Introduction
Osteosarcoma (OS) is the most common malignancy of
bone in early adolescence (1).
Conventional OS, also termed classical OS, is a common type of OS
and is universally life-threatening due to its rapid growth, high
local aggression and metastatic potential (2). During previous years, considerable
progress has been made in identifying the key components in
conventional OS, including genes, pathways and microRNAs (miRNAs).
For example, during osteoblast differentiation, miRNA (miR)-34 is
significantly induced by bone morphogenetic protein 2, and
regulates multiple components of the Notch signalling pathway,
including Notch1, Notch2 and jagged 1, which affects osteoclast
differentiation. This regulatory association may be closely
associated with the pathogenesis of OS (3). In addition, phosphatase and tensin
homolog (PTEN) has been found to be a potent regulator of the
phosphatidylinositol 3-kinase (PI3K) /serine-threonine kinase (Akt)
pathway (4), and the loss of
PTEN is a common occurrence in conventional OS (5). A previous study has showed that the
expression of PTEN can be inhibited by miR-221, which
potentiates the PI3K/Akt pathway in the conventional pathogenesis
of OS (6). PTEN is also a
target of miR-92a, and of members of the miR-17 and miR-130/301
families in OS (7).
In 2010, using genome-wide microarrays,
Fritsche-Guenther et al (8)
found that the aberrant expression of ephrin receptor A2 (EphA2)
and its ligand, EFNA1 in OS can modulate the activation of the
mitogen-activated protein kinase (MAPK) pathway. In addition, it
was found that the expression of CD52 was higher in OS metastases
compared with conventional OS metastases, and CAMPATH-1H, an
antibody directed against CD52, reduced the number of viable OS
cells (9). In 2013, Luo et
al (10) found numerous
differentially expressed genes (DEGs) and regulatory associations
between transcription factors and DEGs in OS using the microarray
data deposited by Fritsche-Guenther et al For example,
interleukin 6 can be regulated by tumour protein p53 (TP53),
nuclear factor I/C (CCAAT-binding transcription factor), retinoic
acid receptor α, and CCAAT/enhancer binding protein β. In 2014,
Yang et al (11) also
identified a number of DEGs, Gene Ontology (GO) terms, including
protein binding, and significant pathways, including focal
adhesion, in OS based on a meta-analysis of eight expression
profiles, including the one deposited by Fritsche-Guenther
(8). However, in these previous
studies, the potential miRNAs and regulatory associations between
miRNAs and DEGs in OS were not examined.
In the present study, to screen and identify
additional DEGs and miRNAs in conventional OS, the microarray data
deposited by Fritsche-Guenther (8)
were downloaded. Following GO and pathway enrichment analyses, and
construction of a protein-protein interaction (PPI) network for the
DEGs, the potential miRNAs in the most significant pathway for the
upregulated DEGs were identified, and a regulatory network for the
miRNAs-DEGs was constructed. The results were expected to assist in
elucidating the aetiology of conventional OS, and provide more
information to assist in the clinical diagnosis and treatment of
this disease.
Materials and methods
Affymetrix microarray data
The GSE14359 (8)
gene expression profile data were acquired from the Gene Expression
Omnibus (http://www.ncbi.nlm.nih.gov/geo/), which was based on
the platform of the GPL96 [HG-U133A] Affymetrix Human Genome U133A
Array. This dataset contains 10 conventional OS samples from the
femur or tibia (two replicates each) from five consenting patients
with grade 2–3 conventional OS between 7 and 74 years of age; eight
OS lung metastasis tumour samples (two replicates each) from four
consenting patients with a grade 1–3 OS lung metastatic tumour; and
two non-neoplastic primary osteoblast cell samples with limited
life span in vitro from one patient (two replicates). These
10 conventional OS samples and two non-neoplastic primary
osteoblast samples were selected for further analysis.
The CEL files and probe annotation files were
downloaded, and the gene expression data of all samples were
preprocessed via background correction, quantile normalization and
probe summarization using the Gene Chip Robust Multi Array
algorithm (12) in the Affy
software package (version 1.32.0; http://www.bioconductor.org/packages/release/bioc/html/affy.html)
(13).
DEG screening
The Linear Models for Microarray Data package
(version 3.10.3; http://www.bioconductor.org/packages/2.9/bioc/html/limma.html)
(14) of R was used to identify
genes, which were significantly differentially expressed in the
conventional OS samples. The raw P-value was adjusted by the
Benjamin and Hochberg method (15), and only genes meeting the cut-off
criteria of |log2 fold-change|>1 and adjusted
P<0.01 were selected as DEGs.
Hierarchical clustering analysis of
the DEGs
Hierarchical clustering is a common method used to
determine clusters of similar data points in multidimensional
spaces (16). The pheatmap package
(version 1.08; https://cran.r-project.org/web/packages/pheatmap/)
(17) was used to perform
hierarchical clustering via joint between-within distances for the
DEGs in the conventional OS and non-neoplastic primary osteoblasts
samples.
GO and pathway enrichment
analyses
The Database for Annotation, Visualization and
Integrated Discovery (DAVID) provides a set of comprehensive
functional annotation tools, which can be used to identify the
biological meanings of abundant genes (18). P<0.01 was used as the cut-off
criterion for GO and Kyoto Encyclopedia of Genes and Genomes
pathway enrichment analysis using DAVID (version 6.7; https://david-d.ncifcrf.gov/), based on the
hypergeometric distribution algorithm.
