Introduction
Wolfram syndrome 1 (WS; OMIM: 222300) is a rare
autosomal recessive neurodegenerative disease. It is characterized
by DIDMOAD; that is, the development of diabetes insipidus,
diabetes mellitus, optic atrophy and deafness (1,2). WS
is caused by mutations in the Wolfram syndrome 1 (WFS1)
gene. WFS1 encodes wolframin, a transmembrane glycoprotein
that is ubiquitously expressed, with the highest levels expressed
in heart, brain, pancreatic β-cells, placenta and lungs. Wolframin
is primarily located in the endoplasmic reticulum (ER) and
Wfs1-deficiency causes ER stress and cellular dysfunction
(1,2). Human WFS1 protein amino acid content
is 87% conserved with the mouse homolog (Wfs1). Wfs1 comprises nine
central transmembrane domains, an extracytoplasmic amino-terminus
and an intracytoplasmic carboxyl-terminus; and nitrogen-linked
glycosylation is essential for its biogenesis and stability
(3). As an ER glycoprotein,
wolframin may participate in membrane trafficking, regulation of
Ca2+ homeostasis and protein processing (4). In patients with WS, pancreatic β-cell
death may be associated with impaired β-cell dysfunction, as seen
in patients with type 2 diabetes (5). The expression of Wfs1 in mouse
pancreatic islets was previously demonstrated to be upregulated
during glucose-induced insulin secretion (6).
WS is related to different pathological conditions
and it is a valuable disease model for identifying biomarkers
associated with ER stress, juvenile-onset diabetes and
neurodegeneration (1,2). In addition, WS arises from mutations
of a single gene; therefore, it may be a good model for
investigating the mechanisms of ER dysfunction compared with
multifactorial conditions like ‘ordinary’ type 1 and type 2
diabetes (7).
ER stress is a situation in which misfolded proteins
accumulate in the lumen of ER; the unfolded protein response (UPR)
that follows upregulates survival related signaling and chaperone
synthesis, and inhibits the synthesis of many other proteins
(8). Oxidative stress is
considered to increase ER stress through the activity of
oxidoreductin-1 and protein disulfide isomerases (9,10).
Protein folding depends on many factors other than chaperones;
among these factors, proper reduction/oxidation (redox) ratio and
glutathione (GSH) levels are directly and indirectly via GSH
sensitive regulatory proteins involved in appropriate disulfide
bridge formation (11). GSH is a
thiol-containing tripeptide comprising γ-glutamate, cysteine and
glycine, and its formation occurs in the cytosol and requires no
folding compared with redox enzymes. ER stress is known to enhance
GSH synthesis through the transcription factors cyclic
AMP-dependent transcription factor 4 and nuclear factor erythroid
2-related factor 2 (12,13). Although the aim of UPR is cell
survival, persistent stress may induce the cells to trigger
apoptosis. It remains unknown how acute and chronic ER stress are
managed by individual cells and organisms as a whole.
GSH is a potent scavenger of free radicals and other
oxidant species in which it is oxidized by selenium-containing GSH
peroxidase (GPx) to oxidized GSH (GSSG) and is reduced back to GSH
by GSH reductase (GR). The antioxidant capacity of cells is mainly
described by the GSSG/GSH redox ratio and has been related to
several pathological states, including neurodegenerative,
cardiovascular and immune system diseases (14). Therefore the present study aimed to
characterize the GSH system in the heart, kidney, liver and
pancreatic tissues of Wfs1-deficient mice.
Materials and methods
Chemicals
All chemicals were from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). The DC Protein Assay was from Bio-Rad
Laboratories, Inc. (Hercules, CA, USA) and Glutathione Assay,
Glutathione Peroxidase Assay and Glutathione Reductase Assay kits
were from Cayman Chemical Company (Ann Arbor, MI, USA).
