Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune
disease characterized by an elevated synovial inflammatory response
with hyperplasia and subsequent destruction or erosion of
cartilage, articular tissue and bone in major joints (1). Epidemiological studies have indicated
that the incidence of RA in developing and developed countries has
been increasing, and almost 0.5–1 in 100 individuals are annually
diagnosed with RA worldwide (2).
RA predominantly affects the elderly, and there is an increased
prevalence in women due to the involvement of hormonal factors
(3). RA is associated with a
negative impact on quality of life due to severe pain, disability,
and deformity, in addition to an association with cardiovascular
and cerebrovascular diseases; therefore, it representsa large
global socioeconomic burden (4).
The pathophysiology of RA is complex, although
inflammation and oxidative stress are the principal contributors
(5). Previous studies have
suggested that inflammatory processes may be elevated during RA,
which may elicit respiratory bursts to produce excessive free
radicals, subsequently resulting in oxidative stress (imbalance
between free radicals and antioxidants), which further intensifies
synovial damage (6,7). At present, a number of types of drugs
are prescribed to manage RA, including non-steroidal
anti-inflammatory drugs (NSAIDs) and disease modifying
anti-rheumatic drugs (DMARDs); however, these exhibit limited
efficacy and are associated with numerous adverse events, including
cardiovascular disease, gastrointestinal disorders, and hepatic and
renal dysfunction (8,9). Therefore, research into novel
phytomedicines or natural medicines for RA is increasing, due to
the decreased risk of adverse events. A natural drug with effective
anti-inflammatory and antioxidant activity may be a better option
for the treatment of RA (10).
20-Hydroxyecdysone (HES), is a polyhydroxylated
steroid hormone produced in insects and plants, including Ajuga
reptans, Vitex negundo, Polypodium vulgare and Achyranthes
japonica (11). HES serves a
role in insect development, metabolism and reproduction by
regulating the expression of various genes (12). In addition, it protects plants
against insect and nematode infection. HES exhibits a broad range
of biological properties in various models (in vitro and
in vivo), including antioxidant, anti-inflammatory,
immunomodulatory, anti-obesity and antidiabetic activities, in
addition to acting as a neuroprotective and hepatoprotective agent
(13–15). HES has been used as a dietary or
nutritional supplement, particularly by body builders, due to its
hormonal influences (16). HES it
has been reported to be an anti-osteoporotic agent in
ovariectomized rats (17,18). According to recent searches in
academic databases and medical search engines (Google, www.google.com; PubMed, www.ncbi.nlm.nih.gov/pubmed; and Medline, www.medline.com), no studies have been conducted to
assess the anti-RA activity of HES. Therefore, the present study
aimed to evaluate the anti-RA activity of HES by measuring paw
swelling (edema), arthritis score, index of spleen and thymus,
antioxidant status and a number of inflammatory markers, in
addition to histological analysis of synovial joint tissue, in the
collagen-induced arthritis (CIA) rat model.
Materials and methods
Chemicals
HES, complete Freund's adjuvant (CFA), bovine type
II collagen (CII) and formalin were purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). Glycerol, NaOH, PBS, EDTA,
hematoxylin and eosin (H&E) stains and
H2O2 were obtained from Kangchen Bio-tech,
Inc. (Shanghai, China). Other reagents and chemicals used in the
present study were of analytical grade.
Experimental animals
A total of 40 healthy male Sprague-Dawley (SD)
strain rats weighing 240–250 g were purchased from local pet animal
dealer (Animal Aid, Heilongjiang, China) and housed in an animal
center at Harbin Medical University, China. Rats were kept in a
cage and maintainedunder standard housing conditions (12-h
light/dark cycle at 23–24°C with 78% humidity) with food and water
ad libitum. The use of animals in the present study was
approved by the Ethical Committee Board of Harbin Medical
University (no. HMU-34051) and all the experimental procedures and
handling of animals were performed according to the guidelines laid
out by the National Institutes of Health (Bethesda, MD, USA).
CIA induction (immunization)
CIA induction was performedusing the method of Liu
et al (19). CII was
combined with 0.05 M acetic acid and emulsified by adding equal
amounts of CFA (with heat-inactivated Mycobacterium
tuberculosis H37Ra) to make a CFA mixture/emulsion. The rats
were induced/immunized with the CFA mixture via intradermal
injection into the base of the tail on day 0, and a booster on the
day 21 with CII and incomplete Freund's adjuvant (IFA;
Sigma-Aldrich; Merck KGaA). Control rats were not administered with
CFA mixture (non-immunized).
Experimental design
A total of 40 healthy male SD rats were selected
arbitrarily and separated into four groups, with n=10 rats in each
group. Rats receiving saline for 28 days without CIA induction
served as control rats or the non-arthritic group (group I); rats
subjected to CIA induction (as described above) served as the
induced or CIA group (group II); and rats induced with CIA and
administered with 10 or 20 mg/kg body-weight HES dissolved in
saline, via intraperitoneal injection for 28 days, served as the
treatment groups, or HES 10/20 + CIA (groups III and IV). On the
initial day (day 0, without CIA induction), the days 14 and 28, the
paw swelling (edema) volume was evaluated using a digital
plethysmometer. The severity of arthritis was measured in the form
of an arthritis score using the method of Zhang et al
(20). Paws (hind- and fore-) were
graded or scored by checking swelling and erythema using a
five-point scale, assigned according to the extent of swelling or
erythema as follows: 0, no sign of swelling or erythema (without
any abnormal condition); 1, signs of swelling or erythema in ankle
or wrist; 2, signs of swelling or erythema in the ankle + tarsal or
wrist + carpal; 3, signs of swelling or erythema extended to the
metatarsals or metacarpals; and 4, signs of swelling or erythema
involving the entire hind-or forepaw. Therefore, each rat was able
to attain a maximum score of 8 (4×2 hind-/forepaw). In addition,
the body weight of each rat was monitored throughout the study.
