using reagent
kits for AST, ALP, ALT and indirect bilirubin levels purchased from
Beckman Coulter, Inc. The levels of all four were measured on the
Beckman Coulter AU5800 automatic analyzer (Beckman Coulter, Inc.),
according to the manufacturer's instructions.
Urinary protein analysis
Specimens were obtained from the 56 patients with
SLE. Urine was collected from each patient over a 24 h period; each
patient collected all urine produced during this period in one
container to generate one complete specimen. Urine specimens were
analyzed using the Beckman Coulter Urine Protein Calibrator
(Beckman Coulter, Inc.), according to the manufacturer's
instructions.
Immunostaining and flow cytometric
analysis
For flow cytometric analysis, RBC lysis was
performed on whole blood samples, using the BD Multitest™ IMK kit
(cat. no. 340503; BD Biosciences, San Jose, CA, USA), according to
the manufacturer's instructions. The resulting cellular components
were washed with phosphate-buffered saline twice and stained with
fluorophore-conjugated antibodies at 4°C in the dark for 30 min.
The following antibodies were purchased from BD Biosciences (the
manufacturer's working dilution was used for all antibodies):
Phycoerythrin (PE)-conjugated anti-CD19 (cat. no. 340364),
fluorescein isothiocyanate-conjugated anti-CD24 (cat. no. 555427),
allophycocyanin-conjugated anti-CD38 (cat. no. 340439) and
PE-conjugated anti-CD210 (IL-10R; cat. no. 308804). Flow cytometry
(16) was performed on a BD
FACSCalibur cytometer (BD Biosciences), and data were analyzed
using WinMDI 2.8 software (Scripps Research Institute, La Jolla,
CA, USA).
Measurement of cytokine
production
The serum levels of TGF-β1 (cat. no. BMS249/4) and
IL-10 (cat. no. BMS215/2) from all participants were measured using
enzyme-linked immunosorbent assay (ELISA) kits (eBioscience, San
Diego, CA, USA), according to the manufacturer's protocols.
Statistical analysis
Statistical analysis was performed using SPSS 18.0
(SPSS, Inc., Chicago, IL, USA) software. Data distribution was
checked for normality using the Kolmogorov-Smirnov test. Normally
distributed data are expressed as the mean ± standard deviation,
and the differences were examined using two-tailed t-tests.
Correlation coefficients and their significance were calculated by
two-tailed Pearson correlation. For nonparametric data, the results
are presented as medians (25–75th percentile) The Mann-Whitney
U-test was used to compare the data between the patient and control
groups. To examine the correlations among the parameters measured
in the present study, multivariate analysis was performed.
Correlation coefficients and their significance were calculated by
two-tailed Spearman's Rho correlation. P<0.05 was considered to
indicate a statistically significant difference.
Results
CD19+CD24highCD38high Breg cells
are enriched in the circulating lymphocytes of patients with
SLE
To determine the potential involvement of Bregs in
SLE development, the present study examined the frequency of
CD19+CD24highCD38high Bregs in
patients with SLE and compared it with that in healthy controls. As
shown in Fig. 1, there was a
higher percentage of
CD19+CD24highCD38high Bregs among
circulating lymphocytes from patients with SLE compared with in
healthy individuals (39.83±21.39 vs. 8.74±3.97%; P<0.001).