Animals and tissue collection
All animal experiments in this study were carried
out in accordance with European Communities Directive (86/609/EEC)
and the study was approved by The Estonian National Board of Animal
Experiments (permit number 36; obtained July 23, 2014). Mice
(weight, 22 g at 2 months old; 28 g at 6 months old) were housed
under standard laboratory conditions: 12-h light/dark cycle (lights
out at 07:00 PM) at 20±2°C, 40–60% humidity, with access to chow
diet and water ad libitum. A total of 48 male (age 2 and 6
months) wild-type (WT; Wfs1+/+) and
Wfs1-deficient [heterozygous (HZ; Wfs1+/−)
and homozygous (knockout, KO; Wfs1−/−] mice were
used throughout this study. The [(129S6/SvEvTac × C57BL/6) ×
(129S6/SvEvTac × C57BL/6)] F2 hybrids Wfs1-deficient mice
were generated in the Institute of Biomedicine and Translational
Medicine, University of Tartu (Tartu, Estonia) and the detailed
generation, breeding and genotyping analysis of the mice was as
described previously (15).
Mice were euthanized by manual cervical dislocation.
Liver, heart, pancreas and kidney tissues were collected, perfused
with ice-cold saline, snap frozen in liquid nitrogen and stored at
−80°C until processing. Each experimental group consisted of eight
animals.
Sample preparation
Tissue samples (15–250 mg) were homogenized in 0.1 M
phosphate buffer (1:10 w/v; pH 7.4) and centrifuged for 15 min at
10,000 × g 4°C. Supernatants were collected and immediately
aliquoted for the measurement of total GSH (tGSH) or the enzymatic
activity of GR or GPx. For the measurement of tGSH and GSSG,
proteins were precipitated with 10% metaphosphoric acid (1:1 w/v)
to avoid interference owing to particulates and sulfhydryl groups
in the assay.
Measurement of total and oxidized
glutathione concentration
tGSH and GSSG levels in heart, liver, kidney and
pancreas were measured using the Glutathione Assay kit according to
manufacturer's protocol, which uses the optimized enzymatic GR
recycling method first described by Tietze (16). Briefly, 50 µl/ml of 4 M
triethanolamine was added to the supernatant to increase the pH of
the samples and the final sample volume was 50 µl per well. Next,
the thiol group of GSH in the sample (50 µl) reacted with 450 µl
5,5-dithiobis-(2-nitrobenzoic acid) (DTNB) resulting in the
formation of the yellow-colored 5-thio-2-nitrobenzoic acid (TNB)
and a mixed disulfide of GSH and DTNB after 25 min incubation. The
latter is reduced by GR to recycle the GSH and produce extra TNB.
The production of total TNB is directly proportional to the
concentration of GSH in the sample. The quantification of GSSG is
accomplished by first derivatizing GSH with 1 M 2-vinylpyridine (10
µl/ml). The tGSH and GSSG samples were measured at 412 nm with a
Tecan Sunrise spectrophotometer (Tecan Group, Ltd., Männedorf,
Switzerland).
Measurement of the activity of GPx and
GR
The mRNA or protein expression levels of an enzyme
do not necessarily result in an increase in activity, therefore the
activity of GPx and GR was measured with a Glutathione Peroxidase
Assay Kit and a Glutathione Reductase Assay Kit according to the
manufacturer's protocols, respectively. GR catalyzes the
NADPH-dependent reduction of GSSG to GSH and therefore maintains
adequate levels of cellular GSH. GPx catalyzes the reduction of
hydrogen peroxide to protect the cell from oxidative stress and
uses GSH as the ultimate electron donor. The assay measures GR
activity by the rate of NADPH oxidation and GPx activity indirectly
by coupled reaction with GR (17,18).
Statistical analysis
Data were analyzed using GraphPad Prism version
5.0.0 for Windows (GraphPad Software, Inc., La Jolla, CA, USA). The
results are presented as the mean ± standard error of the mean.
Comparisons between groups were made using one-way analysis of
variance followed by Tukey's test. P<0.05 was considered to
indicate a statistically significant difference.
Results
The concentration of tGSH and the activities of GPx
and GR were measured in the heart, liver, kidneys and pancreatic
tissue of KO, HZ and WT mice.
Kidney
The 2-month-old KO mice exhibited a significantly
lower level of tGSH concentration in the kidney tissue compared
with tGSH concentration in the WT littermates (1.6-fold;
F2,15=5.9; P<0.05; Fig.
1A). HZ 6-month-old mice exhibited a 1.2-fold higher
concentration of tGSH in the kidney tissue compared to WT mice
(F2,19=8.2; P<0.05; Fig.
1B). The level of GSSG was below the detection limit and could
not be measured. GPx activity was 1.7-fold higher in 2-month-old KO
mice compared with WT mice (F2,21=18.0; P<0.0001;
Fig. 2A). GR activity was 1.4-fold
higher in 2-month-old KO mice, but this was not indicted to be
statistically significant (Fig.
