Introduction
Glomerular podocytes are highly specialized cells
with a complex cellular organization that assist the kidneys in
blood filtration. Podocytes also serve a crucial role in the
synthesis of glomerular basement membrane components, the formation
of the slit membrane and interactions that ensure endothelial cell
viability (1,2). Several studies have revealed that
podocyte injury results in the effacement of foot processes and
proteinuria, and ultimately leads to consequence of acquired
glomerular diseases (1,3). In 2009, Ronconi et al
(4) indicated that the podocyte
damage that occurs in the pathogenesis of glomerulosclerosis could
potentially be repaired through stem cell regeneration in the
kidney. Furthermore, the effacement of podocytes and the decrease
in their density appear to be central to the pathogenesis of
diabetic nephropathy (DN). Injuries sustained because of increased
oxidative stress constitute the most crucial mechanism (5,6).
Recently, Mallipattu and He (7)
reported that podocytes are terminally differentiated and have a
minimal capacity to self-replicate; therefore, they are extremely
sensitive to cellular injury. When podocyte injury occurs, it
directly causes the onset and progression of glomerular diseases
such as focal segmental glomerular sclerosis, minimal change
disease, DN and human immunodeficiency virus-associated
nephropathy. Therefore, understanding the biological mechanisms
involved in podocyte injury may provide novel therapeutic targets
for preventing or mitigating progression to end-stage renal
failure.
Caveolin (CAV)-1 is an essential protein component
of caveolae, which are omega-shaped plasma membrane invaginations
rich in sphingolipids and cholesterol. In addition to maintaining
cholesterol homeostasis, CAV-1 is involved in regulating vesicular
transport, signal transduction and tumor progression (8,9).
Cellular organelles such as mitochondria, nuclei and endoplasmic
reticuli are rich in CAVs, and CAV-1 is highly expressed in
vascular endothelial cells, adipocytes, smooth muscle cells and
fibroblasts (10). In
CAV-1-deficient fibroblasts, >40 upregulated protein biomarkers
have been identified. Most of these biomarkers are associated with
myofibroblast differentiation or oxidative stress hypoxia (11). The absence of CAV-1 causes
cholesterol-dependent mitochondrial dysfunction and apoptotic
susceptibility (12). By contrast,
previous studies have demonstrated that CAV-1 is highly expressed
in podocytes and interacts with the podocyte slit diaphragm protein
nephrin and CD2AP (13,14). Previous studies demonstrated that
angiotensin II induces nephrin dephosphorylation and podocyte
injury through a CAV-1-dependent mechanism; therefore, CAV-1 is
potentially a novel therapeutic target in nephrotic syndrome and
podocyte injury (15–17). Hence, demonstrating the
cell-specific role of CAV-1 in the pathogenesis of renal-associated
disease may be crucial.
The present study used antennapedia-conjugated CAV-1
peptide, which is a Drosophila transcription factor
facilitating CAV-1 translocation across the cell membrane (18,19),
in a H2O2-induced podocyte dysfunction model.
To evaluate CAV-1-induced changes in the
H2O2-dependent mechanism in injured podocyte
cells, the present study examined the mRNA expression levels of
CAV-1, cyclophilin A (CypA) and ATP-binding cassette transporter A1
(ABCA1), as well as the mitochondrial function, oxidative and
antioxidative homeostasis, and apoptosis of E11 podocytes.
Materials and methods
Materials
Antenapedia-CAV-1 (AP-CAV-1) peptide
[RQPKIWEFPNRRKPWKK-DGIWKA SFTTFVTKYWFYR-(OH)] was obtained from
AllBio Science, Inc., (Taiwan). Hydrogen peroxide solution
(H2O2) was purchased from the Sigma-Aldrich;
Merck KGaA (Darmstadt, Germany). Antibodies against monoclonal
anti-CAV-1 (cat no. 1249-1; 1:1,000; Epitomics; Abcam, Cambridge,
MA, USA), monoclonal anti-CyP A (cat no. GTX113520; 1:1,000;
GeneTex, Inc., Irvine, CA, USA), polyclonal anti-superoxide
dismutase 2 (SOD2; cat no. NB100-1992; 1:1,000; Novus Biologicals,
LLC, Littleton, CO, USA), polyclonal anti-catalase (cat no.
ab16731; 1:1,000; Abcam), polyclonal anti-glutamate-cysteine ligase
catalytic subunit (GCLC; cat no. GTX113197; 1:800; GeneTex, Inc.),
mouse anti-optic atrophy 1 (OPA1; cat no. 612607; 1:1,000; BD
Biosciences, San Jose, CA, USA), mouse anti-translocase of the
inner membrane 23 (Tim23; cat no. 611222; 1:1,000; BD Biosciences),
MitoProfile Total oxidative phosphorylation (OXPHOS) rodent
antibody cocktail (cat no. ab110413; 1:800; MitoSciences; Abcam),
and mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; cat
no. ab8245; 1:1,000; Abcam).
Cell culture
The E11 murine kidney podocyte cell line was
obtained from CLS Cell Lines Service GmbH (Germany) and was
maintained in RPMI 1640 medium (Amimed, BioConcept Ltd.,
Switzerland) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and 100
g/ml streptomycin (TOKU-E, Bellingham, WA, USA), 20 U/ml human
recombinant interferon gamma (IFN-γ; ProSpec-Tany TechnoGene Ltd.,
East Brunswick, NJ, USA) at 33°C in a humidified 5% CO2
incubator.
