Introduction
Hypertensive disorders occur in ~7% of all
pregnancies and are the primary factors that lead to maternal-fetal
mortality (1). There is clinical
and experimental evidence suggesting that gestational hypertension
is associated with the induction of acute kidney injury (AKI)
(2,3). A recent literary review indicates
that hypertension during pregnancy (49.2%) and postpartum
hemorrhage (13.8%) are the primary factors resulting in
pregnancy-associated AKI in China (4). Furthermore, gestational hypertension
is associated with proteinuria during pregnancy, which results in
fetal growth restriction and low birth weight (2,5).
Increased proteinuria may become nephrotic at an early stage during
the pregnancy, with an increased risk of intravascular volume
depletion, thromboses, reflux nephropathy and patients are
additionally at a greater risk of developing urinary tract
infections during this period (6).
Therefore, it is important to develop safe and effective
therapeutic drugs to improve AKI and proteinuria during
pregnancy-induced hypertension.
Icariin, the primary active flavonol glucoside in
Epimedium, has been widely used therapeutically, due to its
anti-tumor effects (7). It has
additionally been used in anti-osteoporotic therapy (8,9) and
has been demonstrated to delay cellular senescence (10). Icariin ameliorates chemotherapeutic
drug induced AKI (11) and
protects against 5/6 nephrectomy-induced chronic kidney failure by
increasing the number of renal stem cells in a rat model (12). Furthermore, icariin alleviates high
glucose-induced type IV collagen and fibronectin accumulation in
glomerular mesangial cells (13).
These data suggest that the renoprotective properties of icariin
have been verified by pharmacological experiments in vivo
and in vitro. However, the mechanism of the renoprotection
of icariin in a rat model of pregnancy-induced hypertension has not
been fully elucidated. To the best of our knowledge, this study is
the first to attempt to determine the protective effect of icariin
in a rat model of pregnancy-induced hypertension. The data provide
evidence that icariin improves AKI and proteinuria during pregnancy
accompanied with hypertension.
Materials and methods
Animal treatment
Specific pathogen-free experimental animals were
obtained from Vital River Laboratories Co., Ltd (Beijing, China).
The rats were caged individually under controlled temperature
(23±2°C) and humidity (55±5%) with an artificial 12-h light/dark
cycle, and were given free access to food and tap water. Wistar
rats (40 female, 20 male; age 10–12 weeks; weight 180–220 g) were
used for the present study. All experimental procedures were
carried out in accordance with the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health (14). All protocols were approved by the
Animal Care and Research Committee of Tianjin First Central
Hospital (Tianjin, China; Permit number: E20130825-003A).
Estrous female rats were mated overnight with one
male. The next morning, the presence of a vaginal plug indicated
successful mating and was documented as day 0 of gestation. A total
of 40 female pregnant rats were randomly divided into five groups:
Control pregnant rats, pregnancy-induced hypertension (PIH) rats,
PIH rats + icariin [10 mg/kg, low (L)], PIH rats + icariin [50
mg/kg, medium (M)] and PIH rats + icariin [100 mg/kg, high (H)].
Nitric oxide synthase inhibitor NG-nitro-L-arginine methylester
(L-NAME; Cayman Chemical Company, Ann Arbor, MI, USA); 0.5 g/l
drinking water) was administered from day 12 of gestation to induce
PIH. Icariin was administered intragastrically from day 1 to day 18
of gestation.
Blood pressure measurement
Systolic blood pressure (SBP) and diastolic blood
pressure (DBP) in pregnant rats were measured at day 1 and day 18
of gestation with the CODATM2 non-invasive single
channel blood pressure measuring instrument (Shanghai Zande Medical
Devices Co., Ltd., Shanghai, China).
