Introduction
Pain is one of the most common symptoms in patients
with cancer (1). Morphine is one
of the most commonly used drugs in the treatment of moderate and
severe pain. However, the development of tolerance and dependence
after chronic administration limit its use (2). Although several studies have partly
elucidated the mechanisms of morphine tolerance (3,4), the
exact mechanisms mediating the development of tolerance remain to
be completely understood. Aside from the opioid receptor-based
mechanisms (3), certain studies
have demonstrated that the interactions between opioid and
non-opioid systems, including cannabinoids, also serve roles in the
development of morphine tolerance (4).
Cannabinoid receptors are a family of G
protein-coupled receptors and are classified into two subtypes,
cannabinoid type 1 receptor (CB1) and CB2. Modifying CB1 receptor
activity has limited clinical potential due to adverse neurological
side effects and the development of tolerance (5,6). CB2
receptors are expressed by glia in the dorsal root ganglia (DRG)
and spinal cord (7–9), as well as by neurons in the central
(10,11) and peripheral (12) nervous systems. Certain studies have
suggested that CB2 receptors are involved in the analgesic effects
of repeated morphine administration in naïve and inflammatory
animals (4,13,14).
In addition, several groups have reported that coadministration of
a nonanalgesic dose of cannabinoid receptor agonist with morphine
could reduce morphine antinociceptive tolerance in normal rats and
animal models of neuropathic pain (15–17).
The authors previously observed that intrathecal (i.t.) injection
of a nonanalgetic dose of a CB2 receptor agonist potentiated the
analgesic effect and alleviated tolerance to morphine in
tumor-bearing rats, potentially by regulating µ-opioid receptor
expression in the spinal cord and DRG (18).
Vanilloid receptor 1 (TRPV1) is critical in the
development of thermal and mechanical hyperalgesia in inflammatory,
neuropathic and cancer pain (19,20),
and is involved in morphine tolerance in normal animals (21,22).
A previous study demonstrated the colocalization of CB2 and TRPV1
in human DRG sensory neurons, and observed that CB2 agonists
selectively inhibited capsaicin-induced responses in human DRG
sensory neurons (12). Previous
studies have identified that cannabinoids can evoke
antihyperalgesia and antinociception through cannabinoid receptors,
primarily by inhibiting TRPV1 in peripheral sensory neurons
(23,24). In a cancer pain rat model, whether
coadministration of a CB2 agonist and morphine attenuated TRPV1
expression, and whether this participated in morphine analgesia and
tolerance, is unknown. Therefore, it was hypothesized that
coadministration of a CB2 agonist AM1241 and morphine could reduce
TRPV1 expression in peripheral sensory neurons of the DRG, in
chronic morphine analgesia and tolerance in cancer pain. In the
present study, the effects of coadministration of a CB2 agonist
AM1241 and morphine on TRPV1 expression were investigated following
repeated morphine administration in tumor-bearing rats.
Morphine-mediated analgesia and tolerance was also tested using a
radiant heat stimulation.
Materials and methods
Animals
Adult (6-weeks-old, 160–180 g, n=60) male
specific-pathogen-free Wistar rats (Yisi Experimental Animal
Corporation, Changchun, China) were housed in a climate-controlled
room with a 12-h light/dark cycle, and were provided with food and
water ad libitum. All animal experiments complied with the
policies and recommendations of the International Association for
the Study of Pain, and the National Institutes of Health (NIH)
guidelines for the handling and use of laboratory animals. The
experimental protocol of the present study was approved by the
Animal Care and Use Committee of the Harbin Medical University
(Harbin, China), and all possible steps were taken to avoid animal
suffering at each stage of the experiment. After a 1-week
habituation period, animals received Walker 256 breast carcinoma
cell implantation on the plantar region of the right hind paw of
each rat. The method of Walker 256 cell culture and implantation
was performed as described previously (25).
Drug administration
Drug administration was performed 5 days following
cell injection, when marked proliferation of tumor cells and the
thermal hyperalgesia of the right hind paw could be detected
(20,25) Tumor-bearing rats were randomly
divided into one of six groups using a random number table. For all
experiments, 20 µl 50% dimethyl sulfoxide was used as the vehicle.
The rats in the ‘control’ and ‘morphine’ groups received
subcutaneous (s.c.) injections of normal saline (NS; vehicle +
saline group; n=10) or morphine sulfate (Northeast Pharmaceutical
Group Co., Ltd., Harbin, China); 10 mg/kg/injection; volume, 1
ml/kg; vehicle + morphine group; n=10), respectively, twice daily
(8:00 a.m. and 8:00 p.m.) for 8 days. Furthermore, separate animal
groups received i.t. injections of a nonanalgesic dose of AM1241
(0.07 µg; Cayman Chemical Company, Ann Arbor, MI, USA), the dose
was determined as the authors described previously (18). Rats received injections via lumbar
puncture at the L5-L6 intervertebral space and subcutaneous
morphine (AM1241 + morphine group; n=10), or 10 µg CB2 antagonist
AM630 (Cayman Chemical Company) with AM1241 and morphine (AM1241 +
AM630 + morphine group; n=10), twice daily for 8 days. In addition,
the effects of AM1241 and AM630 were tested separately, the rats
received i.t. injections of AM1241 (AM1241 + saline group; n=10) or
AM630 (AM630 + saline group; n=10), and 30 min subsequently they
received s.c. injections of NS twice daily for 8 days. AM1241 and
AM630 were diluted in 50% dimethyl sulfoxide and a volume of 20 µl
of each was used for the i.t. injection. AM630 was injected 30 min
prior to the agonist AM1241 and the agonist was injected 30 min
prior to morphine. On day 9, the effect of AM1241 on analgesic
tolerance to morphine was measured once all rats had received 5
mg/kg morphine (s.c.).