Western blot analysis and
quantification
Cells were pretreated with the indicated
concentration of H2O2 for 1 h, followed by
treatment with an indicated concentration of AP-CAV-1 peptide for
an addition 48 h. Cells were washed with ice-cold PBS and lysed in
radioimmunoprecipitation assay buffer, and centrifuged at 20,000 ×
g for 20 min at 4°C. The protein concentration was detected using a
Bicinchoninic Acid protein assay kit (Thermo Fisher Scientific,
Inc.). Proteins (20 µg) were separated by 12% SDS-PAGE and then
transferred to polyvinylidene difluoride membranes. The membrane
was probed with the indicated primary antibodies at 4°C overnight,
and then with horseradish peroxidase-conjugated goat anti-mouse
(cat no. 115-035-003; 1:50,000; Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA) and goat anti-rabbit (cat
no. 31460; 1:100,000; Thermo Fisher Scientific, Inc.) secondary
antibodies at room temperature for 1 h, and signals were obtained
using an enhanced chemiluminescence kit (EMD Millipore, Billerica,
MA, USA). Blots were semi-quantified by densitometry using
Fusion-Capt Advance FX7 software versoin 16.08a on a Fusion FX7
imaging system (Labtech International, Inc., Vilber Lourmat,
France).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was prepared using an AllPure Total RNA
Isolation kit (AllBio Science Inc., Taichung, Taiwan) according to
the manufacturer's protocol. Reverse transcription and qPCR were
performed using AllScript First-Strand cDNA Synthesis SuperMix and
AllScript Green qPCR SuperMix UDG (AllBio Science, Inc.) according
to the manufacturer's protocol. qPCR analysis was used to determine
the relative levels of CAV-1, CypA, ATP-binding cassette
transporter A1 (ABCA1), B-cell lymphoma 2 (Bcl2), and
BCL2-associated X protein (Bax) mRNA. β-actin was performed in the
same reaction on all samples tested as an internal control for
variations in RNA amounts. Relative gene expression was quantified
according to the comparative Cq method and normalized to β-actin
mRNA levels (20). The
gene-specific primers are listed in Table I. The thermocycling conditions for
qPCR included an initial phase of 3 min at 50°C, followed by 10 sec
at 94°C and 40 cycles of 5 sec at 94°C, 15 sec at 60°C and 15 sec
at 72°C. Each sample was assayed in duplicate, and fluorescence
spectra were continuously monitored using the LightCycler 480
Detection system (Roche, Basel, Switzerland).
 | Table I.Primers used in reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primers used in reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| Caveolin-1 |