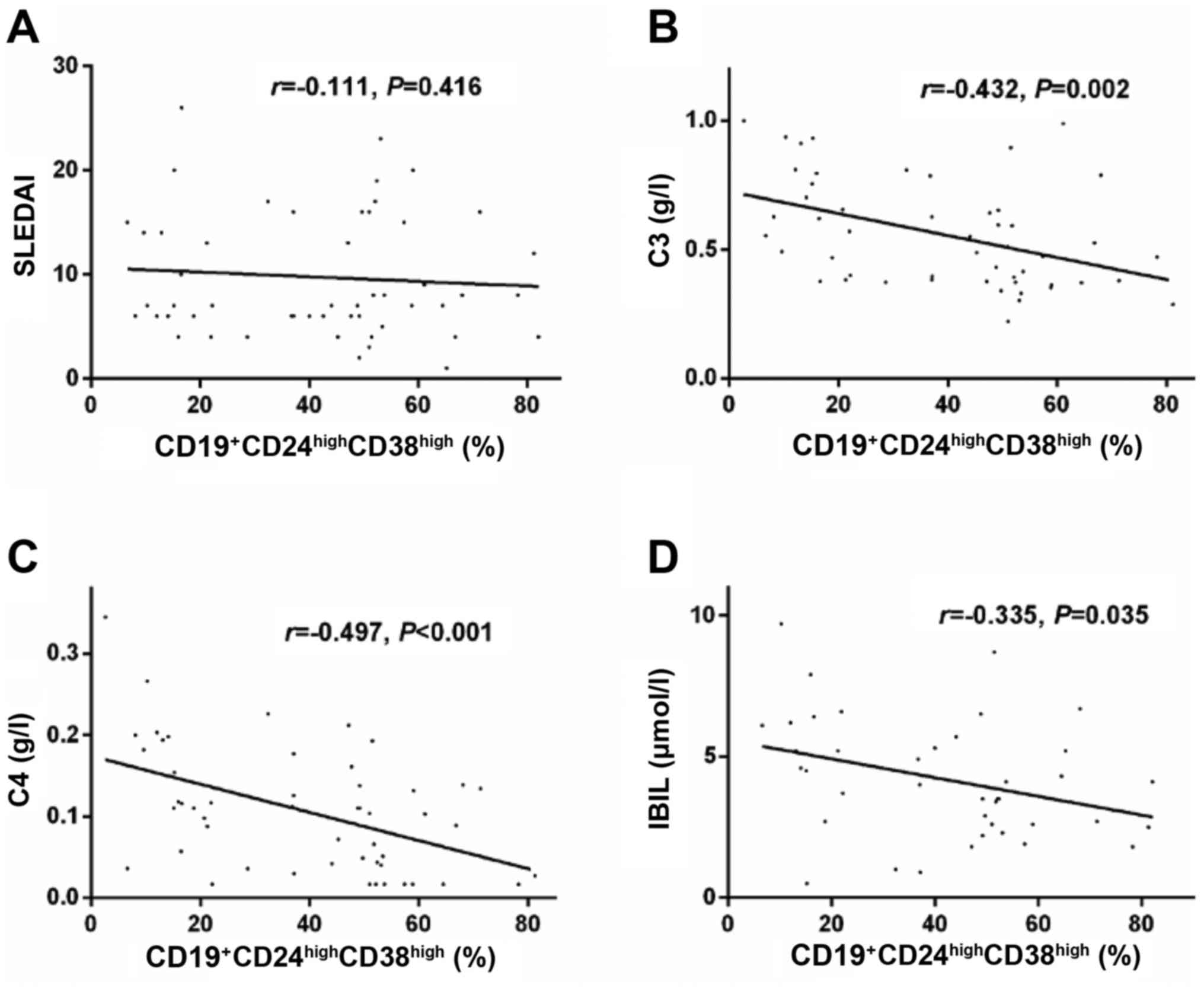
Further analysis on the clinical significance of
Bregs indicated that the frequency of
CD19+CD24highCD38high Bregs was
not significantly correlated with the SLEDAI score (r=−0.111,
P=0.416; Fig. 2A) or the presence
of LN (P>0.05, data not shown) in patients with SLE; however, it
was negatively correlated with blood complement C3 (r=−0.432,
P=0.002; Fig. 2B), complement C4
(r=−0.497, P<0.001; Fig. 2C)
and serum indirect bilirubin (r=−0.335, P=0.035; Fig. 2D) levels.
IL-10R expression in lymphocytes from
patients with SLE is significantly lower compared with in healthy
controls
Bregs are characterized by production of the
immunosuppressive cytokine IL-10 (17), which signals through a receptor
complex consisting of two IL-10R1 and two IL-10R2 molecules on
target cells (18). To determine
whether aberrant IL-10R expression exists in patients with SLE, the
expression of IL-10R was detected on lymphocytes from patients with
SLE and healthy controls by flow cytometry. As shown in Fig. 3A, the percentage of
IL-10R+ cells among circulating lymphocytes was markedly
lower in patients with SLE compared with in healthy individuals
(23.76±0.62 vs. 51.01±16.03%; P<0.001). Furthermore, the
expression levels of IL-10R, based on the mean fluorescence
intensity (MFI) of these cells, were also significantly lower in
patients with SLE compared with in healthy individuals (20.18±3.88
vs. 23.23±3.66; P<0.001; Fig.
3B).
 | Figure 3.IL-10R expression in lymphocytes was
significantly lower in patients with SLE compared with in HCs. (A)
Percentage of IL-10R+ lymphocytes was compared between
the patients with SLE and the HCs. (B) MFI of IL-10R on circulating
lymphocytes was compared between the patients with SLE and the HCs.