Sample preparation
At the end of the experimental period (on the day
29) all rats were sacrificed by cervical decapitation, blood
samples were collected and the serum was separated and centrifuged
at 3,000 × g for 10 min at 4°C. The spleen and thymus glands were
excised immediately and cleaned with saline, and the dry weights
were noted. Synovial joint tissue was obtained from each rat and
fixed in 10% formalin for histological analysis. A portion of joint
tissue (10%) was homogenized using ice-cold PBS and used for
biochemical and molecular analysis. The protein levels were
measured using a Pierce bicinchoninic acid protein assay kit
(Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Thymus and spleen index assay, lipid
peroxidation products and antioxidant enzymes
The thymus and spleen indexes were assayed using the
method of Zhang et al (20)
and were expressed as the ratio of mg/g. Lipid peroxidation
products, including malondialdehyde (MDA) levels, were assessed in
joint tissue using a commercial kit provided by Shanghai Yantuo
Biotechnology Ltd. (Shanghai, China), according to the
manufacturer's protocol. Similarly, catalase (CAT), superoxide
dismutase (SOD), and glutathione (GSH) activity was assayed in
joint tissue homogenate using commercial kits (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) according to the
manufacturer's protocol.
Histomorphological evaluation
Synovial joint tissue wasisolated from each rat and
fixed in 10% buffered formalin (formaldehyde) for 24 h at 37°C and
decalcified using EDTA. The tissues were embedded in liquid
paraffin wax for sectioning by preparing tissue slices of 5-µm
diameter using a microtome, and mounted onto a microscopic slide
followed by staining with H&E for 10 h at 37°C. Subsequently,
the joint tissue was examined under a light microscope from Olympus
Corporation (Tokyo, Japan) to examine any histopathological
changes.
Articular elastase and nitric
oxide
Articular elastase (ELA) activity in articular joint
tissues was assessed as the index of polymorphonuclear cell (PMN)
accumulation (infiltration or activated inflammation) as described
elsewhere by Umar et al (6). ELA was expressed as ng/g protein
(based on the protein content of articular tissue). In the case of
nitric oxide (NO) measurement, the Griess method was used following
the protocols of Sun et al (21).
Anti-collagen II-specific
immunoglobulins (Igs) and inflammatory markers
The levels of serum anti-collagen II IgG,
IgG1, and IgG2a were measured as arbitrary
units using ELISA kits (180128, 180124 and 180125 respectively;
Thermo Fisher Scientific, Inc.). The concentrations of inflammatory
markers, including C-reactive protein (CRP), cytokines
[interleukins (IL-1β, IL-6), tumor necrosis factor (TNF)-α and
cyclooxygenase-2 (COX-2)] in serum samples, and nuclear factor
(NF)-κB p65 subunit from the joint tissue lysate [nuclear fraction
isolated using Nuclear/Cytosolic Fractionation kit (AKR171; Cell
Biolabs Inc., San Diego, CA, USA)], were evaluated using
commercially available ELISA kits (KA1035, KA1502, KA1503,
NBP1-92681, IMK503 respectively; Imgenex; Novus Biologicals, LLC,
Littleton, CO, USA), according to the manufacturer's protocol.
Western blotting
Immunoblotting techniques were used to quantify the
protein expression of inflammatory markers. The protein were
extracted and estimated using a Pierce bicinchoninic acid protein
assay kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA). A
protein extract of 50 µg (from each rat) was resolved using
SDS-PAGE on a 12% gel, and electrotransferred onto a polyvinylidene
difluoride membrane. The membrane was blocked with a mixture of 5%
skimmed milk and Tween-20 in Tris-phosphate buffer solution (TPBS)
for 1 h at 37°C, and incubated with the primary antibodies, mouse
monoclonal anti-inducible nitric oxide synthase (iNOS; 2982) and
anti-cycloxygenase-2 (COX-2; 4842; both 1:1,200; Cell Signaling
Technology, Inc., Danvers, MA, USA) and β-actin (2791; 1:800;
Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) for 10 h at
37°C. Aliquots of secondary antibody conjugated with anti-rabbit
horseradish peroxidase (7074; 1:2,000; Cell Signaling Technology,
Inc.) in TPBS were subsequently added and incubated at 37°C for 1
h. The formed protein bandswere visualized using an enhanced
chemiluminescent image analyzer (ChemiDoc-17001401; Bio-Rad
Laboratories, Inc., Hercules, CA, USA), and the specific proteins
were quantified using ImageJ software version 5.1 (National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Experiments were performed in triplicate. The data
values were expressed as the mean ± standard deviation. The
significant differences between each group were assessed using
one-way analysis of variance followed by Tukey's post hoc test for
multiple comparisons. SPSS software version 21 (IBM Corp., Armonk,
NY, USA) was used for the analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Paw swelling and arthritis scores
The effect of HES on the levels of paw swelling
(edema) and arthritis scores in experimental rats are presented in
Figs. 1 and 2. On days 14 and 28, the paw swelling
(edema) and arthritis scores (swelling and erythema) of CIA-induced
rats were significantly increased (P<0.01) compared with the
control rats. By contrast, supplementation with doses of HES (10
and 20 mg/kg) significantly decreased (P<0.01) the edema and the
signs and symptoms of arthritis (swelling and erythema) compared
with the CIA-induced rats.