2B). GPx and GR activities in 6-month-old mice could not be
measured due to their insufficient activity in the available amount
of renal tissue.
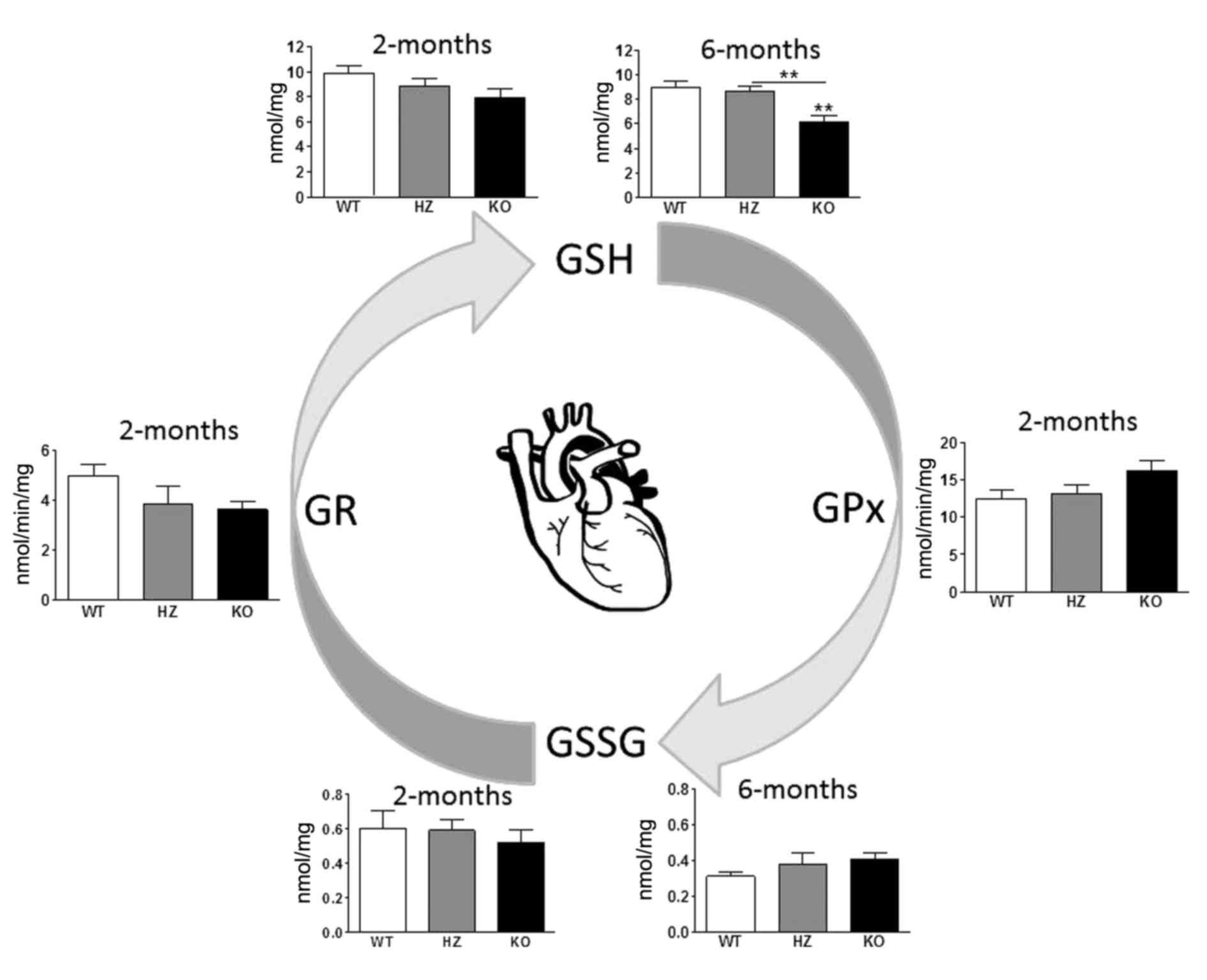
Heart
Analyses on heart tissues identified slightly lower,
albeit not statistically significant, levels of GSSG and GSH
(Fig. 3) compared with WT. In
6-month-old KO mice, the GSH concentration was 1.5-fold lower
(F2,20=10.9; P<0.001; Fig. 3) in the heart tissue compared with
WT littermates. GPx activity was slightly higher and GR activity
lower in KO 2-month-old mice compared with WT mice, but these
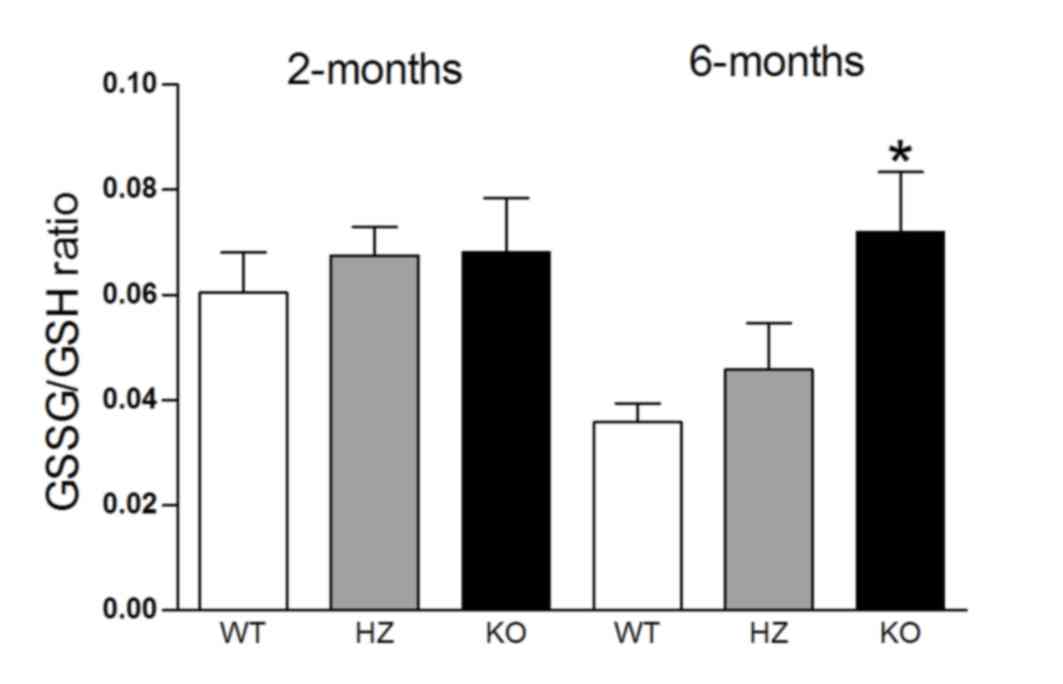
differences were not statistically significant (Fig. 3). The GSSG/GSH ratio was 2-fold
higher (F2,20=4.9; P<0.05) in older and slightly
higher in younger Wfs1-deficient mice compared with WT
(Fig. 4).
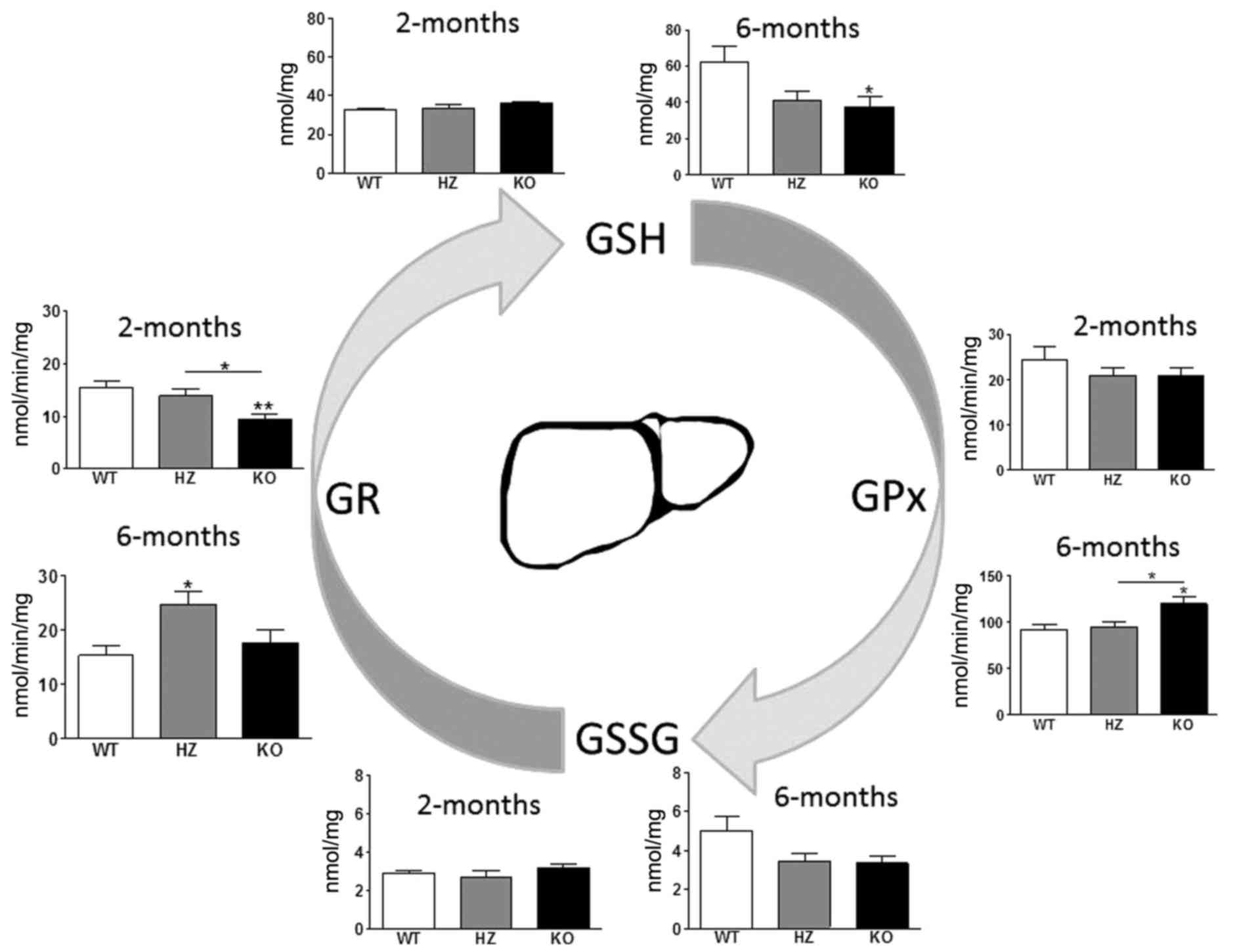
Liver
In the liver, there was a 1.1-fold higher level (not
statistically significant) of GSH in 2-month-old KO mice and a
1.7-fold lower level of GSH in 6-month-old mice compared with WT
littermates (F2,21=4.2; P<0.05; Fig. 5). GR activity was 1.6-fold lower in