Correlation analysis between the MFI of IL-10R+
lymphocytes and (C) SLEDAI, (D) blood ESR, (E) ALT levels and (F)
ALP levels. ALP, alkaline phosphatase; ALT, alanine transaminase;
CD, cluster of differentiation; ESR, erythrocyte sedimentation
rate; HCs, healthy controls; IL-10R, interleukin-10 receptor; MFI,
mean fluorescent intensity; SLE, systemic lupus erythematosus;
SLEDAI, systemic lupus erythematosus disease activity index. |
Further correlation analysis was conducted (Fig. 3C-F). The results indicated that the
MFI of IL10-R1+ lymphocytes was not significantly
correlated with the presence of LN in patients with SLE (P>0.05,
data not shown), the SLEDAI (r=0.009, P=0.951; Fig. 3C), serum ALT (r=0.254, P=0.075;
Fig. 3E) or serum ALP (r=0.252,
P=0.077; Fig. 3F); however, it was
negatively correlated with ESR (r=−0.389, P=0.016; Fig. 3D).
Serum IL-10 levels are elevated in
patients with SLE
To examine whether the increased number of
circulating CD19+CD24highCD38high
Bregs led to elevated IL-10 production, the present study examined
the serum IL-10 levels using ELISA. The results demonstrated that
serum IL-10 concentration was significantly higher in patients with
SLE compared with in healthy controls [3.65 (2.22–9.17) pg/ml in
patients with SLE vs. 1.04 (0.43–1.46) pg/ml in healthy controls;
P<0.001; data not show].
Correlation analysis indicated that serum IL-10
levels were not correlated with the presence of LN in patients with
SLE (P>0.05, data not shown) or the SLEDAI; however, it was
positively correlated with ESR, ANA titer, IgG, IgM and AST, and
was negatively correlated with complement C3 levels, as well as RBC
and lymphocyte counts (Table
I).
 | Table I.Correlation between serum IL-10
levels and clinical parameters. |
Table I.
Correlation between serum IL-10
levels and clinical parameters.
| Variable | SLEDAI | ESR | ANA | ds-DNA | IgG | IgM | IgA | C3 | Lym | RBC | AST |
|---|
| r | 0.160 | 0.411 | 0.401 | 0.228 | 0.376 | 0.334 | 0.045 | −0.466 | −0.385 | −0.337 | 0.367 |
| P-value | 0.240 | 0.008 | 0.014 | 0.188 | 0.007 | 0.017 | 0.752 | 0.002 | 0.005 | 0.015 | 0.007 |