Thymus and spleen index (weight)
assay
Fig. 3 illustrates
the effect of HES on the index of the thymus (Fig. 3A) and spleen (Fig. 3B) in experimental rats. Compared
with control rats, a significant increase (P<0.01) in the thymus
and spleen indexes was observed in the CIA group. By contrast, HES
supplementation significantly (P<0.01) suppressed the thymus and
spleen index compared with the CIA-induced rats.
Joint tissue antioxidants and lipid
peroxidation products
Table I summarizes
the effect of HES on the activities of joint tissue lipid
peroxidation products (MDA) and antioxidants in experimental rats.
The levels of joint tissue MDA were significantly increased
(P<0.01), with a significant decrease (P<0.01) in the
activities of joint tissue antioxidants CAT, SOD and GSH in the CIA
group. However, treatment with HES at 10 and 20 mg/kg reverted the
MDA levels to near-normalcy and ameliorated the activities of CAT,
SOD and GSH compared with the CIA-immunized rats.
 | Table I.Effect of HES on the activities of
joint tissue lipid peroxidation products and antioxidants in
experimental rats. |
Table I.
Effect of HES on the activities of
joint tissue lipid peroxidation products and antioxidants in
experimental rats.
| Group | MDA, nmol/mg
protein | CAT, U/mg
protein | SOD, U/mg
protein | GSH, µg/mg
protein |
|---|
| Control |
0.59±0.10 |
71.46±9.35 |
3.74±0.39 |
8.92±0.80 |
| CIA |
1.88±0.21a |
50.21±6.10a |
2.03±0.25a |
7.23±0.70a |
| HES 10 + CIA |
1.23±0.16b |
59.08±8.82b |
2.81±0.42c |
8.03±0.87b |
| HES 20 + CIA |
0.76±0.09c |
67.82±8.50c |
3.46±0.48c |
8.72±0.96b |
Joint tissue morphology
The effect of HES on the joint tissue sections
stained with H & E (magnification, ×200) in experimental rats
are presented in Fig. 4. The
normal architecture of articular cartilage without any mononuclear
or PMN cell infiltration was observed in control rats (Fig. 4A). The CIA-induced rats (Fig. 4B) exhibited increased PMN
infiltration with eroded articular cartilage and prominent
synovitis, which are indicated by arrows. Rats treated with 10
mg/kg HES (Fig. 4C) displayed
decreased PMN infiltration with reduced cartilage erosion and
synovitis. Rats treated with 20 mg/kg HES (Fig. 4D) exhibited healthy articular
cartilage, with limited PMN infiltration and synovitis.
Joint tissue ELA activity
Fig. 5 demonstrates
the effect of HES on joint tissue ELA activity in experimental
rats. The rats induced with CIA displayed a significant increase
(P<0.01) in the activity of ELA compared with non-immunized rats
(control). HES dosages of 10 (P<0.01) and 20 mg/kg (P<0.01)
in CIA-induced rats significantly decreased the activity of ELA
compared with the CIA group.
Levels of anti-collagen II-specific
Igs
IgG, IgG1, and IgG2a levels
were significantly increased (P<0.01) in CIA-induced rats
compared with control rats (Fig.
6). Doses of 10 and 20 mg/kg HES reversed the elevated
collagen-specific Ig levels almost to those of the control
group.
Inflammatory markers
Table II presents
the effect of HES on the concentration of the inflammatory markers,
NO, COX-2 and CRP, in experimental rats. A significant elevation
(P<0.01) in the levels of NO, COX-2 and CRP was noted in the CIA
group compared with control rats. Therapy with 10 and 20 mg/kg HES
decreased the levels of NO, COX-2 and CRP compared with the CIA
rats. Table III exhibits the
effect of HES on the concentration of pro-inflammatory cytokines in
experimental rats. The concentrations of IL-1β, IL-6, TNF-α (all
serum) and NF-κB p65 subunit (joint tissue) were significantly
increased (P<0.01) in CIA rats. However, treatment with HES (10
and 20 mg/kg) decreased the concentration of IL-1β, IL-6, TNF-α and
NF-κB p65 subunit compared with CIA rats.
 | Table II.Effect of HES on the levels of
inflammatory markers in experimental rats. |
Table II.
Effect of HES on the levels of
inflammatory markers in experimental rats.
| Group | NO, µmol/l | COX-2,
OD450 | CRP, mg/dl |
|---|
| Control |
9.87±0.80 |
0.22±0.03 |
0.86±0.06 |
| CIA |
21.58±3.42a |
0.97±0.08a |
1.97±0.03a |
| HES 10 + CIA |
16.04±2.00b |
0.54±0.06b |
1.30±0.02b |
| HES 20 + CIA |
11.73±2.83b |
0.33±0.03b |
1.01±0.01b |
 | Table III.Effect of HES on the concentration of
pro-inflammatory cytokines in experimental rats. |
Table III.