PPI network construction
The Search Tool for the Retrieval of Interacting
Genes database (version 10.0; http://string-db.org/), which provides experimental
and predicted interaction information (19), was used to analyse the PPIs for
DEGs by calculating the combined score, and a score >0.4 was
selected as the cut-off criterion. Subsequently, the PPI network of
the upregulated and downregulated DEGs was visualized using
Cytoscape (version 3.2.0; http://cytoscape.org/) (20).
Screening and analysis of network
modules
The network modules were obtained based on Molecular
Complex Detection (MCODE) analysis (21) of the original PPI networks. The
default parameters (degree cut-off, 2; node score cut-off, 0.2;
K-core, 2) were used as the cut-off criteria for the network module
screening.
In order to obtain further information on the gene
functions and identify pathways closely associated with the DEGs,
functional annotation analysis and subsequent pathway enrichment
analysis of the network module with the highest MCODE score were
performed using DAVID, with a P<0.01 cut-off.
Integrated miRNA-DEG regulatory
network construction
The potential miRNAs for upregulated DEGs in the
most significant pathway were predicted using the miRDB database
(version 1.24.0; http://www.bioconductor.org/packages/2.8/bioc/html/maDB.html)
(22), with a cut-off for the
target score of ≥60. The binding sites of miRNAs in the human mRNAs
> 800 were abandoned. The integrated miRNA-DEG regulatory
network was then visualised with Cytoscape.
Results
Identification of DEGs
Following the data preprocessing, 11,107 probes were
obtained. Based on the cut-off criteria, a total of 987 DEGs were
screened from the conventional OS samples, including 317
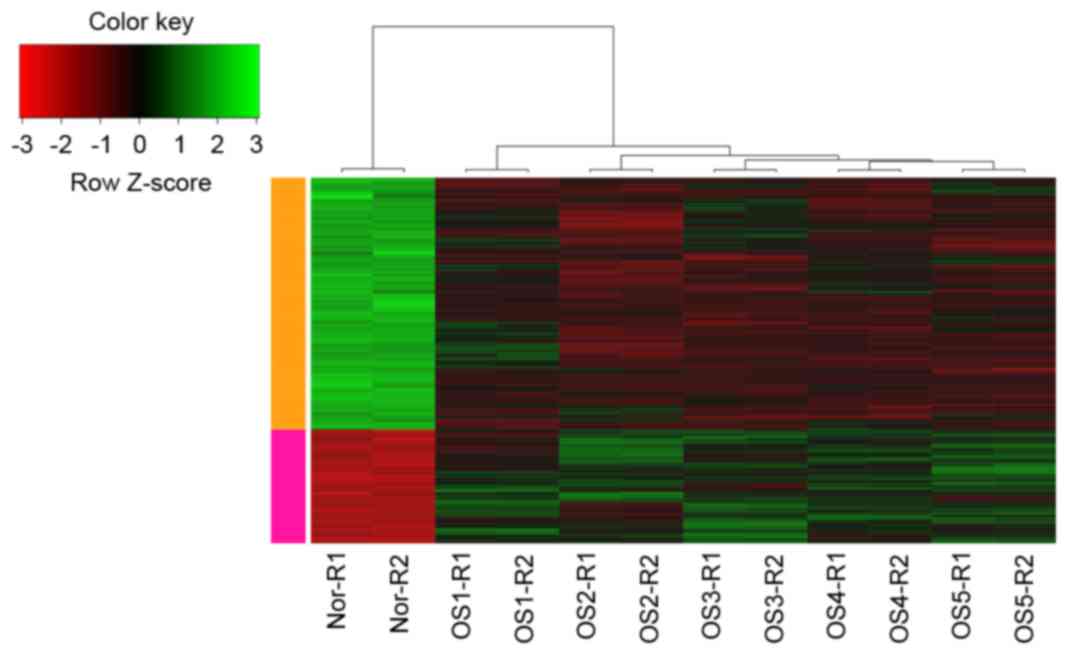
upregulated genes and 670 downregulated genes. The hierarchical
cluster analysis of the data revealed that it was possible to use
the DEGs to accurately distinguish the conventional OS samples from
the non-neoplastic primary osteoblast cell samples (Fig. 1).
Enrichment analysis of upregulated and
downregulated DEGs
According to the GO functional annotation, the
upregulated DEGs were predominantly enriched in GO terms associated
with DNA replication, including MCM3, replication factor C
(RFC)5, replication protein A3 (RPA3) and flap
endonuclease 1 (FEN1), and cell cycle, including
cyclin-dependent kinase 1 (CDK1), NDC80 kinetochore complex
(NDC80), BUB1 mitotic checkpoint serine/threonine-protein
kinase (BUB1) and mitotic arrest deficient 2 like 1
(MAD2L1). A number of downregulated DEGs, including caveolin
1 (CAV1), cadherin 13 (CDH13), vascular endothelial
growth factor C (VEGFC) and transforming growth factor β
receptor 3 (TGFBR3), were relevant to blood vessel
development, whereas epidermal growth factor receptor
(EGFR), TP53, VEGFB and MAPK1 were
associated with the regulation of cell proliferation (Table IA).
 | Table I.GO terms and pathways enriched for
the upregulated and downregulated DEGs. |
Table I.