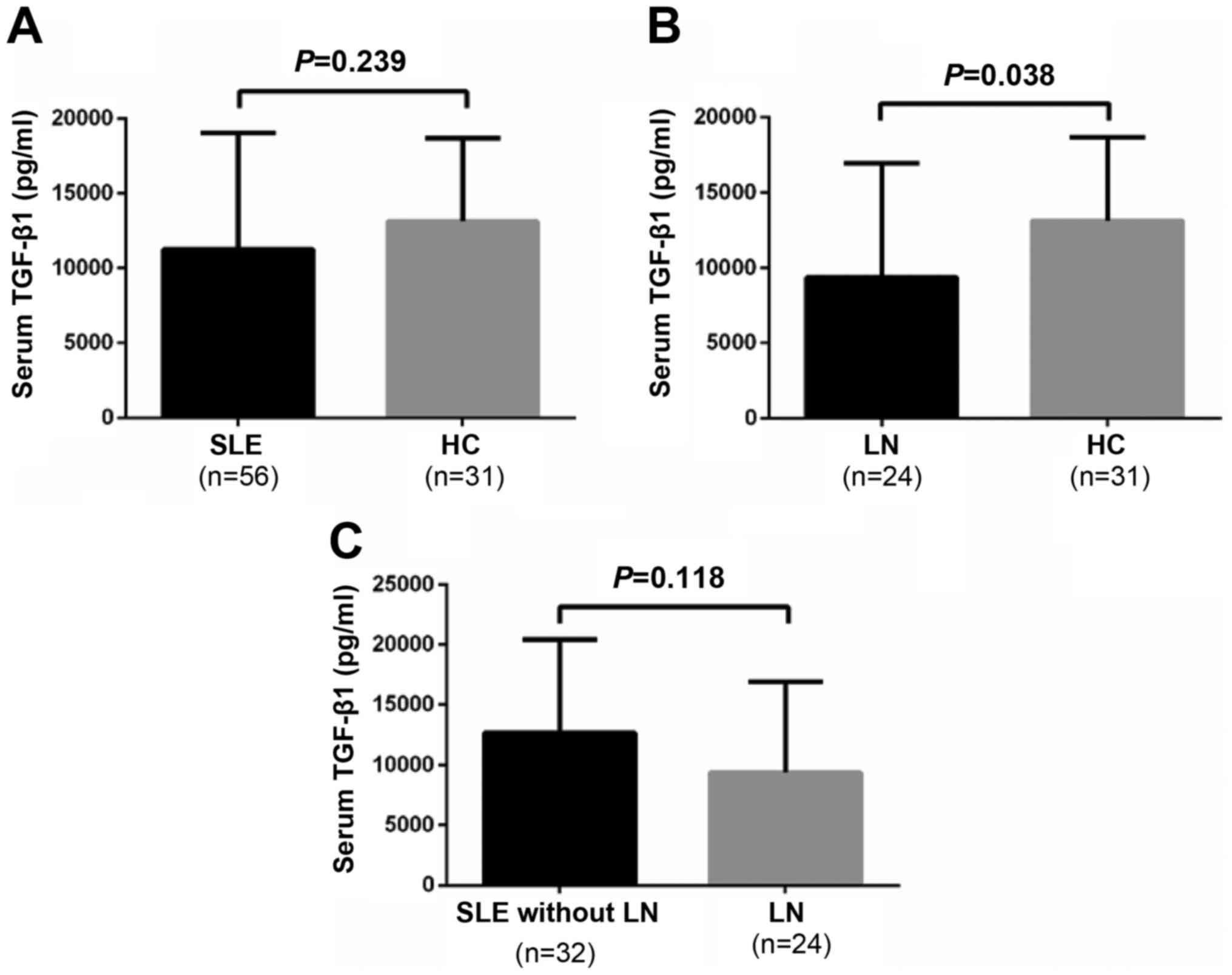
Serum TGF-β1 levels are significantly
lower in the LN group compared with in the healthy control
group
In addition to serum IL-10 levels, the present study
measured TGF-β1 serum levels. As presented in Fig. 4A, serum TGF-β1 levels were not
significantly different between patients with SLE and healthy
controls (11,262.02±7,784.17 vs. 13,143.27±5,559.21 pg/ml;
P=0.239). However, serum TGF-β1 levels in patients with SLE and LN
were significantly lower compared with in healthy controls
(9,382.07±7,558.16 vs. 13,143.27±5,559.21 pg/ml; P=0.038; Fig. 4B). Conversely, there was no
significant difference in serum TGF-β1 levels between the patients
with SLE with or without LN (9,382.07±7,558.16 vs.
12,671.98±7,767.39 pg/ml; P=0.118; Fig. 4C).
Correlation analysis indicated that serum TGF-β1
levels were not significantly correlated with the SLEDAI score;
however, they were positively correlated with RBC count, and
negatively correlated with globulin, 24-h urine protein, and IgM
and AST levels (Table II).
 | Table II.Correlation between serum TGF-β1
levels and clinical parameters. |
Table II.
Correlation between serum TGF-β1
levels and clinical parameters.
| Variable | SLEDAI | Globulin | 24-h up | IgM | RBC | AST | ds-DNA | C3 | ESR | CRP |
|---|
| r | 0.190 | −0.300 | −0.413 | −0.312 | 0.342 | −0.321 | 0.106 | −0.028 | −0.080 | 0.037 |
| P-value | 0.160 | 0.032 | 0.015 | 0.026 | 0.013 | 0.020 | 0.544 | 0.843 | 0.624 | 0.806 |
Correlations among various
measurements
To examine the correlations among the parameters
measured in the present study, a multivariate analysis was
performed. As shown in Table
III, the percentage of
CD19+CD24highCD38high Bregs was
positively correlated with the percentage of IL-10R+
lymphocytes, the MFI of IL-10R+ lymphocytes, and serum
IL-10 levels. In addition, the percentage of IL-10R+
lymphocytes was positively correlated with its expression level
(MFI), whereas serum TGF-β1 levels were negatively correlated with
serum IL-10 levels.
 | Table III.Multivariate correlation
analysis. |
Table III.