AGCCCAACAACAAGGCCAT |
GCAATCACATCTTCAAAGTCAATCTT |
| Cyclophilin A |
TGCTGGACCAAACACAAACG |
GCCTTCTTTCACCTTCCCAAA |
| ABCA1 |
AACAGTTTGTGGCCCTTTTG |
AGTTCCAGGCTGGGGTACTT |
| Bcl2 |
CTGAGTACCTGAACCGGCATC |
GAGCAGCGTCTTCAGAGACAG |
| Bax |
GTTTCATCCAGGATCGAGCAG |
AGCTGAGCGAGTGTCTCCGGCG |
| β-actin |
TGGAATCCTGTGGCATCCATGAAAC |
TAAAACGCAGCTCAGTAACAGTCCG |
Statistical analysis
Statistical analyses were performed using one-way
analysis of variance followed by Bonferroni's post hoc test in SPSS
software version 22.0 (IBM Corp., Armonk, NY, USA). Data are
presented as mean ± standard deviation. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of H2O2
on expression levels of antioxidant-associated proteins, CAV-1 and
CypA in E11 podocytes
To determine the effects of
H2O2 on the antioxidant-associated proteins
of podocytes, E11 cells were treated with various concentrations of
H2O2 for 1 h. The expression of
antioxidant-associated proteins was measured through western blot
analysis. A significant and dose-dependent decrease was observed in
the expression of the antioxidant enzymes GCLC, catalase and SOD2
in the H2O2-treated groups compared with the
vehicle control (Fig. 1A).
Similarly, the H2O2 treatment markedly
reduced the expression of CAV-1; whereas the expression of CypA,
which is an inflammatory marker, was significantly upregulated. The
quantification of the results is presented in Fig. 1B (P<0.001). These results
suggested that H2O2 significantly affects the
antioxidant capacities of podocytes, thus promoting intercellular
inflammation and altering mitochondrial antioxidant capacity.
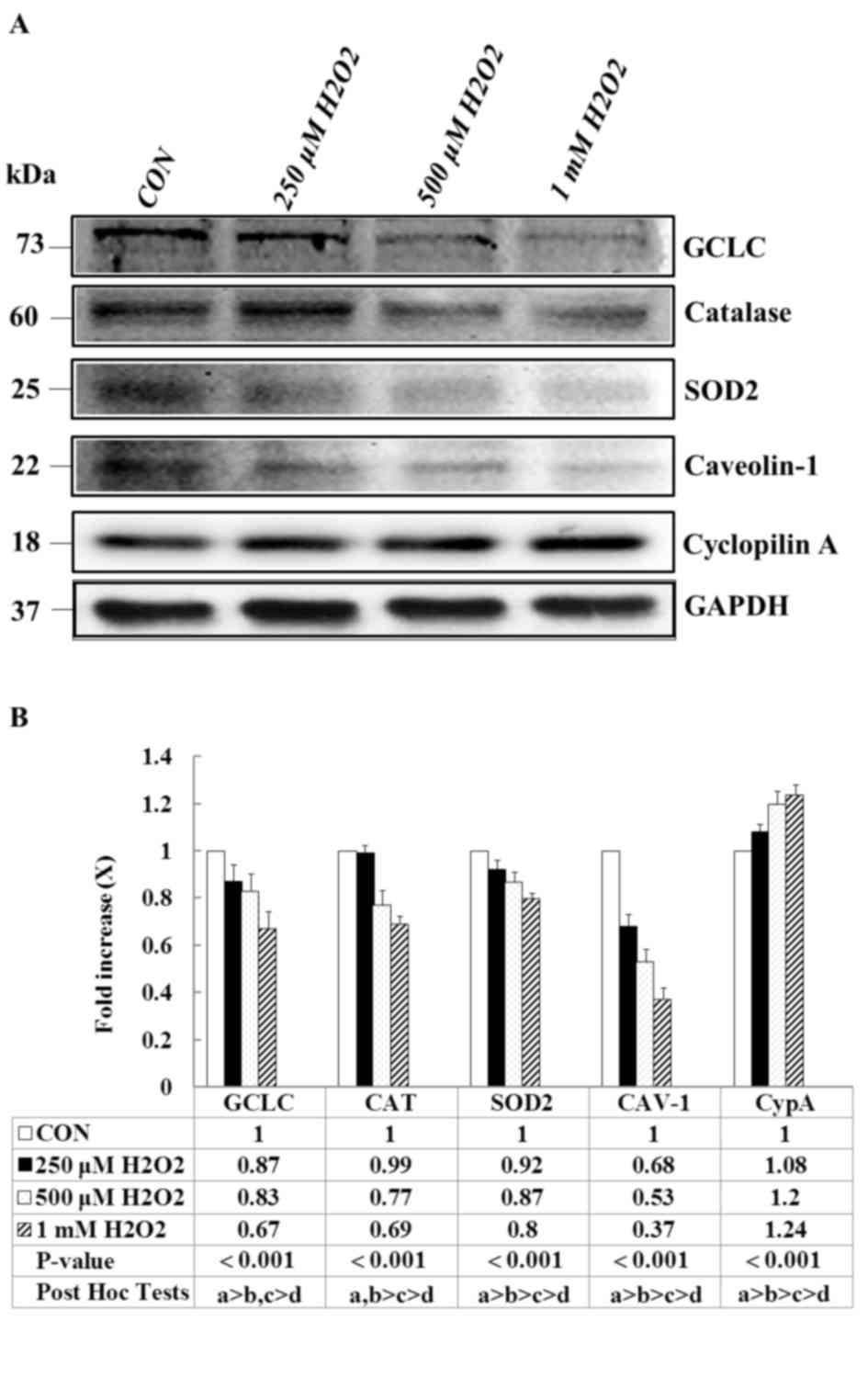 | Figure 1.Effects of H2O2
on antioxidant-associated proteins, CAV-1 and CypA expression
levels. E11 cells, except for the CON group, were treated with the
indicated concentration of H2O2 for 1 h. (A)
Representative western blot images and (B) quantification of GCLC,
catalase, SOD2, CAV-1 and CypA protein expression levels. GAPDH
served as an internal control, Data are presented as the mean ±
standard deviation of at least three independent experiments. a:
CON group, b: 250 µM H2O2 group, c: 500 µM
H2O2 group, d: 1 mM
H2O2 group. CON, control; CAV-1, caveolin-1;
CypA, cyclophilin A; SOD2, superoxide dismutase 2; GCLC,
glutamine-cysteine ligase catalytic subunit. |
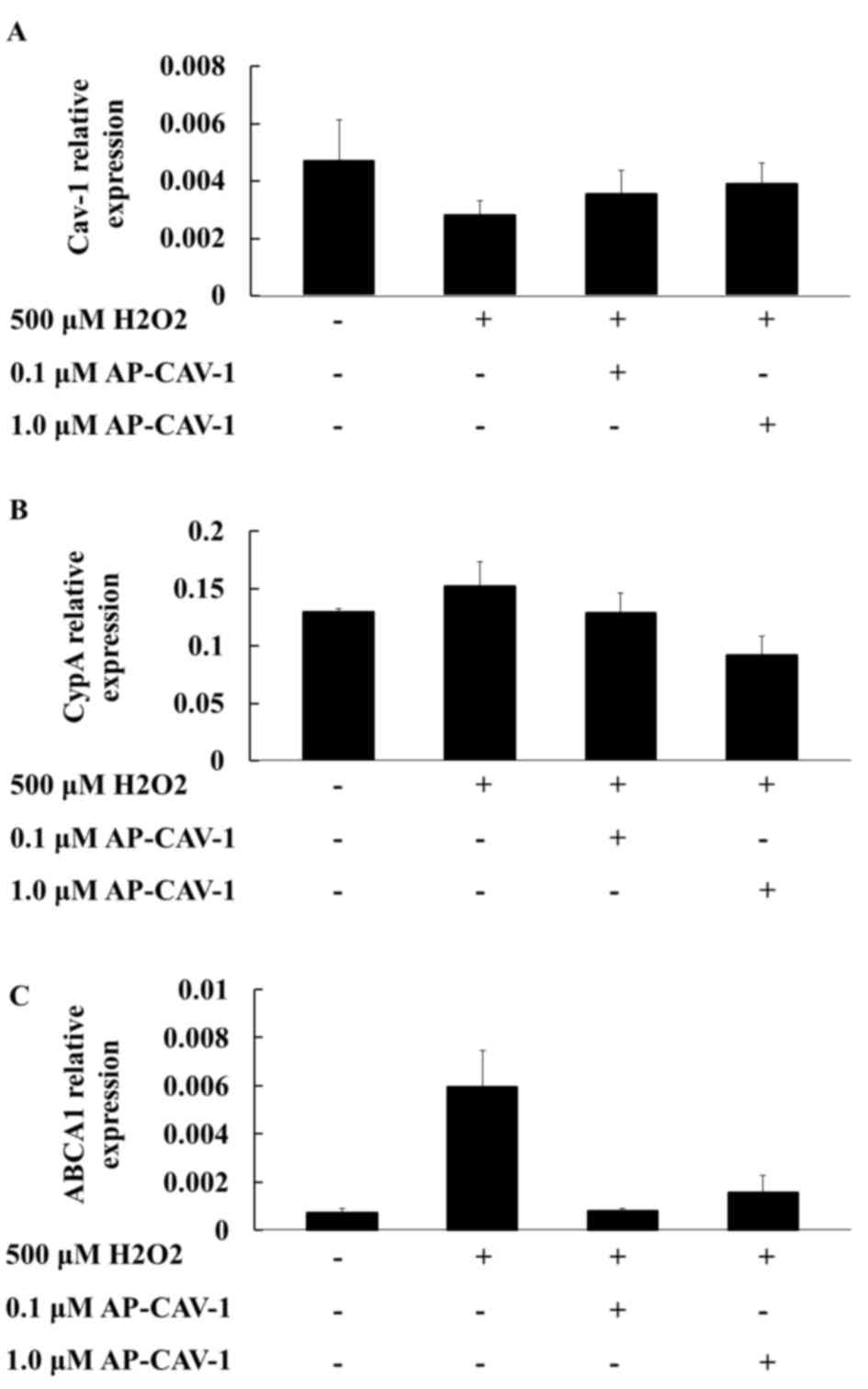
Effects of CAV-1 on the mRNA
expression levels of the CypA and ABCA1 genes
The present study examined whether AP-CAV-1
treatment exerted additional effects on the expression levels of
CAV-1 and CypA in H2O2-treated E11 cells.