2-month-old KO mice compared to WT mice (F2,21=6.4;
P<0.001). Notably, the activity of GPx (1.3-fold;
F2,21=5.6; P<0.05) and GR (1.6-fold;
F2,20=5.2; P<0.05) were significantly increased in
6-month-old KO and HZ mice compared with WT littermates. These data
indicated more intensive usage of GSH by GPx in older mice, whereas
the activity of GR is recovered.
Pancreas
No significant differences were identified in the
GSH system of the pancreas of Wfs1-deficient 2- and
6-month-old mice (Fig. 6A and B,
respectively). GSSG levels were below the detection limit and could
not be measured.
Discussion
Oxidative stress is strongly associated with ER
stress; ER and oxidative stress reduce the GSH capacity and induce
the synthesis of reduced GSH (9,13).
The present study examined the GSH system in Wfs1-deficient
mice to characterize the extent of oxidative stress in several
tissues under chronic ER stress. The results indicated that the GSH
system was not identical in all tissues of Wfs1-deficient
mice. It is particularly complex for the heterozygous mice, in
which the tGSH levels may be up- or downregulated depending on
tissue type and age.
One of the highest expression levels of Wfs1
is found in heart tissue (1). In
the present study, Wfs1 deficiency also exhibited the
greatest effects on the GSH system in the heart. At 2-months old,
when the disease has not yet fully manifested its clinical symptoms
(1,3,6), the
levels of both GSH and GSSG were slightly decreased in KO mice, but
the GSSG/GSH ratio was increased, though not significantly. Enzyme
levels favor the change in the ratio with a tendency of increased
GPx activity and reduced GR activity. As the disease progresses,
the changes become more significant. The HZ is between the WT and
KO. A previous GPx-1-KO study has suggested that GPx may have
anti-ER stress effects (19), and
therefore its upregulation may also be part of the UPR (20). In addition to ER stress response,
particularly at the older age (6 months), the GSH system may be
altered by complications of systemic WS manifestations such as
diabetes. In streptozotocin-induced diabetes, a decrease of GR
activity in heart has been reported (21).