Multivariate correlation
analysis.
| Variable | CD210% | CD210-MFI | IL-10 serum levels
(pg/ml) | TGF-β1 serum levels
(pg/ml) |
|---|
|
CD19+CD24highCD38high
Bregs (%) |
|
|
|
|
| r | 0.364 | 0.312 | 0.265 | 0.069 |
|
P-value | 0.006 | 0.022 | 0.048 | 0.612 |
| CD210+ lymphocytes
(%) |
|
|
|
|
| r |
| 0.397 | −0.097 | 0.118 |
|
P-value |
| 0.003 | 0.479 | 0.388 |
| MFI of CD210 on
lymphocytes |
|
|
|
|
| r |
|
| 0.111 | −0.002 |
|
P-value |
|
| 0.424 | 0.990 |
| Serum IL-10 level
(pg/ml) |
|
|
|
|
| r |
|
|
| −0.306 |
|
P-value |
|
|
| 0.022 |
Discussion
The present study demonstrated that
CD19+CD24highCD38high Bregs were
enriched in the circulating lymphocytes of patients with SLE
compared with in healthy individuals. The increase in
CD19+CD24highCD38high Bregs was
associated with elevated serum IL-10 levels, no significant
difference in serum TGF-β1 levels, and a significant reduction in
IL-10R expression on circulating lymphocytes.
The main function of B cells is to serve as
antigen-presenting cells, which leads to optimal expansion of
antigen-specific CD4+ T cells, memory formation and
cytokine production (19–21). Furthermore, B cells positively
regulate CD8+ T cell responses in murine models of
autoimmune diseases (22,23). Therefore, in addition to producing
antibodies, B cells are able to positively regulate cellular immune
responses. However, specific B cell subsets negatively regulate
immune responses and have been termed Bregs (24). At present, IL-10-producing Bregs
are the most extensively studied, and these cells are characterized
by the surface expression profile
CD19+CD24highCD38high (24). The present study demonstrated that
CD19+CD24highCD38high Bregs were
highly enriched in patients with SLE. For SLE, which is an
autoimmune disease characterized by the persistent activation of
autoreactive B cells, the expansion of immunosuppressive Bregs
seems paradoxical to the well-accepted pathogenesis of the disease.
However, to the best of our knowledge, the present study is not the
first to report Breg expansion in SLE. Sims et al (25) detected significantly higher
percentages of
CD19+CD38highCD24high B cells in
the peripheral blood mononuclear cells of patients with SLE
compared with in healthy individuals. However, a more recent study
reported that a similar number of
CD19+CD38highCD24high B cells were
detected in patients with SLE and healthy individuals, whereas the
CD19+CD38highCD24high B cells in
patients with SLE were functionally impaired (9). The differences in circulating Breg
levels between these studies may reflect variations in patient
geographic lineages, genetic background, clinical characteristics
or received therapies. The biological significance and the
underlying mechanisms for Breg expansion in SLE, if present, still
remain to be elucidated. The negative correlations between the
percentage of Bregs within circulation and other clinical
parameters, including serum C3, C4 and indirect bilirubin levels,
suggest that the Breg level reflects patient characteristics and
disease progression.
Similar to alterations in the percentage of Bregs,
the present study observed an elevation in serum IL-10 levels in
patients with SLE compared with in healthy individuals. Although
not the only source of IL-10, IL-10 is the key functional cytokine
generated by CD19+CD24highCD38high
Bregs. It has previously been reported that the serum levels of
IL-10 are markedly increased in patients with SLE, and are
correlated with the SLEDAI or the production of antibodies
(anti-DNA) (26). However, in the
present study, a significant association was not detected between
serum IL-10 levels and SLEDAI or anti-dsDNA antibody levels.
Conversely, a significant correlation was determined between serum
IL-10 levels and other clinical parameters that reflect disease
activity, including ESR, ANA titer, IgG, IgM and C3, thus
suggesting the significance of serum IL-10 levels in reflecting
disease status. Given the immunosuppressive activities of Bregs and
IL-10, the upregulation of these two factors indicates the body's
efforts to inhibit autoimmunity within patients with SLE, but with
no success. The failed efforts of elevated Bregs and serum IL-10
levels suggest potential defects in downstream signaling from Bregs
and IL-10. A previous study demonstrated that in patients with SLE,
the capacity of IL-10 to suppress the production of inflammatory
cytokines is attenuated and that defects in IL-10 homeostatic
function contribute to SLE pathogenesis (27). Furthermore, IL-10 may have a
deleterious effect on humoral-based autoimmune diseases, such as
SLE, since excessive IL-10 promotes B cell differentiation and
autoantibody production, eventually contributing to SLE
development. IL-10 may also suppress the ability of T cells,
monocytes and natural killer cells to produce TGF-β, thus promoting
the development of SLE (28).