Effect of HES on the concentration of
pro-inflammatory cytokines in experimental rats.
| Group | IL-1β, pg/ml | IL-6, pg/ml | TNF-α, ng/ml | NF-κΒ p65, pg/mg
protein |
|---|
| Control |
1.54±0.15 |
1.45±0.21 |
2.08±0.35 |
6.32±0.84 |
| CIA |
3.34±0.35a |
3.07±0.46a |
7.15±0.60a |
14.85±0.13a |
| HES 10 + CIA |
2.45±0.17b |
2.51±0.55b |
4.62±0.40b |
9.93±0.15b |
| HES 20 + CIA |
1.87±0.20b |
1.92±0.30b |
2.79±0.45b |
7.45±0.10b |
Protein expression of iNOS and
COX-2
Fig. 7 illustrates
the effect of HES on the protein expression of iNOS and COX-2 inthe
joint tissue ofexperimental rats, as determined by immunoblotting.
In the CIA-induced group, the expression of iNOS and COX-2 protein
in the joint tissues was significantly upregulated (P<0.01).
Following 28 days of treatment with HES, the protein expression was
significantly downregulated (P<0.01). The twodosages of HES
exhibited anti-RA effects, although HES 20 exhibited increased
efficacy in all of the above discussed parameters.
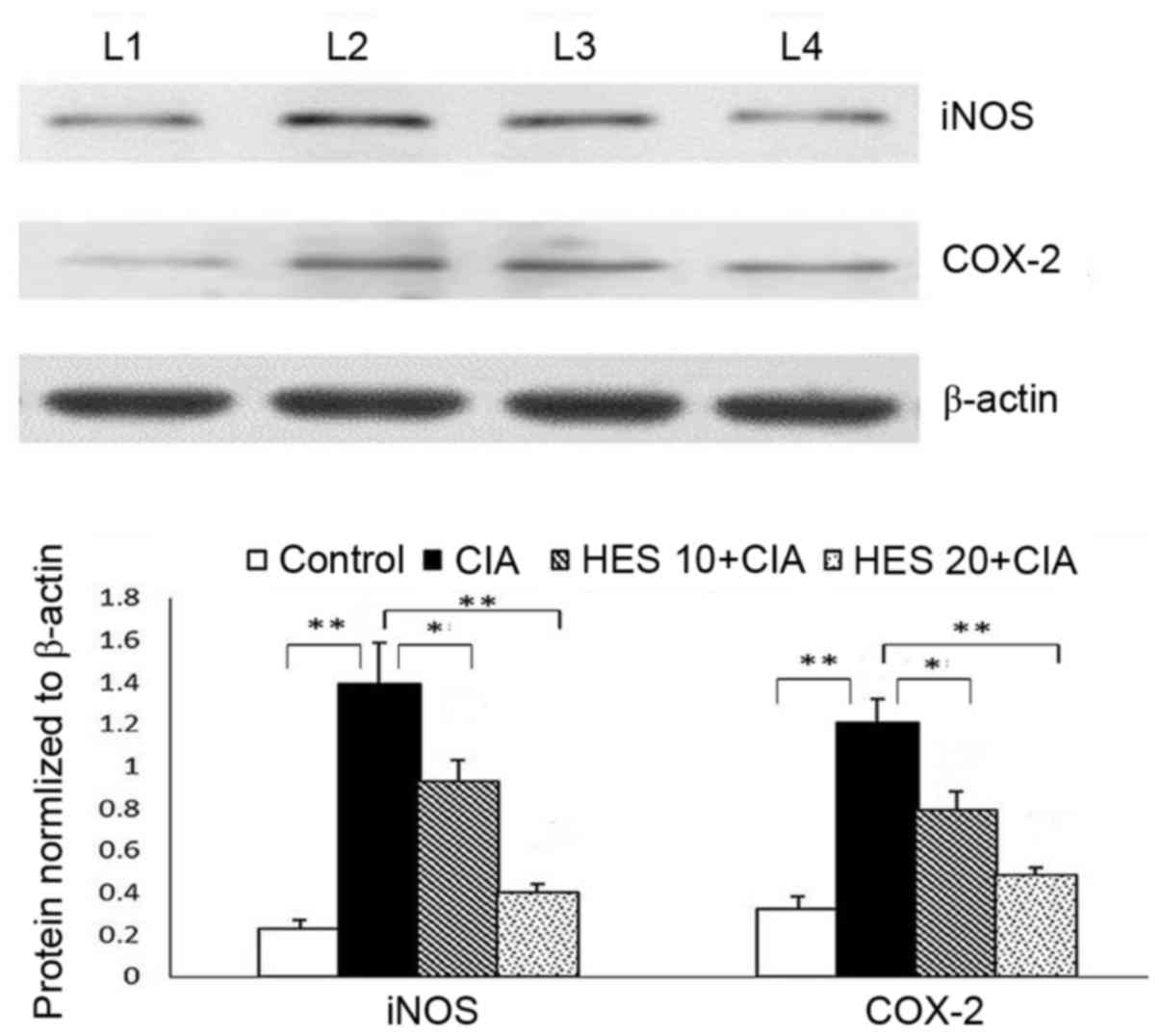 | Figure 7.Effects of HES on the protein
expression of iNOS and COX-2 in joint tissue of experimental rats.
β-actin was used as internal standard. Data are presented as the
mean ± standard deviation for 10 rats in each group. **P<0.01,
CIA vs. control; *P<0.05 HES 10 vs. CIA; **P<0.01 HES 20 vs.