The pancreas is another organ with high levels of
Wfs1 expression. Its exocrine and endocrine functions
require active synthesis of proteins, and makes the pancreas
particularly susceptible to the effects of Wfs1 deficiency
and UPR. Pancreatic β-cells have low levels of antioxidant enzyme
expression and activity, including superoxide dismutases, catalase
and GPx (22). By contrast, the
catalytic subunit of γ-glutamylcysteine ligase, which is the
rate-limiting enzyme for GSH biosynthesis, is highly expressed in
pancreatic islet cells (23). The
inability to properly process insulin is a key event in WS
pathophysiology and the development of diabetes is triggered by the
deficiency of insulin (24). In
the present study, whole pancreas tissue was examined, making the
results more relevant for the larger exocrine function. With no
identified significant changes in tGSH levels and with GSSG under
the detection limit, oxidative stress is seemingly well
controlled.
Liver has lower Wfs1 expression compared with
heart or pancreas (2). The liver
serves a major role in the regulation of carbohydrate metabolism,
such as maintaining the blood glucose level and homeostasis in
general. An entire spectrum of liver diseases have been associated
with type 2 diabetes, including abnormal liver enzymes,
nonalcoholic fatty liver disease, cirrhosis, hepatocellular
carcinoma and acute liver failure (25). A decrease in GSH levels in the
diabetic liver and remarkable increment of GSSG/GSH ratio have been
reported previously (26). In the
present study, 2-month-old Wfs1-KO mice at young age, GSH
expression similar to WT expression levels; at 2 months of age
hyperglycemia and diabetes has not yet manifested itself in mouse
models (27,28), therefore changes from diabetic
complications are not expected. At 6 months of age, however, the
expected decrease was observed. A small GSH increase at young age
could be expected in response to ER stress (12,13),
which at that time is not overwhelming the compensatory mechanisms.
It may be considered that the heart has either stronger stress
owing to higher dependency on Wfs1 or its compensatory
mechanisms are weaker, which may lead to a tendency of GSH
reduction even early on. The activity of the two main GSH redox
enzymes, GPx and GR, also suggest that the early and late liver
tissues are experiencing different situations. Early on, GPx
activity remains unchanged in KO mice compared with ‘healthy’ WT,
although GR activity is reduced. At 6 months old, GR activity in KO
mice returns to similar levels as WT (or even surpasses the WT
activity in HZ), but GPx activity was increased.
Similar to the liver, kidneys express Wfs1 at
low levels. A commonly observed complication of WS and type 2
diabetes is diabetic nephropathy, which is a frequent cause of
mortality in diabetic patients (29). It has been postulated that
oxidative stress may be a key component in the development of
nephropathy (30). Chronic
exposure to high levels of glucose leads to a decrease in GPx
activity in vascular endothelial and kidney cells (31,32).
It has been demonstrated that exposure to oxidative stress inducers
such as carbon tetrachloride increases GPx activity in rat kidneys
(33). Rats treated with ethanol
exhibited increases in both GPx and GR activity in kidneys
(34). High glucose concentration
has been reported to decrease γ-glutamylcysteine ligase expression
and GSH levels in mesangial cell culture (32). Therefore increased GPx activity
indicates an increased rate of GSH usage and the depletion of tGSH
pool as seen in the present results.
In conclusion, the concentration of GSH was
generally decreased in KO Wfs1-deficient mice. A slight, but
not statistically significant increase was seen in liver at young
age. In HZ mice, statistically significant or minimal increases of
tGSH were observed in the kidneys and pancreatic tissue at older
age. The upregulation of GSH in the liver of 2-month-old KO mice is
probably an attempt to control ER stress and depends on the
expected expression of Wfs1.
Acknowledgements
The authors wish to thank the undergraduate students
in the Department of Biochemistry, University of Tartu, Ms. Mariin
Rehe, Ms. Anu Viispert, Ms. Helen Raude, Ms. Alina Lebedeva and Mr.