Urinary protein detection in serum and
plasma
Rats were selected randomly from each group and were
placed in metabolic cages to collect urine for 24 h. The total
volume of urine was recorded and used to detect urinary total
protein and concentration. Urine protein concentration was measured
using the Coomassie Brilliant Blue method, serum creatinine was
measured using the picric acid method and blood urea nitrogen (BUN)
was measured via an enzymatic kinetic method using commercial kits
purchased from Nanjing Jiancheng Biological Engineering Research
Institute (Nanjing, China). Plasma levels of angiotensin II (Ang
II) were detected using a bioactive Ang II ELISA assay (catalog no.
C506065; Sangon Biotech, Co., Ltd., Shanghai, China), according to
the manufacturer's protocol.
Hematoxylin & eosin (H&E) and
immunohistochemical staining
Kidney tissues were collected at day 18 of gestation
by intraperitoneal injection of sodium pentobarbital (2%; 200
mg/kg; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and were
fixed with 4% formalin at room temperature for 24 h and
paraffin-embedded. Tissues were then cut into ~5 µm-thick sections,
which were stained with H&E at room temperature for 1–2 min and
visualized under a microscope (Leica DM 2500; Leica Microsystems
GmbH, Wetzlar, Germany).
The immunohistochemical staining analysis of kidney
tissue was evaluated using anti-rat Ang II (catalog no. sc-20718;
1:50; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). In brief,
the paraffin sections (5 µm) were heated in an oven at 65°C for 24
h, dewaxed with water and rinsed with PBS for 5 min, 3 times.
Paraffin sections were then placed in EDTA buffer (Beyotime
Institute of Biotechnology, Haimen, China) for microwave antigen
retrieval and boiled for 10 min. Following natural cooling, the
sections were washed with PBS 3 times. The sections were placed
into 3% hydrogen peroxide solution and incubated at room
temperature for 10 min, to block endogenous peroxidase activity,
and then washed with PBS 3 times. They were then blocked with 5%
bovine serum albumin (BSA; Beyotime Institute of Biotechnology) for
20 min at room temperature, following drying. Following removal of
BSA liquid, each section was incubated with 50 µl diluted anti-rat
Ang II primary antibody overnight at 4°C, then washed with PBS 3
times. Following the removal of PBS liquid, each slice was
incubated with 50–100 µl goat anti-rabbit horseradish
peroxidase-conjugated secondary antibody (catalog no. sc-2004;
1:2,000; Santa Cruz Biotechnology, Inc.) at 4°C for 50 min, then
washed with PBS 3 times. A total of 50–100 µl freshly prepared DAB
solution was added to each sample. Following washing, sections were
counterstained with hematoxylin at room temperature for 5 min,
rinsed with tap water, dehydrated, mounted and visualized under a
microscope (Leica DM 2500; Leica Microsystems GmbH).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The renal total RNA extraction was performed using
TRIzol®, according to the manufacturer's protocol
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Synthesis of cDNAs was performed by RT reactions with 2 µg total
RNA using moloney murine leukemia virus reverse transcriptase
(Invitrogen; Thermo Fisher Scientific, Inc.) with oligo dT 15
primers (Fermentas; Thermo Fisher Scientific, Inc.) and 4 µl Maxima
5X Reaction Mix (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The RT temperature
protocol was as follows: 37°C for 50 min and 70°C for 15 min. The
first strand cDNAs served as the template for the PCR, performed
using a DNA Engine (ABI 7300; Thermo Fisher Scientific, Inc.).
GAPDH served as an internal control, and was used to normalize the
data to determine the relative expression of the target genes using
the 2−∆∆Cq method (15). Reaction mixtures (20 µl) were
prepared using the TaqMan Universal PCR Master Mix (Thermo Fisher
Scientific, Inc.). The reaction conditions were set according to
the manufacturer's protocol. The PCR primers used in this study
were as follows: Forward, 5′-AGCTCGTGTCTCCCAGAGT-3′ and reverse,
5′-CGTTCACGTTTGCAGAGATGT-3′ for nephrin; forward,
5′-CTGGAGCTAAAGGACACACAGA-3′ and reverse,
5′-GTGAAGGGACCCAAGCTCTC-3′ for angiotensinogen (AGT) and forward,
5′-GGATTTGGTCGTATTGGG-3′ and reverse, 5′-GGAAGATGGTGATGGGATT-3′ for
GAPDH.