CIA. L1, control group; L2 CIA-induced group; L3, HES 10 group; L4,
HES 20 group; CIA, collagen-induced arthritis; HES,
20-hydroxyecdysone; 10, 10 mg/kg HES; 20, 20 mg/kg HES; iNOS,
inducible nitric oxide synthase; COX-2, cyclooxygenase-2. |
Discussion
A number of studies have suggested that the use of
synthetic or semi-synthetic anti-RA drugs, including NSAIDs and
DMARDs, may lead to severe side effects (8,9).
Therefore, the use of natural drugs may be the better option, due
to the decreased risk of adverse events and the possibility of the
identification of effective anti-RA agents. Previous studies have
indicated that polyphenols, terpenoids and steroids (also termed
nutraceuticals) from plants may decrease oxidative stress and
inflammatory responses, and thus act as anti-RA agents (22,23).
Therefore, in the present study, the phytosterol (ecdysteroid) HES
was used to assess anti-RAproperties. The CIA-induced animal model
was employed for the present study, as CIA may alter cellular and
humoral immunity and thus mimic the human RA condition. Previous
studies have demonstrated that attenuation of the CIA-induced
inflammatory process in rats is among the best methods to assess
the anti-RA activity of a drug (24,25).
Paw swelling (edema) and arthritis scores are the
most important factors to consider when examining the
anti-inflammatory and anti-arthritic properties of a drug (26). The paw swelling and arthritis
scores (swelling and erythema) of the CIA-induced rats in the
present study were markedly increased. Treatment with HES decreased
the paw swelling and symptoms of arthritis compared with
CIA-induced rats. Previously, Achyranthes aspera, which is
rich in HES, has been observed to inhibit paw swelling in the
CIA-induced animal model, thus further demonstrating its
anti-arthritic activity (27).
The spleen and thymus are the major lymphoid organs
that regulate the immune system, and their relative weight (index)
indicates their functional properties. Therefore, the indexes of
the thymus and spleen were used in the present study to evaluate
immunoregulatory activity as, during RA, immune organ indexes have
been observed to be altered (28).
A marked increase in the indexes of the thymus and spleen was
observed in CIA rats; however, with the addition of HES, those
indexes were suppressed, demonstrating the immune modulatory
activity of HES. Consistently, previous studies have suggested that
HES may act as an immune modulatory agent in the diet-induced
obesity mouse model (15,29).
Previous experimental studies have demonstrated that
inflammatory processes are markedly increased during RA, which may
stimulate respiratory bursts to produce excessive free radicals,
and subsequently result in oxidative stress and intensified
synovial damage (6,7). Oxidative stress and inflammation are
associated with each other and may interact to directly contribute
to the pathophysiology of RA (1).
The levels of joint tissue MDA were observed to be increased in the
present study, with a decline in the activities of the joint tissue
antioxidants, CAT, SOD, and GSH, indicating the presence of
oxidative stress. However, treatment with 10 and 20 mg/kg HES
significantly suppressed the MDA levels and restored the activities
of CAT, SOD and GSH, nearly to the levels in sham-control rats. The
results of the present study are consistent with the results of
Sundaram et al (30), who
demonstrated that supplementation with HES was able to increase the
activities of antioxidants, including SOD, CAT and GSH, with
reduced lipid peroxidation products in a diabetic rat model.
In order to ascertain the severity of arthritis in
CIA-induced rats and HES-treated rats, histological alterations to
the joint tissue were observed to validate the results of the
biochemical studies. The control rat joint exhibited normal
architecture with an absence of mononuclear or PMN cell
infiltration. Increased PMN infiltration with eroded articular
cartilage, in addition to prominent synovitis, were noted in CIA
rats. Slides from rats treated with 10 mg/kg HES exhibited
decreased PMN infiltration with reduced cartilage erosion and
synovitis. However, the 20 mg/kg HES group displayed fewer
histological alterations with normal articular cartilage, although
limited PMN infiltration and synovitis were observed. Similarly,
administration of HES has been demonstrated to exert a beneficial
effect on cartilage/bone in the joints by decreasing inflammation
and subsequently reducing cartilage or trabecular bone erosion in
ovariectomized rats (18).
As described above, the activity of ELA (a serine
proteinase) in joint tissue was assessed in the present study as
the index of PMN accumulation (infiltration or activated
inflammation). Activated PMNs are the most significant contributor
to free radical generation (reactive oxygen/nitrogen species),
particularly in joint tissue (31). The CIA-induced animals exhibited
increased ELA activity, whereas treatment with HES significantly
decreased the activity of ELA, demonstrating its antioxidant and
anti-inflammatory activity (32).
The results of the present study suggested that treatment with HES
inhibited the infiltration of leukocytes, and thereby dampened the
inflammatory response.
A previous study suggested that the production of
anti-collagen IIIgG, IgG1 and IgG2awas
elevated in the CIA-induced animal model (33). Similarly, during CIA induction in
the present study, IgG, IgG1and IgG2a levels
were observed to be increased. HES supplementation reversed the
elevated levels of the collagen-specific Igs to normalcy. It may be
hypothesized that improved spleen and thymus indexes following
treatment with HES may be associated with its immune regulatory
activity. Kizelsztein et al (29) observed that HES supplementation may
alter the immune system and therebymodulatethe production of
different antibodies in mouse models.