Behavioral testing
Morphine antinociception and tolerance was
determined by measuring paw withdrawal latency to radiant heat
stimulation (26). Rats were
acclimated within plexiglass enclosures on a clear glass plate
maintained at 26±0.5°C. A radiant heat source (Chengdu Technology
and Market Co., Ltd., Chengdu, China) was focused on the plantar
surface of the hind paw. The stimulus shut off automatically when
the hind paw moved. A threshold time of 30 sec was set to prevent
tissue damage. The tests were performed every morning, prior to
drug administration (baseline) on the first test day and 30 min
following drug administration on days 1–8. On day 9, the tests were
performed 30, 60, 90 and 120 min following s.c. 5 mg/kg morphine
injection.
Immunohistochemistry
The rats (n=4 for each group) received
intraperitoneal (i.p.) injections of 100 mg/kg sodium pentobarbital
for anesthesia following the behavioral test on day 9. The rats
were subsequently transcardially perfused with cold saline,
followed by 4% paraformaldehyde in 0.1 M PBS (pH 7.2–7.4, 4°C). The
L4 DRG ipsilateral to the tumor cell implantation site was removed
and post-fixed in the above fixative for 24 h at 4°C, then embedded
in paraffin, and sectioned (4 µm thickness). The sections were
deparaffinized in a series of descending alcohol concentrations and
the antigen was retrieved using 0.01 M sodium citric buffer in high
pressure (120°C), and then incubated with 2 µg/ml polyclonal rabbit
antibodies against TRPV1 (cat. no. PAB14852; Abnova, Taipei,
Taiwan) overnight at 4°C. The sections were incubated with
biotinylated goat anti-rabbit immunoglobulin G (IgG) (cat. no.
BA1003, 1:200; Boster Biological Technology, Pleasanton, CA, USA)
for 20 min at room temperature, mounted with neutral balsam, and
the morphological details were examined under an inverted
microscope (Olympus Corporation, Tokyo, Japan). The positive area
of the images was digitized and subjected to color threshold
analysis using NIH ImageJ software version 2.1 (National Institutes
of Health, Bethesda, MD, USA).
Western blotting
Western blotting was performed to detect the protein
expression of TRPV1 using protein isolated from the lumbar segments
of the DRG. Following the behavioral tests on day 9, rats (n=3 for
each group) were anesthetized using sodium pentobarbital (60 mg/kg)
and were decapitated for tissue harvesting. The L3/L4/L5 DRG
ipsilateral to the tumor cell injection site were dissected, frozen
in liquid nitrogen and stored at −80°C until further use. The DRG
samples were homogenized in a volume of 200 µl in ice-cold Protein
Lysis Buffer (Beyotime Institute of Biotechnology, Haimen, China)
containing the protease inhibitor phenylmethane sulfonyl fluoride
(1 nM; Beyotime Institute of Biotechnology). The homogenate was
centrifuged (14,000 × g for 20 min at 4°C) and collected. Aliquots
of the total protein samples (30 g; measured by BCA method) were
separated by SDS-PAGE (5% stacking gel and 12% separating gel) and
then the protein was transferred onto a polyvinylidene difluoride
membrane. The bands were analyzed using antibodies specific for
TRPV1 (2 µg/ml) and GAPDH (cat. no. TA08, 1:500; OriGene
Technologies, Inc., Beijing, China) was used as a loading control,
and the membranes were incubated at 4°C overnight. The next day,
the membranes were incubated with secondary antibodies
(IgG-horseradish peroxidase, cat. nos. ZB2301 and ZB2305; OriGene
Technologies, Inc.) at room temperature for 1 h and visualized
using the ECL Plus chemiluminescence detection system (Fluorescent
chemiluminescence imaging system; NatureGene Corporation, Medford,
NJ, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
mRNA expression of TRPV1 was analyzed by RT-qPCR
using lumbar L3/L4/L5 of the DRG ipsilateral to tumor cell
injection. A total of three rats from each group were anesthetized
and then sacrificed immediately following behavioral tests. The
L3/L4/L5 DRG were dissected and immediately frozen in liquid
nitrogen and stored at −80°C until further use. Total RNA was
isolated using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and 2 µg aliquots of the samples were used for
cDNA synthesis using the Transcriptor First-Strand cDNA Synthesis
kit (Roche Diagnostics, Basel, Switzerland). The synthesized cDNA
was used as a template for qPCR amplification using Fast Start
Universal SYBR Green Master mix (Roche Diagnostics) and the
following primers: TRPV1 (Invitrogen; Thermo Fisher Scientific,
Inc.) forward, 5′-TCCAAGGCACTTGCTCCATT-3′ and reverse,
5′-TGGAGGTGGCTTGCAGTTAG-3′; and GAPDH forward,
5′-AGATGGTGAAGGTCGGTGTG-3′ and reverse, 5′-AACTTGCCGTGGGTAGAGTC-3′.
PCR amplification was performed according to the manufacturer's
protocol (95°C for 10 min; and 40 cycles of 95°C for 10 sec, 56°C
for 30 sec, 95°C for 15 sec, 60°C for 1 min, 95°C for 30 sec and
60°C for 15 sec) using an ABI 7500 fast real-time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.).
Statistical analysis
Power analysis was based on preliminary experiments
of paw withdrawal latency to radiant heat stimulation and molecular
biological parameters, and yielded a sample size of n=8 for paw
withdrawal latency to heat stimulation and n=3 for TRPV1 protein
and gene expression (α=0.05; 1-β=0.9) for each group. Data were
analyzed using SPSS 21.0 software (version 21.0; IBM Corp., Armonk,
NY, USA). The behavioral testing data were analyzed using two-way
analysis of variance (ANOVA) followed by the Bonferroni post hoc
test. The TRPV1 receptor protein and mRNA expression levels were
analyzed by one-way ANOVA followed by post-hoc analysis using the
Bonferroni test. P<0.05 was considered to indicate a
statistically significant difference. Data are presented as the
mean ± standard deviation.
Results
Effect of coadministration of AM1241
and morphine on morphine analgesia
Morphine administration produced significant
antinociception between days 1 and 3 compared with the vehicle +
saline group (all P<0.001). The effect of morphine analgesia
gradually declined during chronic exposure between days 4 and 7
compared with day 1 (vehicle + morphine group: Day 4, P=0.023; days
5–8, all P<0.001; Fig. 1). On
day 8, the effect of chronic morphine administration on thermal
withdrawal latency did not exhibit a significant difference
compared with that of the vehicle + saline group (12.76±2.78 vs.