Western blotting
The kidney was homogenized and protein extracted
using NP-40 buffer (Beyotime Institute of Biotechnology), followed
by 5–10 min boiling and centrifugation (4°C, 10 min, 12,000 × g) to
obtain the supernatant. Protein concentrations were determined
using the Bicinchoninic Acid kit for Protein Determination
(Sigma-Aldrich; Merck KGaA). Samples containing 50 µg protein were
separated on 10% SDS-PAGE gel and transferred to nitrocellulose
membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Following saturation with 5% (w/v) non-fat dry milk in TBS and 0.1%
(w/v) Tween-20 (TBST), the membranes were incubated with the
following primary antibodies, against nephrin (catalog no.
sc-377246; 1:1,000; Santa Cruz Biotechnology, Inc.) and Ang II
(catalog no. sc-20718; 1:500; Santa Cruz Biotechnology, Inc.) at
4°C overnight. Following 3 washes with TBST, membranes were
incubated with secondary immunoglobulins (donkey anti-goat IgG;
catalog no. sc-2020; 1:10,000; Santa Cruz Biotechnology, Inc.)
conjugated to IRDye 800 CW Infrared Dye (LI-COR Biosciences,
Lincoln, NE, USA). Following a 2 h incubation period at 37°C,
membranes were washed 3 times with TBST. Blots were visualized
using the Odyssey Infrared Imaging System (LI-COR Biosciences).
Signals were densitometrically assessed (Odyssey Application
Software, version 3.0; LI-COR Biosciences) and normalized to the
GAPDH signals to correct for unequal loading (catalog no.
sc-365062; 1:2,000; Santa Cruz Biotechnology, Inc.).
Statistical analysis
The data from these experiments are presented as the
mean ± standard deviation for each group. All statistical analyses
were performed using PRISM version 4.0 (GraphPad Software, Inc., La
Jolla, CA, USA). Inter-group differences were analyzed using
one-way analysis of variance followed by Tukey's multiple
comparison test as a post-hoc test to compare the group means.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Icariin regulates systolic blood
pressure in PIH rats
Firstly, the SBP and DBP were measured in the
control, PIH and icariin treated rats, and it was demonstrated that
no significant differences were present in SBP (Fig. 1A) and DBP (Fig. 1B) among the five experimental
groups on day 1. However, SBP and DBP were significantly increased
in the PIH group compared with control group on day 18. The