Notably, these diverse activities may well explain the phenomenon
that IL-10 appears to serve a protective role in some diseases, but
is harmful in SLE and other autoimmune diseases.
IL-10 signals through the IL-10R, which is a complex
composed of two IL-10R1 and two IL-10R2 molecules, and subsequently
through Jak-signal transducer and activator of transcription
signaling. Cairns et al (29) demonstrated that the expression of
IL-10R on leukocytes is not significantly different between
patients with SLE and controls. Conversely, Li et al
(30) reported that the mRNA
expression of IL-10R on peripheral blood mononuclear cells is
increased in patients with active SLE. Furthermore, other studies
have suggested that the expression of IL-10R on dendritic cells
from patients with SLE is significantly lower compared with in
controls (31,32). The present study demonstrated that
the expression of IL-10R on circulating lymphocytes was
significantly reduced in patients with SLE compared with in healthy
controls. The lower expression was not only determined based on the
percentage of IL-10R+ lymphocytes but also on the MFI of
IL-10R. Functionally, the reduced IL-10R expression was negatively
correlated with ESR, a type of disease activity marker. However, no
significant correlation was detected between IL-10R and the SLEDAI,
ALT or ALP. These data provided a novel mechanism for defective
Breg function in patients with SLE, that is, deficient IL-10R
expression and subsequent IL-10/IL-10R signaling. It is also
possible that Breg expansion and upregulation of serum IL-10 levels
occur as a compensatory mechanism for deficient IL-10R expression.
For further mechanistic studies, it is important to understand the
molecular control of IL-10R expression in lymphocytes and how such
control may be altered in SLE.
TGF-β is another characteristic suppressive cytokine
produced by Bregs. A previous study revealed no significant
differences in the serum levels of bioactive TGF-β between healthy
control subjects and patients with inactive and active SLE
(33). However, a more recent
study reported that reduced serum TGF-β1 levels may be the most
consistent cytokine abnormality in SLE, correlating with disease
activity, a reduction in CD4+, CD8+ and
natural killer cell numbers, and severe organ damage in active SLE
(34). Numerous factors may
contribute to the reduced serum levels of TGF-β in patients with
SLE. One of these factors may be the reduced level of IL-2 in
patients with SLE (35). IL-2
serves an essential role in the development of induced forkhead box
P3+ regulatory T cells, and can induce natural mouse and
human CD4+CD25+, and TGF-β-induced
CD4+CD25+ Treg cells to produce and express
mature TGF-β on the cell surface (35). Another potential factor is IL-10.
IL-10 may suppress the ability of T cells, monocytes and natural
killer cells to produce TGF-β, thus promoting the development of
SLE. Consistently, the present study identified a negative
correlation between serum TGF-β1 levels and IL-10 levels. However,
when considering absolute serum levels, although a trend was
detected for reduced serum TGF-β1 levels in patients with SLE
compared with in healthy individuals, no statistical significance
was noted. Further analysis indicated that TGF-β1 levels in were
significantly lower in patients with LN compared with in healthy
individuals. Consistently, TGF-β1 levels were positively correlated
with the RBC count, but were negatively correlated with globulin,
24-h urinary protein excretion, and IgM and AST levels, thus
suggesting the potential involvement of TGF-β in the development of
nephritis among patients with SLE.
Approximately 25–50% of patients with SLE may
develop abnormal liver function (36). In various liver diseases,
significant correlations have been reported between IL-10 or TGF-β
and liver function (37–39). Therefore, the present study
examined the correlations between circulating Bregs, serum IL-10,
serum TGF-β1 or lymphocyte-associated IL-10R expression, and
various parameters representing liver function, including indirect
bilirubin, CRP, AST, ALT and ALP. The results demonstrated a
significant negative correlation between the number of Bregs and
indirect bilirubin levels, as well as between serum TGF-β1 and AST
levels. In addition, a positive correlation was observed between
serum IL-10 and AST levels, thus suggesting that these immune
regulators may modulate liver function during SLE development.
However, due to the relatively small sample size, it is difficult
to draw a definite conclusion between the immune regulators and
liver function, as well as other clinicopathological parameters.