GO terms and pathways enriched for
the upregulated and downregulated DEGs.
| A, Top 10 GO terms
for the upregulated and downregulated DEGs |
|---|
|
|---|
| Category | Term | Description | P-value | n | Genes |
|---|
| Up | GO:0006259 | DNA metabolic
process | 8.09E-13 | 38 | RPA3,
FANCL, FEN1, DTL, CENPF, MCM3,
RFC5, RFC3, RFC4, RFC2 |
|
| GO:0006260 | DNA
replication | 3.90E-10 | 21 | DTL,
CENPF, MCM3, RPA3, RFC5, RFC3,
RFC4, RFC2, RRM2, FEN1 |
|
| GO:0022403 | Cell cycle
phase | 8.15E-10 | 30 | AURKA,
NCAPG, BUB1, CDK1, KIF11,
DLGAP5, CENPF, BIRC5, NDC80,
MAD2L1 |
|
| GO:0000278 | Mitotic cell
cycle | 1.34E-09 | 28 | NCAPG,
BUB1, CDK1, KIF11, CENPF, BIRC5,
NDC80, CDKN3, MAD2L1, ZWINT |
|
| GO:0051301 | Cell division | 1.36E-09 | 25 | NCAPG,
BUB1, ASPM, CDK1, KIF11, CENPF,
BIRC5, NDC80, MCM5, MAD2L1 |
|
| GO:0000280 | Nuclear
division | 5.16E-09 | 21 | CDK1,
KIF11, CENPF, CDC23, NDC80,
BIRC5, SMC4, MAD2L1, NCAPG,
BUB1 |
|
| GO:0007067 | Mitosis | 5.16E-09 | 21 | CDK1,
KIF11, CENPF, CDC23, NDC80,
BIRC5, SMC4, MAD2L1, NCAPG,
BUB1 |
|
| GO:0000087 | M phase of mitotic
cell cycle | 7.03E-09 | 21 | CDK1,
KIF11, CENPF, CDC23, NDC80,
BIRC5, SMC4, MAD2L1, NCAPG,
BUB1 |
|
| GO:0048285 | Organelle
fission | 1.03E-08 | 21 | CDK1,
KIF11, CENPF, CDC23, NDC80,
BIRC5, SMC4, MAD2L1, NCAPG,
BUB1 |
|
| GO:0000279 | M phase | 1.18E-08 | 25 | NCAPG,
BUB1, CDK1, KIF11, DLGAP5,
CDC23, CENPF, BIRC5, NDC80,
MAD2L1 |
| Down | GO:0001568 | Blood vessel
development | 1.57E-06 | 28 | CAV1,
THBS1, MMP14, PNPLA6, CDH13,
VEGFC, NTRK2, TGFBR3, ENG,
TNFAIP2 |
|
| GO:0042127 | Regulation of cell
proliferation | 1.63E-06 | 60 | EGFR,
CTBP1, TP53, MFGE8, HOXC10,
VEGFB, MAPK1, VEGFC, SMAD3,
SMAD2 |
|
| GO:0007242 | Intracellular
signaling cascade | 2.08E-06 | 84 | RRAS,
TP53, CAV1, MAPKAPK3, FHL2,
TGFBR3, GRK5, ABL1, CRK,
IGFBP5 |
|
| GO:0010033 | Response to organic
substance | 2.28E-06 | 56 | TIMP3,
STAT6, SRR, PPP3CB, COL6A2,
SMAD2, CDH13, ADCY9, SMPD1,
TGFBR3 |
|
| GO:0051270 | Regulation of cell
motion | 2.47E-06 | 24 | SMAD3,
ACTN1, MAPK1, VEGFC, SEMA3F,
TGFBR3, RRAS, THBS1, IGFBP3,
IGFBP5 |
|
| GO:0001944 | Vasculature
development | 2.52E-06 | 28 | CAV1,
MYH9, MMP14, PNPLA6, CDH13,
VEGFC, NTRK2, TGFBR3, ENG,
TNFAIP2 |
|
| GO:0008285 | Negative regulation
of cell proliferation | 7.25E-06 | 34 | CAV1,
TP53, SMAD3, SMAD2, TGFBR3,
ADAMTS1, IGFBP3, ENG, IGFBP5,
TOB1 |
|
| GO:0040007 | Growth | 1.22E-05 | 22 | SMAD2,
LAMB2, NUPR1, DHCR7, SERPINE1,
TGFBR3, BIN3, ADD1, IGFBP5,
ERCC2 |
|
| GO:0030334 | Regulation of cell
migration | 1.24E-05 | 21 | EGFR,
SMAD3, MAPK1, VEGFC, PTP4A1,
RRAS, TGFBR3, THBS1, IGFBP3,
IGFBP5 |
|
| GO:0048514 | Blood vessel
morphogenesis | 3.44E-05 | 23 | CAV1,
CDH13, VEGFC, SEMA3C, PLCD1,
NR2F2, THBS1, TNFAIP2, ENG,
CYR61 |
|
| B, Pathways
enriched for the upregulated and downregulated DEGs |
|
| Category | Term | Description | P-value | n | Genes |
|
| Up | hsa03030 | DNA
replication | 1.50E-06 | 9 | RFC5,
RFC3, RFC4, POLE3, RFC2, MCM3,
FEN1, MCM5, RPA3 |
|
| hsa04110 | Cell cycle | 1.77E-04 | 12 | CDC7,