significant increase in SBP in PIH rats was prevented by icariin at
a high concentration on day 18. However, icariin administration had
no effect on DBP compared with PIH group on day 18 (Fig. 1C and D).
Icariin improves proteinuria in PIH
rats
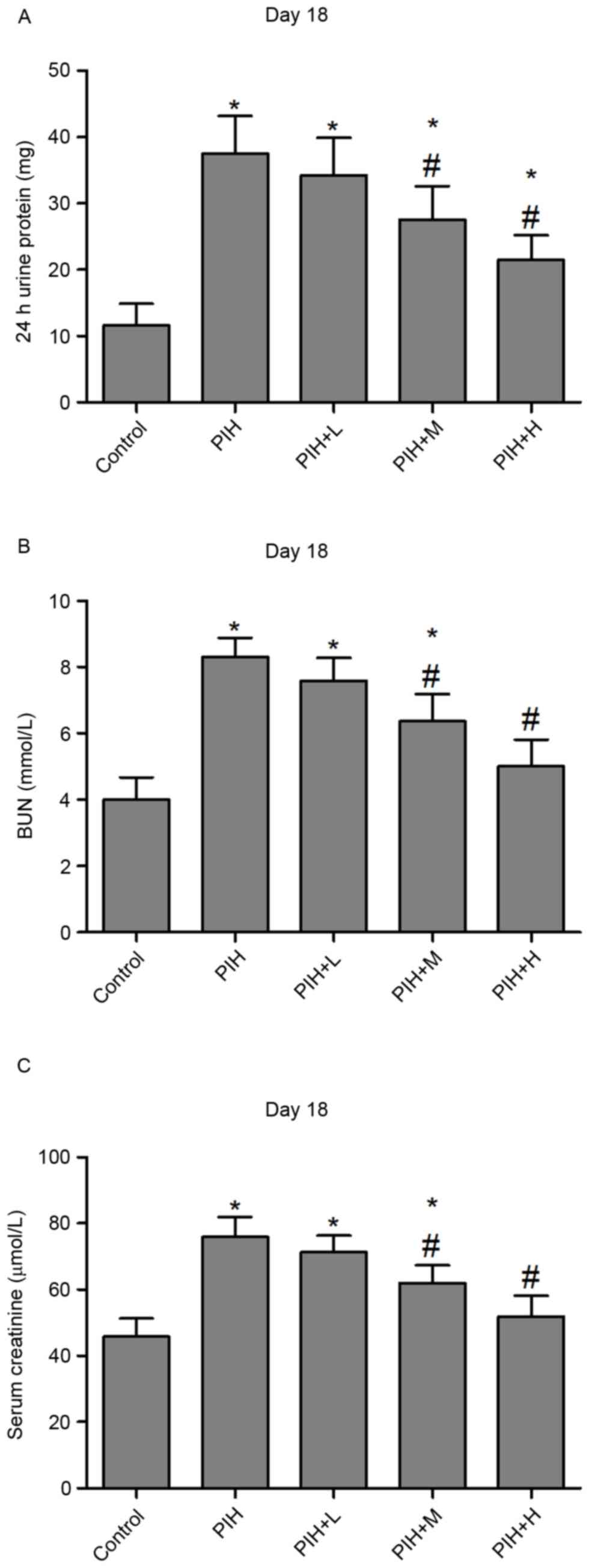
The urinary protein excretion increased ~4-fold in
PIH rats compared with control pregnant rats (Fig. 2A). However, icariin administration
reversed increased urinary protein in PIH rats at medium and high
concentrations on day 18 (Fig.
2A). Furthermore, BUN and serum creatinine were significantly
elevated in PIH rats compared with control pregnant rats. BUN and
serum creatinine levels in PIH rats were attenuated following
treatment with icariin at medium and high concentrations on day 18
(Fig. 2B and C).
Icariin improves reproductive
performance in PIH rats
Litters from PIH rats were significantly smaller in
number compared with control pregnant rats, and icariin prevented
the reduction in litter number induced by PIH (Fig. 3A). Furthermore, L-NAME
administration (PIH group) resulted in a reduction in the average
weight of the pup, however, icariin administration did not result
in any significant weight gain of the pups during pregnancy
(Fig. 3B).
Icariin alleviates the pathological
alterations of the kidney in PIH rats
H&E staining demonstrated that L-NAME
administration resulted in severe mesangial expansion and
significant basement membrane thickening, however, kidney
morphology was well preserved in control pregnant rats. Compared
with the PIH group, rats with L-NAME-induced PIH treated with
icariin (50 or 100 mg/kg) exhibited markedly reduced severity of
glomerular lesions (Fig. 4A). The
podocyte protein nephrin is essential for maintaining the
filtration barrier of the kidney and preventing proteinuria
(16). A previous study indicated
that glomerular expression of nephrin is decreased in kidney
sections from women with pre-eclampsia (17). In the present study, the expression
of nephrin was measured in the kidney from control pregnant and
L-NAME administered PIH rats. The results demonstrated that L-NAME
administration exerted a marked decrease in the mRNA and protein
expression of nephrin in the kidneys, compared with the control
group. However, icariin (50 or 100 mg/kg) treatment significantly
reversed L-NAME-induced downregulation of nephrin levels in the
kidney (Fig. 4B and C).