9.62±2.14 sec; P=0.431; Fig. 1),
indicating that the rats developed tolerance to the analgesic
effects of morphine. Rats pretreated with the nonanalgesic dose of
AM1241 (AM1241 + morphine group) exhibited no statistically
significant difference compared with morphine alone (vehicle +
morphine group) on morphine-induced analgesia between days 1 and 4.
However, on day 5, the effect of morphine was significantly reduced
in the vehicle + morphine group compared with that of the AM1241 +
morphine group (18.58±4.80 vs. 24.56±3.92 sec; P=0.024; Fig. 1). Although the paw withdrawal
latency reduced in the following days in AM1241 + morphine group,
the effects of morphine analgesia remained significantly increased
in the AM1241 + morphine group compared with the vehicle + morphine
treatment on day 8 (21.51±3.44 vs. 12.76±2.78 sec; P<0.001).
Pretreatment with AM630 (AM630 + AM1241 + morphine group) produced
a significant decrease in the effect of AM1241 on morphine
analgesia in the treatment of cancer pain (P<0.001; Fig. 1).
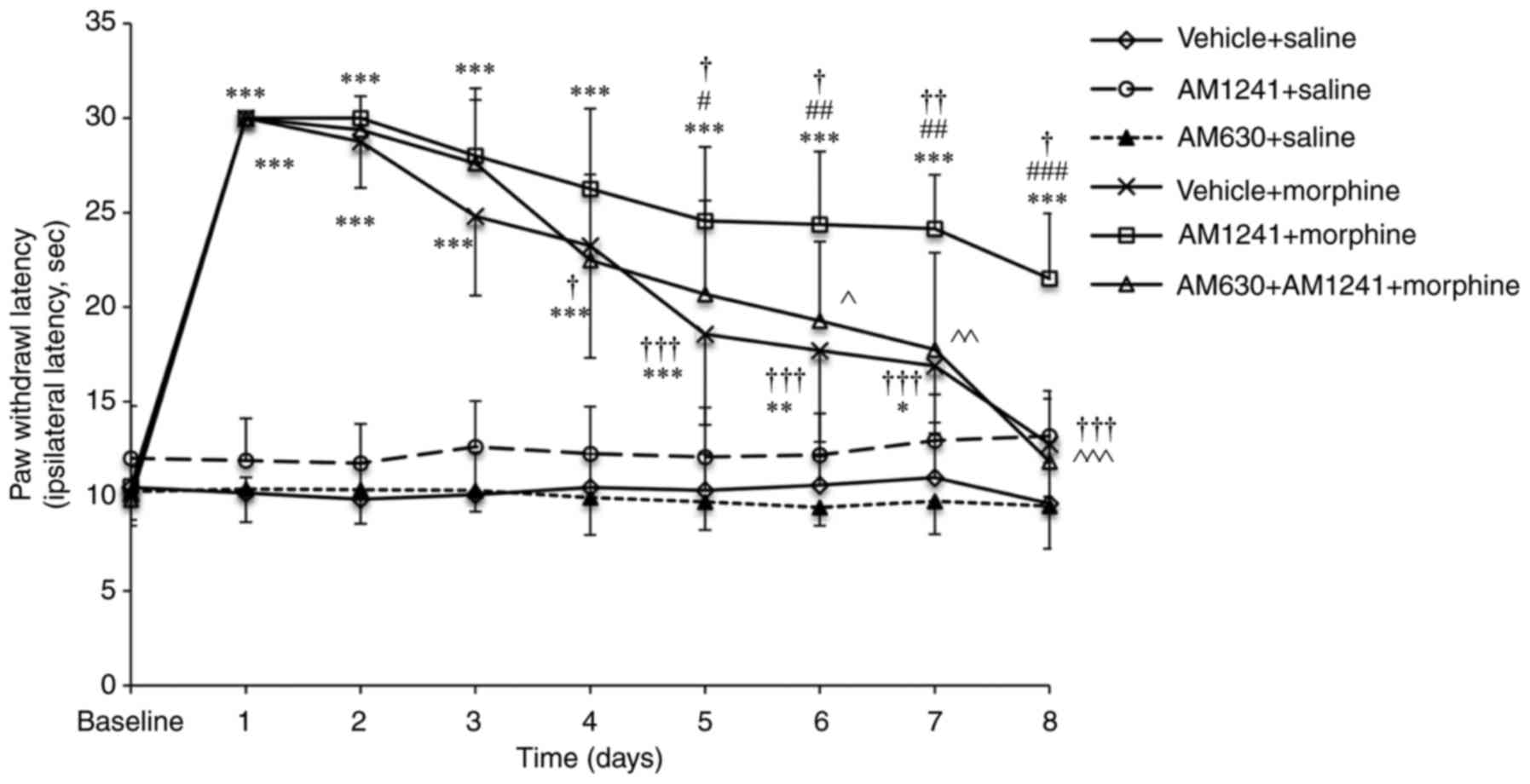 | Figure 1.Effect of coadministration of AM1241
and morphine on morphine-induced antinociception. Rats received
vehicle + saline, vehicle + morphine, AM1241 + saline, AM630 +
saline, AM1241 + morphine or AM630 + AM1241 + morphine (n=10/group)
twice daily for 8 days. The antinociceptive action of the