CDK1, CDKN1B, MAD2L1, CREBBP,
BUB1, PRKDC, CDC23, MCM3,
SMC1A |
|
| hsa03040 | Spliceosome | 1.90E-04 | 12 | RBM22,
HNRNPA3, SF3B1, SNRPA1, MAGOH,
TRA2B, SNRNP200, LSM5, LSM3,
SNRPE |
|
| hsa03430 | Mismatch
repair | 1.96E-03 | 5 | RFC5,
RFC3, RFC4, RFC2, RPA3 |
|
| hsa03420 | Nucleotide excision
repair | 3.76E-03 | 6 | RFC5,
RFC3, RFC4, POLE3, RFC2,
RPA3 |
|
| hsa04114 | Oocyte meiosis | 4.63E-03 | 9 | CDK1,
MAD2L1, SLK, BUB1, CDC23, AURKA,
PPP1CC, SMC1A, PPP1CB |
|
| hsa03410 | Base excision
repair | 9.30E-03 | 5 | HMGB1,
POLE3, TDG, POLB, FEN1 |
| Down | hsa04510 | Focal adhesion | 4.08E-07 | 29 | CAV1,
COL6A1, THBS1, FN1, EGFR, FLNC,
VEGFB, LAMA2, MAPK1, VEGFC |
|
| hsa04142 | Lysosome | 1.74E-04 | 17 | SGSH,
CLN3, NAGLU, PLA2G15, CLTB,
PSAP, CTSA, GLB1, DNASE2,
LAMP1 |
|
| hsa04520 | Adherens
junction | 3.35E-04 | 13 | EGFR,
WASF3, SMAD3, SMAD2, CTNNA1,
TCF7L1, CSNK2A2, MAPK1, FYN,
MAPK3 |
|
| hsa04115 | p53 signaling
pathway | 4.26E-04 | 12 | CCND1,
TNFRSF10B, ZMAT3, SERPINE1, DDB2,
TP53, FAS, PERP, THBS1,
IGFBP3 |
|
| hsa05219 | Bladder cancer | 8.61E-04 | 9 | EGFR,
VEGFB, MAPK1, VEGFC, CCND1,
MAP2K2, MAPK3, TP53, THBS1 |
|
| hsa04540 | Gap junction | 1.28E-03 | 13 | ADCY3,
EGFR, GNAI2, MAP2K2, GNA11,
LPAR1, ITPR3, MAPK1, ADCY9,
CSNK1D |
|
| hsa00980 | Metabolism of
xenobiotics by cytochrome P450 | 2.44E-03 | 10 | GSTM1,
AKR1C3, GSTM2, AKR1C2, CYP1B1,
ADH5, GSTT1, EPHX1, AKR1C1,
ALDH3B1 |
|
| hsa05216 | Thyroid cancer | 2.48E-03 | 7 | MAPK1,
CCND1, MAP2K2, RXRA, MAPK3,
TP53, TCF7L1 |
|
| hsa05212 | Pancreatic
cancer | 2.57E-03 | 11 | EGFR,
VEGFB, MAPK1, VEGFC, CCND1,
RELA, MAPK3, TP53, SMAD3,
SMAD2 |
|
| hsa05220 | Chronic myeloid
leukemia | 3.49E-03 | 11 | MAPK1,
CTBP1, CCND1, MAP2K2, RELA,
MAPK3, TP53, SMAD3, BCL2L1,
ABL1 |
According to the results of the pathway enrichment
analysis, the upregulated DEGs were predominantly enriched in seven
pathways. In accordance with the GO term analysis, the DNA
replication pathway, including RFC2, RFC3,
RFC4 and RFC5, and cell cycle pathway, including
CDK1, minichromosome maintenance complex component 3
(MCM3) and BUB1, were also enriched in the
upregulated genes. The downregulated DEGs were predominantly
enriched in the focal adhesion, including CAV1, collagen
type VI α1 (COL6A1), thrombospondin 1 (THBS1) and
EGFR, and p53 signalling pathways, including TP53,
Fas cell surface death receptor (FAS) and TP53 apoptosis
effector (PERP), as shown in Table IB.
Construction and analysis of the PPI
network
The PPI network for the upregulated and
downregulated DEGs consisted of 442 pairs of PPIs. The degrees of
DEGs, including CDK1, MAD2L1, NDC80, non-SMC
condensin I complex subunit G (NCAPG), BUB1,
centromere protein F (CENPF) and kinesin family member 11
(KIF11), were >17 (Table
II), indicating that they were important genes in OS.
 | Table II.Differentially expressed genes with a
connectivity degree of ≥10 in the protein-protein interaction
network. |
Table II.
Differentially expressed genes with a
connectivity degree of ≥10 in the protein-protein interaction
network.