RT-qPCR assay results revealed that the mRNA expression levels of
CAV-1 and CypA in the AP-CAV-1-treated group were significantly
elevated and diminished, respectively, compared with those of the
H2O2-treated group (Figs. 2A and B, respectively). The
quantification of the results is presented in Table II (CAV-1, P=0.018; CypA,
P<0.001). Furthermore, the ABCA1 mRNA levels in the E11
podocytes were significantly higher in the
H2O2-treated group compared with the control
group. In the AP-CAV-1-treated E11 cells, CAV-1 provided protection
from H2O2-associated damage and the change in
the ABCA1 mRNA expression level was significantly reduced (Fig. 2C). However, no significant
difference was observed for ABCA1 mRNA levels between
AP-CAV-1-treated groups. Overall, these results indicated that
CAV-1 diminished H2O2-induced E11 podocytes
injuries and prevented ABCA1 compensatory action from becoming
excessively active in the H2O2-treated E11
cells.
 | Table II.mRNA expression levels of CAV-1, CypA
and ABCA1 in the control and
AP-CAV-1-H2O2-treated E11 cells. |
Table II.
mRNA expression levels of CAV-1, CypA
and ABCA1 in the control and
AP-CAV-1-H2O2-treated E11 cells.
|
| CONa | 500 µM
H2O2b | 0.1 µM
AP-CAV-1c | 1.0 µM
AP-CAV-1d |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Gene | Mean | SD | Mean | SD | Mean | SD | Mean | SD | P-value | Post Hoc Tests |
|---|
| CAV-1 | 0.0047 | 0.0014 | 0.0028 | 0.0005 | 0.0035 | 0.0008 | 0.0039 | 0.0007 | 0.018 | a>b |
| CypA | 0.1296 | 0.0031 | 0.1519 | 0.0215 | 0.1288 | 0.0170 | 0.0920 | 0.0163 | <0.001 | a,b,c>d |
| ABCA1 | 0.0007 | 0.0005 | 0.0059 | 0.0092 | 0.0008 | 0.0003 | 0.0016 | 0.0021 | 0.515 |
|
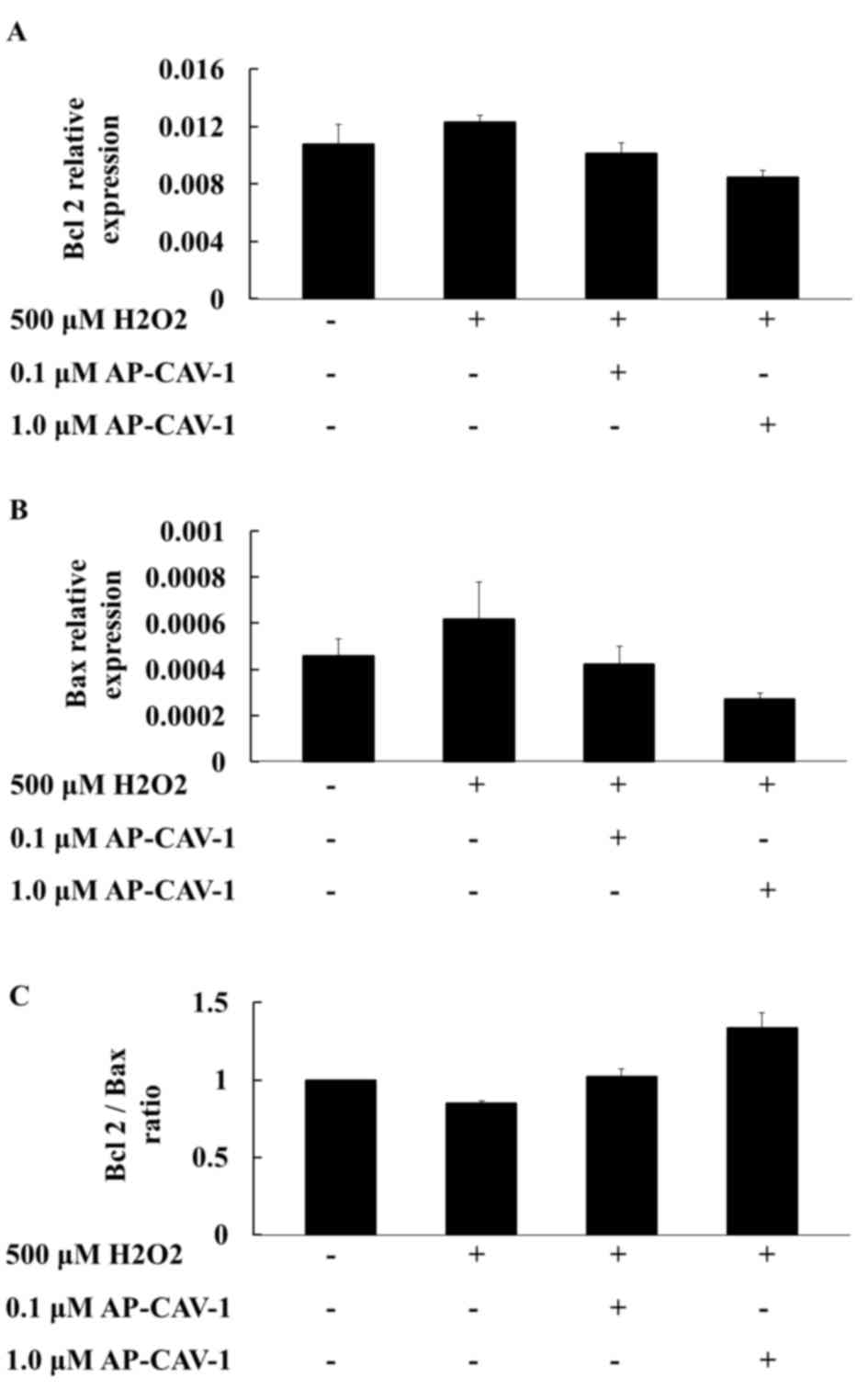
Effects of CAV-1 on
apoptosis-associated gene mRNA expression levels
To investigate whether CAV-1 activity affects cell
survival, the present study examined the expression of
apoptosis-associated gene mRNA expression levels in E11 cells by
RT-qPCR. A higher compensatory mRNA level of Bcl2 was observed in
the H2O2-treated E11 cells. CAV-1 treatment
significantly suppressed Bcl2 mRNA expression in a dose-dependent
manner (Fig. 3A). The Bax mRNA
expression levels were more markedly diminished in the
CAV-1-treated groups than in the H2O2-treated
group (Fig. 3B). The
quantification of the results is presented in Table III. The Bcl2/Bax ratios were also
higher in the CAV-1 groups compared with the
H2O2-treated group. CAV-1 provided podocytes
with resistance to apoptotic stimuli (Fig. 3C). These results suggested that
CAV-1 may prevent apoptotic cell death in E11 podocytes.