Oliver Arg for technical assistance. This research was supported by
Institutional Research Funding (grant no. IUT20-42), The Center of
Excellence for Genomics and Translational Medicine from the
Estonian Ministry of Education and Science, and by the European
Union through the European Regional Development Fund (project no.
2014-2020.4.01.15–0012).
References
|
1
|
Strom TM, Hörtnagel K, Hofmann S, Gekeler
F, Scharfe C, Rabl W, Gerbitz KD and Meitinger T: Diabetes
insipidus, diabetes mellitus, optic atrophy and deafness (DIDMOAD)
caused by mutations in a novel gene (wolframin) coding for a
predicted transmembrane protein. Hum Mol Genet. 7:2021–2028. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Inoue H, Tanizawa Y, Wasson J, Behn P,
Kalidas K, Bernal-Mizrachi E, Mueckler M, Marshall H, Donis-Keller
H, Crock P, et al: A gene encoding a transmembrane protein is
mutated in patients with diabetes mellitus and optic atrophy
(Wolfram syndrome). Nat Genet. 20:143–148. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hofmann S, Philbrook C, Gerbitz KD and
Bauer MF: Wolfram syndrome: Structural and functional analyses of
mutant and wild-type wolframin, the WFS1 gene product. Hum Mol
Genet. 12:2003–2012. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takeda K, Inoue H, Tanizawa Y, Matsuzaki
Y, Oba J, Watanabe Y, Shinoda K and Oka Y: WFS1 (Wolfram syndrome
1) gene product: Predominant subcellular localization to
endoplasmic reticulum in cultured cells and neuronal expression in
rat brain. Hum Mol Genet. 10:477–484. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fonseca SG, Ishigaki S, Oslowski CM, Lu S,
Lipson KL, Ghosh R, Hayashi E, Ishihara H, Oka Y, Permutt MA and
Urano F: Wolfram syndrome 1 gene negatively regulates ER stress
signaling in rodent and human cells. J Clin Invest. 120:744–755.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fonseca SG, Fukuma M, Lipson KL, Nguyen
LX, Allen JR, Oka Y and Urano F: WFS1 is a novel component of the
unfolded protein response and maintains homeostasis of the
endoplasmic reticulum in pancreatic beta-Cells. J Biol Chem.
280:39609–39615. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Urano F: Wolfram syndrome iPS cells: The
first human cell model of endoplasmic reticulum disease. Diabetes.
63:844–846. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Walter P and Ron D: The unfolded protein
response: From stress pathway to homeostatic regulation. Science.
334:1081–1086. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zeeshan H, Lee G, Kim HR and Chae HJ:
Endoplasmic reticulum stress and associated ROS. Int J Mol Sci.
17:3272016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Delaunay-Moisan A and Appenzeller-Herzog
C: The antioxidant machinery of the endoplasmic reticulum:
Protection and signaling. Free Radic Biol Med. 83:341–351. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ellgaard L and Ruddock LW: The human
protein disulphide isomerase family: Substrate interactions and
functional properties. EMBO Rep. 6:28–32. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cullinan SB, Zhang D, Hannink M, Arvisais
E, Kaufman RJ and Diehl JA: Nrf2 is a direct PERK substrate and
effector of PERK-dependent cell survival. Mol Cell Biol.
23:7198–7209. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harding HP, Zhang Y, Zeng H, Novoa I, Lu
PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, et al: An
integrated stress response regulates amino acid metabolism and
resistance to oxidative stress. Mol Cell. 11:619–633. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ballatori N, Krance SM, Notenboom S, Shi
S, Tieu K and Hammond CL: Glutathione dysregulation and the
etiology and progression of human diseases. Biol Chem. 390:191–214.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luuk H, Plaas M, Raud S, Innos J, Sütt S,
Lasner H, Abramov U, Kurrikoff K, Kõks S and Vasar E:
Wfs1-deficient mice display impaired behavioural adaptation in
stressful environment. Behav Brain Res. 198:334–45. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tietze F: Enzymic method for quantitative
determination of nanogram amounts of total and oxidized
glutathione: Applications to mammalian blood and other tissues.
Anal Biochem. 27:502–522. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ursini F, Maiorino M and Gregolin C: The
selenoenzyme phospholipid hydroperoxide glutathione peroxidase.