| ID | Degree |
|---|
| CDK1 | 29 |
| MAD2L1 | 23 |
| NDC80 | 20 |
| NCAPG | 20 |
| BUB1 | 19 |
| CENPF | 19 |
| KIF11 | 18 |
| DLGAP5 | 17 |
| CREBBP | 17 |
| BIRC5 | 17 |
| RFC4 | 16 |
| RRM2 | 16 |
| TP53 | 16 |
| AURKA | 16 |
| SF3A2 | 14 |
| ASPM | 14 |
| SNRPG | 13 |
| MAPK1 | 12 |
| HMMR | 11 |
| NUP107 | 11 |
| CDKN3 | 11 |
| PPP1CC | 11 |
| SRSF1 | 11 |
| RACGAP1 | 10 |
| NUP160 | 10 |
| ZWINT | 10 |
| SRSF3 | 10 |
Analysis of network modules
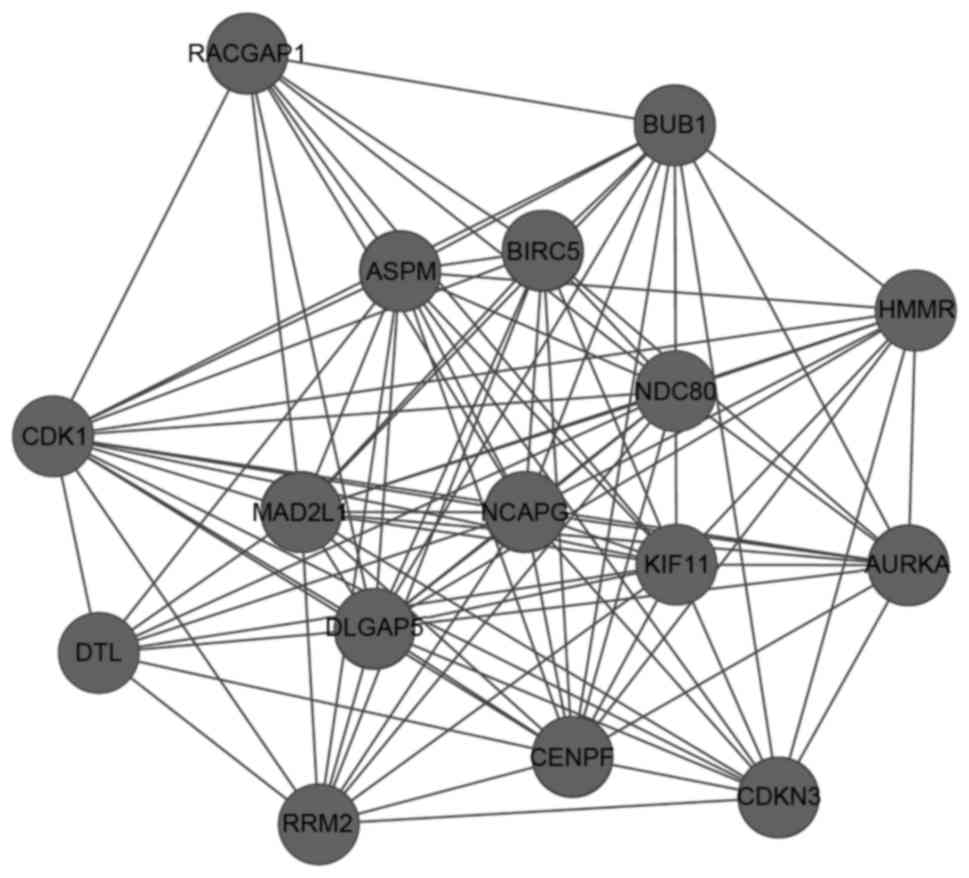
A total of 10 network modules were obtained from the
PPI network using the default criteria, and the module with the
highest score contained 16 nodes and 102 edges. In this module,
CDK1 interacted with other DEGs, including MAD2L1,
BUB1, NCAPG, NDC80 and CENPF (Fig. 2).
The functional enrichment analysis of the module
with the highest score showed that the majority of the DEGs in this
module were predominantly associated with the cell cycle. Certain
DEGs, including CDK1, MAD2L1, BUB1 and
NDC80, were implicated in mitosis and the M phase of mitotic
cell cycle; other DEGs, including Rac GTPase-activating protein 1
(RACGAP1) and MAD2L1, were correlated with cell cycle
process (Table IIIA).
CDK1, MAD2L1, BUB1 and aurora kinase A
(AURKA) were significantly enriched in the oocyte meiosis
pathway (Table IIIB).
 | Table III.GO terms and pathways enriched for
the DEGs in the most significant module. |
Table III.
GO terms and pathways enriched for
the DEGs in the most significant module.
| A, Top 10 GO terms
enriched for the DEGs in the most significant module |
|---|
|
|---|
| Term | Description | P-value | n | Genes |
|---|
| GO:0000280 | Nuclear
division | 9.92E-16 | 11 | CDK1,
MAD2L1, KIF11, NCAPG, DLGAP5, BUB1,
CENPF, BIRC5, NDC80, AURKA |
| GO:0007067 | Mitosis | 9.92E-16 | 11 | CDK1,
MAD2L1, KIF11, NCAPG, DLGAP5, BUB1,
CENPF, BIRC5, NDC80, AURKA |
| GO:0000087 | M phase of mitotic
cell cycle | 1.19E-15 | 11 | CDK1,
MAD2L1, KIF11, NCAPG, DLGAP5, BUB1,
CENPF, BIRC5, NDC80, AURKA |
| GO:0048285 | Organelle
fission | 1.49E-15 | 11 | CDK1,