Further studies including a larger number of patients are required
to verify the data from the present study, and to explore the
underlying molecular mechanisms.
In conclusion, the present study demonstrated that
in the patient population examined, the percentage of circulating
CD19+CD24highCD38high Bregs and
serum IL-10 levels were significantly increased, whereas the
expression of IL-10R in lymphocytes was markedly decreased, in
patients with SLE compared with in healthy individuals. Conversely,
serum TGF-β1 levels were only significantly different between
patients with SLE and LN, and healthy individuals. In addition,
these four factors all exhibited correlations with other laboratory
parameters reflecting SLE activities. These findings indicated that
the ‘Bregs/IL-10/IL-10R’ system may serve an important role in the
pathogenesis of SLE. Therefore, deficient IL-10R expression on
lymphocytes may provide a novel mechanism for the impaired function
of Bregs and IL-10. These factors may not only serve as markers for
SLE progression but may also provide future therapeutic targets for
SLE and other autoimmune disorders.
References
|
1
|
Cojocaru M, Cojocaru IM, Silosi I and
Vrabie CD: Manifestations of systemic lupus erythematosus. Maedica
(Buchar). 6:330–336. 2011.PubMed/NCBI
|
|
2
|
Bombardier C, Gladman DD, Urowitz MB,
Caron D and Chang CH: Derivation of the SLEDAI. A disease activity
index for lupus patients. The Committee on Prognosis Studies in
SLE. Arthritis Rheum. 35:630–640. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Manson JJ and Rahman A: Systemic lupus
erythematosus. Orphanet J Rare Dis. 1:62006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lipsky PE: Systemic lupus erythematosus:
An autoimmune disease of B cell hyperactivity. Nat Immunol.
2:764–766. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
LeBien TW and Tedder TF: B lymphocytes:
how they develop and function. Blood. 112:1570–1580. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
DiLillo DJ, Matsushita T and Tedder TF:
B10 cells and regulatory B cells balance immune responses during
inflammation, autoimmunity and cancer. Ann N Y Acad Sci.
1183:38–57. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fillatreau S: Novel regulatory functions
for Toll-like receptor-activated B cells during intracellular
bacterial infection. Immunol Rev. 240:52–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mauri C: Regulation of immunity and
autoimmunity by B cells. Curr Opin Immunol. 22:761–767. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blair PA, Norena LY, Flores-Borja F,
Rawlings DJ, Isenberg DA, Ehrenstein MR and Mauri C: CD19
(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy
individuals but are functionally impaired in systemic Lupus
Erythematosus patients. Immunity. 32:129–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yanaba K, Bouaziz JD, Matsushita T, Magro
CM, St Clair EW and Tedder TF: B-lymphocyte contributions to human
autoimmune disease. Immunol Rev. 223:284–299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mauri C and Bosma A: Immune regulatory
function of B cells. Annu Rev Immunol. 30:221–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pistoia V: Production of cytokines by
human B cells in health and disease. Immunol Today. 18:343–350.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang M, Rui K, Wang S and Lu L: Regulatory
B cells in autoimmune diseases. Cell Mol Immunol. 10:122–132. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pillai S, Mattoo H and Cariappa A: B cells
and autoimmunity. Curr Opin Immunol. 23:721–731. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hochberg MC: Updating the American College
of Rheumatology revised criteria for the classification of systemic
lupus erythematosus. Arthritis Rheum. 40:17251997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Davies R, Vogelsang P, Jonsson R and Appel
S: An optimized multiplex flow cytometry protocol for the analysis
of intracellular signaling in peripheral blood mononuclear cells. J
Immunol Methods. 436:58–63. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Candando KM, Lykken JM and Tedder TF: B10
cell regulation of health and disease. Immunol Rev. 259:259–272.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mosser DM and Zhang X: Interleukin-10: New
perspectives on an old cytokine. Immunol Rev. 226:205–218. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Linton PJ, Harbertson J and Bradley LM: A
critical role for B cells in the development of memory CD4 cells. J
Immunol. 165:5558–5565. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Crawford A, Macleod M, Schumacher T,
Corlett L and Gray D: Primary T cell expansion and differentiation
in vivo requires antigen presentation by B cells. J Immunol.