 | Table III.mRNA expression levels of Bcl2 and
Bax in the control and AP-CAV-1-H2O2-treated
E11 cells. |
Table III.
mRNA expression levels of Bcl2 and
Bax in the control and AP-CAV-1-H2O2-treated
E11 cells.
|
| CONa | 500 µM
H2O2b | 0.1 µM
AP-CAV-1c | 1.0 µM
AP-CAV-1d |
|
|---|
|
|
|
|
|
|
|
|---|
| Gene | Mean | SD | Mean | SD | Mean | SD | Mean | SD | P-value |
|---|
| Bcl2 | 0.0108 | 0.0041 | 0.0123 | 0.0014 | 0.0101 | 0.0023 | 0.0085 | 0.0015 | 0.388 |
| Bax | 0.0005 | 0.0002 | 0.0006 | 0.0005 | 0.0004 | 0.0002 | 0.0003 | 0.0001 | 0.585 |
Effects of CAV-1 on
H2O2-induced changes
To determine whether AP-CAV-1 treatment was
associated with a local decrease in oxidative stress, the present
study measured the expression levels of the antioxidant enzymes
GCLC, catalase and SOD2 in E11 podocytes. A significant elevation
of three markers was observed in the AP-CAV-1-treated E11 cells
(Fig. 4; P<0.001). The
expressions of the pro-inflammatory markers CypA and CAV-1 were
significantly lower and higher, respectively, in the
AP-CAV-1-treated groups compared with the
H2O2-treated groups (Fig. 4; P<0.001). Thus, AP-CAV-1 may
have increased antioxidant enzyme activity and attenuated local
oxidative damage in the CAV-1-treated E11 cells.
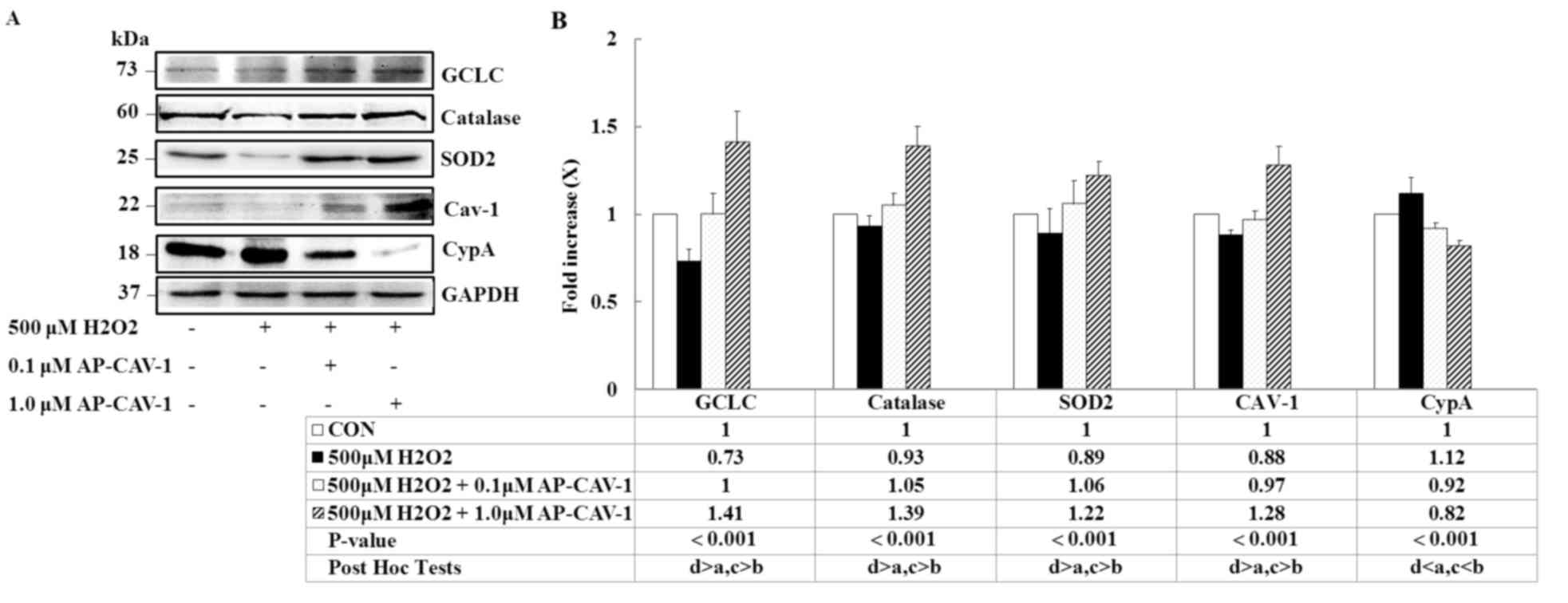 | Figure 4.AP-CAV-1 reverses
H2O2-reduced antioxidant-associated protein
and CAV-1 expression, and prevents CypA expression. E11 cells were
pretreated with 500 µM H2O2 for 1 h, followed
by treatment with the indicated concentration of AP-CAV-1 peptide