Icariin inhibits Ang II activity in
PIH rats
Previous studies suggest that the circulating and
local renin-angiotensin systems (RAS) are activated during rat
pregnancy (18,19). However, the role of local RAS
activity in the kidney of PIH rats remains to be elucidated. The
present study investigated the renoprotective effects of icariin in
PIH rats, and the association between icariin and the local renal
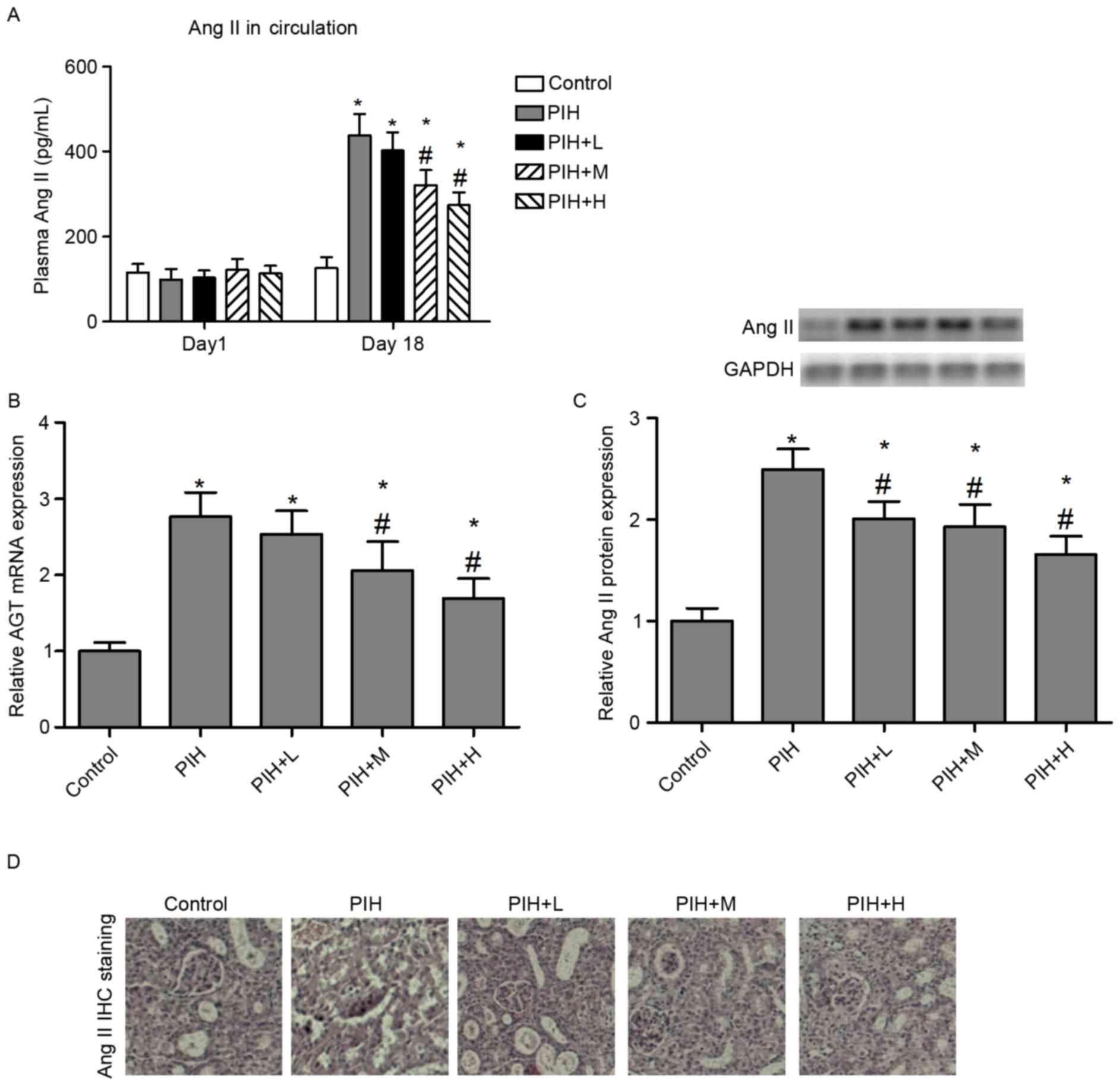
RAS in the progression of PIH was examined. The expression of Ang
II, as a key active peptide in the RAS, was measured in the kidney
of control pregnant and PIH rats. The results demonstrated that
circulating Ang II and AGT mRNA expression levels were
significantly elevated in PIH rats compared with control pregnant
rats. Rats that received icariin at the two greater doses (50 or
100 mg/kg), exhibited alleviated circulating Ang II levels
(Fig. 5A) and AGT mRNA expression
(Fig. 5B), compared with the PIH
group. Furthermore, the protein expression of Ang II was
significantly increased in the kidneys of PIH rats compared with
control group. However, icariin administration significantly
reversed the L-NAME-induced upregulation of Ang II levels in the
kidney (Fig. 5C). These effects of
icariin on Ang II expression were verified by immunohistochemical
staining (Fig. 5D).