NO is a signaling or messenger molecule that is
produced from L-arginine, catalyzed by NOS enzymes. During RA (with
activated PMNs), iNOS is upregulated, leading to an abundance of NO
and contributing to further inflammatory responses due to its
conversion to peroxynitrite and subsequent damage to cartilage
(34,35). Prostaglandins are important lipid
mediators, which are highly expressed in synovial joints in RA
through upregulation of the inducible isoform of COX-2 (36). Plant et al (37) demonstrated that CRP is an important
inflammatory mediator in RA which is secreted during infection or
altered immunological conditions, with levels increasing in the
liver and, therefore, in the serum. Therefore, evaluating
inflammatory markers, including NO, COX-2 and CRP, may demonstrate
whether HES is able to effectively act as an anti-rheumatoid agent
in the CIA-induced rat model. In the present study, the levels of
NO, COX-2 and CRP were increased in the CIA group compared with
control rats. A significant decrease in the levels of NO, COX-2,
and CRP was observed in HES-treated rats. HES has been reported to
be an anti-inflammatory agent, due to its inactivation of NF-κB and
subsequent inhibition of the secretion of CRP, in addition to the
expression of inflammatory enzymes, including iNOS and COX-2
(16,38).
The pro-inflammatory cytokines TNF-α, IL-1β and
IL-6, and NF-κB p65, have been observed to be markedly upregulated
in synovial joints, and serve a role in the physiopathology of RA
by evoking an inflammatory response (39,40).
The concentrations of IL-1β, IL-6, TNF-α and NF-κΒp65 were
significantly increased in CIA rats in the present study. During
the CIA-induced/RA condition, PMNs are activated, and promote the
expression of major histocompatibility complex class II molecules
in inflamed joint tissues, thereby producing free radicals and
pro-inflammatory cytokines by triggering the NF-κB signaling
pathway (41). The
pro-inflammatory cytokines assayed in the present study were
markedly downregulated following treatment with HES. Xia et
al (42), demonstrated that
supplementation with HES attenuated the production of
pro-inflammatory cytokines in a streptozotocin-induced rat
model.
In order to examine the mechanism underlying the
anti-rheumatoid and anti-inflammatory activity of HES, the present
study quantified the protein expression of various inflammatory
enzymes, including iNOS and COX-2. The gene expression of iNOS and
COX-2 is regulated by the pro-inflammatory cytokines that are
activated by TNF-α and the NF-κB pathway (28). Jia et al (35) indicated that inhibition of the
COX-2 and iNOS signaling pathways may promote anti-RA activity in
the CIA-induced rat model (35).
The protein expression of iNOS and COX-2 in the joint tissues of
CIA-induced animals were upregulated in the present study. The
elevated levels of pro-inflammatory cytokines in CIA rats further
stimulate the production of chemokines and chemotactic molecules
and upregulate the expression of inflammatory enzymes, including
iNOS and COX-2. However, 28 days of treatment with 10 and 20 mg/kg
HES downregulated the protein expression of iNOS and COX-2 compared
with the CIA rats, thereby further demonstrating the
anti-inflammatory activity of HES. The western blotting results of
the present study are consistent with the results of the assay of
NO and COX-2 activity in the serum. Hu et al (32) observed that treatment with HES may
effectively downregulate the expression of iNOS, and thereby hamper
the production of NO (32). It may
be hypothesized that HES markedly inhibited NF-κB activation by
hampering the translocation of the NF-κΒp65 subunit from the
cytosol into the nucleus. Therefore HES (particularly at a
concentration of 20 mg/kg) may inactivate the NF-κB signaling
pathway and downregulate the expression of inflammation-associated
genes, particularly pro-inflammatory cytokines (IL-1β, IL-6 and
TNF-α) and inflammatory enzymes, including iNOS and COX-2. The
present study exhibits certain limitations, however, including a
lack of investigation into the underlying mechanism behind the
association between HES and any specific signaling pathways, in
addition to not utilizing a standard, for example, dexamethasone or
methotrexate, to compare with the anti-RA activity of HES.
In conclusion, the results of the present study
indicated that treatment with HES (particularly 20 mg/kg) may
protect the joint tissue against excessive production of free
radicals by augmenting endogenous antioxidant levels, and may
thereby maintain the integrity of synovial joint tissue or
cartilage by decreasing paw edema/swelling, arthritis score, ELA
activity and auto-antibodies, in addition to inhibiting the
activation of PMNs. Treatment with HES inhibited the NF-κB
signaling pathway and its inflammation-associated genes,
particularly inflammatory markers (NO, COX-2 and CRP),
pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α), and the
inflammatory enzymes iNOS and COX-2. Future studies will evaluate
the underlying mechanism of the anti-RA and anti-inflammatory
activities of HES in different models.
Acknowledgements
The present study was funded by the National Natural
Science Fund Project (grant no. 81202339) and Educating General
Projects of Heilongjiang Province (grant no. 11511238).