administered drugs was evaluated 30 min following the first
injection (morning) on days 1–8 of the experiment using a radiant
heat stimulation. The values are presented as the mean ± standard
deviation. *P<0.05, **P<0.01, ***P<0.001 vs. the saline +
vehicle group; #P<0.05, ##P<0.01,
###P<0.001 vs. the morphine + vehicle group;
†P<0.05, ††P<0.01,
†††P<0.001 vs. day 1 in corresponding group;
^P<0.05, ^^P<0.01,
^^^P<0.001 AM1241 + morphine group vs. the AM630 +
AM1241 + morphine group. |
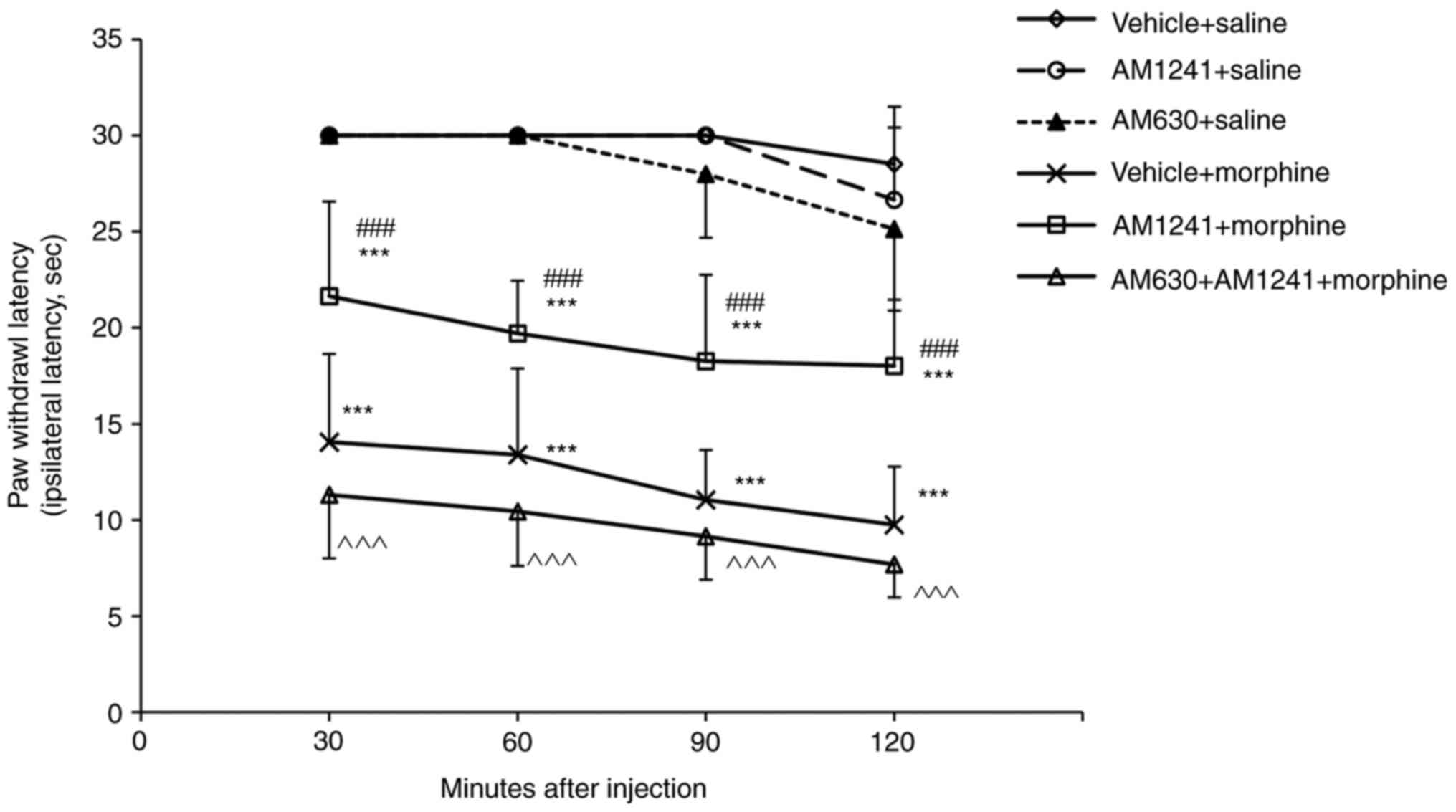
Effect of coadministration of AM1241
and morphine on the development of morphine tolerance
The tumor-bearing rats treated with morphine for 8
days (10 mg/kg twice daily) exhibited significantly decreased
analgesic responses at 30, 60, 90 and 120 min compared with the
vehicle + saline treatment group (all P<0.001) when challenged
with 5 mg/kg of morphine on day 9 (Fig. 2). The antinociceptive responses of
5 mg/kg morphine were significantly increased in the rats
pretreated with the non-analgetic dose of AM1241 (AM1241 + morphine
group) compared with those of the morphine-tolerant rats (vehicle +
morphine group) at 30, 60, 90 and 120 min (all P<0.001; Fig. 2). The selective CB2 antagonist
AM630 completely reversed the effects of AM1241 on morphine
tolerance (all P<0.001; Fig.
2).