Discussion
In the present study, the urinary protein excretion
was significantly increased in PIH rats, and PIH-induced AKI
resulted in profound renal histological alterations, including
mesangial expansion and glomerular lesions, accompanied by
increased BUN and serum creatinine. The protective effects of
icariin in PIH-induced nephropathy were assessed. The results of
the present study demonstrated that icariin administration
suppressed urinary protein excretion and renal tissue damage in PIH
rats. Furthermore, upregulation of circulating and renal Ang II
levels in PIH rats appeared to be reversed by icariin
administration.
Pregnancy-induced hypertension has been accompanied
by renal injury and proteinuria. Renal regeneration is at the
center of treating AKI and other renal diseases (12). Previous studies suggest that
icariin possesses the ability to promote the regeneration and
differentiation of nephrocytes (12,20).
The present study hypothesized that icariin may improve PIH-induced
renal injury and proteinuria. Histomorphological examinations
demonstrated that icariin markedly improved glomerular lesions and
renal interstitial fibrosis in PIH rats. Increased levels of BUN
and serum creatinine in PIH rats were significantly reduced by
icariin administration. Notably, icariin treatment significantly
reversed the downregulation of nephrin mRNA and protein expression
in the kidneys of PIH rats. Nephrin, a cytoskeletal protein which
localizes to the slit pore of podocytes, may be important in
proteinuria (21). An experimental
model of diabetes and hypertension suggests that downregulation of
nephrin levels in the kidney is associated with the development of
albuminuria (22). The nephrin
gene is specifically expressed in the kidney, brain and pancreas of
rodents, and inactivation of nephrin results in proteinuria and
neonatal death (23). Nephrin as a
biomarker reflects podocyte dysfunction in various kidney disease
models (24). The present study
verified that L-NAME treatment in pregnant rats resulted in AKI,
evidenced by severe proteinuria and BUN and serum creatinine
elevation, which may potentially be associated with a decrease in
nephrin expression in the kidney. Icariin suppressed the elevation
of proteinuria, BUN and serum creatinine and increased the mRNA and
protein expression levels of nephrin. The renoprotective effects of
icariin may therefore be mediated via the upregulation of nephrin
levels in the kidney.
RAS activation has previously been demonstrated to
be important in the pathology of hypertension (25). However, its involvement in the
pathogenesis of PIH remains to be fully elucidated. A previous
study indicates that pregnant women with high susceptibility to
increased Ang II levels had a high risk of developing pre-eclampsia
(26). Notably, the circulatory
RAS in pre-eclampsia appears to be suppressed (27), however the underlying mechanisms
remain to be investigated. Various animal models demonstrate an
increase in circulating maternal renin during gestation (28). A continuous infusion of high-dose
Ang II during pregnancy in mice induces hypertension, proteinuria,
intrauterine growth restriction and kidney injury (29). Consistent with the previous
observations in rat pregnancy, the present study demonstrated that
circulating and local renal Ang II expression were significantly
elevated in PIH rats compared with control pregnant rats. These
results suggest that increased Ang II activity may promote PIH in
pregnant rats. Icariin administration significantly reversed the
upregulation of Ang II levels in the plasma and kidney of PIH
rats.