for an additional 48 h. (A) Representative western blot images and
(B) quantification of GCLC, catalase, SOD2, CAV-1 and CypA protein
expression levels. GAPDH served as a loading control. Data are
presented as the mean ± standard deviation of three independent
experiments. a: CON group, b: 500 µM H2O2
group, c: 500 µM H2O2 + 0.1 µM AP-CAV-1
group, d: 500 µM H2O2 + 1.0 µM AP-CAV-1
group. CAV-1, caveolin-1; CypA, cyclophilin A; AP-CAV-1,
antenapedia-caveolin-1; SOD2, superoxide dismutase 2; GCLC,
glutamine-cysteine ligase catalytic subunit; CON, control. |
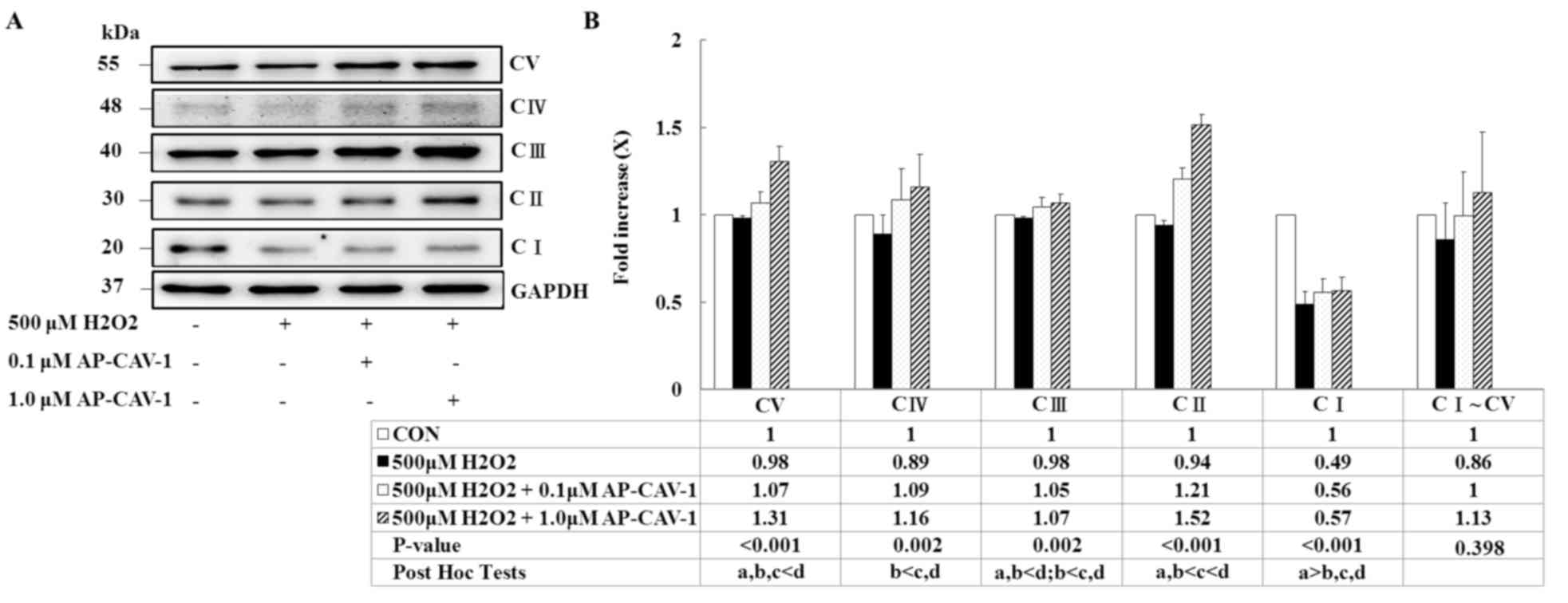
CAV-1 preserves mitochondrial
respiratory function by upregulating OXPHOS expression
The present study used western blot analysis to
examine mitochondrial OXPHOS complexes because they directly affect
mitochondrial function and antioxidative capacity. The expression
levels of the ATP synthase α-subunit (complex V), cytochrome c
oxidase subunit 1 (MTCO1; complex IV), core 2 protein (complex
III), Succinate dehydrogenase [ubiquinone] iron-sulfur subunit,
mitochondrial (SDHB; complex II) and NADH dehydrogenase
[ubiquinone] 1 β subcomplex subunit 8 (NDUFB8; complex I) were
significantly enhanced in a dose-dependent manner in the
AP-CAV-1-treated groups compared with the
H2O2-treated-group (P<0.001 vs. P=0.002).
AP-CAV-1 treatment resulted in a significantly increased expression
of electron transport chain complex I–V protein, suggesting that
CAV-1 treatment preserves mitochondrial respiratory function in
H2O2-treated E11 podocytes (Fig. 5).