Effect of coadministration of AM1241
and morphine on the morphine-mediated TRPV1 protein expression
level in the lumbar DRG
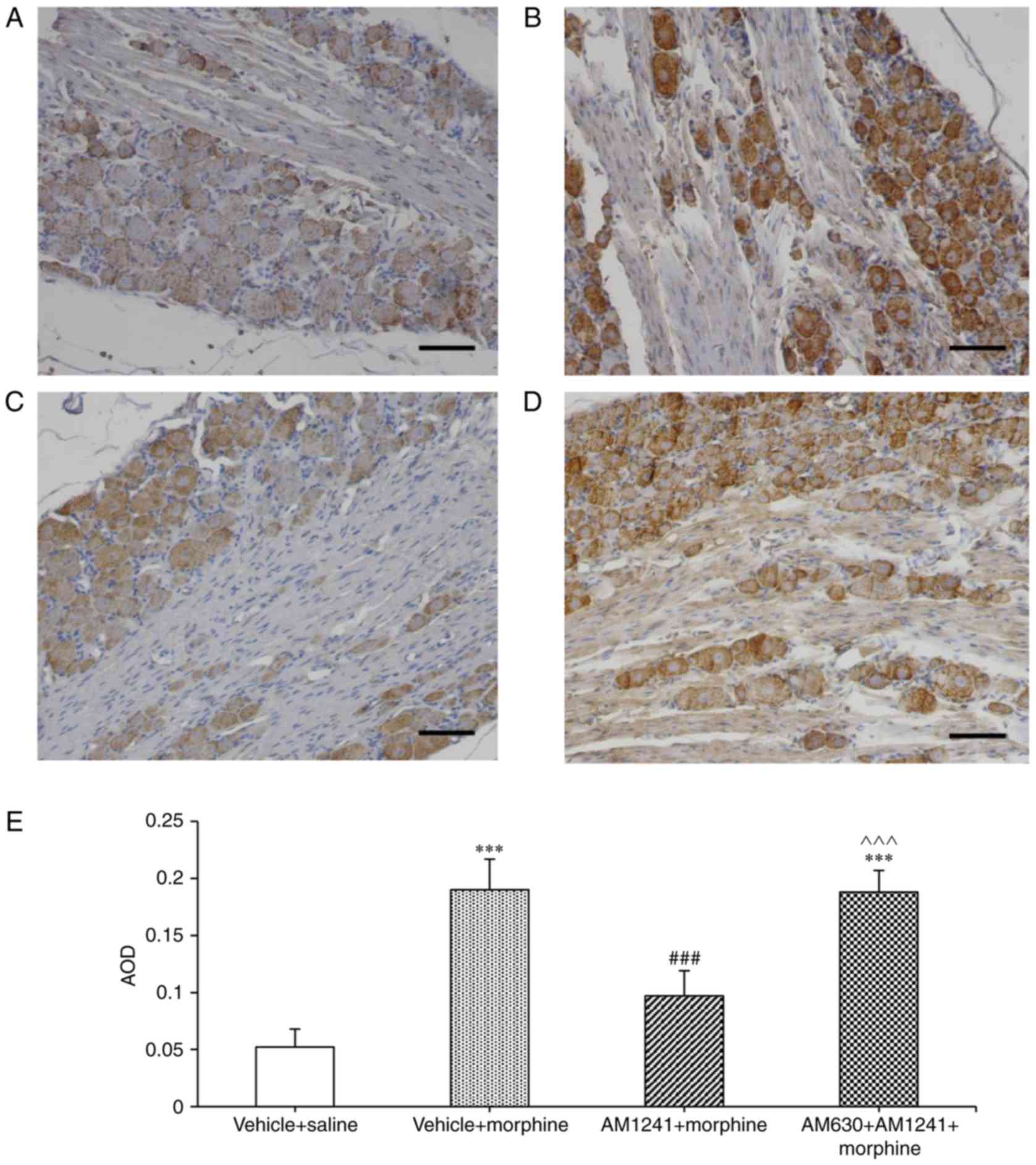
Immunohistochemistry revealed TRPV1 protein
expression in DRG sensory neurons ipsilateral to the site of tumor
cell injection (Fig. 3). Chronic
treatment with morphine for 8 days significantly increased TRPV1
protein expression predominantly in sensory neurons compared with
vehicle + saline group (P<0.001; Fig. 3A, B and E). Pretreatment with
AM1241 (AM1241 + morphine group) significantly reduced
morphine-induced TRPV1 protein expression in DRG sensory neurons
(P<0.001; Fig. 3C and E). The
average optical density in the DRG sections significantly decreased
in AM1241 + morphine group compared with vehicle + morphine group
(P<0.001; Fig. 3E). AM630
reversed the effect of AM1241 on morphine-induced TRPV1 expression
(P<0.001; Fig. 3D and E).
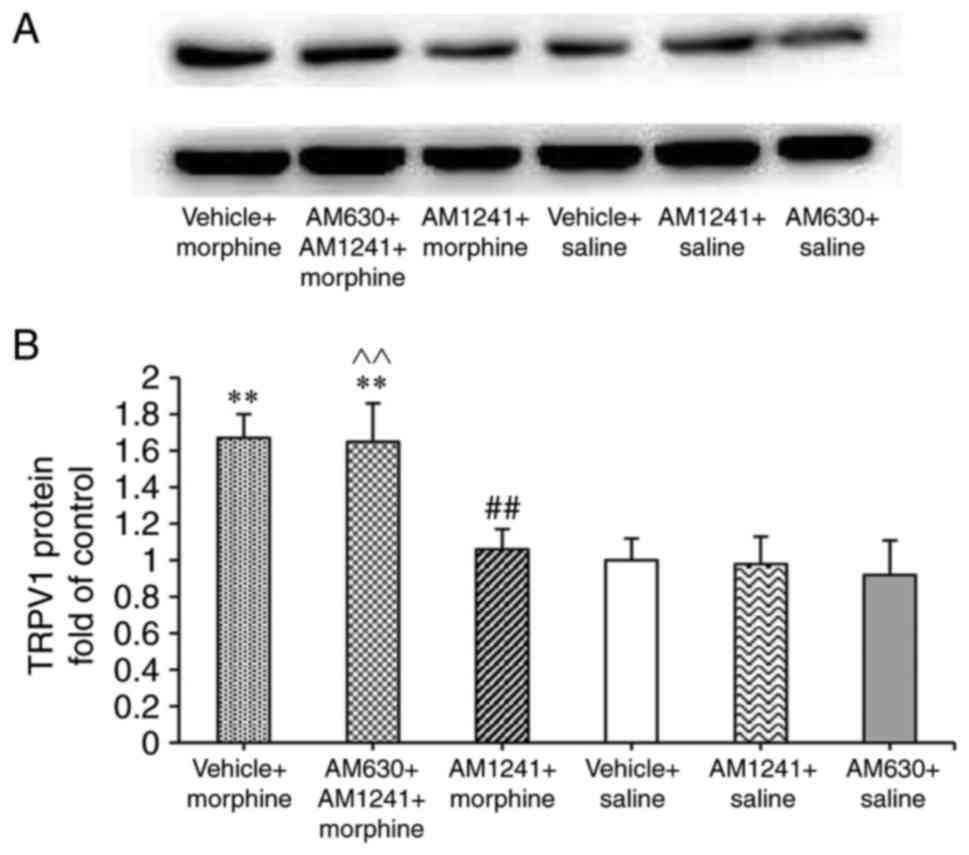
Western blotting was further performed to assess the
total protein levels of TRPV1 in the DRG. The total protein levels
of TRPV1 in the DRG increased significantly after 8 days of
treatment with morphine (P=0.003), AM1241-pretreated group
significantly decreased the TRPV1 expression compared with
treatment with morphine alone (AM1241 + morphine group vs. vehicle
+ morphine group; P=0.007; Fig.