References
|
1
|
Umar S, Umar K, Sarwar AH, Khan A, Ahmad
N, Ahmad S, Katiyar CK, Husain SA and Khan HA: Boswellia serrata
extract attenuates inflammatory mediators and oxidative stress in
collagen induced arthritis. Phytomedicine. 21:847–856. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alamanos Y and Drosos AA: Epidemiology of
adult rheumatoid arthritis. Autoimmun Rev. 4:130–136. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Symmons D, Turner G, Webb R, Asten P,
Barrett E, Lunt M, Scott D and Silman A: The prevalence of
rheumatoid arthritis in the United Kingdom: New estimates for a new
century. Rheumatology (Oxford). 41:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jacobs P, Bissonnette R and Guenther LC:
Socioeconomic burden of immune-mediated inflammatory diseases -
focusing on work productivity and disability. J Rheumatol Suppl.
88:55–61. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Umar S, Sarwar AH Golam, Umar K, Ahmad N,
Sajad M, Ahmad S, Katiyar CK and Khan HA: Piperine ameliorates
oxidative stress, inflammation and histological outcome in collagen
induced arthritis. Cell Immunol. 284:51–59. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Umar S, Zargan J, Umar K, Ahmad S, Katiyar
CK and Khan HA: Modulation of the oxidative stress and inflammatory
cytokine response by thymoquinone in the collagen induced arthritis
in Wistar rats. Chem Biol Interact. 197:40–46. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mateen S, Moin S, Khan AQ, Zafar A and
Fatima N: Increased reactive oxygen species formation and oxidative
stress in rheumatoid arthritis. PLoS One. 11:e01529252016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Negrei C, Bojinca V, Balanescu A, Bojinca
M, Baconi D, Spandidos DA, Tsatsakis AM and Stan M: Management of
rheumatoid arthritis: Impact and risks of various therapeutic
approaches. Exp Ther Med. 11:1177–1183. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singh JA, Saag KG, Bridges SL Jr, Akl EA,
Bannuru RR, Sullivan MC, Vaysbrot E, McNaughton C, Osani M,
Shmerling RH, et al: 2015 American college of rheumatology
guideline for the treatment of rheumatoid arthritis. Arthritis
Rheumatol. 68:1–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adhikary R, Majhi A, Mahanti S and Bishayi
B: Protective effects of methanolic extract of Adhatoda vasica Nees
leaf in collagen-induced arthritis by modulation of synovial
toll-like receptor-2 expression and release of pro-inflammatory
mediators. J Nutr Intermed Metab. 3:1–11. 2016. View Article : Google Scholar
|
|
11
|
Boo KH, Lee D, Jeon GL, Ko SH, Cho SK, Kim
JH, Park SP, Hong Q, Lee SH, Lee DS and Riu KZ: Distribution and
biosynthesis of 20-hydroxyecdysone in plants of Achyranthes
japonica Nakai. Biosci Biotechnol Biochem. 74:2226–2231. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tarrant AM, Reitzel AM, Blomquist CH,
Haller F, Tokarz J and Adamski J: Steroid metabolism in cnidarians:
Insights from Nematostella vectensis. Mol Cell Endocrinol.
301:27–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumpun S, Girault JP, Dinan L, Blais C,
Maria A, Dauphin-Villemant C, Yingyongnarongkul B, Suksamrarn A and
Lafont R: The metabolism of 20-hydroxyecdysone in mice: Relevance
to pharmacological effects and gene switch applications of
ecdysteroids. J Steroid Biochem Mol Biol. 126:1–9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xia XC, Gui GX, Li YF, Hua CX, Liu JH and
Li HW: Anti-oxidative ability study of 20-hydroxyecdysone on acute
liver injury of mice. J Liaoning Univ Tradit Chin Med. 10:81–83.
2012.
|
|
15
|
Foucault AS, Even P, Lafont R, Dioh W,
Veillet S, Tomé D, Huneau JF, Hermier D and Quignard-Boulangé A:
Quinoa extract enriched in 20-hydroxyecdysone affects energy
homeostasis and intestinal fat absorption in mice fed a high-fat
diet. Physiol Behav. 128:226–231. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lafont R and Dinan L: Practical uses for
ecdysteroids in mammals including humans: An update. J Insect Sci.
3:72003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seidlova-Wuttke D, Christel D, Kapur P,
Nguyen BT, Jarry H and Wuttke W: Beta-ecdysone has bone protective
but no estrogenic effects in ovariectomized rats. Phytomedicine.
17:884–889. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kapur P, Wuttke W, Jarry H and
Seidlova-Wuttke D: Beneficial effects of beta-Ecdysone on the
joint, epiphyseal cartilage tissue and trabecular bone in
ovariectomized rats. Phytomedicine. 17:350–355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Q, Zhu XZ, Feng RB, Liu Z, Wang GY,
Guan XF, Ou GM, Li YL, Wang Y, Li MM and Ye WC: Crude triterpenoid
saponins from Anemone flaccida (Di Wu) exert anti-arthritic effects
on type II collagen-induced arthritis in rats. Chin Med. 10:202015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang LL, Wei W, Yan SX, Hu XY and Sun WY:
Therapeutic effects of glucosides of Cheanomeles speciosa on
collagen-induced arthritis in mice. Acta Pharmacol Sin.