MAD2L1, KIF11, NCAPG, DLGAP5, BUB1,
CENPF, BIRC5, NDC80, AURKA |
| GO:0000278 | Mitotic cell
cycle | 1.86E-15 | 12 | CDK1,
MAD2L1, KIF11, NCAPG, DLGAP5, BUB1,
CENPF, BIRC5, NDC80, AURKA |
| GO:0022402 | Cell cycle
process | 2.11E-15 | 13 | CDK1,
KIF11, DLGAP5, CENPF, AURKA, NDC80,
BIRC5, MAD2L1, NCAPG, BUB1 |
| GO:0022403 | Cell cycle
phase | 6.44E-15 | 12 | CDK1,
MAD2L1, KIF11, NCAPG, DLGAP5, BUB1,
BIRC5, NDC80, AURKA, ASPM |
| GO:0000279 | M phase | 5.81E-14 | 11 | CDK1,
MAD2L1, KIF11, NCAPG, DLGAP5, BUB1,
CENPF, BIRC5, NDC80, AURKA |
| GO:0007049 | Cell cycle | 9.57E-14 | 13 | CDK1,
KIF11, DLGAP5, CENPF, AURKA, NDC80,
BIRC5, MAD2L1, NCAPG, BUB1 |
| GO:0051301 | Cell division | 1.80E-12 | 10 | CDK1,
MAD2L1, KIF11, NCAPG, BUB1, CENPF,
BIRC5, NDC80, RACGAP1, ASPM |
|
| B, Pathways
enriched for the DEGs in the most significant module |
|
| Term | Description | P-value | n | Genes |
|
| hsa04114 | Oocyte meiosis | 1.88E-04 | 4 | CDK1,
MAD2L1, BUB1, AURKA |
| hsa04914 |
Progesterone-mediated oocyte
maturation | 4.06E-03 | 3 | CDK1,
MAD2L1, BUB1 |
| hsa04110 | Cell cycle | 8.43E-03 | 3 | CDK1,
MAD2L1, BUB1 |
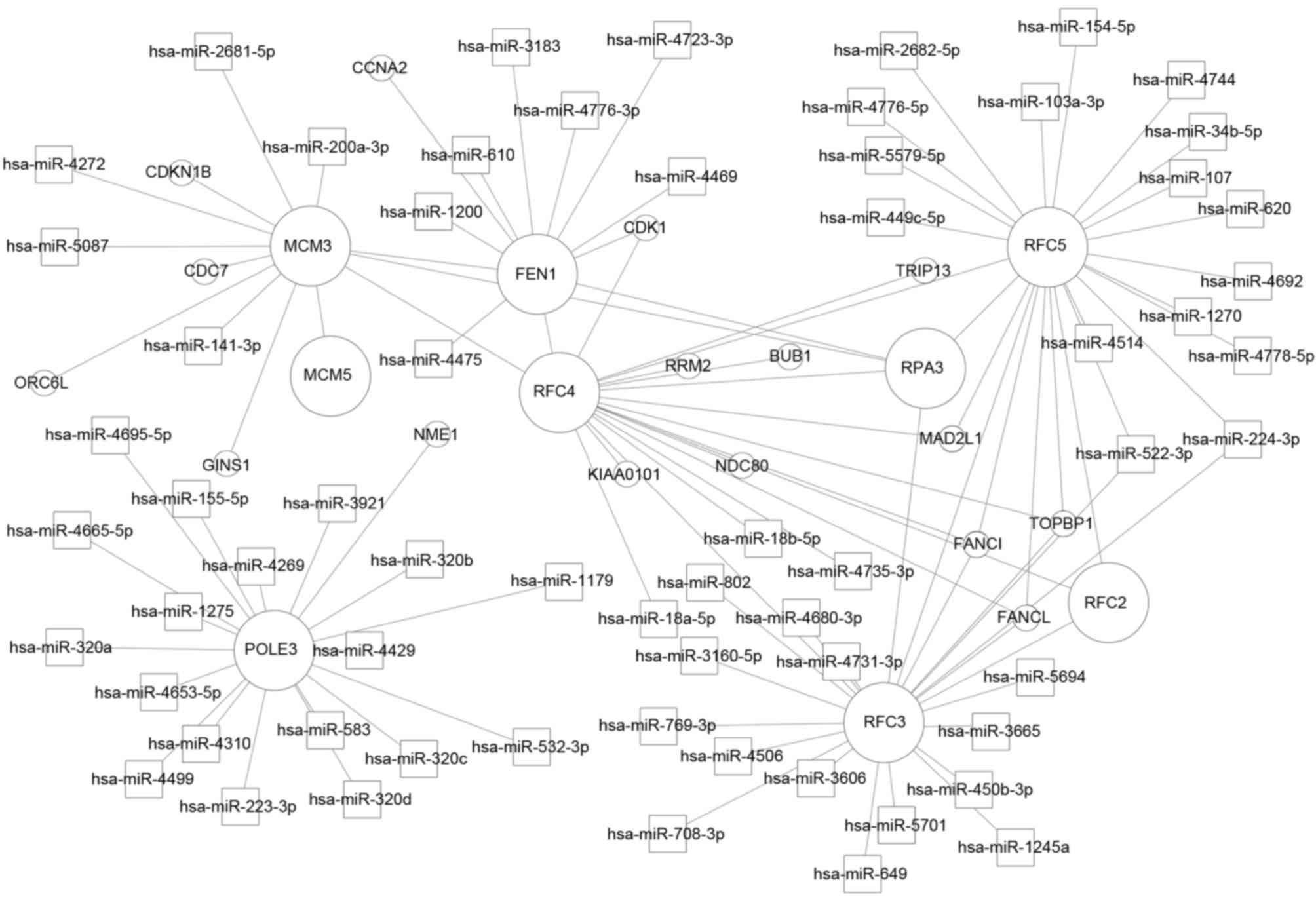
Analysis of the miRNA-DEG regulatory
network
The miRNA-DEG regulatory network contained 63
miRNAs, nine of their corresponding DEGs and 16 DEGs, which
interacted with these nine DEGs. DNA polymerase ζ subunit 3
(POLE3) was regulated by 18 miRNAs, including miR-4310,
miR-4680-3p, miR-583 and miR-4269; RFC3 was regulated by 16
miRNAs, including miR-802 and miR-649; RFC3 and RFC5
were modulated by miR-522-3p and miR-224-3p. In addition,
RFC2, RFC3, RFC4 and RFC5 interacted
with each other (Fig. 3).
Discussion
In the present study, 317 DEGs were found to be
significantly upregulated and 670 were significantly downregulated
in the conventional OS samples. A majority of the DEGs were
associated with cell cycle. According to the miRNA-DEG regulatory
network for the DEGs enriched in DNA replication, RFC2,
RFC3, RFC4 and RFC5 were found to interact
with each other.