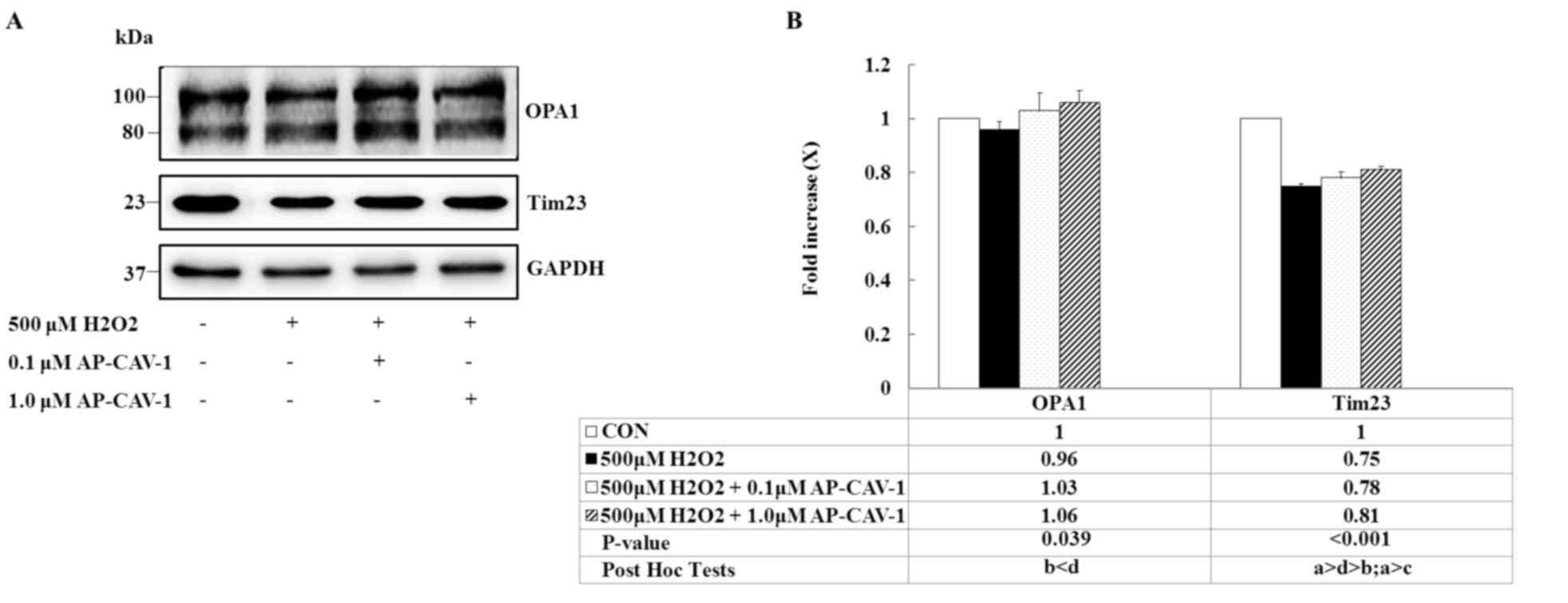
CAV-1 prevents OPA1 and Tim23
degradation
The present study determined whether OPA1 and Tim23
expression levels that are upregulated by CAV-1 treatment prevent
H2O2-induced apoptosis in E11 podocytes. OPA1
is a protein required for inner mitochondrial membrane fusion.
H2O2 exposure decreased the intensity levels
of both bands, whereas OPA1 levels markedly increased after CAV-1
treatment. Similarly, CAV-1 prevented a substantial decrease in the
expression of the inner-membrane protein Tim23 from being induced
by the H2O2 insult (Fig. 6, P=0.039 and P<0.001,
respectively). These data suggested that CAV-1 prevents the
mitochondrial fusion mechanism from undergoing OPA1 deregulation,
and preserves the integrity of Tim23 content, mediating the
translocation of proteins into the mitochondrial matrix in response
to in vitro H2O2-induced toxicity.
Discussion
The present study demonstrated that
H2O2 induces reactive oxygen species (ROS)
production, oxidative stress, inflammation, cell apoptosis and
mitochondrial dysfunction in podocytes, and that treatment with
CAV-1 prevents H2O2-induced ROS production,
oxidative stress, inflammation, cell apoptosis and mitochondrial
dysfunction, indicating that CAV-1 functions as a positive
regulator in podocyte injury.
ROS overexpression has been observed in glomerular
endothelial and epithelial cells and has been demonstrated to
disrupt normal glomerular permselectivity, thus leading to
proteinuria (21,22). The inhibition of ROS generation
through the use of NADPH oxidase inhibitors,
renin-angiotensin-aldosterone system inhibitors, statins,
antidiabetic drugs and antioxidant vitamins may ameliorate the
renal damage caused by diabetic nephropathy (5,6).
CAV-1 has been associated with oxidative regulating pathways. Chen
et al (23) demonstrated
that CAV-1 is a negative regulator of NADPH oxidase-induced ROS in
endothelial cells, and Sun et al (24) demonstrated that CAV-1 significantly
reduces ROS production and apoptosis in podocytes. Similarly, the
results of the present study revealed that increased CAV-1
expression not only promotes GCLC, SOD2 and catalase expression to
increase antioxidant defensive capacity, but also downregulates the
Bcl2/Bax expression ratio, thus preventing podocyte cell death.
However, Volonte et al (25) revealed that the CAV-1 interaction
with nuclear factor erythroid 2-related factor 2-GCLC proteins
negatively regulated antioxidant defenses in fibroblasts. Hence,
the differences observed in the roles of CAV-1 in oxidative
regulating pathways are cell-type specific.
Crucially, the CAV-1 expression level was markedly
affected by the addition of H2O2 to the
culture medium; the CAV-1 level decreased by nearly 50–70%.
Similarly, previous studies demonstrated that the expression of
CAV-1 is significantly decreased after H2O2
treatment in cardiomyocytes and skeletal muscle cells (26,27).
Several studies have suggested that the rapid degradation of CAV-1
protein could be caused by the ubiquitin-proteasome pathway,
particularly after oxidative injury (28,29).
The present study revealed that H2O2 had a
reversible effect on CAV-1 expression when podocyte cells were
incubated with an AP-CAV-1 peptide, and that CAV-1 protein
degradation could then trigger cellular mitochondrial function and
antioxidant defense.
In conclusion, the results of the present study
demonstrated that CAV-1 provides protection against the
H2O2-induced oxidative stress response, as
demonstrated by an increase in the activity of the antioxidant
enzymes GCLC, SOD2 and catalase. CAV-1 also attenuated the
expression of the proinflammatory marker CypA, altered Bcl2/Bax
mRNA expression levels, suppressed apoptotic cell death and
preserved mitochondrial functions such as upregulated OXPHOS, OPA-1
and Tim23 protein expression levels. Therefore, targeting enhanced
CAV-1 expression levels in podocyte injury may have potential as a
therapeutic strategy for the treatment of glomerular injury.
Acknowledgements
The present study was supported by the Changhua
Christian Hospital (grant. no. 105-CCH-IRP-133).
References
|
1
|
Pavenstädt H, Kriz W and Kretzler M: Cell
biology of the glomerular podocyte. Physiol Rev. 83:253–307. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nagata M: Podocyte injury and its
consequences. Kidney Int. 89:1221–1230. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Asanuma K and Mundel P: The role of
podocytes in glomerular pathobiology. Clin Exp Nephrol. 7:255–259.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ronconi E, Mazzinghi B, Sagrinati C,
Angelotti ML, Ballerini L, Parente E, Romagnani P, Lazzeri E and
Lasagni L: The role of podocyte damage in the pathogenesis of
glomerulosclerosis and possible repair mechanisms. G Ital Nefrol.