In conclusion, the findings of the present study
suggest that icariin improves proteinuria and renal injury, and the
underlying mechanism is mediated, in part, via the upregulation of
nephrin expression and downregulation of Ang II. These results may
therefore provide a novel therapeutic strategy for the treatment of
PIH in the future.
Acknowledgements
The present study was supported by the Science and
Technology Fund of Tianjin Municipal Health Bureau (grant no.
2013KZ024).
References
|
1
|
Veerbeek JH, Hermes W, Breimer AY, van
Rijn BB, Koenen SV, Mol BW, Franx A, de Groot CJ and Koster MP:
Cardiovascular disease risk factors after early-onset preeclampsia,
late-onset preeclampsia and pregnancy-induced hypertension.
Hypertension. 65:600–606. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shortliffe LM, Hammam O, Han X, Kouba E,
Tsao PS and Wang B: Dietary fructose in pregnancy induces
hyperglycemia, hypertension, and pathologic kidney and liver
changes in a rodent model. Pregnancy Hypertens. 5:308–314.
2015.PubMed/NCBI
|
|
3
|
Kendrick J, Sharma S, Holmen J, Palit S,
Nuccio E and Chonchol M: Kidney disease and maternal and fetal
outcomes in pregnancy. Am J Kidney Dis. 66:55–59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu YM, Bao HD, Jiang ZZ, Huang YJ and
Wang NS: Pregnancy-related acute kidney injury and a review of the
literature in China. Intern Med. 54:1695–1703. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carty DM, Delles C and Dominiczak AF:
Preeclampsia and future maternal health. J Hypertens. 28:1349–1355.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haase N, Golic M, Herse F, Rugor J, Linz
D, Solano ME, Müller DN and Dechend R: Relaxin treatment in an
Ang-II-based transgenic preeclamptic-rat model. PLoS One.
11:e01507432016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fan C, Yang Y, Liu Y, Jiang S, Di S, Hu W,
Ma Z, Li T, Zhu Y and Xin Z: Icariin displays anticancer activity
against human esophageal cancer cells via regulating endoplasmic
reticulum stress-mediated apoptotic signaling. Sci Rep.
6:211452016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang J, Tao Y, Ping Z, Zhang W, Hu X, Wang
Y, Wang L, Shi J, Wu X and Yang H: Icariin attenuates
titanium-particle inhibition of bone formation by activating the
Wnt/β-catenin signaling pathway in vivo and in vitro. Sci Rep.
6:238272016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang G, Qin L and Shi Y:
Epimedium-derived phytoestrogen flavonoids exert beneficial effect
on preventing bone loss in late postmenopausal women: A 24-month
randomized, double-blind and placebo-controlled trial. J Bone Miner
Res. 22:1072–1079. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiao-Hong D, Chang-Qin X, Jian-Hua H,
Wen-Jiang Z and Bing S: Icariin delays homocysteine-induced
endothelial cellular senescence involving activation of the
PI3K/AKT-eNOS signaling pathway. Pharm Biol. 51:433–440. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma P, Zhang S, Su X, Qiu G and Wu Z:
Protective effects of icariin on cisplatin-induced acute renal
injury in mice. Am J Transl Res. 7:2105–2114. 2015.PubMed/NCBI
|
|
12
|
Huang Z, He L, Huang D, Lei S and Gao J:
Icariin protects rats against 5/6 nephrectomy-induced chronic
kidney failure by increasing the number of renal stem cells. BMC
Complement Altern Med. 15:3782015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li YC, Ding XS, Li HM and Zhang C: Icariin
attenuates high glucose-induced type IV collagen and fibronectin
accumulation in glomerular mesangial cells by inhibiting
transforming growth factor-β production and signalling through G
protein-coupled oestrogen receptor 1. Clin Exp Pharmacol Physiol.
40:635–643. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
National Research Council (US) Committee
for the Update of the Guide for the C and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th.