4).
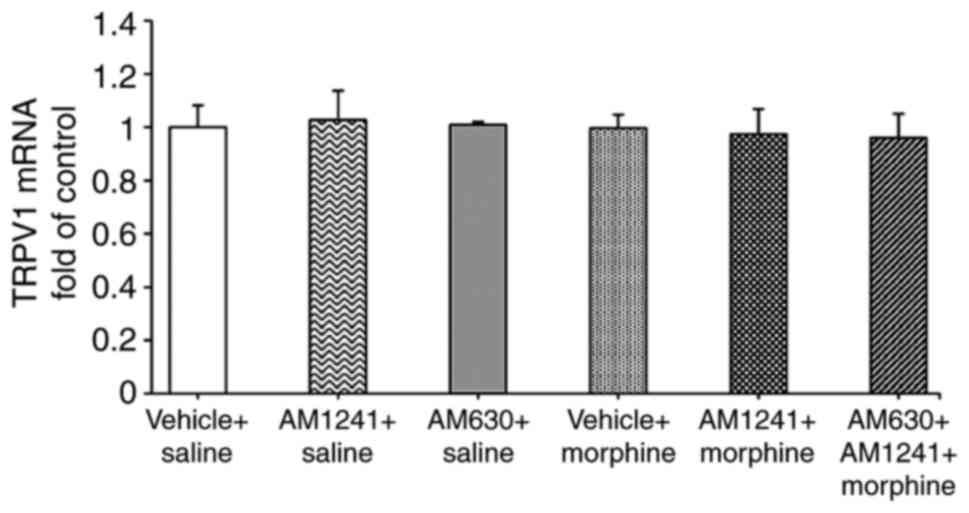
Effect of coadministration of AM1241
and morphine on the morphine-mediated TRPV1 mRNA expression level
in the lumbar DRG
RT-qPCR revealed no significant change in the TRPV1
mRNA levels in the DRG among six groups (all P=0.913; Fig. 5).
Discussion
In the present study, it was observed that
coadministration of a non-analgesic dose of the CB2 agonist AM1241
and morphine attenuated TRPV1 protein expression in the DRG of
morphine-tolerant tumor-bearing rats. These results suggest that
downregulation of TRPV1 protein expression in the peripheral
sensory neurons of the DRG may be one of the mechanisms by which
coadministration of a CB2 agonist increased chronic morphine
analgesia and attenuated tolerance in cancer pain.
In the present study, it was demonstrated that i.t.
administration of a nonanalgetic dose of the CB2 agonist AM1241
potentiated morphine antinociception and alleviated morphine
tolerance in a cancer pain rat model by measuring paw withdrawal
latency to radiant heat stimulation. These results are consistent
with the results of the authors' previous study using von Frey
filament and hot plate test in Walker 256 tumor-bearing rats
(18). It further confirmed that
CB2 agonists may contribute to increased morphine antinociception
and inhibit the development of tolerance associated with repeated
treatment with morphine in cancer pain.
In primary sensory neurons, TRPV1 serves an
important role in nociceptive conduction, including models of
cancer, inflammatory and neuropathic pain (20,24,27).
It has been suggested that neuropathy or inflammation-induced
hyperalgesia and morphine tolerance may share similar mechanisms
(28–30). Following chronic treatment with
morphine, the percentage of TRPV1-immunoreactive DRG neurons was
increased (21), and activation of
TRPV1 contributed to morphine tolerance in normal rats (21,22).
In the present study, the results revealed that chronic treatment
with morphine resulted in increased expression levels of TPRV1
protein. These results implied that involvement of the TRPV1 in
morphine tolerance in cancer pain. Previous studies reported that
there is no increase in TRPV1 mRNA expression levels in the DRG in
inflammation and normal rats with morphine tolerance (21,31).
In the present study, no increase in TRPV1 mRNA expression was
detected in the lumbar DRG of morphine-tolerant rats in the rat
model of cancer pain. The results suggested that chronic treatment
with morphine enhances TRPV1 protein expression at the
translational or post-translational level in DRG neurons (21).
Combination of morphine and TRPV1 antagonist
potentiated the analgesic effects of morphine in a mouse model of
bone cancer pain (32). I.t. and
i.p. injection of a selective TRPV1 antagonist decreased
TRPV1-immunoreactivity, and attenuated morphine tolerance and
dependence in normal rats (21). A
previous study demonstrated the colocalization of CB2 and TRPV1 in
human DRG sensory neurons (12).
The cannabinoids inhibited capsaicin responses in cultured human
DRG neurons and in rat threonine ganglia (12,23,33),
and regulated TRPV1 in the animal model of inflammatory and
neuropathic pain (24). However,
little is known about the effect of CB2 agonist on TRPV1 expression
on morphine tolerance in cancer pain. In the present study, it was
observed that coadministration of a nonanalgetic dose of AM1241 and
morphine significantly decreased TRPV1 immunostaining and total
protein expression in the DRG following repeated treatment with
morphine in the rat model of cancer pain, and the effect of the CB2
agonist AM1241 was abolished by the CB2 antagonist AM630. These
results suggested a potential role for the CB2 agonist on TRPV1
expression in morphine tolerance in cancer pain.