26:660–669. 2009.PubMed/NCBI
|
|
5
|
Kashihara N, Haruna Y, Kondeti VK and
Kanwar YS: Oxidative stress in diabetic nephropathy. Curr Med Chem.
17:4256–4269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bhatti AB and Usman M: Drug Targets for
Oxidative Podocyte Injury in Diabetic Nephropathy. Cureus.
7:e3932015.PubMed/NCBI
|
|
7
|
Mallipattu SK and He JC: The podocyte as a
direct target for treatment of glomerular disease? Am J Physiol
Renal Physiol. 311:F46–F51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Panneerselvam M, Patel HH and Roth DM:
Caveolins and Heart DiseasesCaveolins and Caveolae: Roles in
Signaling and Disease Mechanisms. Jasmin JF, Frank PG and Lisanti
MP: Springer US; New York, NY: pp. 145–156. 2012, View Article : Google Scholar
|
|
9
|
Frank PG, Pavlides S, Cheung MW, Daumer K
and Lisanti MP: Role of caveolin-1 in the regulation of lipoprotein
metabolism. Am J Physiol Cell Physio. 295:C242–C248. 2008.
View Article : Google Scholar
|
|
10
|
Mercier I, Jasmin JF, Pavlides S, Minetti
C, Flomenberg N, Pestell RG, Frank PG, Sotgia F and Lisanti MP:
Clinical and translational implications of the caveolin gene
family: Lessons from mouse models and human genetic disorders. Lab
Invest. 89:614–623. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Trimmer C, Sotgia F, Whitaker-Menezes D,
Balliet RM, Eaton G, Martinez-Outschoorn UE, Pavlides S, Howell A,
Iozzo RV, Pestell RG, et al: Caveolin-1 and mitochondrial SOD2
(MnSOD) function as tumor suppressors in the stromal
microenvironment: A new genetically tractable model for human
cancer associated fibroblasts. Cancer Biol Ther. 11:383–394. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bosch M, Marí M, Herms A, Fernández A,
Fajardo A, Kassan A, Giralt A, Colell A, Balgoma D, Barbero E, et
al: Caveolin-1 deficiency causes cholesterol-dependent
mitochondrial dysfunction and apoptotic susceptibility. Curr Biol.
21:681–686. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sörensson J, Fierlbeck W, Heider T,
Schwarz K, Park DS, Mundel P, Lisanti M and Ballermann BJ:
Glomerular endothelial fenestrae in vivo are not formed from
caveolae. J Am Soc Nephrol. 13:2639–2647. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ostalska-Nowicka D, Nowicki M, Zachwieja
J, Kasper M and Witt M: The significance of caveolin-1 expression
in parietal epithelial cells of Bowman's capsule. Histopathology.
51:611–621. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ren Z, Liang W, Chen C, Yang H, Singhal PC
and Ding G: Angiotensin II induces nephrin dephosphorylation and
podocyte injury: Role of caveolin-1. Cell Signal. 24:443–450. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang L, Ren Z, Yang Q and Ding G: Csk
regulates angiotensin II-induced podocyte apoptosis. Apoptosis.
21:846–855. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wan X, Chen Z, Choi WI, Gee HY,
Hildebrandt F and Zhou W: Loss of epithelial membrane protein 2
aggravates podocyte injury via upregulation of caveolin-1. J Am Soc
Nephrol. 27:1066–1075. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Derossi D, Calvet S, Trembleau A,
Brunissen A, Chassaing G and Prochiantz A: Cell internalization of
the third helix of the Antennapedia homeodomain is
receptor-independent. J Biol Chem. 271:18188–11893. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bucci M, Gratton JP, Rudic RD, Acevedo L,
Roviezzo F, Cirino G and Sessa WC: In vivo delivery of the
caveolin-1 scaffolding domain inhibits nitric oxide synthesis and
reduces inflammation. Nat Med. 6:1362–1367. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nath KA, Fischereder M and Hostetter TH:
The role of oxidants in progressive renal injury. Kidney Int Suppl.
45:S111–S115. 1994.PubMed/NCBI
|
|
22
|
Johnson RJ, Lovett D, Lehrer RI, Couser WG
and Klebanoff SJ: Role of oxidants and protease in glomerular
injury. Kidney Int. 45:352–359. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen F, Barman S, Yu Y, Haigh S, Wang Y,
Black SM, Rafikov R, Dou H, Bagi Z, Han W, et al: Caveolin-1 is a
negative regulator of NADPH oxidase-derived reactive oxygen
species. Free Radic Biol Med. 73:201–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun LN, Liu XC, Chen XJ, Guan GJ and Liu
G: Curcumin attenuates high glucose-induced podocyte apoptosis by
regulating functional connections between caveolin-1
phosphorylation and ROS. Acta Pharmacol Sin. 37:645–655. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Volonte D, Liu Z, Musille PM, Stoppani E,
Wakabayashi N, Di YP, Lisanti MP, Kensler TW and Galbiati F:
Inhibition of nuclear factor-erythroid 2-related factor (Nrf2) by
caveolin-1 promotes stress-induced premature senescence. Mol Biol
Cell. 24:1852–1862. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hsieh SR, Hsu CS, Lu CH, Chen WC, Chiu CH
and Liou YM: Epigallocatechin-3-gallate-mediated cardioprotection
by Akt/GSK-3β/caveolin signalling in H9c2 rat cardiomyoblasts. J
Biomed Sci. 20:862013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mougeolle A, Poussard S, Decossas M,
Lamaze C, Lambert O and Dargelos E: Oxidative stress induces
caveolin 1 degradation and impairs caveolae functions in skeletal
muscle cells. PLoS One. 10:e01226542015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shang F and Taylor A: Ubiquitin-proteasome
pathway and cellular responses to oxidative stress. Free Radic Biol
Med. 51:5–16. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pickering AM and Davies KJ: Degradation of
damaged proteins: The main function of the 20S proteasome. Prog Mol
Biol Transl Sci. 109:227–248. 2012. View Article : Google Scholar : PubMed/NCBI
|