Biochim Biophys Acta. 839:62–70. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carlberg I and Mannervik B: Glutathione
reductase. Methods Enzymol. 113:484–90. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Geraghty P, Baumlin N, Salathe MA, Foronjy
RF, D'Armiento JM and Armiento JM: Glutathione Peroxidase-1
suppresses the unfolded protein response upon cigarette smoke
exposure. Mediators Inflamm. 2016:94612892016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eletto D, Chevet E, Argon Y and
Appenzeller-Herzog C: Redox controls UPR to control redox. J Cell
Sci. 127:3649–3658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li S, Li X, Li YL, Shao CH, Bidasee KR and
Rozanski GJ: Insulin regulation of glutathione and contractile
phenotype in diabetic rat ventricular myocytes. Am J Physiol Hear
Circ Physiol. 292:H1619–H1629. 2007. View Article : Google Scholar
|
|
22
|
Tiedge M, Lortz S, Drinkgern J and Lenzen
S: Relation between antioxidant enzyme gene expression and
antioxidative defense status of insulin-producing cells. Diabetes.
46:1733–1742. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tran PO, Parker SM, LeRoy E, Franklin CC,
Kavanagh TJ, Zhang T, Zhou H, Vliet P, Oseid E, Harmon JS and
Robertson RP: Adenoviral overexpression of the glutamylcysteine
ligase catalytic subunit protects pancreatic islets against
oxidative stress. J Biol Chem. 279:53988–53993. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shang L, Hua H, Foo K, Martinez H,
Watanabe K, Zimmer M, Kahler DJ, Freeby M, Chung W, LeDuc C, et al:
β-Cell dysfunction due to increased ER stress in a stem cell model
of wolfram syndrome. Diabetes. 63:923–933. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tolman KG, Fonseca V, Dalpiaz A and Tan
MH: Spectrum of liver disease in type 2 diabetes and management of
patients with diabetes and liver disease. Diabetes Care.
30:734–743. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Furfaro AL, Nitti M, Marengo B,
Domenicotti C, Cottalasso D, Marinari UM, Pronzato MA and Traverso
N: Impaired synthesis contributes to diabetes-induced decrease in
liver glutathione. Int J Mol Med. 29:899–905. 2012.PubMed/NCBI
|
|
27
|
Ishihara H, Takeda S, Tamura A, Takahashi
R, Yamaguchi S, Takei D, Yamada T, Inoue H, Soga H, Katagiri H, et
al: Disruption of the WFS1 gene in mice causes progressive
beta-cell loss and impaired stimulus-secretion coupling in insulin
secretion. Hum Mol Genet. 13:1159–1170. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Noormets K, Kõks S, Muldmaa M, Mauring L,
Vasar E and Tillmann V: Sex differences in the development of
diabetes in mice with deleted wolframin (Wfs1) gene. Exp Clin
Endocrinol Diabetes. 119:271–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ibrahim HN and Hostetter TH: Diabetic
nephropathy. J Am Soc Nephrol. 8:487–493. 1997.PubMed/NCBI
|
|
30
|
Kashihara N, Haruna Y, Kondeti VK and
Kanwar YS: Oxidative stress in diabetic nephropathy. Curr Med Chem.
17:4256–69. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Urata Y, Yamamoto H, Goto S, Tsushima H,
Akazawa S, Yamashita S, Nagataki S and Kondo T: Long exposure to
high glucose concentration impairs the responsive expression of
gamma-glutamylcysteine synthetase by interleukin-1beta and tumor
necrosis factor-alpha in mouse endothelial cells. J Biol Chem.
271:15146–15152. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Catherwood MA, Powell LA, Anderson P,
McMaster D, Sharpe PC and Trimble ER: Glucose-induced oxidative
stress in mesangial cells. Kidney Int. 61:599–608. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Szymonik-Lesiuk S, Czechowska G,
Stryjecka-Zimmer M, Słomka M, Maldro A, Celiński K and Wielosz M:
Catalase, superoxide dismutase, and glutathione peroxidase
activities in various rat tissues after carbon tetrachloride
intoxication. J Hepatobiliary Pancreat Surg. 10:309–315. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jurczuk M, Moniuszko-Jakoniuk J and
Brzóska MM: Involvement of some low-molecular thiols in the
peroxidative mechanisms of lead and ethanol action on rat liver and
kidney. Toxicology. 219:11–21. 2006. View Article : Google Scholar : PubMed/NCBI
|