CB2 and TRPV1 belong to the G-protein-coupled
receptor family, Anand et al (12) demonstrated colocalization of CB2
and TRPV1 in human and rat DRG small/medium-diameter sensory
neurons. A previous study reported that the increase in TRPV1
immunoreactivity contributes to morphine tolerance in a
mitogen-activated protein kinase (MAPK)-dependent manner (21). Inhibition of MAPK phosphorylation
reduced the increased TRPV1 protein expression that is normally
associated with chronic treatment with morphine (21), and reduced morphine tolerance in
normal rats and mice (21,34). It has been demonstrated that CB2
agonists reduce the phosphorylated forms of MAPKs, including
phosphorylated (p)-p38 and p-extracellular-regulated kinase 1/2
in vitro and in a rat model of neuropathic pain (35,36),
which may subsequently regulate TRPV1 downstream of the DRG neurons
of rats exhibiting morphine tolerance. In addition, CB2 agonists
have been demonstrated to indirectly mediate TRPV1 phosphorylation
by inhibiting adenylyl cyclase and depleting cAMP in cells,
including cultured human DRG neurons, the BV2 microglial cell line,
CB2-transfected human embryonic kidney cells and Chinese hamster
ovary cells (12,36), which possibly participates in CB2
agonist-associated morphine tolerance.
The mechanism of opioid tolerance remains unclear.
CB2 agonist-associated reduced morphine tolerance may involve
multiple aspects. The authors previously reported that a
nonanalgetic dose of CB2 agonist AM1241 reduced morphine tolerance
by upregulating µ-opioid receptor protein and mRNA expression in a
rat model of cancer pain (18). In
addition, the activation of glial cells by chronic morphine
administration contributes to morphine tolerance (2,37).
It is possible that the CB2 agonist alleviates morphine tolerance
by reducing glial activation (4).
There are certain limitations to the present study.
Specific TRPV1 inhibitors were not used to further elucidate the
association between CB2 agonists and TRPV1 in morphine tolerance in
the treatment of cancer pain; therefore, no direct proof of this
result can be drawn from the data. The pathway between CB2 and
TRPV1 was not investigated. Therefore, the molecular and cellular
mechanisms involved in CB2 agonists-modulated TRPV1 expression in
regards to analgesia and tolerance of morphine in cancer pain
remain to be determined. Further studies are required to address
these limitations.
In conclusion, the data indicated that
coadministration of a nonanalgetic dose of a CB2 agonist and
morphine reduced morphine tolerance in a rat model of cancer pain,
and the effect of the CB2 agonist on morphine tolerance may involve
the suppression of TRPV1 protein expression. The results provide
further evidence that CB2 agonists may assist in the reduction of
morphine tolerance in cancer pain, and also provide a novel
strategy to strengthen morphine analgesia and improved clinical
treatment of cancer pain.
Acknowledgements
The present study was supported by the Translational
Medicine Special Foundation of China Russia Medical Research Center
(grant nos. 201519 and CR1418), Natural Science Foundation of China
(grant no. 81571885) and Haiyan Research Fund of Cancer Hospital of
Harbin Medical University (grant no. JJQN2014-09).
Glossary
Abbreviations
Abbreviations:
|
CB2
|
cannabinoid type 2 receptor
|
|
DRG
|
dorsal root ganglion
|
|
MAPK
|
mitogen-activated protein kinase
|
|
TRPV1
|
vanilloid receptor 1
|
|
s.c.
|
subcutaneous
|
|
i.t.
|
intrathecal
|
|
i.p.
|
intraperitoneal
|
References
|
1
|
Mercadante S: The use of opioids for
treatment of cancer pain. Expert Opin Pharmacother. 16:389–394.
2015.PubMed/NCBI
|
|
2
|
Watkins LR, Hutchinson MR, Johnston IN and
Maier SF: Glia: Novel counter-regulators of opioid analgesia.
Trends Neurosci. 28:661–669. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nagi K and Piñeyro G: Regulation of opioid
receptor signalling: Implications for the development of analgesic
tolerance. Mol Brain. 4:252011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tumati S, Largent-Milnes TM, Keresztes A,
Ren J, Roeske WR, Vanderah TW and Varga EV: Repeated morphine
treatment-mediated hyperalgesia, allodynia and spinal glial
activation are blocked by co-administration of a selective
cannabinoid receptor type-2 agonist. J Neuroimmunol. 244:23–31.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ledent C, Valverde O, Cossu G, Petitet F,
Aubert JF, Beslot F, Böhme GA, Imperato A, Pedrazzini T, Roques BP,
et al: Unresponsiveness to cannabinoids and reduced addictive
effects of opiates in CB1 receptor knockout mice. Science.
283:401–404. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Vry J, Jentzsch KR, Kuhl E and Eckel G:
Behavioral effects of cannabinoids show differential sensitivity to
cannabinoid receptor blockade and tolerance development. Behav
Pharmacol. 15:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pertwee RG: Cannabinoid receptors and
pain. Prog Neurobiol. 63:569–611. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Beltramo M, Bernardini N, Bertorelli R,
Campanella M, Nicolussi E, Fredduzzi S and Reggiani A: CB2
receptor-mediated antihyperalgesia: Possible direct involvement of
neural mechanisms. Eur J Neurosci. 23:1530–1538. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Romero-Sandoval A, Nutile-McMenemy N and
DeLeo JA: Spinal microglial and perivascular cell cannabinoid
receptor type 2 activation reduces behavioral hypersensitivity
without tolerance after peripheral nerve injury. Anesthesiology.