176:3498–3506. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bouaziz JD, Yanaba K, Venturi GM, Wang Y,
Tisch RM, Poe JC and Tedder TF: Therapeutic B cell depletion
impairs adaptive and autoreactive CD4+ T cell activation
in mice. Proc Natl Acad Sci USA. 104:20878–20883. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Homann D, Tishon A, Berger DP, Weigle WO,
von Herrath MG and Oldstone MB: Evidence for an underlying CD4
helper and CD8 T-cell defect in B-cell-deficient mice: Failure to
clear persistent virus infection after adoptive immunotherapy with
virus-specific memory cells from muMT/muMT mice. J Virol.
72:9208–9216. 1998.PubMed/NCBI
|
|
23
|
Bergmann CC, Ramakrishna C, Kornacki M and
Stohlman SA: Impaired T cell immunity in B cell-deficient mice
following viral central nervous system infection. J Immunol.
167:1575–1583. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miyagaki T, Fujimoto M and Sato S:
Regulatory B cells in human inflammatory and autoimmune diseases:
From mouse models to clinical research. Int Immunol. 27:495–504.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sims GP, Ettinger R, Shirota Y, Yarboro
CH, Illei GG and Lipsky PE: Identification and characterization of
circulating human transitional B cells. Blood. 105:4390–4398. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peng H, Wang W, Zhou M, Li R, Pan HF and
Ye DQ: Role of interleukin-10 and interleukin-10 receptor in
systemic lupus erythematosus. Clin Rheumatol. 32:1255–1266. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yuan W, DiMartino SJ, Redecha PB, Ivashkiv
LB and Salmon JE: Systemic lupus erythematosus monocytes are less
responsive to interleukin-10 in the presence of immune complexes.
Arthritis Rheum. 63:212–218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng SG: Transforming growth factor-beta
level: Indicator for severity of disease and organ damage in
patients with systemic lupus erythematosus. J Rheumatol.
37:1983–1985. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cairns AP, Crockard AD and Bell AL:
Interleukin-10 receptor expression in systemic lupus erythematosus
and rheumatoid arthritis. Clin Exp Rheumatol. 21:83–86.
2003.PubMed/NCBI
|
|
30
|
Li YM, Chen ZQ, Yao X, Yang AZ, Li AS, Liu
DM and Gong JQ: mRNA expression of chemokine receptors on
peripheral blood mononuclear cells and correlation with clinical
features in systemic lupus erythematosus patients. Chin Med Sci J.
25:162–168. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cui HD, Qi ZM, Yang LL, Qi L, Zhang N,
Zhang XL, Du SY and Jiang Y: Interleukin-10 receptor expression and
signalling were down-regulated in CD4 (+) T cells of lupus
nephritis patients. Clin Exp Immunol. 165:163–171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Figueroa-Vega N, Galindo-Rodriguez G,
Bajaña S, Portales-Pérez D, Abud-Mendoza C, Sánchez-Torres C and
González-Amaro R: Phenotypic analysis of IL-10-treated,
monocyte-derived dendritic cells in patients with systemic lupus
erythematosus. Scand J Immunol. 64:668–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bennett AL, Chao CC, Hu S, Buchwald D,
Fagioli LR, Schur PH, Peterson PK and Komaroff AL: Elevation of
bioactive transforming growth factor-beta in serum from patients
with chronic fatigue syndrome. J Clin Immunol. 17:160–166. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Becker-Merok A, Eilertsen GØ and Nossent
JC: Levels of transforming growth factor-beta are low in systemic
lupus erythematosus patients with active disease. J Rheumatol.
37:2039–2045. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng SG, Wang J, Wang P, Gray JD and
Horwitz DA: IL-2 is essential for TGF-beta to convert naive
CD4+CD25+ cells to
CD25+Foxp3+ regulatory T cells and for
expansion of these cells. J Immunol. 178:2018–2027. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
van Hoek B: The spectrum of liver disease
in systemic lupus erythematosus. Neth J Med. 48:244–253. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
El-Emshaty HM, Nasif WA and Mohamed IE:
Serum Cytokine of IL-10 and IL-12 in Chronic Liver Disease: The
Immune and Inflammatory Response. Dis Markers. 2015:7072542015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Taylor A, Verhagen J, Blaser K, Akdis M
and Akdis CA: Mechanisms of immune suppression by interleukin-10
and transforming growth factor-beta: The role of T regulatory
cells. Immunology. 117:433–442. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
el-Houseini ME, Mohammed MS, Elshemey WM,
Hussein TD, Desouky OS and Elsayed AA: Enhanced detection of
hepatocellular carcinoma. Cancer Control. 12:248–253. 2005.
View Article : Google Scholar : PubMed/NCBI
|