RFC2, RFC3, RFC4 and
RFC5 encode members of RFC family, also termed activator 1.
These DEGs were enriched in DNA replication, which agreed with the
results of previous studies (23,24).
DNA replication is an essential event in tumour growth (25). The deregulation of protein
complexes involved in the initiation of DNA replication can lead to
cancer (26). Several DEGs in the
network module, including CDK1, MAD2L1, NDC80
and BUB1, which had higher degrees in the PPI network, were
found to interact with RFC4. These four DEGs were
predominantly enriched in cell mitosis and cell cycle. Alterations
in cell cycle regulation occur in several types of cancer,
including OS (27).
Cyclin-dependent kinase 1 (CDK1) is an important G2/M checkpoint
protein (28), and its inhibitor,
SCH 727965 (dinacliclib) can trigger the apoptosis of U-2 OS cells
(29). MAD2L1, BUB1
and NDC80 are involved in the spindle checkpoint pathway
(30,31). MAD2 has been reported to be
commonly overexpressed in human conventional OS (32), and BUB1 has been found to be
ectopically expressed in SAOS and U-2 OS cell lines (33). In addition, CDK1,
MAD2L1 and BUB1 have been found to be significantly
enriched in the pathway of oocyte meiosis, which was found to be
markedly altered in high-grade OS cell lines when compared with
osteoblasts (34). RFC3 was
also modulated by a cluster of miRNAs, including miR-802. The
expression of miR-802 has been reported to be upregulated in OS
tissues, and to promote cell proliferation by targeting p27 in U-2
OS and MG-63 cells (35).
RFC3 and RFC5 are also modulated by miR-224-3p and
miR-522-3p. There is no previous evidence indicating that
miR-224-3p and miR-522-3p are associated with conventional OS.
Therefore, miR-224-3p and miR-522-3p are predicted to be novel
biomarkers in conventional OS. Therefore, RFC2-5, together
with certain DEGs, including CDK1, MAD2L1,
NDC80 and BUB1, and a series of miRNAs, including
miR-802, miR-224-3p and miR-522-3p, may be responsible for the
initiation and development of conventional OS.
In conclusion, the present study found that the
majority of DEGs, including CDK1, MAD2L1,
NDC80 and BUB1, were associated with the cell cycle.
Other DEGs, including RFC2, RFC3, RFC4 and
RFC5, were associated with DNA replication. These, in
addition to a number of miRNAs, including miR-802, miR-224-3p and
miR-522-3p, may be essential in the pathogenesis of conventional
OS, providing novel information to assist in the clinical diagnosis
of this disease. However due to limitations in the present study,
additional experiments are required to shed light on the molecular
mechanisms involved in this life-threatening disease.
References
|
1
|
Messerschmitt PJ, Garcia RM, Abdul-Karim
FW, Greenfield EM and Getty PJ: Osteosarcoma. J Am Acad Orthop
Surg. 17:515–527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mohseny AB, Tieken C, Van Der Velden PA,
Szuhai K, de Andrea C, Hogendoorn PC and Cleton-Jansen AM: Small
deletions but not methylation underlie CDKN2A/p16 loss of
expression in conventional osteosarcoma. Genes Chromosomes Cancer.
49:1095–1103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bae Y, Yang T, Zeng HC, Campeau PM, Chen
Y, Bertin T, Dawson BC, Munivez E, Tao J and Lee BH: miRNA-34c
regulates Notch signaling during bone development. Hum Mol Genet.
21:2991–3000. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Silva A, Yunes JA, Cardoso BA, Martins LR,
Jotta PY, Abecasis M, Nowill AE, Leslie NR, Cardoso AA and Barata
JT: PTEN posttranslational inactivation and hyperactivation of the
PI3K/Akt pathway sustain primary T cell leukemia viability. J Clin
Invest. 118:3762–3774. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Freeman SS, Allen SW, Ganti R, Wu J, Ma J,
Su X, Neale G, Dome JS, Daw NC and Khoury JD: Copy number gains in
EGFR and copy number losses in PTEN are common events in
osteosarcoma tumors. Cancer. 113:1453–1461. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao G, Cai C, Yang T, Qiu X, Liao B, Li
W, Ji Z, Zhao J, Zhao H, Guo M, et al: MicroRNA-221 induces cell
survival and cisplatin resistance through PI3K/Akt pathway in human
osteosarcoma. PLoS One. 8:e539062013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Namløs HM, Meza-Zepeda LA, Barøy T,
Østensen IH, Kresse SH, Kuijjer ML, Serra M, Bürger H,
Cleton-Jansen AM and Myklebost O: Modulation of the osteosarcoma
expression phenotype by microRNAs. PLoS One. 7:e480862012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fritsche-Guenther R, Noske A, Ungethüm U,
Kuban RJ, Schlag PM, Tunn PU, Karle J, Krenn V, Dietel M and Sers
C: De novo expression of EphA2 in osteosarcoma modulates activation
of the mitogenic signalling pathway. Histopathology. 57:836–850.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fritsche-Guenther R, Gruetzkau A, Noske A,
Melcher I, Schaser KD, Schlag PM, Kasper HU, Krenn V and Sers C:
Therapeutic potential of CAMPATH-1H in skeletal tumours.
Histopathology. 57:851–861. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo Y, Deng Z and Chen J: Pivotal
regulatory network and genes in osteosarcoma. Arch Med Sci.