National Academies Press (US); Washington (DC): 2011
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin CL, Lee PH, Hsu YC, Lei CC, Ko JY,
Chuang PC, Huang YT, Wang SY, Wu SL and Chen YS: MicroRNA-29a
promotion of nephrin acetylation ameliorates hyperglycemia-induced
podocyte dysfunction. J Am Soc Nephrol. 25:1698–1709. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Garovic VD, Wagner SJ, Petrovic LM, Gray
CE, Hall P, Sugimoto H, Kalluri R and Grande JP: Glomerular
expression of nephrin and synaptopodin, but not podocin, is
decreased in kidney sections from women with preeclampsia. Nephrol
Dial Transplant. 22:1136–1143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hering L, Herse F, Geusens N, Verlohren S,
Wenzel K, Staff AC, Brosnihan KB, Huppertz B, Luft FC and Muller
DN: Effects of circulating and local uteroplacental angiotensin II
in rat pregnancy. Hypertension. 56:311–318. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shah DM: Role of the renin-angiotensin
system in the pathogenesis of preeclampsia. Am J Physiol Renal
Physiol. 288:F614–625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang JH, Cai WJ, Zhang XM and Shen ZY:
Icariin promotes self-renewal of neural stem cells: An involvement
of extracellular regulated kinase signaling pathway. Chin J Integr
Med. 20:107–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takahashi A, Fukusumi Y, Yamazaki M,
Kayaba M, Kitazawa Y, Tomita M and Kawachi H: Angiotensin II type 1
receptor blockade ameliorates proteinuria in puromycin
aminonucleoside nephropathy by inhibiting the reduction of NEPH1
and nephrin. J Nephrol. 27:627–634. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Forbes JM, Bonnet F, Russo LM, Burns WC,
Cao Z, Candido R, Kawachi H, Allen TJ, Cooper ME, Jerums G and
Osicka TM: Modulation of nephrin in the diabetic kidney:
Association with systemic hypertension and increasing albuminuria.
J Hypertens. 20:985–992. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Putaala H, Soininen R, Kilpeläinen P,
Wartiovaara J and Tryggvason K: The murine nephrin gene is
specifically expressed in kidney, brain and pancreas: Inactivation
of the gene leads to massive proteinuria and neonatal death. Hum
Mol Genet. 10:1–8. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wada Y, Abe M, Moritani H, Mitori H, Kondo
M, Tanaka-Amino K, Eguchi M, Imasato A, Inoki Y and Kajiyama H:
Original research: Potential of urinary nephrin as a biomarker
reflecting podocyte dysfunction in various kidney disease models.
Exp Biol Med (Maywood). 241:1865–1876. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Solomon SD, Janardhanan R, Verma A,
Bourgoun M, Daley WL, Purkayastha D, Lacourcière Y, Hippler SE,
Fields H and Naqvi TZ: Effect of angiotensin receptor blockade and
antihypertensive drugs on diastolic function in patients with
hypertension and diastolic dysfunction: A randomised trial. Lancet.
369:2079–2087. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seki H: The role of the renin-angiotensin
system in the pathogenesis of preeclampsia - new insights into the
renin-angiotensin system in preeclampsia. Med Hypotheses.
82:362–367. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weir RJ, Paintin DB, Brown JJ, Fraser R,
Lever AF, Robertson JI and Young J: A serial study in pregnancy of
the plasma concentrations of renin, corticosteroids, electrolytes
and proteins and of haematocrit and plasma volume. J Obstet
Gynaecol Br Commonw. 78:590–602. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Irani RA and Xia Y: Renin angiotensin
signaling in normal pregnancy and preeclampsia. Semin Nephrol.
31:47–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shirasuna K, Karasawa T, Usui F, Kobayashi
M, Komada T, Kimura H, Kawashima A, Ohkuchi A, Taniguchi S and
Takahashi M: NLRP3 deficiency improves angiotensin II-induced
hypertension but not fetal growth restriction during pregnancy.
Endocrinology. 156:4281–4292. 2015. View Article : Google Scholar : PubMed/NCBI
|