25:1495–1501. 2004.PubMed/NCBI
|
|
21
|
Sun J, Zhang X, Broderick M and Fein H:
Measurement of nitric oxide production in biological systems by
using Griess reaction assay. Sensors. 3:276–284. 2003. View Article : Google Scholar
|
|
22
|
Khanna D, Sethi G, Ahn KS, Pandey MK,
Kunnumakkara AB, Sung B, Aggarwal A and Aggarwal BB: Natural
products as a gold mine for arthritis treatment. Curr Opin
Pharmacol. 7:344–351. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Al-Okbi SY: Nutraceuticals of
anti-inflammatory activity as complementary therapy for rheumatoid
arthritis. Toxicol Ind Health. 30:738–749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kargutkar S and Brijesh S: Anti-rheumatic
activity of Ananas comosus fruit peel extract in a complete
Freund's adjuvant rat model. Pharm Biol. 54:2616–2622. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Talaat RM, Abo-El-Atta AS, Farou SM and
El-Dosoky KI: Therapeutic effect of dimethyl dimethoxy biphenyl
dicarboxylate on collagen-induced arthritis in rats. Chin J Integr
Med. 21:846–854. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Petchi RR and Vijaya C: Anti-diabetic and
anti-arthritic potential of Glycosmis pentaphylla stem bark in FCA
induced arthritis and streptozotocin induced diabetic rats.
Pharmacol Int J Pharma Biol Sci. 3:328–336. 2012.
|
|
27
|
Srivastav S, Singh P, Mishra G, Jha KK and
Khosa RL: Achyranthes aspera-An important medicinal plant: A
review. J Nat Prod Plant Resour. 1:1–4. 2011.
|
|
28
|
Jiang CP, He X, Yang XL, Zhang SL, Li H,
Song ZJ, Zhang CF, Yang ZL, Li P, Wang CZ and Yuan CS:
Anti-rheumatoid arthritic activity of flavonoids from Daphne
genkwa. Phytomedicine. 21:830–837. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kizelsztein P, Govorko D, Komarnytsky S,
Evans A, Wang Z, Cefalu WT and Raskin I: 20-Hydroxyecdysone
decreases weight and hyperglycemia in a diet-induced obesity mice
model. Am J Physiol Endocrinol Metab. 296:E433–E439. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sundaram R, Naresh R, Shanthi P and
Sachdanandam P: Ameliorative effect of 20-OH ecdysone on
streptozotocin induced oxidative stress and β-cell damage in
experimental hyperglycemic rats. Process Biochem. 47:2072–2080.
2012. View Article : Google Scholar
|
|
31
|
Campo GM, Avenoso A, Campo S, Ferlazzo AM,
Altavilla D and Calatroni A: Efficacy of treatment with
glycosaminoglycans on experimental collagen-induced arthritis in
rats. Arthritis Res Ther. 5:R122–R131. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu J, Luo CX, Chu WH, Shan YA, Qian ZM,
Zhu G, Yu YB and Feng H: 20-Hydroxyecdysone protects against
oxidative stress-induced neuronal injury by scavenging free
radicals and modulating NF-κB and JNK pathways. PLoS One.
7:e507642012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Choi JH, Lee JH, Roh KH, Seo SK, Choi IW,
Park SG, Lim JG, Lee WJ, Kim MH, Cho KR and Kim YJ: Gallium nitrate
ameliorates type II collagen-induced arthritis in mice. Int
Immunopharmacol. 20:269–275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Breitbach K, Klocke S, Tschernig T, van
Rooijen N, Baumann U and Steinmetz I: Role of inducible nitric
oxide synthase and NADPH oxidase in early control of Burkholderia
pseudomallei infection in mice. Infect Immun. 74:6300–6309. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jia N, Chu W, Li Y, Ding L, Duan J, Cui J,
Cao S, Zhao C, Wu Y and Wen A: Iridoid glycosides from the flowers
of Gentiana macrophylla Pall. ameliorate collagen-induced arthritis
in rats. J Ethnopharmacol. 189:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jia Z and He J: Paeoniflorin ameliorates
rheumatoid arthritis in rat models through oxidative stress,
inflammation and cyclooxygenase 2. Exp Ther Med. 11:655–659. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Plant MJ, Williams AL, O'Sullivan MM,
Lewis PA, Coles EC and Jessop JD: Relationship between
time-integrated C-reactive protein levels and radiologic
progression in patients with rheumatoid arthritis. Arthritis Rheum.
43:1473–1477. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Feng CY, Huang XR and Qi MX: Effects of
ecdysterone on the expression of NF-kappaB p65 in
H2O2 induced oxidative damage of human lens
epithelial cells. Zhongguo Zhong Xi Yi Jie He Za Zhi. 32:76–79.
2012.(In Chinese). PubMed/NCBI
|
|
39
|
Cooles FA and Isaacs JD: Pathophysiology
of rheumatoid arthritis. Curr Opin Rheumatol. 23:233–240. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McInnes IB and Schett G: Cytokines in the
pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 7:429–442.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cohen S, Hurd E, Cush J, Schiff M,
Weinblatt ME, Moreland LW, Kremer J, Bear MB, Rich WJ and McCabe D:
Treatment of rheumatoid arthritis with anakinra, a recombinant
human interleukin-1 receptor antagonist, in combination with
methotrexate: Results of a twenty-four-week, multicenter,
randomized, double-blind, placebo-controlled trial. Arthritis
Rheum. 46:614–624. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xia X, Zhang Q, Liu R, Wang Z, Tang N, Liu
F, Huang G, Jiang X, Gui G, Wang L and Sun X: Effects of
20-hydroxyecdysone on improving memory deficits in
streptozotocin-induced type 1 diabetes mellitus in rat. Eur J
Pharmacol. 740:45–52. 2014. View Article : Google Scholar : PubMed/NCBI
|





