9:569–575. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang Z, Chen Y, Fu Y, Yang Y, Zhang Y,
Chen Y and Li D: Meta-analysis of differentially expressed genes in
osteosarcoma based on gene expression data. BMC Med Genet.
15:802014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu Z, Irizarry RA, Gentleman R,
Martinez-Murillo F and Spencer F: A model-based background
adjustment for oligonucleotide expression arrays. Journal of the
American Statistical Association. 99:909–917. 2004. View Article : Google Scholar
|
|
13
|
Seo J and Hoffman EP: Probe set
algorithms: Is there a rational best bet? BMC bioinformatics.
7:3952006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smyth GK: Linear models and empirical
bayes methods for assessing differential expression in microarray
experiments. Stat Appl Genet Mol Biol. 3:Article32004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Benjamini Y and Hochberg Y: Controlling
the false discovery rate: A practical and powerful approach to
multiple testing. Journal of the Royal Statistical Society Series B
(Methodological). 57:289–300. 1995.
|
|
16
|
Olson CF: Parallel algorithms for
hierarchical clustering. Parallel Computing. 21:1313–1325. 1995.
View Article : Google Scholar
|
|
17
|
Kolde R: pheatmap: Pretty Heatmaps. R
package. version 0.7. 7. 2012.
|
|
18
|
Huang DW, Sherman BT, Tan Q, Collins JR,
Alvord WG, Roayaei J, Stephens R, Baseler MW, Lane HC and Lempicki
RA: The DAVID gene functional classification tool: A novel
biological module-centric algorithm to functionally analyze large
gene lists. Genome Biol. 8:R1832007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Von Mering C, Huynen M, Jaeggi D, Schmidt
S, Bork P and Snel B: STRING: A database of predicted functional
associations between proteins. Nucleic Acids Res. 31:258–261. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kohl M, Wiese S and Warscheid B:
Cytoscape: Software for visualization and analysis of biological
networks. Methods Mol Biol. 696:291–303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bader GD and Hogue CW: An automated method
for finding molecular complexes in large protein interaction
networks. BMC bioinformatics. 4:22003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X: miRDB: A microRNA target
prediction and functional annotation database with a wiki
interface. RNA. 14:1012–1017. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reynolds N, Fantes PA and MacNeill SA: A
key role for replication factor C in DNA replication checkpoint
function in fission yeast. Nucleic Acids Res. 27:462–469. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Redondo-Muñoz J, Rodríguez MJ, Silió V,
Pérez-García V, Valpuesta JM and Carrera AC: Phosphoinositide
3-kinase beta controls replication factor C assembly and function.
Nucleic Acids Res. 41:855–868. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Loeb LA, Springgate CF and Battula N:
Errors in DNA replication as a basis of malignant changes. Cancer
Res. 34:2311–2321. 1974.PubMed/NCBI
|
|
26
|
Tsaniras S Champeris, Kanellakis N,
Symeonidou IE, Nikolopoulou P, Lygerou Z and Taraviras S: Licensing
of DNA replication, cancer, pluripotency and differentiation: An
interlinked world? Semin Cell Dev Biol. 30:174–180. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
PosthumaDeBoer J, van Royen B and Helder
M: Mechanisms of therapy resistance in osteosarcoma: A review.
Oncol Discov. 1:82013. View Article : Google Scholar
|
|
28
|
Kim MJ, Lee JY and Lee SJ: Transient
suppression of nuclear Cdc2 activity in response to ionizing
radiation. Oncol Rep. 19:1323–1329. 2008.PubMed/NCBI
|
|
29
|
Fu W, Ma L, Chu B, Wang X, Bui MM, Gemmer
J, Altiok S and Pledger WJ: The cyclin-dependent kinase inhibitor
SCH 727965 (dinacliclib) induces the apoptosis of osteosarcoma
cells. Mol Cancer Ther. 10:1018–1027. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Doak SH, Jenkins GJ, Parry EM, Griffiths
AP, Baxter JN and Parry JM: Differential expression of the MAD2,
BUB1 and HSP27 genes in Barrett's oesophagus-their association with
aneuploidy and neoplastic progression. Mutat Res. 547:133–144.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Giantin M, Granato A, Baratto C, Marconato
L, Vascellari M, Morello EM, Vercelli A, Mutinelli F and Dacasto M:
Global gene expression analysis of canine cutaneous mast cell
tumor: Could molecular profiling be useful for subtype
classification and prognostication? PLoS One. 9:e954812014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu L, Guo WC, Zhao SH, Tang J and Chen JL:
Mitotic arrest defective protein 2 expression abnormality and its
clinicopathologic significance in human osteosarcoma. Apmis.
118:222–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Trougakos IP, Chondrogianni N, Amarantos
I, Blake J, Schwager C, Wirkner U, Ansorge W and Gonos ES:
Genome-wide transcriptome profile of the human osteosarcoma Sa OS
and U-2 OS cell lines. Cancer Genet Cytogenet. 196:109–118. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kuijjer ML, Peterse EF, van den Akker BE,
Briaire-de Bruijn IH, Serra M, Meza-Zepeda LA, Myklebost O, Hassan
AB, Hogendoorn PC and Cleton-Jansen AM: IR/IGF1R signaling as
potential target for treatment of high-grade osteosarcoma. BMC
Cancer. 13:2452013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cao ZQ, Shen Z and Huang WY: MicroRNA-802
promotes osteosarcoma cell proliferation by targeting p27. Asian
Pac J Cancer Prev. 14:7081–7084. 2013. View Article : Google Scholar : PubMed/NCBI
|