108:722–734. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Onaivi ES, Ishiguro H, Gong JP, Patel S,
Perchuk A, Meozzi PA, Myers L, Mora Z, Tagliaferro P, Gardner E, et
al: Discovery of the presence and functional expression of
cannabinoid CB2 receptors in brain. Ann N Y Acad Sci. 1074:514–536.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Onaivi ES, Ishiguro H, Gong JP, Patel S,
Meozzi PA, Myers L, Perchuk A, Mora Z, Tagliaferro PA, Gardner E,
et al: Functional expression of brain neuronal CB2 cannabinoid
receptors are involved in the effects of drugs of abuse and in
depression. Ann N Acad Sci. 1139:434–449. 2008. View Article : Google Scholar
|
|
12
|
Anand U, Otto WR, Sanchez-Herrera D, Facer
P, Yiangou Y, Korchev Y, Birch R, Benham C, Bountra C, Chessell IP
and Anand P: Cannabinoid receptor CB2 localisation and
agonist-mediated inhibition of capsaicin responses in human sensory
neurons. Pain. 138:667–680. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lim G, Wang S and Mao J: Central
glucocorticoid receptors modulate the expression of spinal
cannabinoid receptors induced by chronic morphine exposure. Brain
Res. 1059:20–27. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Desroches J, Bouchard JF, Gendron L and
Beaulieu P: Involvement of cannabinoid receptors in peripheral and
spinal morphine analgesia. Neuroscience. 261:23–42. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cichewicz DL and Welch SP: Modulation of
oral morphine antinociceptive tolerance and naloxone-precipitated
withdrawal signs by oral Delta 9-tetrahydrocannabinol. J Pharmacol
Exp Ther. 305:812–817. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cichewicz DL: Synergistic interactions
between cannabinoid and opioid analgesics. Life Sci. 74:1317–1324.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bushlin I, Rozenfeld R and Devi LA:
Cannabinoid-opioid interactions during neuropathic pain and
analgesia. Curr Opin Pharmacol. 10:80–86. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang M, Wang K, Ma M, Tian S, Wei N and
Wang G: Low-dose cannabinoid type 2 receptor agonist attenuates
tolerance to repeated morphine administration via regulating
µ-Opioid Receptor expression in Walker 256 tumor-bearing rats.
Anesth Analg. 122:1031–1037. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma W and Quirion R: Inflammatory mediators
modulating the transient receptor potential vanilloid 1 receptor:
Therapeutic targets to treat inflammatory and neuropathic pain.
Expert Opin Ther Targets. 11:307–320. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Z, Wang C, Gu G, Li H, Zhao H, Wang
K, Han F and Wang G: The effects of electroacupuncture at the ST36
(Zusanli) acupoint on cancer pain and transient receptor potential
vanilloid subfamily 1 expression in Walker 256 tumor-bearing rats.
Anesth Analg. 114:879–885. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen Y, Geis C and Sommer C: Activation of
TRPV1 contributes to morphine tolerance: Involvement of the
mitogen-activated protein kinase signaling pathway. J Neurosci.
28:5836–5845. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nguyen TL, Nam YS, Lee SY, Kim HC and Jang
CG: Effects of capsazepine, a transient receptor potential
vanilloid type 1 antagonist, on morphine-induced antinociception,
tolerance, and dependence in mice. Br J Anaesth. 105:668–674. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Patwardhan AM, Jeske NA, Price TJ, Gamper
N, Akopian AN and Hargreaves KM: The cannabinoid WIN 55,212-2
inhibits transient receptor potential vanilloid 1 (TRPV1) and
evokes peripheral antihyperalgesia via calcineurin. Proc Natl Acad
Sci USA. 103:11393–11398. 2006; View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Devesa I and Ferrer-Montiel A:
Neurotrophins, endocannabinoids and thermo-transient receptor
potential: A threesome in pain signalling. Eur J Neurosci.
39:353–362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brigatte P, Sampaio SC, Gutierrez VP,
Guerra JL, Sinhorini IL, Curi R and Cury Y: Walker 256
tumor-bearing rats as a model to study cancer pain. J Pain.
8:412–421. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang ZB, Gan Q, Rupert RL, Zeng YM and
Song XJ: Thiamine, pyridoxine, cyanocobalamin and their combination
inhibit thermal, but not mechanical hyperalgesia in rats with
primary sensory neuron injury. Pain. 114:266–277. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Szallasi A, Cortright DN, Blum CA and Eid
SR: The vanilloid receptor TRPV1: 10 years from channel cloning to
antagonist proof-of-concept. Nat Rev Drug Discov. 6:357–372. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ossipov MH, Lai J, King T, Vanderah TW and
Porreca F: Underlying mechanisms of pronociceptive consequences of
prolonged morphine exposure. Biopolymers. 80:319–324. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mao J, Price DD and Mayer DJ: Mechanisms
of hyperalgesia and opiate tolerance: A current view of their
possible interactions. Pain. 62:259–2274. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mayer DJ, Mao J, Holt J and Price DD:
Cellular mechanisms of neuropathic pain, morphine tolerance, and
their interactions. Proc Natl Acad Sci USA. 96:7731–7736. 1999;
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Endres-Becker J, Heppenstall PA, Mousa SA,
Labuz D, Oksche A, Schäfer M, Stein C and Zöllner C: Mu-opioid
receptor activation modulates transient receptor potential
vanilloid 1 (TRPV1) currents in sensory neurons in a model of
inflammatory pain. Mol Pharmacol. 71:12–18. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Niiyama Y, Kawamata T, Yamamoto J, Furuse
S and Namiki A: SB366791, a TRPV1 antagonist, potentiates analgesic
effects of systemic morphine in a murine model of bone cancer pain.
Br J Anaesth. 102:251–258. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jeske NA, Patwardhan AM, Gamper N, Price
TJ, Akopian AN and Hargreaves KM: Cannabinoid WIN 55,212-2
regulates TRPV1 phosphorylation in sensory neurons. J Biol Chem.
281:32879–32890. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen Y and Sommer C: The role of
mitogen-activated protein kinase (MAPK) in morphine tolerance and
dependence. Mol Neurobiol. 40:101–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Landry RP, Martinez E, DeLeo JA and
Romero-Sandoval EA: Spinal cannabinoid receptor type 2 agonist
reduces mechanical allodynia and induces mitogen-activated protein
kinase phosphatases in a rat model of neuropathic pain. J Pain.
13:836–848. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dhopeshwarkar A and Mackie K: CB2
Cannabinoid receptors as a therapeutic target-what does the future
hold? Mol Pharmacol. 86:430–437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cui Y, Chen Y, Zhi JL, Guo RX, Feng JQ and
Chen PX: Activation of p38 mitogen-activated protein kinase in
spinal microglia mediates morphine antinociceptive tolerance. Brain
Res. 1069:235–243. 2006. View Article : Google Scholar : PubMed/NCBI
|